Shade-Induced Effects on Essential Oil Yield, Chemical Profiling, and Biological Activity in Some Lamiaceae Plants Cultivated in Serbia
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material and Growing Conditions
2.2. Clevenger-Hydrodistillation
2.3. Gas Chromatography/Mass Spectrometry (GC/MS) and Gas Chromatography/Flame Ionization Detection (GC/FID) Analysis
2.4. DPPH Assay
2.5. Antimicrobial Activity
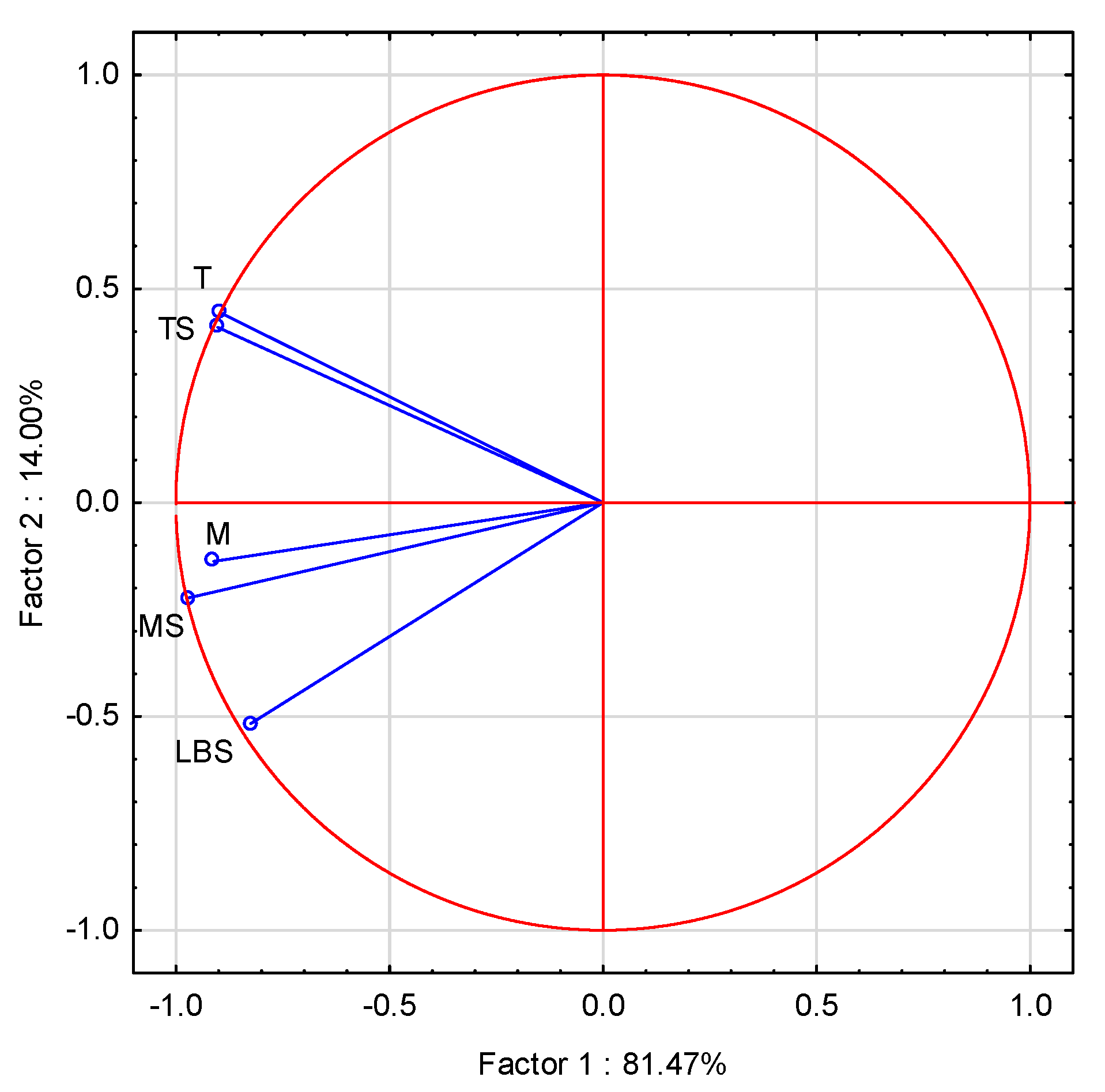
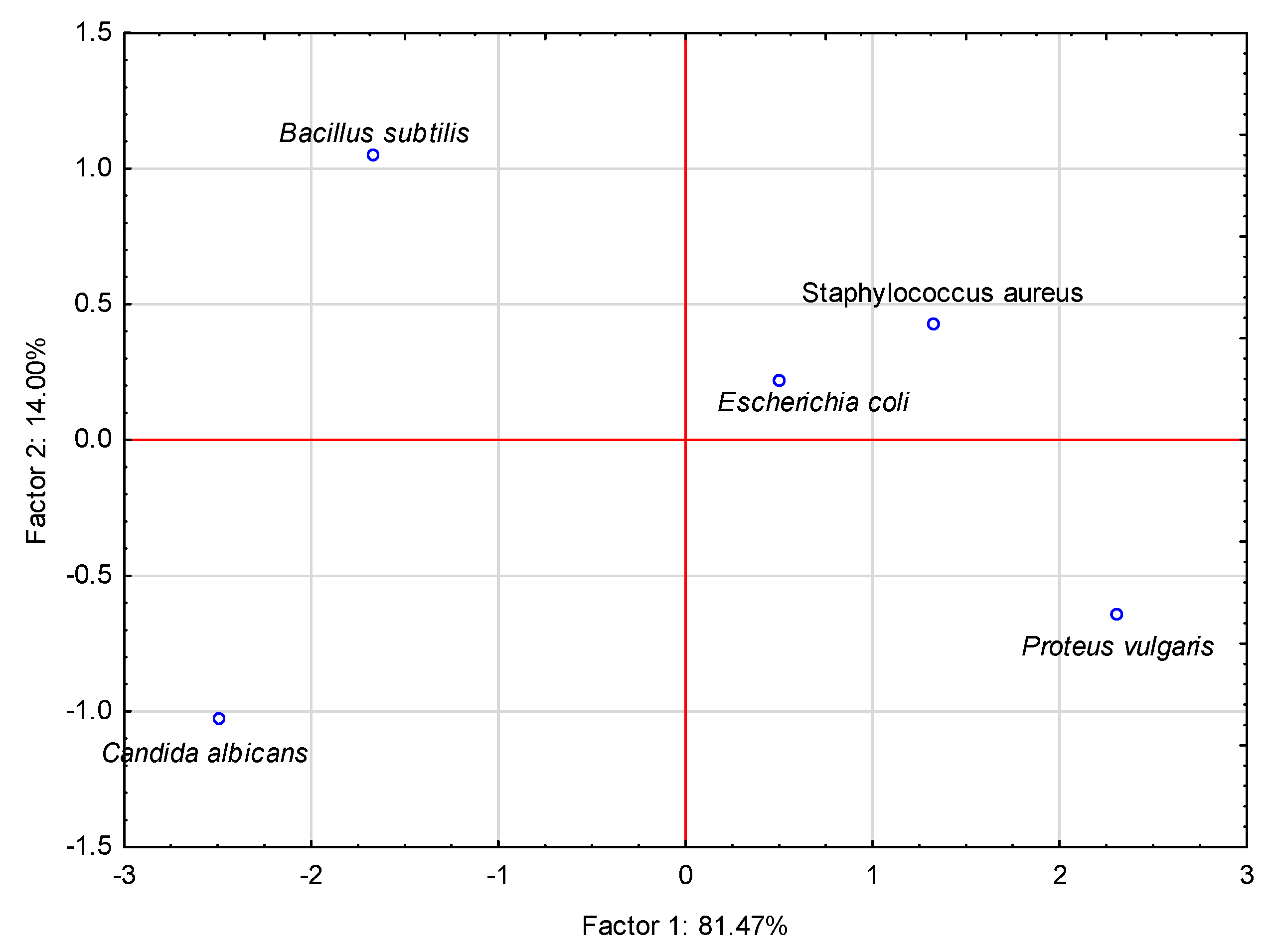
2.6. Statistical Methods
3. Results and Discussion
3.1. Climatic Condition
3.2. Yield of Essential Oils
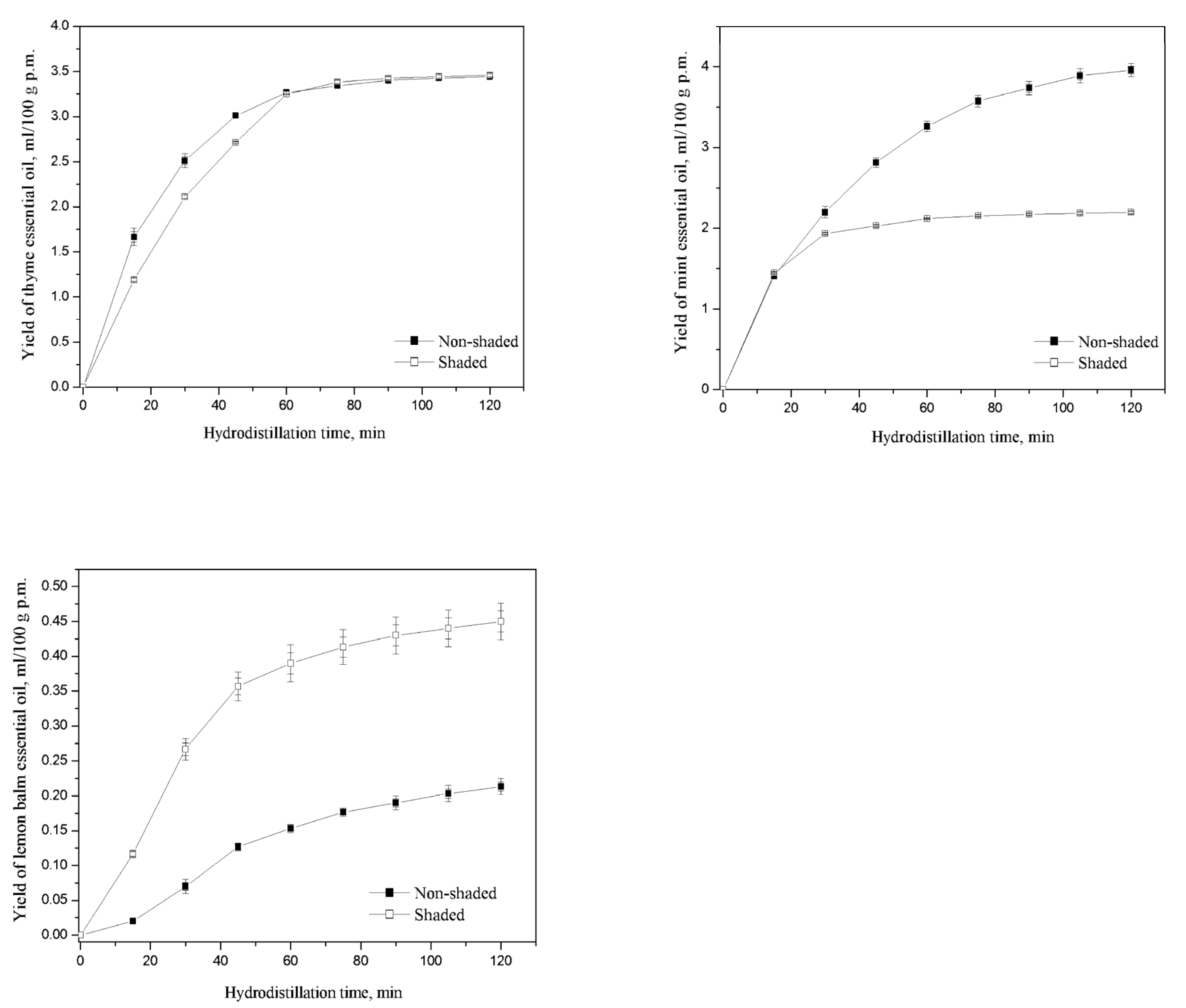
3.2.1. Thyme Essential Oil (TEO) Yield
3.2.2. Mint Essential Oil (MEO) Yield
3.2.3. Lemon Balm Essential Oil (LEO) Yield
3.3. Essential Oils Composition
3.3.1. Thyme Essential Oil (TEO) Composition
3.3.2. Mint Essential Oils (MEOs) Composition
3.3.3. Lemon Balm Essential Oil (LEO) Composition
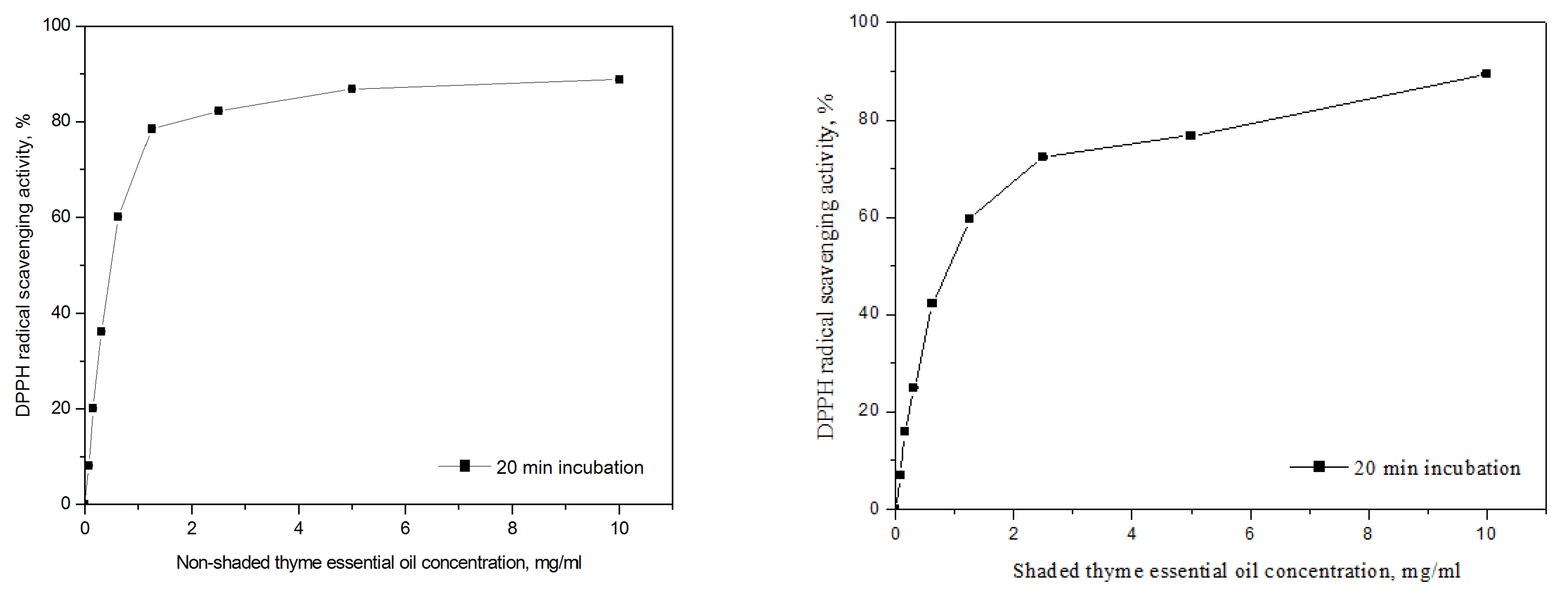
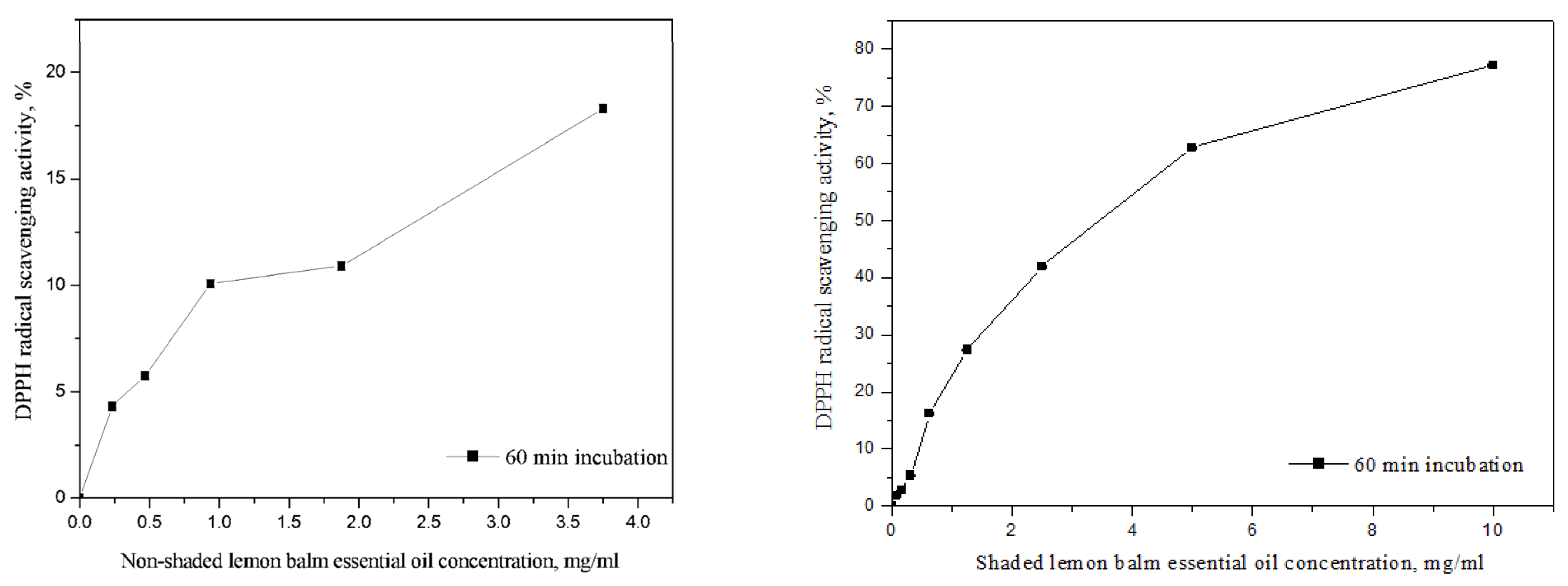
3.4. Antioxidant Activity
3.5. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tmušić, N.; Ilić, Z.S.; Milenković, L.; Šunić, L.; Lalević, D.; Kevrešan, Ž.; Mastilović, J.; Stanojević, L.; Cvetković, D. Shading of medical plants affects the phytochemical quality of herbal extracts. Horticulturae 2021, 7, 437. [Google Scholar] [CrossRef]
- Stojanović, N.M.; Samardžić, L.; Ranđelović, P.J.; Radulović, N.S. Prevalence of self-medication practice with herbal products among nonpsychiatric patients from southearstern Serbia: A cross-secional study. Saudi Pharm. J. 2017, 25, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Ait Bouzid, A.; Oubannin, S.; Ibourki, M.; Bijla, L.; Hamdouch, A.; Sakar, E.H.; Harhar, H.; Majourhat, K.; Koubachi, J.; Gharby, S. Comparative evaluation of chemical composition, antioxidant capacity, and some contaminants in six Moroccan medicinal and aromatic plants. Biocatal. Agric. Biotechnol. 2023, 47, 102569. [Google Scholar] [CrossRef]
- Galovičová, L.; Borotová, P.; Valková, V.; Vukovic, N.L.; Vukic, M.; Štefániková, J.; Ďúranová, H.; Kowalczewski, P.Ł.; Čmiková, N.; Kačániová, M. Thymus vulgaris essential oil and its biological activity. Plants 2021, 19, 1959. [Google Scholar] [CrossRef] [PubMed]
- Efe Ertürk, N.; Ta¸scı, S. The Effects of peppermint oil on nausea, vomiting and retching in cancer patients undergoing chemotherapy: An open label quasi-randomized controlled pilot study. Complement. Ther. Med. 2021, 56, 102587. [Google Scholar] [CrossRef]
- Petrisor, G.; Motelica, L.; Craciun, L.N.; Oprea, O.C.; Ficai, D.; Ficai, A. Melissa officinalis: Composition, pharmacological effects and derived release systems-A review. Int. J. Mol. Sci. 2022, 23, 3591. [Google Scholar] [CrossRef]
- Ilić, S.Z.; Milenković, L.; Tmušić, N.; Stanojević, L.J.; Cvetković, D. Essential oils content, composition and antioxidant activity of lemon balm, mint and sweet basil from Serbia. LWT Food Sci. Technol. 2022, 153, 112210. [Google Scholar] [CrossRef]
- Milenković, L.; Stanojević, J.; Cvetković, D.; Stanojević, L.; Lalević, D.; Šunić, L.; Fallik, E.; Ilić, S.Z. New technology in basil production with high essential oil yield and quality. Ind. Crops Prod. 2019, 140, 111718. [Google Scholar] [CrossRef]
- Ilić, Z.; Stanojević, L.; Milenković, L.; Šunić, L.; Milenković, A.; Stanojević, J.; Cvetković, D. The yield, chemical composition, and antioxidant activities of essential oils from different plant parts of the wild and cultivated oregano (Origanum vulgare L.). Horticulturae 2022, 8, 1042. [Google Scholar] [CrossRef]
