Role of Spectrum-Light on Productivity, and Plant Quality over Vertical Farming Systems: Bibliometric Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
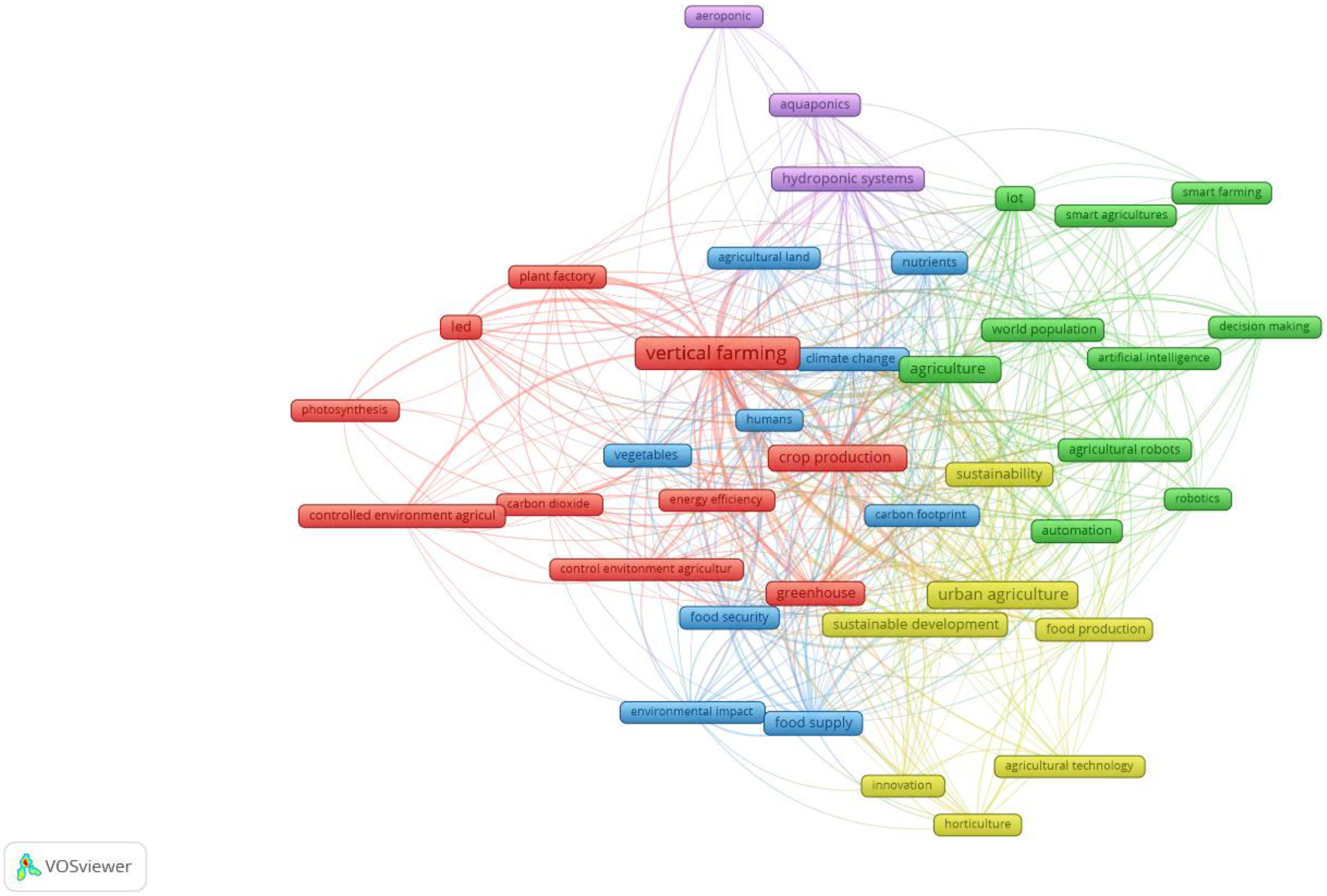
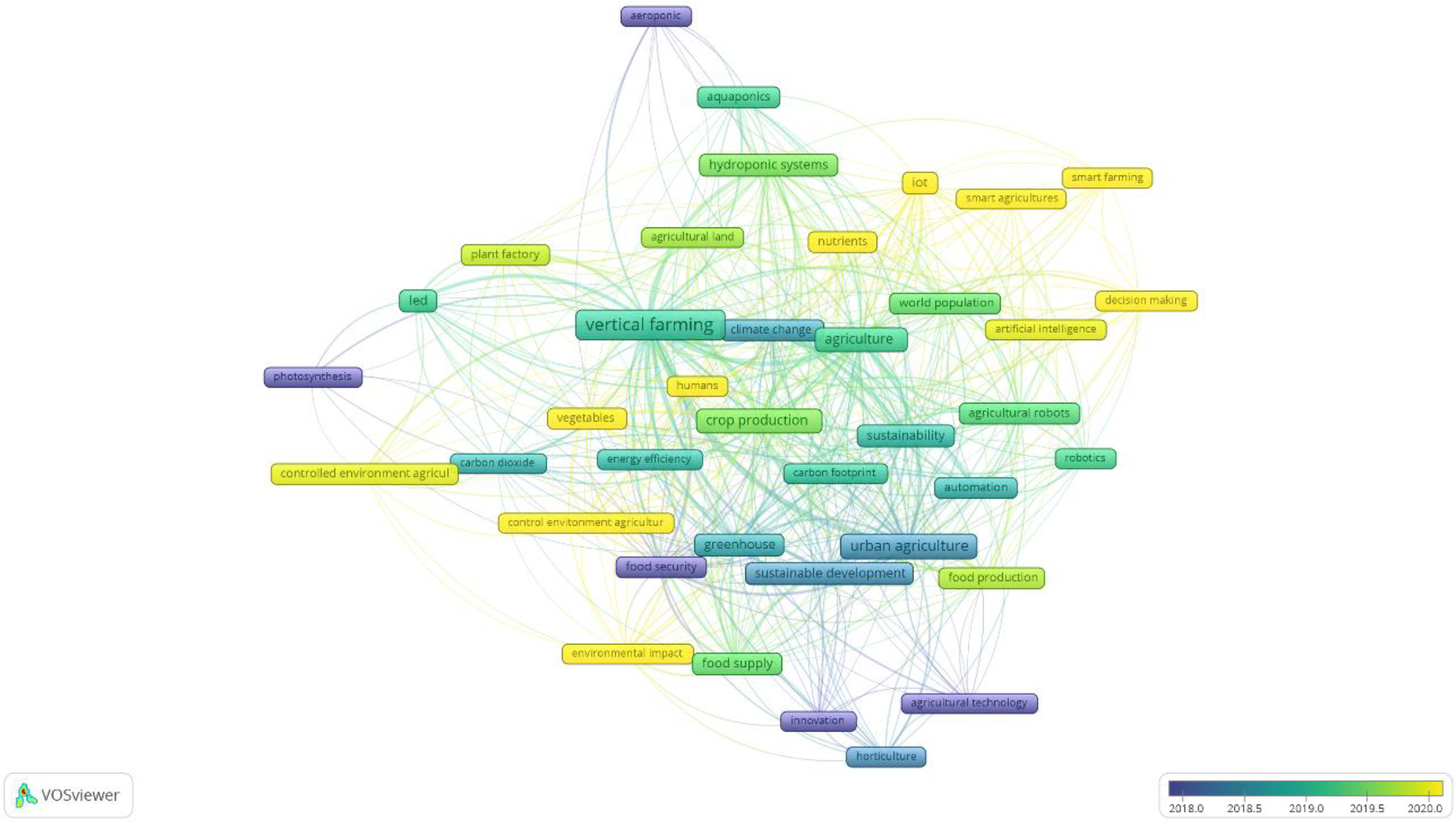
3.1. Bibliometric Study: Clustering
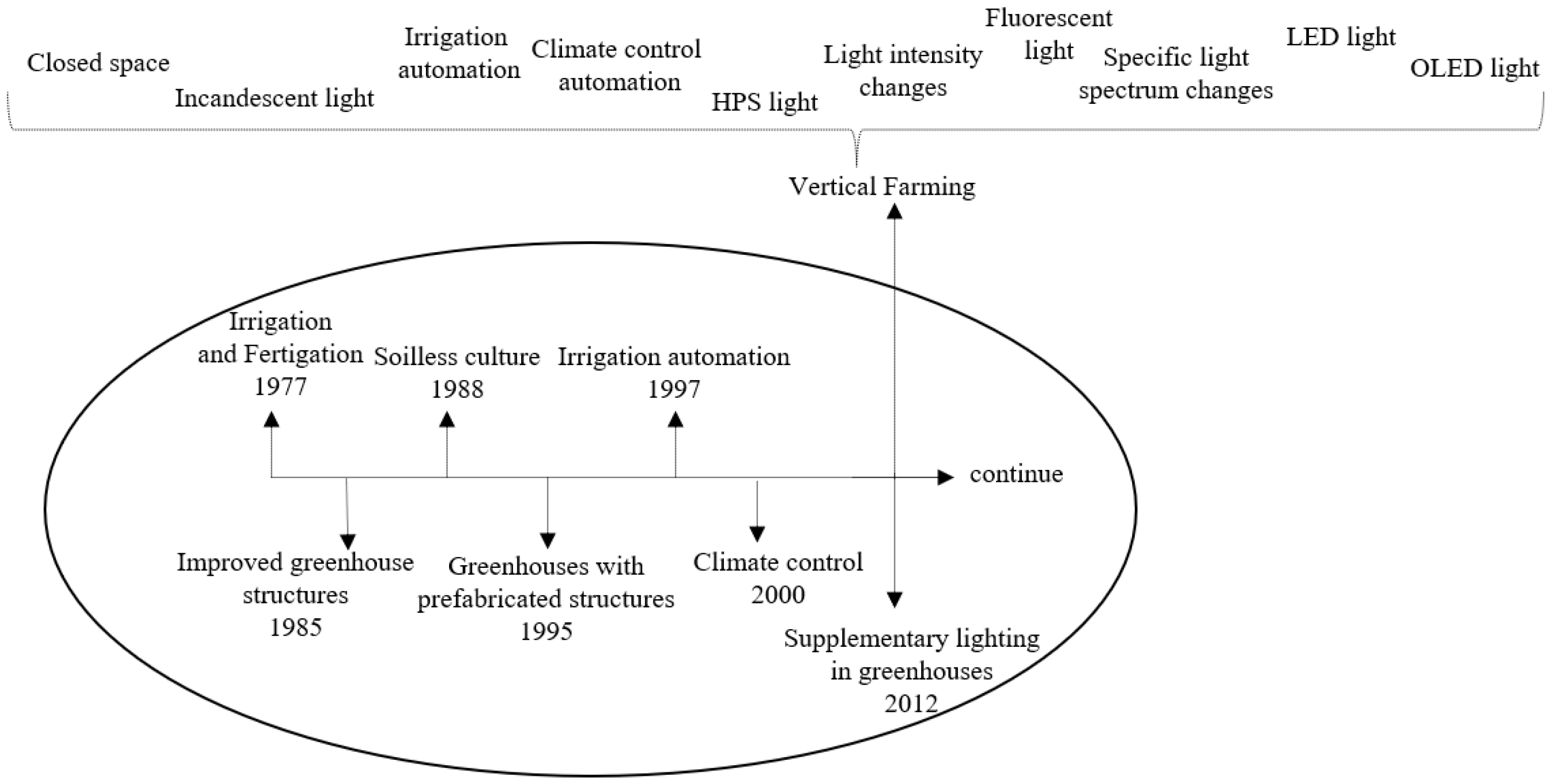
3.2. Development of VF Systems
3.3. Characteristics of VF Systems
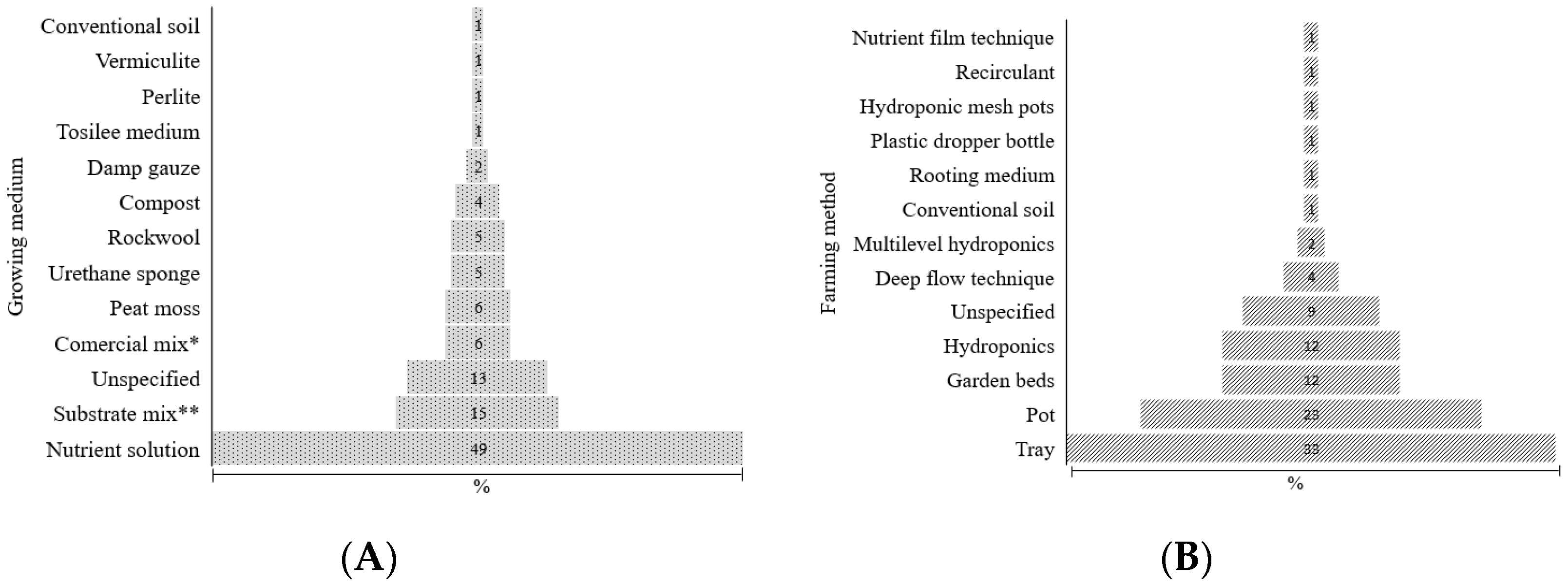
3.3.1. Methods and Culture Media
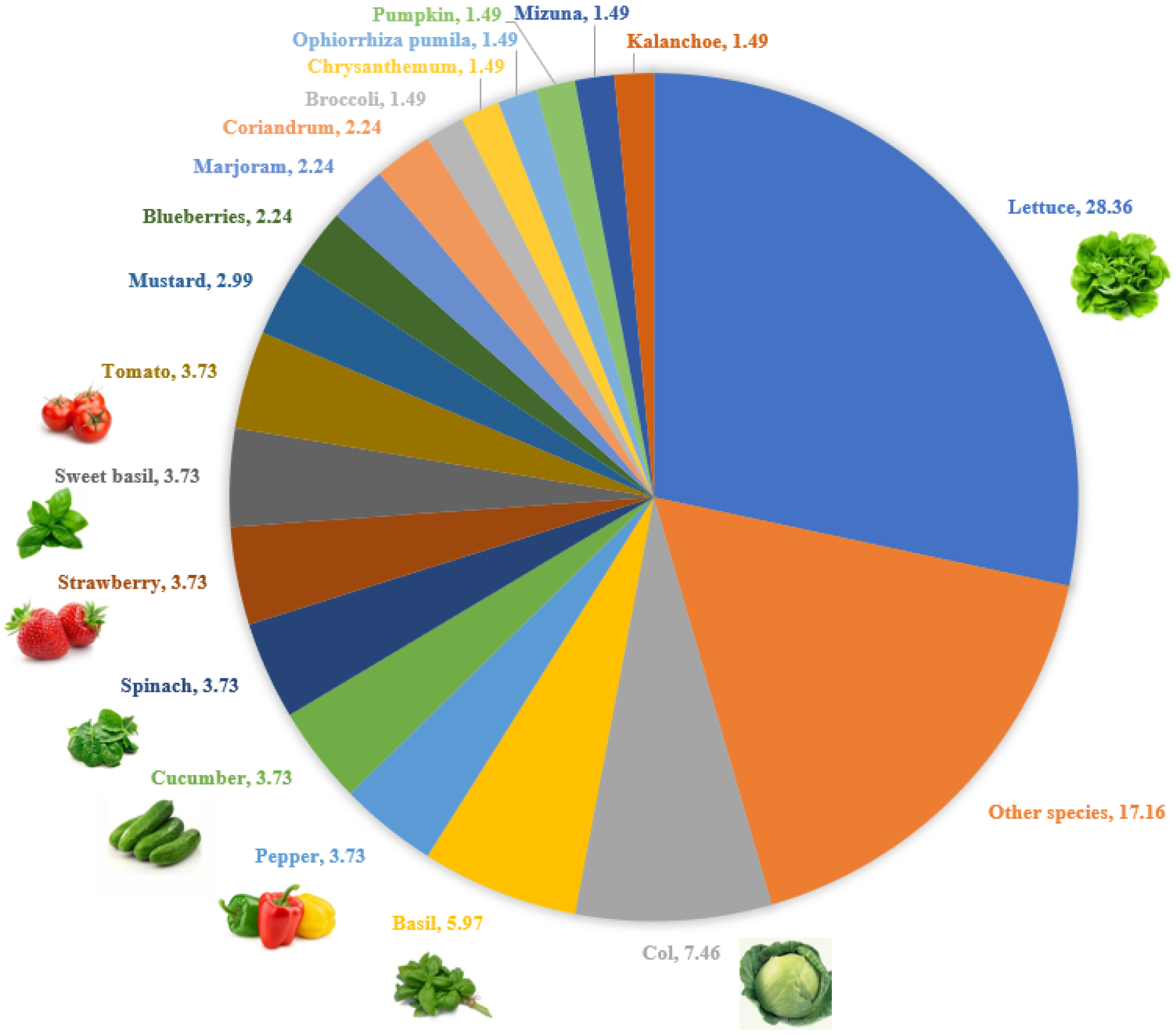
3.3.2. Cultivated Plant Species
3.4. Effect of Light in VF on Productivity, Quality, and Nutraceutical Values
3.4.1. Light Spectrum
3.4.2. Light Intensity
3.4.3. Photoperiod
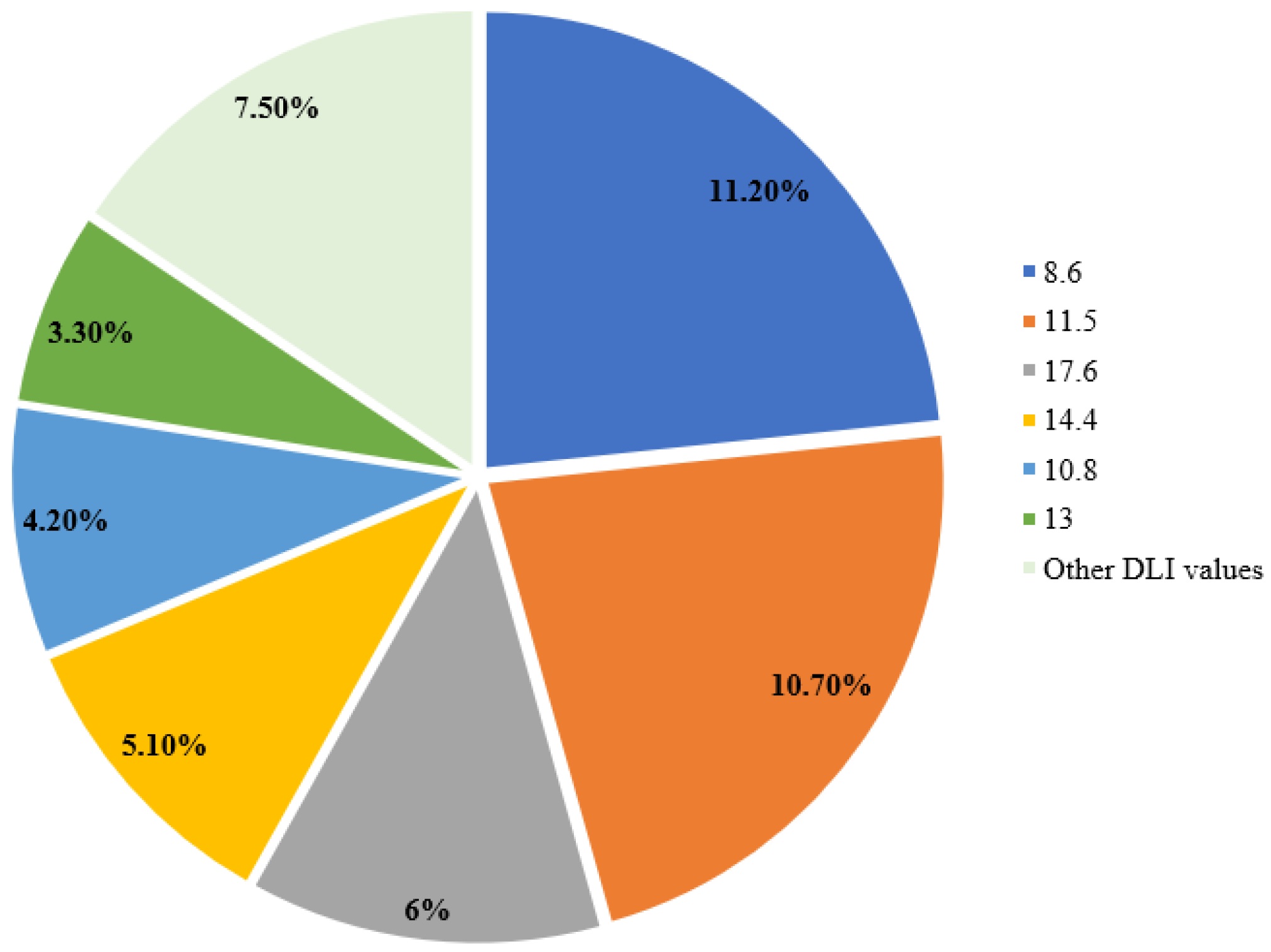
3.4.4. Daily Light Integral (DLI)
| Crop | Variety | Lamp Type | PPFD (μmol∙m−2·s−1) | Hours Per Day (h·day−1) | Best Results | Reference |
|---|---|---|---|---|---|---|
| Amaranth (Amaranthus cruentus) | Three-color cv. Red Aztec | LED R/B | 180 | 16 | Higher blue light, higher phenolic compounds. Chlorophyll decreases with R/B ratio 1:9. | Bantis [102] |
| Arabidopsis (Arabidopsis thaliana) | * | LED R/G/B | 140 | 16 | Red or blue light alone, they had opposite effects on tested parameters, but when of green light was mixed, mediate the effects caused, benefiting growth. | Zou et al. [90] |
| Asparagus (Asparagus aethiopicus) | Aethiopicus | LED B/R | * | 8 | Blue-red light combination improves yield by 1.5:1 ratio. | Rihan et al. [103] |
| Basil (Ocimum basilicum) | Dolly Emily | LED W/R; B | 150; 300 | 16; 18 | The plant fresh mass did not respond to the blue light while plant dry matter content was reduced at the combination high fraction of blue and a high PPFD. | Larsen et al. [75] |
| Genovese | LED R/B | 215; 250 | 16 | Best growth with light intensity 250 μmol·m−2·s−1. | Pennisi et al. [76,77] | |
| Red Rubin | LED R/W; G/R/W/B | 224 | 16 | Green spectrum showed positive effects on phytochemical accumulation. | Dou et al. [79] | |
| Blueberries (Vaccinium sect. Cyanococcus) | Emerald Snowchaser | LED W/R; W/UVA/FR | 35; 70; 105; 140 | 20 | Transplants under W/R produced higher shoot and root dry matter that W/UVA/FR demonstrating the important use of white light. | Gómez et al. [104] |
| Borage (Borago officinalis) | Blue | LED R/B | 180 | 16 | Higher blue light, higher phenolic compounds. Chlorophyll decreases with R/B ratio 1:9. | Bantis [102] |
| Broccoli (Brassica oleracea) | Italica cv. Lvhua | LED UVA | 30 | 16 | UVA treatment increased the contents of total chlorophylls, total soluble proteins, total phenolic compounds, and ferric reducing antioxidant power. | Gao et al. [105] |
| LED G/B/R | 30; 50; 70; 90 | 12 | 50 μmol·m−2·s−1 (red: green: blue = 1:1:1:1) optimum light intensity and spectrum for growth. 70 μmol·m−2·s−1 optimal for phytochemical accumulation. | Gao et al. [106] | ||
| Canola (Brassica napus) | Kizakino-natane | Fluorescent W | 200 | 16 | 200 μmol·m−2·s−1 was not sufficient illumination to reach the entire canopy. | Saito et al. [107] |
| LED W/UVB | 200 | 16 + 0.25; 16 + 0.5; 16 + 1; 16 + 2; 16 + 5; 16 + 8; 16 + 12; 16 + 16; 16 + 24 additional UV-B light | At 200 μmol·m−2·s−1 the concentrations of all antioxidants increased Short-term UV-B irradiation could augment antioxidant biosynthesis without sacrificing crop yield or quality. | Lee et al. [72] | ||
| Capica (Stylosanthes Capitata) | * | LED R/W/B | 150.89 | 16 | Light R and FR improve reproductive responses. | Park et al. [108] |
| Choy Sum (Brassica rapa) | Parachinensis | LED W | 50 to 500 | 12 | Optimum illumination range is 400 μmol·m−2·s−1 for greater leaf area, stem thickness, root system and photosynthesis | Huang et al. [109] |
