Proton Nuclear Magnetic Resonance (1H NMR) Metabolic Profiles Discriminate Two Monovarietal Extra Virgin Olive Oils, Cultivars Arbequina and Koroneiki, with Different Geographical Origin
Abstract
1. Introduction
2. Materials and Methods
2.1. Olive Sampling and Oil Extraction
2.2. 1H NMR Spectroscopy Analysis
2.3. Multivariate Statistical Analysis
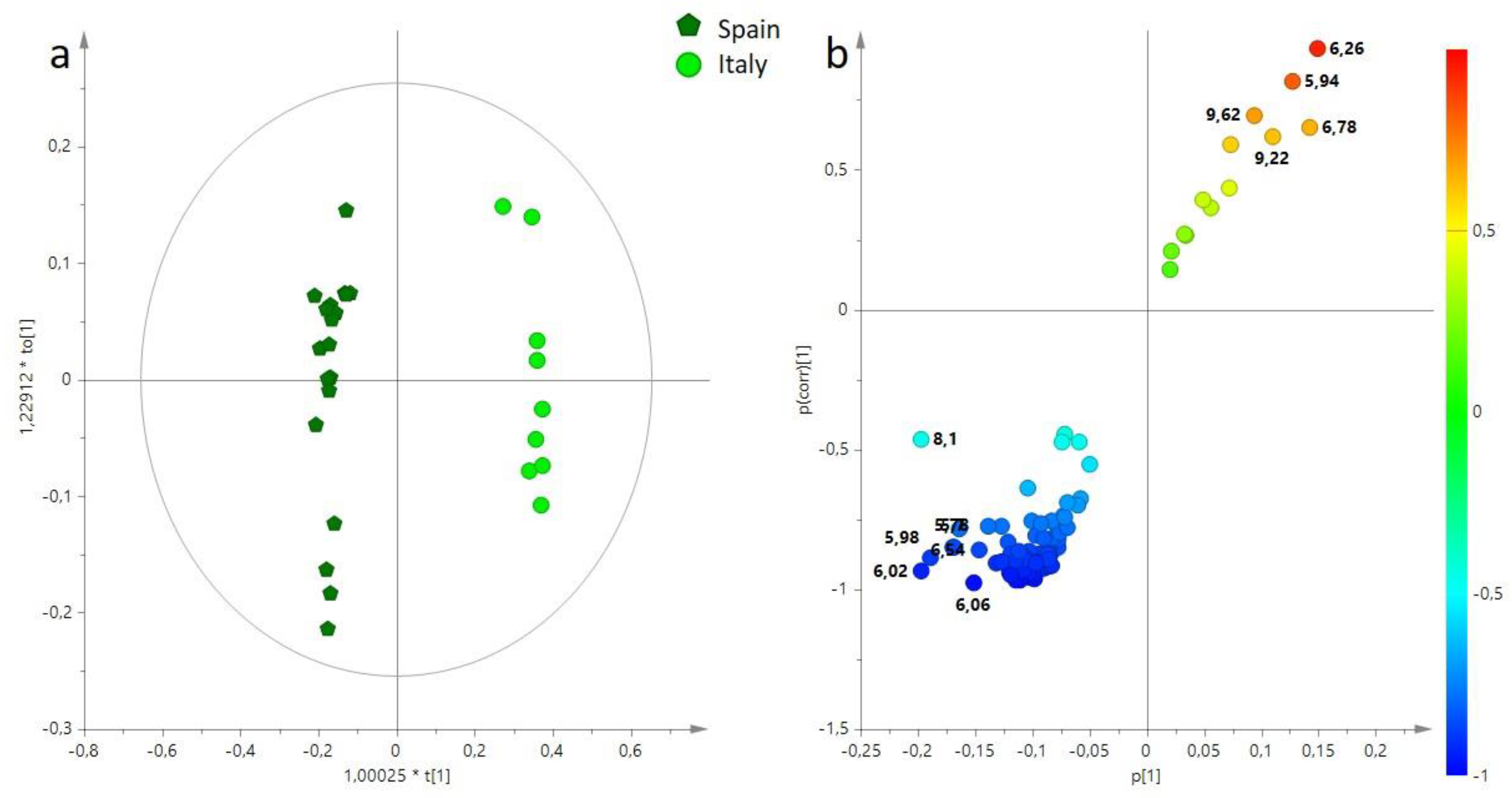
3. Results
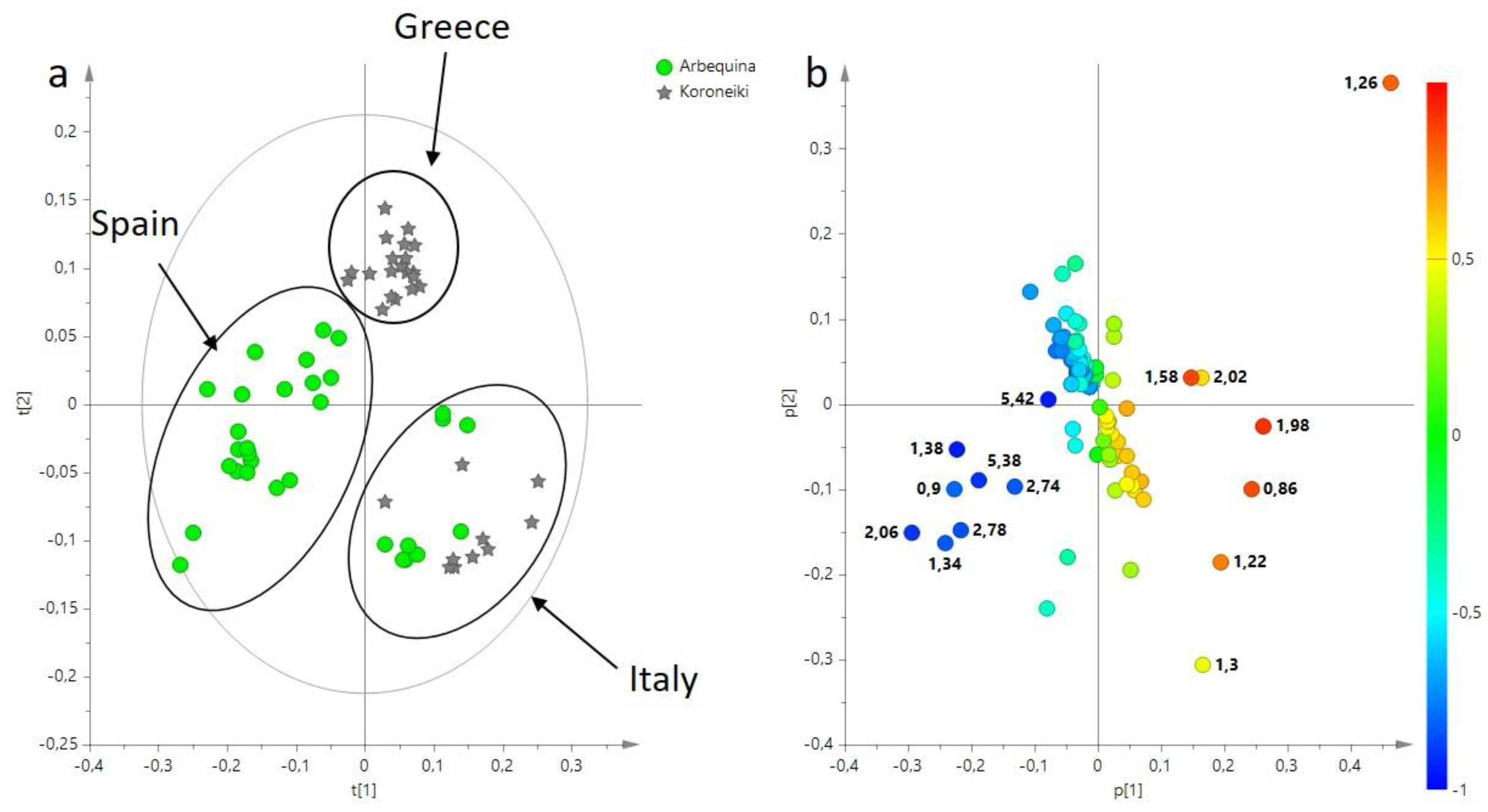
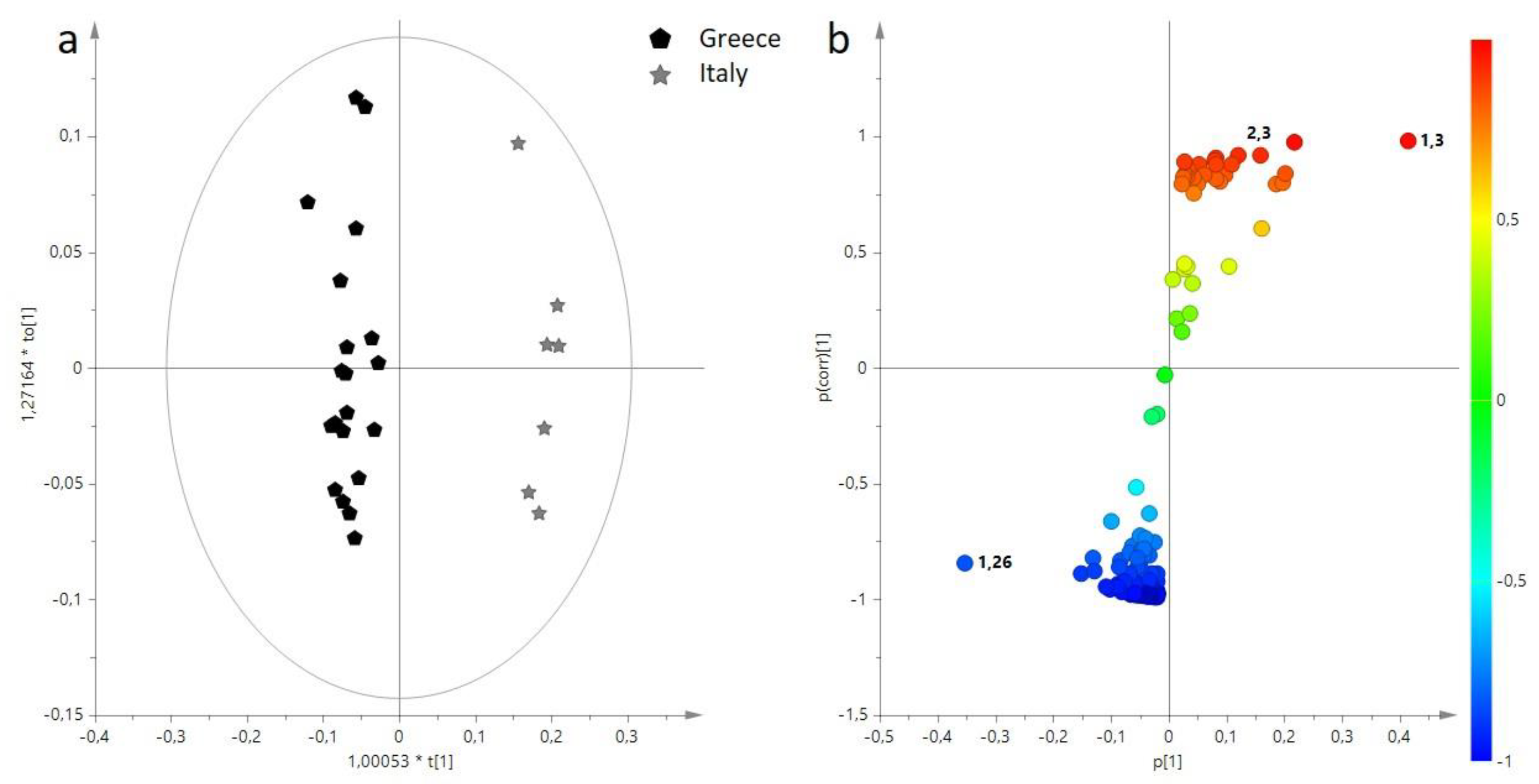
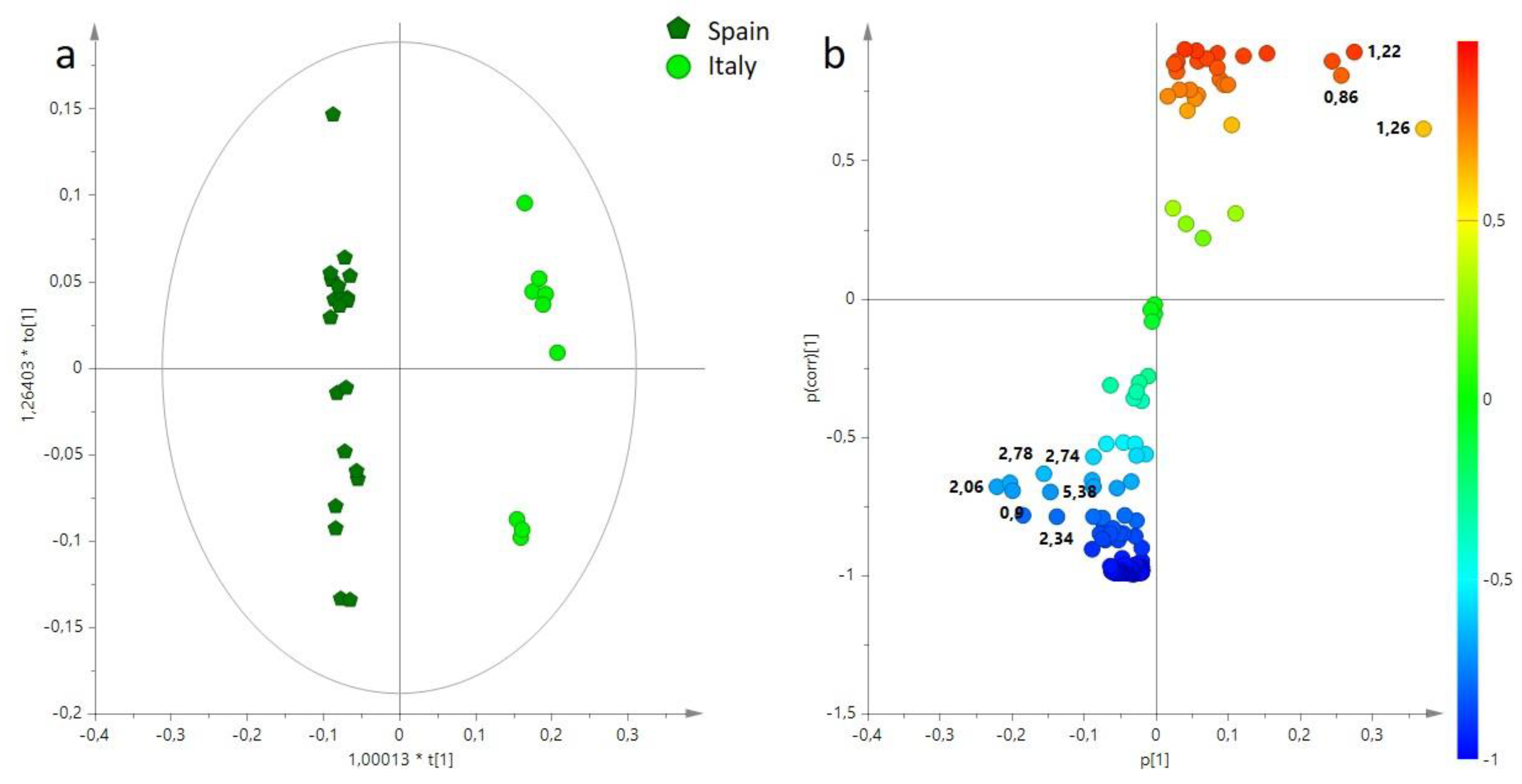
3.1. Multivariate Statistical Analysis of Main Components (BUCKET-1) of EVOO
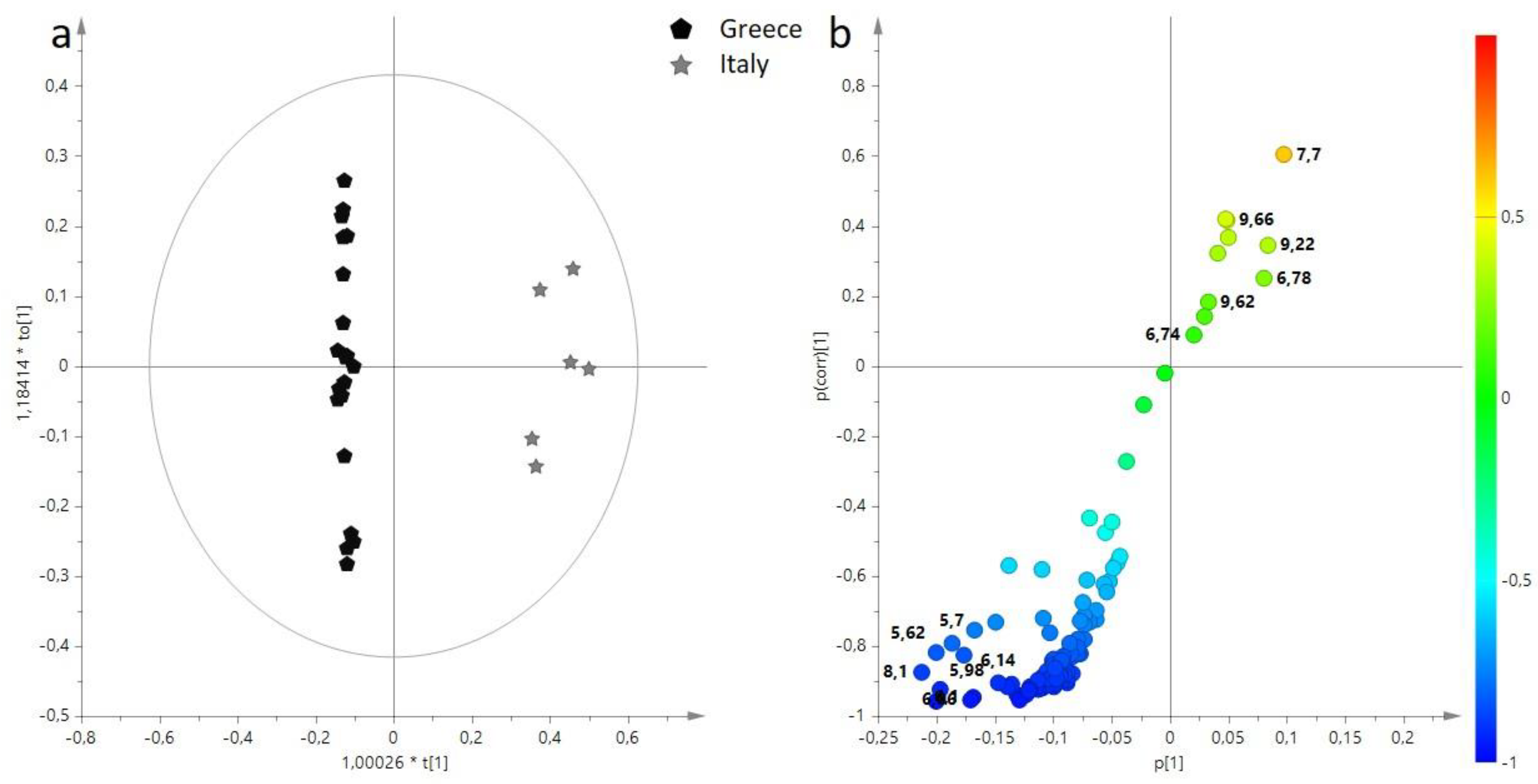