- Milenković, L.; Ilić, S.Z.; Šunić, L.; Tmušić, N.; Stanojević, L.; Cvetković, D. Modification of light intensity influence essential oils content, composition and antioxidant activity of thyme, marjoram and oregano. Saudi J. Biol. Sci. 2021, 28, 6532–6543. [Google Scholar] [CrossRef]
- Giannoulis, K.D.; Kamvoukou, C.A.; Gougoulias, N.; Wogiatzi, E. Matricaria chamomilla L. (German chamomile) flower yield and essential oil affected by irrigation and nitrogen fertilization. Emir. J. Food Agric. 2020, 32, 328–335. [Google Scholar] [CrossRef]
- Ninou, E.; Cook, C.M.; Papathanasiou, F.; Aschonitis, V.; Avdikos, I.; Tsivelikas, A.L.; Stefanou, S.; Ralli, P.; Mylonas, I. Nitrogen effects on the essential oil and biomass production of field grown Greek oregano (Origanum vulgare subsp. hirtum) populations. Agronomy 2021, 11, 1722. [Google Scholar] [CrossRef]
- Pino, J.A.; Rosado, A.; Fuentes, V. Composition of the Essential Oil of Melissa officinalis L. from Cuba. J. Essen Oil Res. 1999, 11, 363–364. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, I.; Silva-Espinoza, B.A.; Ortega-Ramirez, L.A.; Leyva, J.M.; Siddiqui, M.W.; Cruz-Valenzuela, M.R.; Gonzalez- 454 Aguilar, G.A.; Ayala-Zavala, J.F. Oregano essential oil as an antimicrobial and antioxidant additive in food products. Crit. Rev. Food Sci. Nutr. 2016, 56, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Milenković, L.; Šunić, L.J.; Mastilović, J.; Kevrešan, Ž.; Kovač, R.; Cvetković, D.; Stanojević, L.; Danilović, B.; Stanojević, J.; Ilić, S.Z. Antimicrobial activity of essential oils from medical plants grown in light modified environment. In Proceedings of the Food, Nutrients and Nutrition of the Future: 31st Food Technology Days 2022 dedicated to Prof. F. Bitenc, Ljubljana, Slovenia, 15 June 2022; pp. 73–89. [Google Scholar]
- Shanaida, M.; Hudz, N.; Białon, M.; Kryvtsowa, M.; Svydenko, L.; Filipska, A.; Wieczorek, P. Chromatographic profiles and antimicrobial activity of the essential oils obtained from some species and cultivars of the Mentheae tribe (Lamiaceae). Saudi J. Biol. Sci. 2021, 28, 6145–6152. [Google Scholar] [CrossRef]
- Kosakowska, O.; Czupa, W. Morphological and chemical variability of common oregano (Origanum vulgare L. subsp. vulgare) occurring in eastern Poland. Herba Polon. 2013, 64, 11–21. [Google Scholar] [CrossRef]
- Ilić, S.Z.; Milenković, L.; Šunić, L.; Tmušić, N.; Mastilović, J.; Kevrešan, Ž.; Stanojević, L.; Danilović, B.; Stanojević, J. Efficiency of basil essential oil antimicrobial agents under different shading treatments and harvest times. Agronomy 2021, 11, 1574. [Google Scholar] [CrossRef]
- Helal, I.M.; El-Bessoumy, A.; Al-Bataineh, E.; Joseph, M.R.P.; Rajagopalan, P.; Chandramoorthy, H.C.; Ben Hadj Ahmed, S. Antimicrobial efficiency of essential oils from traditional medicinal plants of Asir Region, Saudi Arabia, over drug resistant isolates. BioMed Res. Int. 2019, 2019, 8928306. [Google Scholar] [CrossRef]
- Sparkman, D.O.; Penton, Z.E.; Fulton, K.G. Gas Chromatography and Mass Spectrometry: A Practical Guide, 2nd ed.; Elsevier Inc.: Oxford, UK, 2011. [Google Scholar]
- Stanojević, J.S.; Stanojević, L.P.; Cvetković, D.J.; Danilović, B.R. Chemical composition, antioxidant and antimicrobial activity of the turmeric essential oil (Curcuma longa L.). Adv. Technol. 2015, 4, 19–25. [Google Scholar] [CrossRef]
- Stanojevic, L.J.; Marjanovic-Balaban, Z.; Kalaba, V.; Stanojevic, J.; Cvetkovic, D.; Cakic, M. Chemical composition, antioxidant and antimicrobial activity of basil (Ocimum basilicum L.) essential oil. J. Essent. Oil Bear. Plants 2017, 20, 1557–1569. [Google Scholar] [CrossRef]