| Chrysanthemum (Chrysanthemum) | Gaya Yellow Radost | LED W | 180 | 4; 9; 10; 13 | Supplementing with light B for 4 h·day−1 promoted flowering and increased the number of flower buds. | Park and Jeong [110] |
| Col (Brassica alboglabra) | Bailey | LED W; R/W/B/UVA/FR | 300 | 10 (W); 12 | LED R/B/W/UVA/FR improved biomass, morphology, antioxidant compounds and tender leaf production. | He et al. [78] |
| Capitata | LED G | 0; 24; 48 | Chlorophyl concentration increased with intensity 50 μmol·m−2·s−1 with green light spectrum at 48 h·day−1. | Amagai et al. [111] | ||
| Chinensis | LED R; W | 200 | 16 | Increased growth and development with red LED. | Harun et al. [88] | |
| Lvbao | LED R/W + UVA | 250 | 12 | UVA was benefited to produce functional substances, while FR was conducive to a significant increase in crop yield. | He et al. [78] | |
| Pabularia Scarlet | FR LED R/W; R/W/G | 224 | 16 | Inclusion of G wavelengths decreased shoot biomass compared to that of plants grown under combinations of R and B light. | Dou et al. [79] | |
| Coriandrum (Coriandrum sativum L.) | Green Aroma | LED R/B; R/B/G | 150 | 16 | Increasing the spectral range increases the concentration coriander aromatics E-(2)-decenal and E-(2)-hexenal. Plants grown under LED R acquired the greatest biomass in the same period. | McAusland et al. [112] |
| * | LED R/B | * | 8 | The combination of blue-red light improves yield. | Rihan et al. [103] | |
| * | LED W | 300 | 16 | Growing coriander at 15 °C for 6 days increases the amount of dry biomass, antioxidant capacity, and a high content of secondary metabolites. | Nguyen et al. [113] | |
| Cucumber (Cucumis sativus L.) | Joeunbaegdadagi | LED W | 50; 100; 150; 200; 250 | 12; 16; 20 | PPFD 150 μmol∙m−2·s−1, 16 h·day−1 improved plant growth and energy efficiency. | An et al. [114] |
| Heukjong | LED W; FR | 200 | 16 | FR light positively supports plant morphological growth compared to light W. | Hwang et al. [115] | |
| Yuexiu No.3 | LED R/B | 12 to 16 | Optimal PPFD of 110 to 125 μmol∙m−2·s−1, in 14 to 16 h·day−1. | Cui et al. [19] | ||
| Garlic (Alium schoenoprasum) | Thick sheet | LED R/B | 180 | 16 | Higher blue light, higher phenolic compounds. Chlorophyll decreases with R/B ratio 1:9. | Bantis [102] |
| Gingeng (Panax ginseng) | Meyer | LED R/G/B | 80 | 16 | R light improved growth and photosynthesis, and B-light had a positive effect on bioactive compounds. | Kim et al. [89] |
| Kalanchoe (Kalanchoe blossfeldiana) | Lipstick Spain | LED W | 250 | 160 | Night Interruption Light affects morphogenesis and flowering depending on the cultivar. | Kang et al. [116] |
| Lemon balm (Melissa officinalis) | * | LED B/G/R/W | * | 16 | For blue spectra, the development and yield were lower despite having a significant impact on the photosynthesis activity. White and red-light spectra gave the best outputs in terms of impact on the growth and yield. | Rihan et al. [103] |
| Lettuce (Lactuca sativa L.) | Batavia Othilie | * | 200; 400; 750 | 16 | Dry mass increased with increasing photon flux to a PPFD of 750, but the highest fresh weight efficiency was achieved at 200 μmol·m−2·s−1. | Carotti et al. [117] |
| Best growth effect with LED W without combination. | Nguyen et al. [118] | |||||
| Butterhead cv. Asia Cherokee | LED W; W/R; W/B | 150 | 12 | Addition of constant FR increased weight and growth and reduced chlorophyll. | Meng and Runkle [81] | |
| Rex | LED B/R; B/R/FR | 180 | 24 | Optimal PPFD at 360 μmol·m−2·s−1 with LUE 6.5 g MJ−1. | Kim et al. [89] | |
| Romaine cv. Asia | LED R/B | 200 | 16 | Tipburn showed positive relationship with light intensity and the relative growth rate occurred during 23~27 days after sowing. | Xu et al. [119] | |
| Romaria Summer Surge | LED W LED W/R/B | 85; 125; 187 80; 120 | 16 12 | Increased growth under alternating R/B lights. The R/B combination is recommended for short-cycle crops to reduce autotoxin secretion and guarantee yields. | Ohtake et al. [120] | |