3.2. Multivariate Statistical Analysis of Minor Components (BUCKET-2) of EVOO
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazzocchi, A.; Leone, L.; Agostoni, C.; Pali-Schöll, I. The Secrets of the Mediterranean Diet. Does [Only] Olive Oil Matter? Nutrients 2019, 11, 2941. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.; Casal, S.; Pinho, T.; Cruz, R.; Baptista, P.; Martín, H.; Asensio-S-Manzanera, M.C.; Peres, A.M.; Pereira, J.A. Olive Oil Characteristics of Eleven Cultivars Produced in a High-Density Grove in Valladolid Province (Spain). Eur. Food Res. Technol. 2021, 247, 3113–3122. [Google Scholar] [CrossRef]
- Famiani, F.; Farinelli, D.; Rollo, S.; Camposeo, S.; Di Vaio, C.; Inglese, P. Evaluation of Different Mechanical Fruit Harvesting Systems and Oil Quality in Very Large Size Olive Trees. Span. J. Agric. Res. 2014, 12, 960–972. [Google Scholar] [CrossRef]
- Rodrigues, N.; Casal, S.; Peres, A.M.; Baptista, P.; Bento, A.; Martín, H.; Asensio-S-Manzanera, M.C.; Pereira, J.A. Effect of Olive Trees Density on the Quality and Composition of Olive Oil from Cv. Arbequina. Elsevier Enhanc. Reader. 2018, 238, 222–233. [Google Scholar] [CrossRef]