- Kiehlbauch, J.A.; Hannett, G.E.; Salfinger, M.; Archinal, W.; Monserrat, C.; Carlin, C. Use of the national committee for clinical laboratory standards guidelines for disk diffusion susceptibility testing in New York State Laboratories. J. Clin. Microb. 2000, 38, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- Hudaib, M.; Aburjai, T. Volatile components of Thymus vulgaris L. from wild-growing and cultivated plants in Jordan. Flavour Fragr. J. 2007, 22, 322–327. [Google Scholar] [CrossRef]
- Choi, I.Y.; Song, Y.J.; Choi, D.C.; Lee, W.H. A comparative study for obtaining maximum essential oil from six herbs on the basis of harvesting time, cultivation regions & type, and drying methods. Korean J. Hortic. Sci. Technol. 2010, 28, 492–496. [Google Scholar]
- Zani, F.; Massimo, G.; Benvenuti, S.; Bianchi, A.; Albasini, A.; Melegari, M.; Vampa, G.; Bellotti, A.; Mazza, P. Studies on the genotoxic properties of essential oils with Bacillus subtilis recassay and salmonella/microsome reversion assay. Planta Med. 1991, 57, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Cioni, P.L.; Tomei, P.E.; Catalano, S.; Morelli, I. Study of variation in individual essential oils in a micropopulation of Thymus vulgaris L. plants. Riv. Ital. 1990, 1, 3–6. [Google Scholar]
- Najar, B.; Pistelli, L.; Ferri, B.; Angelini, L.G.; Tavarini, S. Crop yield and essential oil composition of two Thymus vulgaris chemotypes along three years of organic cultivation in a hilly area of central Italy. Molecules. 2021, 23, 5109. [Google Scholar] [CrossRef]
- Andolfi, L.; Flamini, G.; Ceccarini, L.; Cioni, P.L.; Macchia, M.; Morelli, I. Indagine agronomica su un ecotipo di Thymus vulgaris L. e composizione dell’olio essenziale. Agric. Ric. 2000, 186, 3–10. [Google Scholar]
- Ozguven, M.; Tansi, S. Drug yield and essential oil of Thymus vulgaris L. as in influenced by ecological and onto genetical variation. Turk. J. Agric. For. 1998, 22, 537–542. [Google Scholar]
- Carlen, C.; Schaller, M.; Carron, C.A.; Vouillamoz, J.F.; Baroffio, C.A. The new Thymus vulgaris L. hybrid cultivar (Varico 3) compared to five established cultivars from Germany, France and Switzerland. Acta Hort. 2018, 60, 161–166. [Google Scholar] [CrossRef]
- Borugă, O.; Jianu, C.; Mişcă, C.; Goleţ, I.; Gruia, A.T.; Horhat, F.G. Thymus vulgaris essential oil: Chemical composition and antimicrobial activity. J. Med. Life 2014, 7, 56–60. [Google Scholar]
- Iscan, G.; KIrimer, N.; Kürkcüoglu, M.; Baser, H.C.; Demirci, F. Antimicrobial screening of Mentha piperita essential oils. J. Agric. Food Chem. 2002, 50, 3943–3946. [Google Scholar] [CrossRef] [PubMed]
- Németh-Zámboriné, É.; Seidler-Łożykowska, K.; Szabó, K. Effect of harvest date on yield and secondary compounds of lemon balm (Melissa officinalis L.). J. Appl. Bot. Food Qual. 2019, 92, 81–87. [Google Scholar]
- Patora, J.; Majda, T.; Gora, J.; Klimek, B. Variability in the content and composition of essential oil from lemon balm (Melissa officinalis L.) cultivated in Poland. J. Endocrinol. Invest. 2003, 26, 950–955. [Google Scholar]
- Oliveira, G.C.; Vieira, W.L.; Bertolli, S.C.; Pacheco, A.C. Photosynthetic behavior, growth and essential oil production of Melissa officinalis L. cultivated under colored shade nets. Chil. J. Agric. Res. 2016, 76, 123. [Google Scholar] [CrossRef]
- Li, Y.; Craker, L.E.; Potter, T. Effect of light level on the essential oil production of sage (Salvia officinalis) and thyme (Thymus vulgaris). Acta Hortic. 1996, 426, 419–426. [Google Scholar] [CrossRef]
- Kim, M.; Sowndhararajan, K.; Kim, S. The chemical composition and biological activities of essential oil from Korean native thyme Bak-Ri-Hyang (Thymus quinquecostatus Celak.). Molecules 2022, 27, 4251. [Google Scholar] [CrossRef]
- Karousou, R.; Balta, M.; Hanlidou, E.; Kokkini, S. Mints, smells and traditional uses in Thessaloniki (Greece) and other Mediterranean countries. J. Ethnopharmac. 2007, 109, 248–257. [Google Scholar] [CrossRef]