| Tiberius | LED W; R/B | 200 | 16 | Light quality with different R/B ratios showed pronounced effects on organic carbon and autotoxin secretion. | Zhou et al. [121] | |
| Yanzhi | LED W; W/FR; W/B | 250 | 10 | W/B produced higher contents of pigments, anthocyanins, vit C-A, phenolics and total flavonoids. With W/FR increase in fresh and dry weight. | Li et al. [122] | |
| Lychnis coronaria (Silene coronaria) | * | LED R/B | * | 8 | The combination of blue-red light improves yield. | Rihan et al. [103] |
| Marjoram (Origanum majorana) | * | LED W/FR; W; W/B | 362 | 4 | W/FR and W LEDs increased plant growth, dry matter, and light use efficiency. | Wittmann et al. [123] |
| Mentha (Mentha spicata) | * | LED R/W | * | 8 | B/R light combination improves yield. | Rihan et al. [103] |
| Mizuna (Brassica rapa) | Japonica Little Gem | LED R + B pulses LED W | 154 444; 370; 318; 278; 247; 222 | Various 10; 12; 14; 16; 18; 20 | Environmental stress in low light, higher water use efficiency. Biomass increased 16% in response to increasing the photoperiod from 10 to 20 h. Extending the photoperiod and reducing PPFD increased plant growth and reduced the instantaneous heat generated by the lamps. | Park et al. [124] Palmer et al. [125] |
| Mustard (Brassica juncea) | Red Lion | LED R/B | 180 | 16 | Increasing the blue light portion caused less phenolic compounds and total antioxidants. | Bantis [102] |
| Nasturtium (Tropaeolum majus L.) | * | LED W | 200; 300 | 16; 24 | 24 h improved dry weight, antioxidant capacity and total phenolic content. | Xu et al. [126] |
| Onion (Allium cepa) | Victory | LED R/G/B | 69–77 | * | Light R promoted leaf width and area, starch accumulation in shoots, but reduced concentrations of flavonoids and total saponins. | Zhou et al. [127] |
| Pea (Pisum sativum) | Dun | LED R/B | 180 | 16 | Increasing the blue light portion caused less phenolic compounds and total antioxidants. | Bantis [102] |
| Pepper (Capsicum annuum L.) | Serrano | LED W; R/B pulses | 50; 110; 180 | * | No difference between continuous and LED pulsed in production or capsaisinoid is shown. | Olvera-Gonzalez et al. [16] |
| Shinhong Tantan | LED W; FR | 200 | 16 | FR-enriched supplemental lighting for improved plant growth and morphology. | Hwang et al. [115] | |
| Pumpkin (Cucurbita mostacha) | Bulrojangsaeng | LED W; FR | 200 | 16 | Supplemental lighting enriched with FR improved plant growth and plant morphology. | Hwang et al. [115] |
| Heukjong | LED W | 150 | 16 | Scion and rootstock production in a Plant Factory with Artificial Light improves productivity and uniformity. | An et al. [128] | |
| Radish (Raphanus raphanistrum) | Saxa | LED R/B | 180 | 16 | Increasing the blue light portion caused less phenolic compounds and total antioxidants. | Bantis [102] |
| Saffron (Crocus sativus) | * | LED W | * | * | Decreases starch content. | Natsuhara et al. [15] |
| Sesame (Sesamum indicum) | Gomazou | Fluorescent | 235 | 12 | At 28 °C leaf browning was induced. At 15ºC the fresh weight of shoots was higher. | Date et al. [129] |
| Spinach (Spinacia oleraciea L.) | BJC009 | LED W/B/R | 145 | 14 | Increase productivity by 160%. | Fernández-Cabanás et al. [130] |
| Disease-resistant 388 | LED R/B/G | 100; 150 | 9; 13 | 150 μmol∙m−2∙s−1 and 9 h is optimal for best quality. | Zou et al. [90] | |
| Geant D Hiver | LED FR/R/G/B/UV | 150 | 16 | Photosynthesis developed best with a high ratio of R or FR light with B. | Bantis et al. [131] | |
| Ssamchoo (Brassica Lee) | Namai | LED W | 300 | 14 | Causes stunted growth due to photorespiration. | Noh and Jeong [132] |