- Polari, J.J.; Mori, M.; Wang, S.C. Virgin Olive Oils from Super-High-Density Orchards in California: Impact of Cultivar, Harvest Time, and Crop Season on Quality and Chemical Composition. Eur. J. Lipid Sci. Technol. 2021, 123, 2000180. [Google Scholar] [CrossRef]
- Camposeo, S.; Vivaldi, G.A.; Montemurro, C.; Fanelli, V.; Canal, M.C. Lecciana, a New Low-Vigour Olive Cultivar Suitable for Super High Density Orchards and for Nutraceutical EVOO Production. Agronomy 2021, 11, 2154. [Google Scholar] [CrossRef]
- Pellegrini, G.; La Sala, P.; Camposeo, S.; Contò, F. Economic Sustainability of the Olive Oil High and Super-High Density Cropping Systems in Italy. Glob. Bus. Econ. Rev. 2017, 19, 553–569. [Google Scholar] [CrossRef]
- Pellegrini, G.; Ingrao, C.; Camposeo, S.; Tricase, C.; Contò, F.; Huisingh, D. Application of Water Footprint to Olive Growing Systems in the Apulia Region: A Comparative Assessment. J. Clean. Production 2016, 112, 2407–2418. [Google Scholar] [CrossRef]
- Camposeo, S.; Vivaldi, G.A.; Russo, G.; Melucci, F.M. Intensification in Olive Growing Reduces Global Warming Potential under Both Integrated and Organic Farming. Sustainability 2022, 14, 6389. [Google Scholar] [CrossRef]
- Home Blog. Available online: https://www.agromillora.com/olint/en/ (accessed on 6 December 2022).