- Mounira, M.; Mohamed, B.; Najeh, B.F. Variability of volatiles in Tunisian Mentha pulegium L. (Lamiaceae). J. Essent. Oil Res. 2007, 19, 211–214. [Google Scholar]
- Telci, I.; Demirtas, I.; Bayram, E.; Arabaci, O.; Kacar, O. Environmental variation on aroma components of pulegone/piperitone rich spearmint (Mentha spicata L.). Ind. Crops Prod. 2010, 3, 588–592. [Google Scholar] [CrossRef]
- Lu, C.G.; Liang, C.Y.; Li, W.L. Analysis on chemical components of volatile oil from Mentha piperita L. J. Anhui Agric. Sci. 2008, 36, 400–425. [Google Scholar]
- Yadegarinia, D.; Gachkar, L.; Rezaei, M.B.; Taghizadeh, M.; Astaneh, S.A.; Rasooli, I. Biochemical activities of Iranian Mentha piperita L. and Myrtus communis L. essential oils. Phytochemistry 2006, 67, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.K.; Malik, A. Antimicrobial potential and chemical composition of Mentha piperita oil in liquid and vapour phase against food spoilage microorganisms. Food Control 2011, 22, 1707–1714. [Google Scholar] [CrossRef]
- Eddaya, T. Gestion Intégrée des Ravageurs de la Menthe Verte (Mentha spicata L. ou Huds) au Centre-Sud du Maroc. Ph.D. Thesis, l’université de Moulay Ismail, Meknes, Morocco, 2015; 285p. [Google Scholar]
- Soilhi, Z.; Rhimi, A.; Heuskin, S.; Fauconnier, M.L.; Mekki, M. Essential oil chemical diversity of Tunisian Mentha spp. collection. Ind. Crops Prod. 2019, 131, 330–340. [Google Scholar] [CrossRef]
- Alizadeh Behbahani, B.; Shahidi, F. Melissa officinalis essential oil: Chemical compositions, antioxidant potential, total phenolic content and antimicrobial activity. Nutr. Food Sci. Res. 2019, 6, 17–25. [Google Scholar] [CrossRef]
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis L.—A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharm. 2016, 188, 204–228. [Google Scholar] [CrossRef]
- Sharopov, F.S.; Wink, M.; Khalifaev, D.R.; Zhang, H.; Dosoky, N.S.; Setzer, W.N. Composition and bioactivity of the essential oil of Melissa officinalis L. growing wild in Tajikistan. Int. J. Tradit. Nat. Med. 2013, 2, 86–96. [Google Scholar]
- Moradkhani, H.; Sargsyan, E.; Bibak, H.; Naseri, B.; Fayazi-Barjin, A.; Meftahizade, H. Melissa officinalis L., a valuable medicine plant: A review. J. Med. Plants Res. 2010, 4, 2753–2759. [Google Scholar]
- Verma, R.S.; Padalia, R.C.; Chauhan, A. Evaluation of essential oil quality of lemon balm (Melissa officinalis L.) grown in two locations of northern India. J. Essent. Oil Res. 2015, 27, 412–416. [Google Scholar] [CrossRef]
- León-Fernández, M.; Sánchez-Govín, E.; Quijano-Celis, C.E.; Pino Jorge, A. Effect of planting practice and harvest time in oil content and its composition in Melissa officinalis L. cultivated in Cuba. J. Essent. Oil Bear. Plants 2008, 11, 62–68. [Google Scholar] [CrossRef]
- Mohamadpoor, H.; Pirbalouti, A.G.; Malekpoor, F.; Hamedi, B. Chemical composition and bioactivity of the essential oil of Melissa officinalis L., cultivated in southwestern Iran. J. Herb. Drug. 2018, 8, 213–218. [Google Scholar] [CrossRef]
- Basta, A.; Tzakou, O.; Couladis, M. Composition of the leaves essential oil of Melissa officinalis L. from Greece. Flavour Fragr. J. 2005, 20, 642–644. [Google Scholar] [CrossRef]
- Salvaneschi, S.; Iriti, M.; Vitalini, S.; Vallone, L. Thymus vulgaris L. as a possible effective substitute for nitrates in meat products. Ital. J. Food Saf. 2020, 9, 7739. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, M.; Jovanović, K.K.; Marković, T.; Marković, D.; Gligorijević, N.; Radulović, S.; Soković, M. Chemical composition, antimicrobial, and cytotoxic properties of five Lamiaceae essential oils. Ind. Crops Prod. 2014, 61, 225–232. [Google Scholar] [CrossRef]