| Strawberry (Fragaria × ananassa Duch.) | Benihoppe | LED R/W | 30; 90; 150; 210 | 16 | PPFD 90 is recommended at the rooting stage and 210 at the seedling stage. | Zheng et al. [83] |
| LED R/W | 200; 250; 300; 350 | 12; 16 | DLI 11.5–17.3 mol·m−2·day−1 is beneficial for propagation. | Zheng et al. [84] | ||
| Elan | LED W | 200; 300; 400; 500 | 16; 24 | At 24 h and 300 μmol∙m−2∙s−1 higher anthocyanin and productivity. | Maeda and Ito [91] | |
| Maehyang | Fluorescent | 230 | 16 | VF is a suitable method to improve the propagation system. | Park and Jeong [110] | |
| Sachinoka | Fluorescent LED W | 65 150 | 10 10 | High potential to produce flowers and fruits. Method and conditions suitable for growth. | Le et al. [21] | |
| Sweet basil (Ocimum basilicum) | Compact | LED W/R | 90 | 16 | Increased light B increased the content of pigments and phenolic compounds. | Azad et al. [133] |
| Tomato (Solanum lycopersicum L.) | Dongfeng | Fluorescent; LED W; W/R | 50; 100; 150 | 14 | LED light showed 110% energy saving, promote dry matter, leaf thickness Increase growth and production. | Zheng et al. [134] |
| Ingar F1 | LED W/B/G/A/R/FR | 210 | 15 | The combination of plant and architecture and spectrum-dependent photosynthesis results in the highest rate of crop photosynthesis under red light in plants initially grown under green light. | Dieleman et al. [135] | |
| Watercress (Nasturtium officinale L.) | Brassicaceae | LED R/B | 133; 160; 200; 266 | 12; 16; 20; 24 | 20 h with PPFD 160 enhanced glucosinolate and plant biomass. | Lam et al. [97] |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borrelli, P.; Robinson, D.A.; Panagos, P.; Lugato, E.; Yang, J.E.; Alewell, C.; Wuepper, D.; Montanarella, L.; Ballabio, C. Land use and climate change impacts on global soil erosion by water (2015–2070). Proc. Natl. Acad. Sci. USA 2020, 117, 21994–22001. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.; Niu, G. Introduction. In Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production, 2nd ed.; Kozai, T., Niu, G., Takagaki, M., Eds.; Elsevier Inc.: London, UK, 2020; pp. 3–5. [Google Scholar]
- Darko, E.; Heydarizadeh, P.; Schoefs, B.; Sabzalian, M.R. Photosynthesis under artificial light: The shift in primary and secondary metabolism. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130243. [Google Scholar] [CrossRef] [PubMed]
- SharathKumar, M.; Heuvelink, E.; Marcelis, L.F.M. Vertical Farming: Moving from Genetic to Environmental Modification. Trends Plant Sci. 2020, 25, 724–727. [Google Scholar] [CrossRef]
- Kozai, T. Why LED lighting for urban Agriculture? In LED Lighting for Urban Agriculture, 1st ed.; Kosai, T., Fujiwara, K., Runkle, E., Eds.; Springer: Singapore, 2016; pp. 3–18. [Google Scholar]
- Beacham, A.M.; Vickers, L.H.; Monaghan, J.M. Vertical farming: A summary of approaches to growing skywards. J. Hortic. Sci. Biotechnol. 2019, 94, 277–283. [Google Scholar] [CrossRef]
- Van Gerrewey, T.; Boon, N.; Geelen, D. Vertical farming: The only way is up? Agronomy 2022, 12, 2. [Google Scholar] [CrossRef]
- Ebel, R. Chinampas: An Urban Farming Model of the Aztecs and a Potential Solution for Modern Megalopolis. HortTechnology 2020, 30, 13–19. [Google Scholar] [CrossRef]
- van Delden, S.H.; SharathKumar, M.; Butturini, M.; Graamans, L.J.A.; Heuvelink, E.; Kacira, M.; Kaiser, E.; Klamer, R.S.; Klerkx, L.; Kootstra, G.; et al. Current status and future challenges in implementing and upscaling vertical farming systems. Nat. Food 2021, 2, 944–956. [Google Scholar] [CrossRef]
- Al-Kodmany, K. The Vertical Farm: A Review of developments and implications for the vertical city. Buildings 2018, 8, 24. [Google Scholar] [CrossRef]
- de Carbonnel, M.; Stormonth-Darling, J.M.; Liu, W.; Kuziak, D.; Jones, M.A. Realising the environmental potential of vertical farming systems through advances in plant photobiology. Biology 2022, 11, 922. [Google Scholar] [CrossRef]