- Camposeo, S.; Godini, A. Preliminary observations about the performance of 13 varieties according to the super high density oliveculture training system in Apulia (southern Italy). Adv. Hortic. Sci. 2010, 24, 16–20. [Google Scholar] [CrossRef]
- Trentacoste, E.R.; Connor, D.J.; Gómez-Del-Campo, M. Response of Oil Production and Quality to Hedgerow Design in Super-High-Density Olive Cv. Arbequina Orchards. Agron. 2021, 11, 1632. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, L.; Kranjac, M.; Marijanović, Z.; Jerković, I.; Corell, M.; Moriana, A.; Carbonell-Barrachina, Á.A.; Sendra, E.; Hernández, F. Quality Attributes and Fatty Acid, Volatile and Sensory Profiles of “Arbequina” HydroSOStainable Olive Oil. Molecules 2019, 24, 2148. [Google Scholar] [CrossRef] [PubMed]
- Lo Bianco, R.; Proietti, P.; Regni, L.; Caruso, T. Planting Systems for Modern Olive Growing: Strengths and Weaknesses. Agriculture 2021, 11, 494. [Google Scholar] [CrossRef]
- Connor, D.J.; Gomez-Del-Campo, M.; Rousseaux, M.C.; Searles, P.S. Structure, Management and Productivity of Hedgerow Olive Orchards: A Review. Sci. Hortic. 2014, 169, 71–93. [Google Scholar] [CrossRef]
- Vivaldi, G.; Strippoli, G.; Pascuzzi, S.; Stellacci, A.; Camposeo, S. Olive Genotypes Cultivated in an Adult High-Density Orchard Respond Differently to Canopy Restraining by Mechanical and Manual Pruning. Sci. Hortic. 2015, 192, 391–399. [Google Scholar] [CrossRef]
- Caruso, T.; Campisi, G.; Marra, F.; Camposeo, S.; Vivaldi, G.; Proietti, P.; Nasini, L. Growth and Yields of “Arbequina” High-Density Planting Systems in Three Different Olive Growing Areas In Italy. Acta Hortic. 2014, 1057, 341–348. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Camposeo, S.; De Gennaro, B.; Pascuzzi, S.; Roselli, L. In the Ancient World, Virgin Olive Oil Was Called “Liquid Gold” by Homer and “the Great Healer” by Hippocrates. Why Has This Mythic Image Been Forgotten? Food Res. Int. 2014, 62, 1062–1068. [Google Scholar] [CrossRef]
- Lechhab, T.; Lechhab, W.; Cacciola, F.; Salmoun, F. Sets of Internal and External Factors Influencing Olive Oil (Olea europaea L.) Composition: A Review. Eur. Food Res. Technol. 2022, 248, 1069–1088. [Google Scholar] [CrossRef]
- Romero, N.; Saavedra, J.; Tapia, F.; Sepúlveda, B.; Aparicio, R. Influence of Agroclimatic Parameters on Phenolic and Volatile Compounds of Chilean Virgin Olive Oils and Characterization Based on Geographical Origin, Cultivar and Ripening Stage. J. Sci. Food Agric. 2016, 96, 583–592. [Google Scholar] [CrossRef]
- Beltrán, M.; Sánchez-Astudillo, M.; Aparicio, R.; García-González, D.L. Geographical Traceability of Virgin Olive Oils from South-Western Spain by Their Multi-Elemental Composition. Food Chem. 2015, 169, 350–357. [Google Scholar] [CrossRef]
- The EU Food Fraud Network. Available online: https://food.ec.europa.eu/safety/agri-food-fraud/eu-food-fraud-network_en (accessed on 6 December 2022).
- No. 1019/2002; Marketing Standards for Olive Oil. The Commission of the European Communities: Brussels, Belgium, 2002.