- Kot, B.; Wierzchowska, K.; Piechota, M.; Czerniewicz, P.; Chrzanowski, G. Antimicrobial activity of five essential oils from lamiaceae against multidrug-resistant Staphylococcus aureus. Nat. Prod. Res. 2019, 33, 3587–3591. [Google Scholar] [CrossRef] [PubMed]
- Soković, M.D.; Vukojević, J.; Marin, P.D.; Brkić, D.D.; Vajs, V.; Van Griensven, L.J.L.D. Chemical composition of essential oils of Thymus and Mentha species and their antifungal activities. Molecules 2009, 14, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Oussalah, M.; Caillet, S.; Saucier, L.; Lacroix, M. Inhibitory effects of selected plant essential oils on the growth of four pathogenic bacteria: E. coli O157: H7, Salmonella typhimurium, Staphylococcus aureus and Listeria monocytogenes. Food Control 2007, 18, 414–420. [Google Scholar] [CrossRef]
- Castanon, J.I. History of the use of antibiotic as growth promoters in European poultry feeds. Poult Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef]
- El Abed, N.; Kaabi, B.; Smaali, M.I.; Chabbouh, M.; Habibi, K.; Mejri, M.; Mazouki, M.N.; Ahmed, S.B.H. Chemical composition and atibacterial activities of Thymus capitata essential oil with its preservative effects against Listeria monocytogenesis inoculated in minced beef meat. Evid.-Based Complement. Altern. Med. 2014, 2014, 152487. [Google Scholar] [CrossRef]

| CaCO₃ (%) | pH in KCl | Humus (%) | N—Total % | P2O5 mg/100 g | K2O mg/100 g |
|---|---|---|---|---|---|
| 2.36 | 6.94 | 3.69 | 0.18 | 16.8 | 30.8 |
| Month | TS 2020. 2021. | TOD 2020. 2021. | TX 2020. 2021. | TM 2020. 2021. | MSR 2020. 2021. | MSRA | RR 2020. 2021. | RO |
|---|---|---|---|---|---|---|---|---|
| May | 16.4 17.2 | −0.2 0.6 | 22.9 24.5 | 11.0 11.0 | 173.2 226.8 | 219.0 | 67.0 29.4 | 66.7 |
| June | 19.8 21.5 | 0.3 2.0 | 25.7 28.9 | 14.9 14.6 | 183.7 245.7 | 237.2 | 186.7 30.3 | 69.7 |
| July | 22.3 25.5 | 1.0 4.2 | 29.1 32.8 | 15.9 18.6 | 290.9 293.2 | 289.0 | 78.6 39.8 | 43.6 |
| August | 22.6 23.5 | 1.5 2.4 | 30.0 31.8 | 16.5 16.1 | 265.5 302.1 | 276.0 | 78.6 39.6 | 43.3 |
| September | 20.3 18.0 | 3.1 0.8 | 28.0 25.8 | 13.6 14.1 | 224.8 200.9 | 210.0 | 14.9 21.0 | 43.6 |
| PAR * (μmol m−2 s−1) | Solar Radiation (W m−2) | Temperature °C | Relative Humidity% | |||||
|---|---|---|---|---|---|---|---|---|
| Time (h) | Non- Shading | Shading Reduction% | Non- Shading | Shading | Non- Shading | Shading Reduction °C | Non- Shading% | Shading % |
| 6:00 | 172.4 | 39.2 | 167.2 | 48.4 | 17.0 | 0.0 | 69.0 | 70.1 |
| 9:00 | 1349.1 | 48,8 | 520.1 | 276.3 | 27.2 | 0.4 | 41.2 | 41.6 |
| 12:00 | 2085.7 | 45.7 | 864.9 | 463.2 | 34.9 | 2.0 | 36.3 | 36.6 |
| 15:00 | 1747.2 | 48.8 | 770.5 | 343.7 | 37.1 | 2.5 | 19.8 | 20.6 |
| 18:00 | 513 | 50.3 | 354.3 | 92.1 | 34.2 | 0.9 | 26.2 | 26.6 |
| Sample | Essential Oil Yield, mL/100 g p.m. * |
|---|---|
| Thyme—non-shaded | 3.44 a ± 0.01 |
| Thyme—shaded | 3.46 a ± 0.03 |
| Mint—non-shaded | 3.96 a ± 0.08 |
| Mint—shaded | 2.20 b ± 0.02 |
| Lemon balm—non-shaded | 0.21 c ± 0.01 |
| Lemon balm—shaded | 0.45 c ± 0.02 |
| N0 | tret., min | Compound | RIexp | RIlit | Method of Identification | c% | |
|---|---|---|---|---|---|---|---|
| Non-Shaded | Shaded | ||||||
| 1. | 6.70 | α-Thujene | 923 | 924 | RI, MS | 1.3 ± 0.010 | 1.4 ± 0.010 |
| 2. | 6.92 | α-Pinene | 931 | 932 | RI, MS | 0.8 ± 0.003 | 0.9 ± 0.002 |
| 3. | 7.40 | Camphene | 947 | 946 | RI, MS | 0.4 ± 0.002 | 0.4 ± 0.002 |
| 4. | 8.15 | Sabinene | 972 | 969 | RI, MS | tr | Tr |
| 5. | 8.28 | β-Pinene | 977 | 974 | RI, MS, Co-I | 0.3 ± 0.001 | 0.3 ± 0.001 |
| 6. | 8.61 | 1-Octen-3-ol | 977 | 974 | RI, MS | 0.8 ± 0.003 | 1.1 ± 0.003 ** |
| 7. | 8.70 | Myrcene | 991 | 988 | RI, MS | 2.4 ± 0.008 | 2.6 ± 0.007 * |
| 8. | 9.12 | 3-Octanol | 994 | 988 | RI, MS | tr | Tr |