- Butturini, M.; Marcelis, L.M. Vertical farming in Europe: Present status and outlook. In Plant Factory, 2nd ed.; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: London, UK, 2020; pp. 77–91. [Google Scholar]
- Johkan, M.; Shoji, K.; Goto, F.; Hahida, S.; Yoshihara, T. Effect of green light wavelength and intensity on photomorphogenesis and photosynthesis in Lactuca sativa. Environ. Exp. Bot. 2012, 75, 128–133. [Google Scholar] [CrossRef]
- Urrestarazu, M.; Nájera, C.; del Mar Gea, M. Effect of the spectral quality and intensity of light-emitting diodes on several horticultural crops. HortScience 2016, 51, 268–271. [Google Scholar] [CrossRef]
- Natsuhara, R.; Uno, Y.; Kuroki, S.; Kajikawa, N.; Umaba, K.; Zako, K.; Nishimura, T.; Itoh, H. Development of a non-destructive starch concentration measurement technique in saffron (Crocus sativus L.) corms using light scattering image analysis. Environ. Control Biol. 2020, 58, 105–113. [Google Scholar] [CrossRef]
- Olvera-Gonzalez, E.; Escalante-Garcia, N.; Myers, D.; Ampim, P.; Obeng, E.; Alaniz-Lumbreras, D.; Castaño, V. Pulsed LED-lighting as an alternative energy savings technique for vertical farms and plant factories. Energies 2021, 14, 1603. [Google Scholar] [CrossRef]
- Liu, N.; Ji, F.; Xu, L.; He, D. Effects of LED light quality on the growth of pepper seedling in plant factory. Int. J. Agric. Biol. Eng. 2019, 12, 44–50. [Google Scholar] [CrossRef]
- Ke, X.; Yoshida, H.; Hikosaka, S.; Goto, E. Optimization of photosynthetic photon flux density and light quality for increasing radiation-use efficiency in dwarf tomato under LED light at the vegetative growth stage. Plants 2022, 11, 121. [Google Scholar] [CrossRef]
- Cui, J.; Song, S.; Yu, J.; Liu, H. Effect of daily light integral on cucumber plug seedlings in artificial light plant factory. Horticulturae 2021, 7, 139. [Google Scholar] [CrossRef]
- Iwao, T.; Murakami, T.; Akaboshi, O.; Cho, H.Y.; Yamada, M.; Takahashi, S.; Kato, M.; Horiuchi, N.; Ogiwara, I. Possibility of Harvesting June-bearing Strawberries in a Plant Factory with Artificial Light during Summer and Autumn by Re-Using Plants Cultivated by Forcing Culture. Environ. Control Biol. 2021, 59, 99–105. [Google Scholar] [CrossRef]
- Le, L.T.; Dinh, H.T.; Takaragawa, H.; Watanabe, K.; Ureshino, K.; Kawamitsu, Y. Photosynthetic responses and reproductive ability of strawberry following sunlight application in a plant factory closed system in subtropical Okinawa. Eur. J. Hortic. Sci. 2021, 86, 590–598. [Google Scholar] [CrossRef]
- Nájera, C.; Gallegos-Cedillo, V.M.; Ros, M.; Pascual, J.A. LED Lighting in Vertical Farming Systems Enhances Bioactive Compounds and Productivity of Vegetables Crops. Biol. Life Sci. Forum 2022, 16, 24. [Google Scholar] [CrossRef]
- Lazzarin, M.; Meisenburg, M.; Meijer, D.; van Ieperen, W.; Marcelis, L.F.M.; Kappers, I.F.; van der Krol, A.R.; van Loon, J.J.A.; Dicke, M. LEDs make it resilient: Effects on plant growth and defense. Trends Plant Sci. 2021, 26, 496–508. [Google Scholar] [CrossRef]
- Smit, J.; Nasr, J.; Ratta, A. Benefits of Urban Agriculture. In Urban Agriculture: Food, Jobs and Sustainable Cities; United Nations Development Programme: New York, NY, USA, 2001. [Google Scholar]
- Chen, Y.; Li, T.; Yang, Q.; Zhang, Y.; Zou, J.; Bian, Z.; Wen, X. UVA radiation is beneficial for yield and quality of indoor cultivated lettuce. Front. Plant Sci. 2019, 10, 1563. [Google Scholar] [CrossRef] [PubMed]
- Stanghellini, C.; Oosfer, B.; Heuvelink, E. Greenhouse Horticulture: Technology for Optimal Crop Production; Wageningen Academic Publishers: Wageningen, The Netherlands, 2019; pp. 1–311. [Google Scholar]