- Masetti, O.; Sorbo, A.; Nisini, L. NMR Tracing of Food Geographical Origin: The Impact of Seasonality, Cultivar and Production Year on Data Analysis. Separations 2021, 8, 230. [Google Scholar] [CrossRef]
- Hlima, H.B.; Ayed, R.B.; Ennouri, K.; Smaoui, S. Geographical Discrimination of Virgin Olive Oils from the Tunisian Coasts by Combining Fatty Acids and Phenolic Acids Profiles within a Multivariate Analysis. J. Oleo Sci. 2017, 66, 963–971. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klikarová, J.; Česlová, L.; Kalendová, P.; Dugo, P.; Mondello, L.; Cacciola, F. Evaluation of Italian Extra Virgin Olive Oils Based on the Phenolic Compounds Composition Using Multivariate Statistical Methods. Eur. Food Res. Technol. 2020, 246, 1241–1249. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 182/2009 of 6 March 2009 Amending Regulation (EC) No 1019/2002 on Marketing Standards for Olive Oil. Off. J. Eur. Union. 2009, 63, 6–8. [Google Scholar]
- Calò, F.; Girelli, C.R.; Wang, S.C.; Fanizzi, F.P. Geographical Origin Assessment of Extra Virgin Olive Oil via NMR and MS Combined with Chemometrics as Analytical Approaches. Foods 2022, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Maestrello, V.; Solovyev, P.; Bontempo, L.; Mannina, L.; Camin, F. Nuclear Magnetic Resonance Spectroscopy in Extra Virgin Olive Oil Authentication. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4056–4075. [Google Scholar] [CrossRef]
- Mannina, L.; Patumi, M.; Proietti, N.; Bassi, D.; Segre, A.L. Geographical Characterization of Italian Extra Virgin Olive Oils Using High-Field 1 H NMR Spectroscopy. J. Agric. Food Chem. 2001, 49, 2687–2696. [Google Scholar] [CrossRef]
- Girelli, C.R.; Calò, F.; Angilè, F.; Del Coco, L.; Papadia, P.; Biagianti, A.; Barbini, D.; Mazzi, L.; Fanizzi, F.P. 1H NMR–metabolomics for PDO and PGI EVOOsAssessment. In Proceedings of the Virtual 2020 AOCS Annual Meeting & Expo, Urbana, IL, USA, 29 June–3 July 2020. [Google Scholar] [CrossRef]
- Calò, F. Metabolomic Techniques Applied To Extra Virgin Olive Oils For Geographical Certification. Ph.D. Thesis, University of Salento, Lecce, Italy, 2022. [Google Scholar]
- Camposeo, S.; Vivaldi, G.A.; Gattullo, C.E. Ripening Indices and Harvesting Times of Different Olive Cultivars for Continuous Harvest. Sci. Hortic. 2013, 151, 1–10. [Google Scholar] [CrossRef]
- Girelli, C.R.; Del Coco, L.; Zelasco, S.; Salimonti, A.; Conforti, F.L.; Biagianti, A.; Barbini, D.; Fanizzi, F.P. Traceability of “Tuscan PGI” Extra Virgin Olive Oils by 1H NMR Metabolic Profiles Collection and Analysis. Metabolites 2018, 8, 60. [Google Scholar] [CrossRef]
- Girelli, C.R.; Calò, F.; Angilè, F.; Mazzi, L.; Barbini, D.; Fanizzi, F.P. 1H NMR Spectroscopy to Characterize Italian Extra Virgin Olive Oil Blends, Using Statistical Models and Databases Based on Monocultivar Ref. Oils. Foods 2020, 9, 1797. [Google Scholar] [CrossRef]
- van den Berg, R.A.; Hoefsloot, H.C.; Westerhuis, J.A.; Smilde, A.K.; van der Werf, M.J. Centering, Scaling, and Transformations: Improving the Biological Information Content of Metabolomics Data. BMC Genom. 2006, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Mohammadkhani, L.G.; Khoshkam, M.; Kompany-Zareh, M. Effect of Different Pretreatment Methods on Classification of Serum Samples Measured with 1H-NMR. Research Square 2022. [CrossRef]
- Angilè, F.; Vivaldi, G.A.; Girelli, C.R.; Del Coco, L.; Caponio, G.; Lopriore, G.; Fanizzi, F.P.; Camposeo, S. Treated Unconventional Waters Combined with Different Irrigation Strategies Affect 1H NMR Metabolic Profile of a Monovarietal Extra Virgin Olive Oil. Sustainability 2022, 14, 1592. [Google Scholar] [CrossRef]