| 9. | 9.25 | α-Phellandrene | 1007 | 1002 | RI, MS | 0.2 ± 0.001 | 0.2 ± 0.001 |
| 10. | 9.40 | δ-3-Carene | 1011 | 1008 | RI, MS | tr | 0.1 ± 0.000 |
| 11. | 9.67 | α-Terpinene | 1018 | 1014 | RI, MS | 2.3 ± 0.009 | 2.4 ± 0.009 |
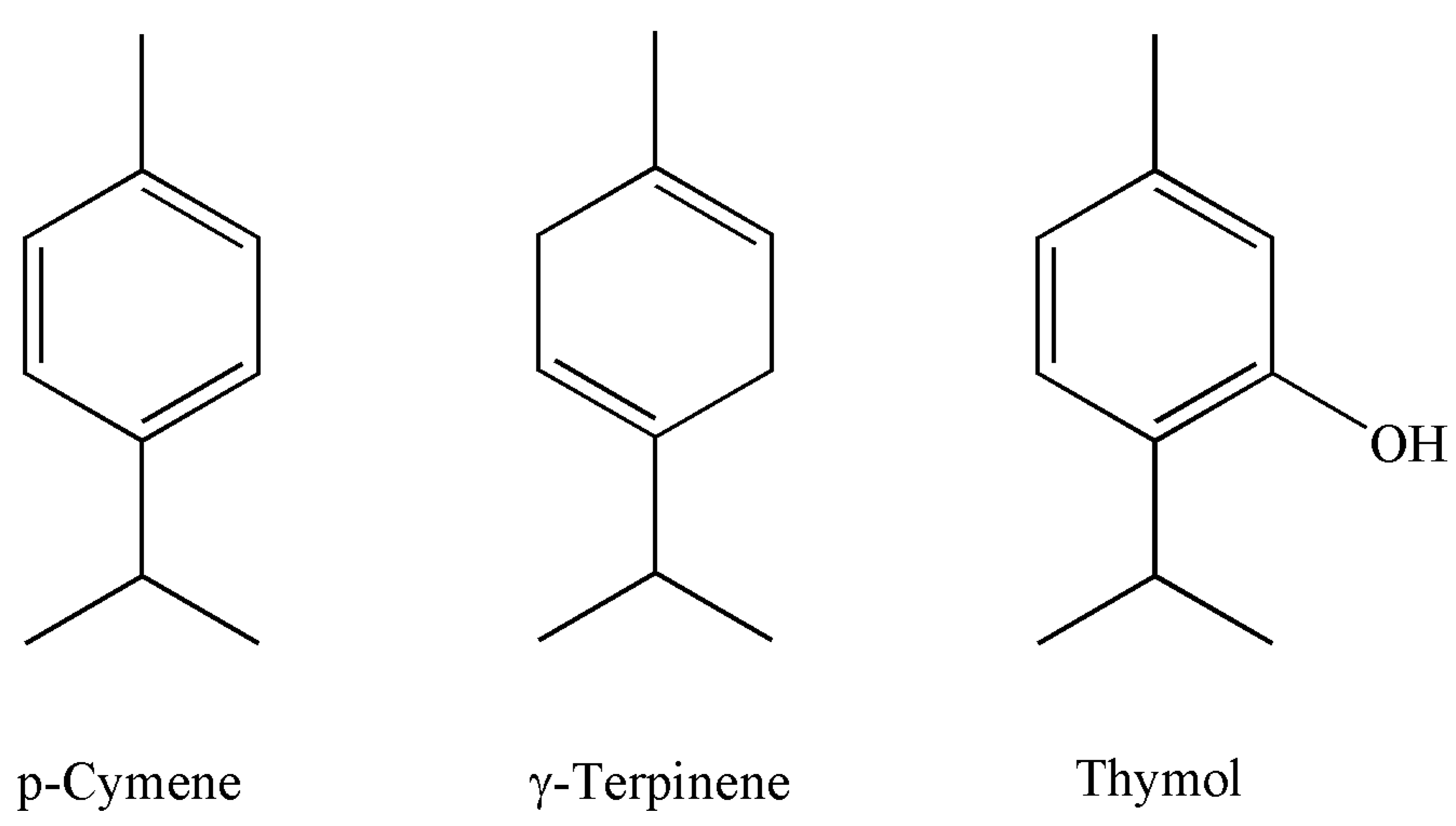
| 12. | 10.08 | p-Cymene * | 1021 | 1020 | RI, MS | 16.5 ± 0.05 | 17.4 ± 0.06 |
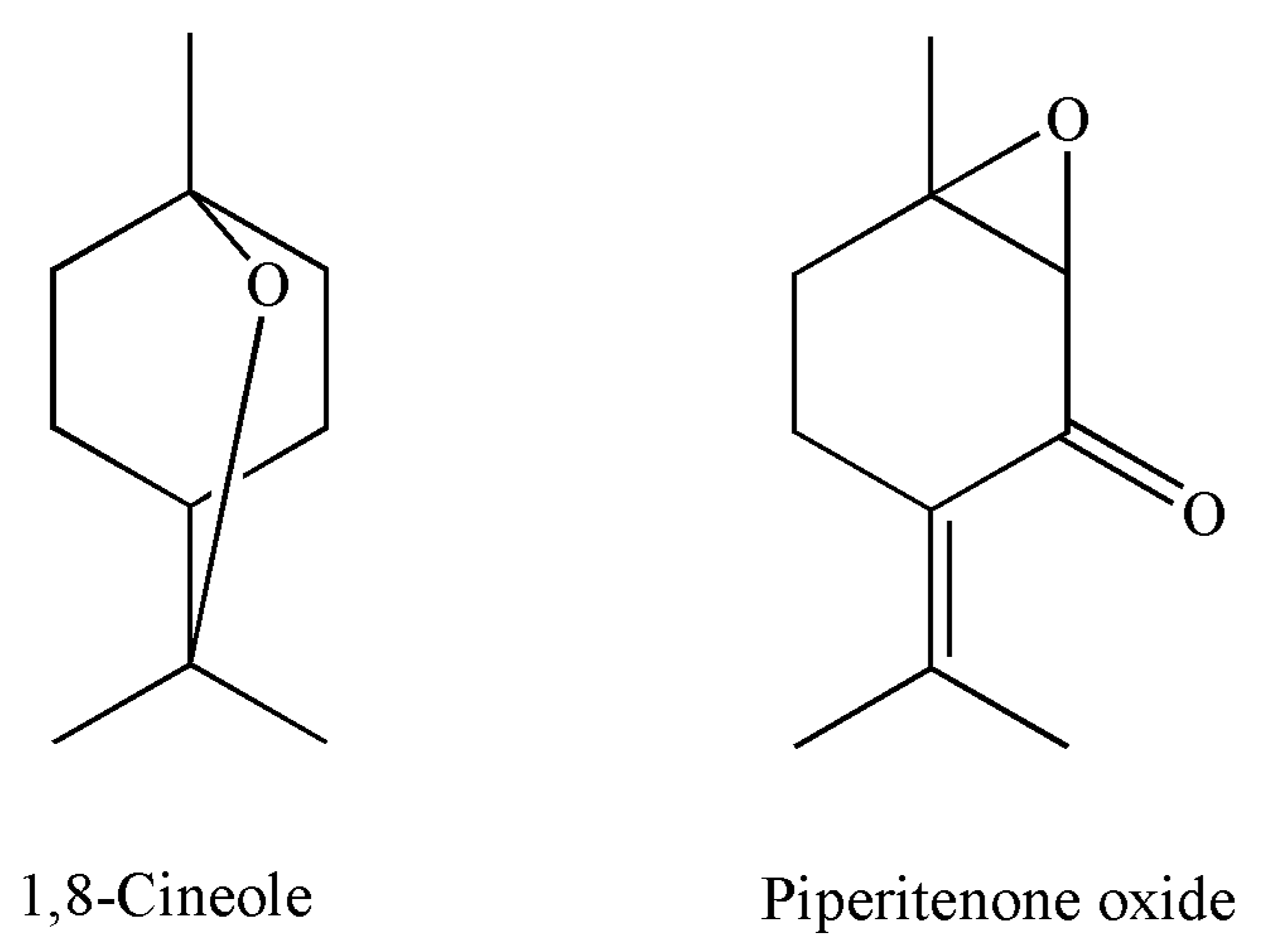
| 13. | 10.18 | 1,8-Cineole * | 1023 | 1026 | RI, MS, Co-I | tr | Tr |
| 14. | 10.85 | (E)-β-Ocimene | 1041 | 1044 | RI, MS | tr | Tr |
| 15. | 11.33 | γ-Terpinene | 1054 | 1054 | RI, MS | 18.3 ± 0.06 | 16.8 ± 0.05 |
| 16. | 11.90 | cis-Sabinene hydrate | 1069 | 1065 | RI, MS | 1.0 ± 0.008 | 0.6 ± 0.004 ** |
| 17. | 12.43 | Terpinolene | 1083 | 1086 | RI, MS | 0.1 ± 0.000 | 0.1 ± 0.000 |
| 18. | 13.22 | Linalool | 1103 | 1095 | RI, MS, Co-I | 2.3 ± 0.008 | 2.2 ± 0.008 |
| 19. | 14.85 | Camphor | 1142 | 1141 | RI, MS, Co-I | 0.1 ± 0.000 | Tr |
| 20. | 16.15 | Borneol | 1173 | 1165 | RI, MS, Co-I | 0.8 ± 0.003 | 0.9 ± 0.004 |
| 21. | 16.49 | Terpinen-4-ol | 1182 | 1174 | RI, MS | 0.8 ± 0.003 | 1.0 ± 0.004 * |
| 22. | 17.28 | α-Terpineol | 1200 | 1196 | RI, MS | 0.3 ± 0.002 | 0.3 ± 0.002 |
| 23. | 17.56 | Octanol acetate | 1207 | 1211 | RI, MS | 0.2 ± 0.002 | - |
| 24. | 18.74 | Thymol, methyl ether | 1235 | 1232 | RI, MS | 0.4 ± 0.003 | 0.7 ± 0.006 ** |
| 25. | 19.13 | Carvacrol, methyl ether | 1244 | 1241 | RI, MS | 0.5 ± 0.003 | 0.4 ± 0.003 |
| 26. | 20.75 | Isobornyl acetate | 1283 | 1283 | RI, MS | tr | - |
| 27. | 22.59 | Thymol | 1299 | 1289 | RI, MS, Co-I | 44.2 ± 0.120 | 43.9 ± 0.110 |
| 28. | 22.96 | Carvacrol | 1307 | 1298 | RI, MS | 4.7 ± 0.015 | 4.4 ± 0.015 |
| 29. | 26.49 | (E)-Caryophyllene | 1421 | 1417 | RI, MS | 0.9 ± 0.004 | 1.4 ± 0.008 ** |
| 30. | 28.59 | Geranyl propanoate | 1474 | 1476 | RI, MS | 0.2 ± 0.002 | 0.1 ± 0.001 * |
| 31. | 33.13 | Caryophyllene oxide | 1592 | 1582 | RI, MS | 0.2 ± 0.002 | 0.2 ± 0.002 |
| Total identified | 100.0 ± 0.322 | 99.8 ± 0.300 | |||||
| Grouped components (%) | |||||||
| Monoterpene hydrocarbons (1–5, 7, 9–11, 14, 15, 17) | 26.1 | 25.2 | |||||
| Oxygen-containing monoterpenes (13, 16, 18–22, 26, 30) | 5.5 | 5.1 | |||||
| Sesquiterpene hydrocarbons (29) | 0.9 | 1.4 | |||||
| Oxygenated sesquiterpenes (31) | 0.2 | 0.2 | |||||
| Aromatic compounds (12, 24, 25, 27, 28) * Phenolics (24, 25, 27, 28) | 66.3 * 49.8 | 66.8 * (49.4) | |||||
| Others (6, 8, 23) | 1.0 | 1.1 | |||||
| N0 | tret., min | Compound | RIexp | RIlit | Method of Identification | c% | |
|---|---|---|---|---|---|---|---|
| Non-Shaded | Shaded | ||||||
| 1. | 6.70 | α-Thujene | 924 | 924 | RI, MS | tr | - |
| 2. | 6.92 | α-Pinene | 932 | 932 | RI, MS | 1.4 ± 0.009 | 0.8 ± 0.006 ** |
| 3. | 7.40 | Camphene | 947 | 946 | RI, MS | tr | - |
| 4. | 8.15 | Sabinene | 972 | 969 | RI, MS | 1.4 ± 0.008 | 0.9 ± 0.006 ** |
| 5. | 8.28 | β-Pinene | 976 | 974 | RI, MS, Co-I | 2.8 ± 0.008 | 1.9 ± 0.009 ** |
| 6. | 8.61 | 1-Octen-3-ol | 977 | 974 | RI, MS | tr | Tr |
| 7. | 8.70 | Myrcene | 980 | 988 | RI, MS | 7.1 ± 0.021 | 6.2 ± 0.018 * |
| 8. | 9.12 | 3-Octanol | 994 | 988 | RI, MS | 0.4 ± 0.003 | 0.3 ± 0.003 |
| 9. | 9.67 | α-Terpinene | 1010 | 1014 | RI, MS | tr | Tr |
| 10. | 10.08 | p-Cymene | 1021 | 1020 | RI, MS | tr | Tr |
| 11. | 10.18 | Limonene | 1023 | 1024 | RI, MS, Co-I | tr | Tr |
| 12. | 10.23 | 1,8-Cineole | 1025 | 1026 | RI, MS, Co-I | 25.9 ± 0.080 | 16.3 ± 0.051 ** |
| 13. | 10.44 | (Z)-β-Ocimene | 1030 | 1032 | RI, MS | 0.8 ± 0.010 | 0.7 ± 0.010 |
| 14. | 10.85 | (E)-β-Ocimene | 1041 | 1044 | RI, MS | tr | Tr |
| 15. | 11.28 | γ-Terpinene | 1054 | 1054 | RI, MS | 0.2 ± 0.002 | 0.2 ± 0.002 |
| 16. | 11.90 | cis-Sabinene hydrate | 1069 | 1065 | RI, MS | tr | Tr |