- Hipps, L.E.; Asrar, G.; Kanemasu, E.T. Assessing the interception of photosynthetically active radiation in winter wheat. Agric. Meteorol. 1983, 28, 253–259. [Google Scholar] [CrossRef]
- McCree, K.J. The action spectrum, absorbance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1972, 9, 191–216. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Ntagkas, N.; Siebenkäs, A.; Mäenpää, M.; Matsubara, S.; Pons, T. A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol. 2019, 223, 1073–1105. [Google Scholar] [CrossRef]
- McClung, C.R. Plant Circadian Rhythms. Plant Cell 2006, 18, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Craufurd, P.Q.; Wheeler, T.R. Climate change and the flowering time of annual crops. J. Exp. Bot. 2009, 60, 2529–2539. [Google Scholar] [CrossRef] [PubMed]
- Faust, J.E.; Logan, J. Daily Light Integral: A Research Review and High-resolution Maps of the United States. HortScience 2018, 53, 1250–1257. [Google Scholar] [CrossRef]
- Hatfield, J.L. Radiation use efficiency: Evaluation of cropping and management systems. Agron. J. 2014, 106, 1820–1827. [Google Scholar] [CrossRef]
- Slattery, R.A.; VanLoocke, A.; Bernacchi, C.J.; Zhu, X.-G.; Ort, D.R. Photosynthesis, light use efficiency, and yield of reduced-chlorophyll soybean mutants in field conditions. Front. Plant Sci. 2017, 8, 549. [Google Scholar] [CrossRef]
- Farquhar, G.D.; von Caemmerer, S.B.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef]
- Urban, O.; Janouš, D.; Acosta, M.; Czerný, R.; Marková, I.; Navrátil, M.; Pavelka, M.; Pokorný, R.; Šprtová, M.; Zhang, R.; et al. Ecophysiological controls over the net ecosystem exchange of mountain spruce stand. Comparison of the response in direct vs. diffuse solar radiation. Glob. Chang. Biol. 2007, 13, 157–168. [Google Scholar] [CrossRef]
- Gallegos-Cedillo, V.M.; Diánez, F.; Nájera, C.; Santos, M. Plant agronomic features can predict quality and field performance: A bibliometric analysis. Agronomy 2021, 11, 2305. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, X.; Jeong, B.R. Carbon dioxide enrichment combined with supplemental light improve growth and quality of plug seedlings of Astragalus membranaceus Bunge and Codonopsis lanceolata Benth. et Hook. f. Agronomy 2019, 9, 715. [Google Scholar] [CrossRef]
- Paulus, D.; Zorzzi, I.C.; Rankrape, F.; Nava, G.A. Wastewater from fish farms for producing Eucalyptus grandis seedlings. Floresta Ambient 2019, 26, e2017580. [Google Scholar] [CrossRef]
- Rocha, J.S.; Calzavara, A.K.; Bianchini, E.; Pimenta, J.A.; Stolf-Moreira, R.; Oliveira, H.C. Nitrogen supplementation improves the high-light acclimation of Guazuma ulmifolia Lam. seedlings. Trees 2019, 33, 421–431. [Google Scholar] [CrossRef]
- Aimi, S.C.; Araujo, M.M.; Fermino, M.H.; Tabaldi, L.A.; Zavistanovicz, T.C.; Mieth, P. Substrate and fertilization in the quality of Myrocarpus frondosus seedlings. Floresta 2019, 49, 831–840. [Google Scholar] [CrossRef]
- Marcelis, L.F.M.; Heuvelink, E.; Goudriaan, J. Modelling biomass production and yield of horticultural crops: A review. Sci. Hortic. 1998, 74, 83–111. [Google Scholar] [CrossRef]
- Mangon, H. Production de la matière verte des feuilles sous l’influence de la lumière électrique. Compt. Rend. Acad. Sci. Paris 1861, 53, 243–244. (In French) [Google Scholar]
- Pfeiffer, N.E. Microchemical and morphological studies of effect of light on plants. Bot. Gaz. 1926, 81, 173–195. [Google Scholar] [CrossRef]
- Siemens, C.W. III. On the influence of electric light upon vegetation, and on certain physical principles involved. Proc. R. Soc. Lond. 1880, 30, 210–219. [Google Scholar] [CrossRef]
- Murdoch, J.B. Illuminating Engineering—From Edison’s Lamp to the Laser; Macmillan Publishing Company: New York, NY, USA, 1985. [Google Scholar]