- Grace, S.C.; Hudson, D.A. Processing and Visualization of Metabolomics Data Using R. In Metabolomics-Fundamentals and Applications; IntechOpen: London, UK; BoD–Books on Demand: Norderstedt, Germany, 2016; pp. 63–94. [Google Scholar]
- Ruiz-Aracama, A.; Goicoechea, E.; Guillén, M.D. Direct Study of Minor Extra-Virgin Olive Oil Components without Any Sample Modification. 1H NMR Multisupression Experiment: A Powerful Tool. Food Chem. 2017, 228, 301–314. [Google Scholar] [CrossRef]
- Del Coco, L.; Girelli, C.; Angilè, F.; Mascio, I.; Montemurro, C.; Distaso, E.; Tamburrano, P.; Chiurlia, S.; Clodoveo, M.; Corbo, F.; et al. NMR-Based Metabolomic Study of Apulian Coratina Extra Virgin Olive Oil Extracted with a Combined Ultrasound and Thermal Conditioning Process in an Industrial Setting. Food Chem. 2021, 345, 128778. [Google Scholar] [CrossRef]
- Martínez-Yusta, A.; Goicoechea, E.; Guillén, M.D. A Review of Thermo-Oxidative Degradation of Food Lipids Studied by 1H NMR Spectroscopy: Influence of Degradative Conditions and Food Lipid Nature. Compr. Rev. Food Sci. Food Saf. 2014, 13, 838–859. [Google Scholar] [CrossRef]
- Mansouri, F.; Ben Moumen, A.; Aazza, S.; Belhaj, K.; Fauconnier, M.; Sindic, M.; Caid, H.S.; Elamrani, A. Quality and Chemical Profiles of Virgin Olive Oils of Three European Cultivars Suitable for Super-High-Density Planting Conditions in Eastern Morocco. Mater. Today Proc. 2019, 13, 998–1007. [Google Scholar] [CrossRef]
- Temime, S.B.; Manai, H.; Methenni, K.; Baccouri, B.; Abaza, L.; Daoud, D.; Casas, J.S.; Bueno, E.O.; Zarrouk, M. Sterolic Composition of Chétoui Virgin Olive Oil: Influence of Geographical Origin. Food Chem. 2008, 110, 368–374. [Google Scholar] [CrossRef]
- Girelli, C.R.; Del Coco, L.; Papadia, P.; De Pascali, S.A.; Fanizzi, F.P. Harvest year effects on Apulian EVOOs evaluated by 1H NMR based metabolomics. Peer. J. 2016, 4, e2740. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angilè, F.; Coco, L.D.; Girelli, C.R.; Calò, F.; Mazzi, L.; Fanizzi, F.P.; Vivaldi, G.A.; Camposeo, S. Proton Nuclear Magnetic Resonance (1H NMR) Metabolic Profiles Discriminate Two Monovarietal Extra Virgin Olive Oils, Cultivars Arbequina and Koroneiki, with Different Geographical Origin. Horticulturae 2023, 9, 66. https://doi.org/10.3390/horticulturae9010066
Angilè F, Coco LD, Girelli CR, Calò F, Mazzi L, Fanizzi FP, Vivaldi GA, Camposeo S. Proton Nuclear Magnetic Resonance (1H NMR) Metabolic Profiles Discriminate Two Monovarietal Extra Virgin Olive Oils, Cultivars Arbequina and Koroneiki, with Different Geographical Origin. Horticulturae. 2023; 9(1):66. https://doi.org/10.3390/horticulturae9010066
Chicago/Turabian StyleAngilè, Federica, Laura Del Coco, Chiara Roberta Girelli, Francesca Calò, Lucia Mazzi, Francesco Paolo Fanizzi, Gaetano Alessandro Vivaldi, and Salvatore Camposeo. 2023. "Proton Nuclear Magnetic Resonance (1H NMR) Metabolic Profiles Discriminate Two Monovarietal Extra Virgin Olive Oils, Cultivars Arbequina and Koroneiki, with Different Geographical Origin" Horticulturae 9, no. 1: 66. https://doi.org/10.3390/horticulturae9010066
APA StyleAngilè, F., Coco, L. D., Girelli, C. R., Calò, F., Mazzi, L., Fanizzi, F. P., Vivaldi, G. A., & Camposeo, S. (2023). Proton Nuclear Magnetic Resonance (1H NMR) Metabolic Profiles Discriminate Two Monovarietal Extra Virgin Olive Oils, Cultivars Arbequina and Koroneiki, with Different Geographical Origin. Horticulturae, 9(1), 66. https://doi.org/10.3390/horticulturae9010066