| 17. | 12.43 | Terpinolene | 1083 | 1086 | RI, MS | 0.2 ± 0.002 | 0.1 ± 0.001 |
| 18. | 13.12 | Isopentyl 2-methyl butanoate | 1101 | 1100 | RI, MS | tr | Tr |
| 19. | 13.22 | Linalool | 1103 | 1095 | RI, MS, Co-I | tr | 0.3 ± 0.002 |
| 20. | 13.75 | 3-Octanol acetate | 1116 | 1120 | RI, MS | tr | tr |
| 21. | 16.22 | δ-Terpineol | 1172 | 1162 | RI, MS | 0.7 ± 0.003 | 0.6 ± 0.003 |
| 22. | 16.49 | Terpinen-4-ol | 1182 | 1174 | RI, MS | 0.2 ± 0.001 | 0.2 ± 0.001 |
| 23. | 17.04 | Myrtenal | 1195 | 1195 | RI, MS | 0.2 ± 0.001 | 0.2 ± 0.001 |
| 24. | 17.28 | α-Terpineol | 1200 | 1196 | RI, MS | 0.6 ± 0.005 | 0.6 ± 0.005 |
| 25. | 18.58 | (3Z)-Hexenyl 3-methyl butanoate | 1232 | 1232 | RI, MS | tr | tr |
| 26. | 20.14 | (4E)-Decen-1-ol | 1268 | 1259 | RI, MS | 0.6 ± 0.005 | 0.7 ± 0.006 |
| 27. | 20.48 | (+)-Isopiperitenone | 1276 | - | MS | 0.3 ± 0.002 | 0.3 ± 0.002 |
| 28. | 22.57 | Thymol | 1299 | 1289 | RI, MS, Co-I | 1.4 ± 0.005 | 0.3 ± 0.002 ** |
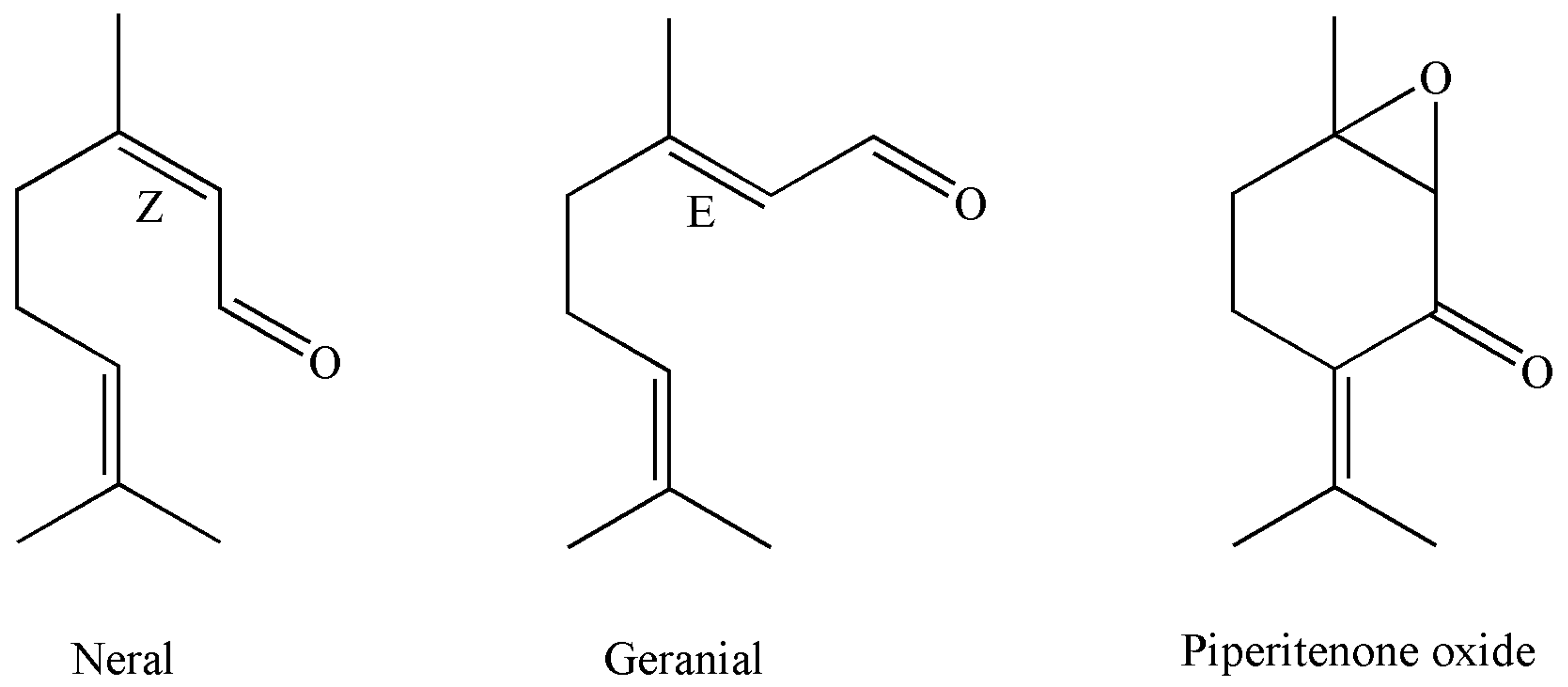
| 29. | 24.74 | Piperitenone oxide | 1376 | 1366 | RI, MS | 52.6 ± 0.120 | 64.8 ± 0.139 * |
| 30. | 25.94 | Nepetalactone | 1403 | 1393 | MS | 0.5 ± 0.003 | 0.9 ± 0.006 * |
| 31. | 26.49 | (E)-Caryophyllene | 1421 | 1417 | RI, MS | 1.1 ± 0.008 | 1.4 ± 0.009 * |
| 32. | 27.92 | α-Humulene | 1457 | 1452 | RI, MS | tr | 0.2 ± 0.001 |
| 33. | 29.04 | Germacrene D | 1485 | 1484 | RI, MS | 1.1 ± 0.007 | 1.5 ± 0.008 * |
| 34. | 29.62 | Bicyclogermacrene | 1500 | 1500 | RI, MS | tr | tr |
| Total identified | 99.5 ± 0.296 | 100.0 ± 0.841 | |||||
| Grouped components (%) | |||||||
| Monoterpene hydrocarbons (1–5, 7, 9, 11, 13–15, 17) | 13.9 | 10.8 | |||||
| Oxygen-containing monoterpenes (12, 16, 19, 21–24, 27, 29, 30) | 81.0 | 84.9 | |||||
| Sesquiterpene hydrocarbons (31–34) | 2.2 | 3.3 | |||||
| Aromatic compounds (10, 28) * Phenolics (28) | 1.4 * 1.4 | tr | |||||
| Others (6, 8, 18, 20, 25, 26) | 1.0 | 1.0 | |||||
| N0 | tret., min | Compound | RIexp | RIlit | Method of Identification | c% | |
|---|---|---|---|---|---|---|---|
| Non-Shaded | Shade | ||||||
| 1. | 8.17 | Sabinene | 962 | 969 | RI, MS | tr | 0.4 ± 0.003 |
| 2. | 8.28 | β-Pinene | 967 | 974 | RI, MS, Co-I | tr | 0.5 ± 0.003 |
| 3. | 8.61 | 6-Methyl-5-hepten-2-one | 977 | 981 | RI, MS | 1.5 ± 0.009 | 1.3 ± 0.008 |
| 4. | 8.70 | Myrcene | 980 | 988 | RI, MS | 0.5 ± 0.003 | 2.0 ± 0.008 ** |
| 5. | 10.18 | 1,8-Cineole | 1023 | 1026 | RI, MS, Co-I | 1.1 ± 0.007 | 4.4 ± 0.018 ** |
| 6. | 10.43 | (Z)-β-Ocimene | 1030 | 1032 | RI, MS | tr | 0.3 ± 0.002 |
| 7. | 10.85 | (E)-β-Ocimene | 1041 | 1044 | RI, MS | tr | 0.2 ± 0.002 |
| 8. | 10.97 | Benzene acetaldehyde | 1044 | 1036 | RI, MS | 0.3 ± 0.002 | Tr |
| 9. | 13.22 | Linalool | 1103 | 1095 | RI, MS, Co-I | 0.7 ± 0.006 | 0.6 ± 0.006 |
| 10. | 14.62 | 1-Terpineol | 1130 | 1137 | RI, MS | 0.2 ± 0.001 | - |
| 11. | 15.17 | Citronellal | 1150 | 1148 | RI, MS | 4.1 ± 0.016 | 2.4 ± 0.009 ** |
| 12. | 15.69 | (Z)-Isocitral | 1162 | 1160 | RI, MS | 0.3 ± 0.002 | 0.9 ± 0.004 ** |
| 13. | 16.43 | (E)-Isocitral | 1180 | 1177 | RI, MS | 0.4 ± 0.003 | 1.6 ± 0.009 ** |
| 14. | 17.26 | α-Terpineol | 1186 | 1196 | RI, MS | 0.4 ± 0.003 | 0.3 ± 0.002 |
| 15. | 18.28 | Isobornyl formate | 1225 | 1235 | RI, MS | 0.3 ± 0.002 | - |
| 16. | 18.71 | 2,3-Epoxygeranial | 1235 | - | MS | 1.1 ± 0.007 | - |
| 17. | 19.11 | Neral | 1244 | 1235 | RI, MS, Co-I | 21.3 ± 0.080 | 24.9 ± 0.085 |
| 18. | 19.72 | cis-Piperitone epoxide | 1258 | 1250 | RI, MS | 3.5 ± 0.014 | 0.1 ± 0.000 ** |
| 19. | 20.44 | Geranial | 1274 | 1264 | RI, MS, Co-I | 34.0 ± 0.070 | 32.8 ± 0.060 |
| 20. | 21.32 | Undecanal | 1296 | 1305 | RI, MS | 1.1 ± 0.007 | - |
| 21. | 22.48 | Methyl geranate | 1324 | 1322 | RI, MS | 0.7 ± 0.006 | 0.5 ± 0.003 * |