- Morrow, R.C.; Tibbitts, T.W. Evidence for involvement of phytochrome in tumor development on plants. Plant Physiol. 1988, 88, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Bula, R.J.; Morrow, R.C.; Tibbitts, T.W.; Barta, D.J.; Ignatius, R.W.; Martin, T.S. Light-emitting diodes as a radiation source for plants. HortScience 1991, 26, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, R.M. A historical background of plant lighting: An introduction to the workshop. HortScience 2008, 43, 1942–1943. [Google Scholar] [CrossRef]
- Moghimi, F. Vertical farming economics in 10 minutes. Rutgers Bus. Rev. 2021, 6, 122–131. [Google Scholar]
- GlobeNewswire. Straits Research: Microgreens Market Size. 2022. Available online: https://www.globenewswire.com/en/news-release/2022/07/18/2481309/0/en/Microgreens-Market-Size-is-projected-to-reach-USD-3-69-Billion-by-2030-growing-at-a-CAGR-of-11-Straits-Research.html (accessed on 1 December 2022).
- O’Sullivan, C.A.; McIntyre, C.L.; Dry, I.B.; Hani, S.M.; Hochman, Z.; Bonnett, G.D. Vertical farms bear fruit. Nat. Biotechnol. 2020, 38, 160–162. [Google Scholar] [CrossRef]
- Frías-Moreno, M.N.; Parra-Quezada, R.A.; González-Aguilar, G.; Ruíz-Canizales, J.; Molina-Corral, F.J.; Sepulveda, D.R.; Salas-Salazar, N.; Olivas, G.I. Quality, Bioactive Compounds, Antioxidant Capacity, and Enzymes of Raspberries at Different Maturity Stages, Effects of Organic vs. Conventional Fertilization. Foods 2021, 10, 953. [Google Scholar] [CrossRef]
- Azevedo, L.; de Araujo Ribeiro, P.F.; de Carvalho Oliveira, J.A.; Correia, M.G.; Ramos, F.M.; de Oliveira, E.B.; Barros, F.; Stringheta, P.C. Camu-camu (Myrciaria dubia) from commercial cultivation has higher levels of bioactive compounds than native cultivation (Amazon Forest) and presents antimutagenic effects in vivo. J. Sci. Food Agric. 2019, 99, 624–631. [Google Scholar] [CrossRef]
- Hallmann, E. The influence of organic and conventional cultivation systems on the nutritional value and content of bioactive compounds in selected tomato types. J. Sci. Food Agric. 2012, 92, 2840–2848. [Google Scholar] [CrossRef]
- Ionică, M.E.; Nour, V.; Trandafir, I. Bioactive compounds and antioxidant activity of hot pepper fruits at different stages of growth and ripening. J. Appl. Bot. Food Qual. 2017, 90, 232–237. [Google Scholar]
- Nurzynska-Wierdak, R.; Zawislak, G. Herb yield and bioactive compounds of tarragon (Artemisia dracunculus L.) as influenced by plant density. Acta Sci. Pol. Hortorum Cultus 2014, 13, 207–221. [Google Scholar]
- Jędrszczyk, E.; Kopeć, A.; Bucki, P.; Ambroszczyk, A.M.; Skowera, B. The enhancing effect of plants growth biostimulants in garlic cultivation on the chemical composition and level of bioactive compounds in the garlic leaves, stems and bulbs. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 81–91. [Google Scholar] [CrossRef]
- Pék, Z.; Daood, H.; Nagyné, M.; Neményi, A.; Helyes, L. Effect of environmental conditions and water status on the bioactive compounds of broccoli. Open Life Sci. 2013, 8, 777–787. [Google Scholar] [CrossRef]
- Liu, R.H. Health-Promoting Components of Fruits and Vegetables in the Diet. Adv. Nutr. 2013, 4, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Van Eck, N.J.; Waltman, L. Visualizing bibliometric networks. In Measuring Scholarly Impact: Methods and Practice; Ding, Y., Rousseau, R., Wolfram, D., Eds.; Springer: Cham, Switzerland, 2014; pp. 285–320. [Google Scholar]
- Chen, X.; Chen, J.; Wu, D.; Xie, Y.; Li, J. Mapping the Research Trends by Co-Word Analysis Based on Keywords from Funded Project. Procedia Comput. Sci. 2016, 91, 547–555. [Google Scholar] [CrossRef]
- FAOSTAT. Macro Indicators. 2022. Available online: https://www.fao.org/faostat/en/#data/MK (accessed on 13 May 2022).