| 22. | 24.56 | Piperitenone oxide | 1374 | 1366 | RI, MS | 17.2 ± 0.100 | 16.7 ± 0.008 |
| 23. | 24.91 | Geranyl acetate | 1383 | 1379 | RI, MS | 2.3 ± 0.008 | 1.2 ± 0.008 ** |
| 24. | 25.96 | Nepetalactone | 1403 | 1393 | RI, MS | 0.5 ± 0.003 | 0.4 ± 0.003 |
| 25. | 26.49 | (E)-Caryophyllene | 1421 | 1417 | RI, MS | 4.0 ± 0.017 | 4.1 ± 0.016 |
| 26. | 27.92 | α-Humulene | 1457 | 1452 | RI, MS | 0.4 ± 0.003 | 0.3 ± 0.002 |
| 27. | 29.03 | Germacrene D | 1485 | 1484 | RI, MS | 0.4 ± 0.003 | 0.7 ± 0.006 ** |
| 28. | 33.13 | Caryophyllene oxide | 1592 | 1582 | RI, MS | 3.6 ± 0.015 | 2.6 ± 0.010 * |
| Total identified | 100.0 ± 0.387 | 100.0 ± 0.271 | |||||
| Grouped components (%) | |||||||
| Monoterpene hydrocarbons (1, 2, 4, 6, 7) | 0.5 | 3.6 | |||||
| Oxygen-containing monoterpenes (5, 9–19, 21–24) | 88.1 | 87.2 | |||||
| Sesquiterpene hydrocarbons (25–27) | 4.8 | 5.1 | |||||
| Oxygenated sesquiterpenes (28) | 3.6 | 2.6 | |||||
| Aromatic compounds (8) | 0.3 | tr | |||||
| Others (3, 20) | 2.6 | 1.7 | |||||
| Species/Production Methods | EC50, mg/mL Incubation Time | |||
|---|---|---|---|---|
| Without Incubation | 20 min | 40 min | 60 min | |
| Thyme—non-shaded | / | 0.54 c ± 0.003 | ||
| Thyme—shaded | / | 0.92 c ± 0.014 | ||
| Mint—non-shaded | / | 3.03 b ± 0.027 | ||
| Mint—shaded | / | 5.43 b ± 0.237 | ||
| Lemon balm—non-shaded | / | 12.85 a ± 0.199 | ||
| Lemon balm—shaded | / | 3.43 b ± 0.010 | ||
| Species/Production Methods | Escherichia coli | Pseudomonas aeruginosa | Proteus vulgaris | Staphylococcus aureus | Bacillus cereus | Bacillus subtilis | Klebsiella pneumoniae | Listeria monocytogenes | Candida albicans |
|---|---|---|---|---|---|---|---|---|---|
| Thyme—non-shaded | 44.3 a | 0.0 | 26.0 a | 38.6 a | 0.0 | 62.0 a | 0.0 | 0.0 | 51.6 a |
| Thyme—shaded | 41.3 b | 0.0 | 23.3 a | 33.3 b | 0.0 | 64.0 a | 0.0 | 0.0 | 51.3 a |
| Mint—non-shaded | 19.3 c | 0.0 | 0.0 e | 23.3 c | 0.0 | 30.3 b | 0.0 | 0.0 | 55.0 a |
| Mint—shaded | 20.6 c | 0.0 | 15.7 c | 17.0 d | 0.0 | 32.3 b | 0.0 | 0.0 | 42.3 b |
| Lemon balm—shaded | 14.6 d | 0.0 | 12.3 d | 0.0 e | 0.0 | 20.6 c | 0.0 | 0.0 | 42.0 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lalević, D.; Ilić, Z.S.; Stanojević, L.; Milenković, L.; Šunić, L.; Kovač, R.; Kovačević, D.; Danilović, B.; Milenković, A.; Stanojević, J.; et al. Shade-Induced Effects on Essential Oil Yield, Chemical Profiling, and Biological Activity in Some Lamiaceae Plants Cultivated in Serbia. Horticulturae 2023, 9, 84. https://doi.org/10.3390/horticulturae9010084
Lalević D, Ilić ZS, Stanojević L, Milenković L, Šunić L, Kovač R, Kovačević D, Danilović B, Milenković A, Stanojević J, et al. Shade-Induced Effects on Essential Oil Yield, Chemical Profiling, and Biological Activity in Some Lamiaceae Plants Cultivated in Serbia. Horticulturae. 2023; 9(1):84. https://doi.org/10.3390/horticulturae9010084
Chicago/Turabian StyleLalević, Dragana, Zoran S. Ilić, Ljiljana Stanojević, Lidija Milenković, Ljubomir Šunić, Renata Kovač, Dragan Kovačević, Bojana Danilović, Aleksandra Milenković, Jelena Stanojević, and et al. 2023. "Shade-Induced Effects on Essential Oil Yield, Chemical Profiling, and Biological Activity in Some Lamiaceae Plants Cultivated in Serbia" Horticulturae 9, no. 1: 84. https://doi.org/10.3390/horticulturae9010084
APA StyleLalević, D., Ilić, Z. S., Stanojević, L., Milenković, L., Šunić, L., Kovač, R., Kovačević, D., Danilović, B., Milenković, A., Stanojević, J., & Cvetković, D. (2023). Shade-Induced Effects on Essential Oil Yield, Chemical Profiling, and Biological Activity in Some Lamiaceae Plants Cultivated in Serbia. Horticulturae, 9(1), 84. https://doi.org/10.3390/horticulturae9010084