- Ros, M.; Hurtado-Navarro, M.; Giménez, A.; Fernández, J.A.; Egea-Gilabert, C.; Lozano-Pastor, P.; Pascual, J.A. Spraying agro-industrial compost tea on baby spinach crops: Evaluation of yield, plant quality and soil health in field experiments. Agronomy 2020, 10, 440. [Google Scholar] [CrossRef]
- Hernández, D.; Ros, M.; Carmona, F.; Saez-Tovar, J.A.; Pascual, J.A. Composting spent mushroom substrate from Agaricus bisporus and Pleurotus ostreatus production as a growing media component for baby leaf lettuce cultivation under Pythium irregulare biotic stress. Horticulturae 2021, 7, 13. [Google Scholar] [CrossRef]
- Wahome, P.K.; Oseni, T.O.; Masarirambi, M.T.; Shongwe, V.D. Effects of different hydroponics systems and growing media on the vegetative growth, yield and cut flower quality of Gypsophila (Gypsophila paniculata L.). World J. Agric. Sci. 2011, 7, 692–698. [Google Scholar]
- Khandaker, M.; Kotzen, B. The potential for combining living wall and vertical farming systems with aquaponics with special emphasis on substrates. Aquac. Res. 2018, 49, 1454–1468. [Google Scholar] [CrossRef]
- Ullah, I.; Mao, H.; Rasool, G.; Gao, H.; Javed, Q.; Sarwar, A.; Khan, M.I. Effect of deficit irrigation and reduced n fertilization on plant growth, root morphology and water use efficiency of tomato grown in soilless culture. Agronomy 2021, 11, 228. [Google Scholar] [CrossRef]
- Frasetya, B.; Harisman, K.; Ramdaniah, N.A.H. The effect of hydroponics systems on the growth of lettuce. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1098, 042115. [Google Scholar] [CrossRef]
- Santos, J.F.; Coelho, M.A.; Cruz, J.L.; Soares, T.M.; Cruz, A.M.L. Growth, water consumption and basil production in the hydroponic systems under salinity. Rev. Ceres 2019, 66, 45–53. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmed, S.; Kumar Roy, S.; Hee Woo, S.; Dashrath Sonawane, K.; Mohammad Shohael, A. Effect of salinity on the morphological, physiological and biochemical properties of lettuce (Lactuca sativa L.) in Bangladesh. Open Agric. 2019, 4, 361–373. [Google Scholar] [CrossRef]
- Lee, M.; Rivard, C.; Wang, W.; Pliakoni, E.; Gude, K.; Rajashekar, C.B. Spectral blocking of solar radiation in high tunnels by poly covers: Its impact on nutritional quality regarding essential nutrients and health-promoting phytochemicals in lettuce and tomato. Horticulturae 2021, 7, 524. [Google Scholar] [CrossRef]
- Ptushenko, O.S.; Ptushenko, V.V.; Solovchenko, A.E. Spectrum of Light as a Determinant of Plant Functioning: A Historical Perspective. Life 2020, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Folta, K.M.; Carvalho, S.D. Photoreceptors and control of horticultural plant traits. HortScience 2015, 50, 1274–1280. [Google Scholar] [CrossRef]
- Larsen, D.H.; Woltering, E.J.; Nicole, C.C.S.; Marcelis, L.F.M. Response of basil growth and morphology to light intensity and spectrum in a vertical farm. Front. Plant Sci. 2020, 11, 597906. [Google Scholar] [CrossRef]
- Pennisi, G.; Pistillo, A.; Orsini, F.; Cellini, A.; Spinelli, F.; Nicola, S.; Fernández, J.A.; Crepaldi, A.; Gianquinto, G.; Marcelis, L.F.M. Optimal light intensity for sustainable water and energy use in indoor cultivation of lettuce and basil under red and blue LEDs. Sci. Hortic. 2020, 272, 109508. [Google Scholar] [CrossRef]
- Pennisi, G.; Orsini, F.; Landolfo, M.; Pistillo, A.; Crepaldi, A.; Nicola, S.; Fernández, J.A.; Marcelis, L.F.M.; Gianquinto, G. Optimal photoperiod for indoor cultivation of leafy vegetables and herbs. Eur. J. Hortic. Sci. 2020, 85, 329–338. [Google Scholar] [CrossRef]
- He, R.; Gao, M.; Li, Y.; Zhang, Y.; Song, S.; Su, W.; Liu, H. Supplemental UV-A affects growth and antioxidants of Chinese kale baby-leaves in artificial light plant factory. Horticulturae 2021, 7, 294. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J. Morphological and physiological responses in basil and Brassica species to different proportions of red, blue, and green wavelengths in indoor vertical farming. J. Am. Soc. Hortic. Sci. 2020, 145, 267–278. [Google Scholar] [CrossRef]
- Rihan, H.Z.; Aljafer, N.; Jbara, M.; McCallum, L.; Lengger, S.; Fuller, M.P. The impact of LED lighting spectra in a plant factory on the growth, physiological traits and essential oil content of Lemon Balm (Melissa officinalis). Plants 2022, 11, 342. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Runkle, E.S. Far-red radiation interacts with relative and absolute blue and red photon flux densities to regulate growth, morphology, and pigmentation of lettuce and basil seedlings. Sci. Hortic. 2019, 255, 269–280. [Google Scholar] [CrossRef]
- Li, Y.; Wu, L.; Jiang, H.; He, R.; Song, S.; Su, W.; Liu, H. Supplementary Far-Red and blue lights influence the biomass and phytochemical profiles of two lettuce cultivars in plant factory. Molecules 2021, 26, 7405. [Google Scholar] [CrossRef]
- Zheng, J.; He, D.; Ji, F. Effect of light intensity on rooting and growth of hydroponic strawberry runner plants in a led plant factory. Agronomy 2019, 9, 875. [Google Scholar] [CrossRef]
- Zheng, J.F.; He, D.X.; Ji, F. Effects of light intensity and photoperiod on runner plant propagation of hydroponic strawberry transplants under LED lighting. Int. J. Agric. Biol. 2019, 12, 26–31. [Google Scholar] [CrossRef]
- Bantis, F.; Smirnakou, S.; Ouzounis, T.; Koukounaras, A.; Ntagkas, N.; Radgoglou, K. Current status and recent achievements in the field of horticulture with the use of light-emitting diodes (LEDs). Sci. Hortic. 2018, 235, 437–451. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, L.; Dai, J.; Liu, Y.; Lin, D.; Yang, Y. Morphological and physiological responses of cucumber seedlings to different combinations of light intensity and photoperiod with the same daily light integral. HortScience 2021, 56, 1430–1438. [Google Scholar] [CrossRef]
- He, D.; Yan, Z.; Sun, X.; Yang, P. Leaf development and energy yield of hydroponic sweet potato seedlings using single-node cutting as influenced by light intensity and LED spectrum. J. Plant Physiol. 2020, 254, 153274. [Google Scholar] [CrossRef]
- Harun, A.N.; Ahmad, R.; Mohamed, N.; Rahim, A.R.A.; Kaidi, H.M. Morphological and physiological responses of Brassica chinensis on different Far-Red (FR) light treatments using Internet-of-Things (IoT) technology. Agriculture 2021, 11, 728. [Google Scholar] [CrossRef]
- Kim, J.; Kang, W.H.; Son, J.E. Interpretation and evaluation of electrical lighting in plant factories with ray-tracing simulation and 3D plant modeling. Agronomy 2020, 10, 1545. [Google Scholar] [CrossRef]
- Zou, T.; Huang, C.; Wu, P.; Ge, L.; Xu, Y. Optimization of artificial light for spinach growth in plant factory based on orthogonal test. Plants 2020, 9, 490. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Ito, Y. Effect of Different PPFDs and Photoperiods on Growth and Yield of Everbearing Strawberry ‘Elan’ in Plant Factory with White LED Lighting. Environ. Control Biol. 2020, 58, 99–104. [Google Scholar] [CrossRef]
- Liu, Y.; Tikunov, Y.; Schouten, R.; Marcelis, L.F.M.; Visser, R.G.F.; Bovy, A. Anthocyanin Biosynthesis and Degradation Mechanisms in Solanaceous Vegetables: A Review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Ort, N.W.W.; Morrison, M.J.; Cober, E.R.; Samanfar, B.; Lawley, Y.E. Photoperiod Affects Node Appearance Rate and Flowering in Early Maturing Soybean. Plants 2022, 11, 871. [Google Scholar] [CrossRef]
- Kim, J.A.; Kim, H.-S.; Choi, S.-H.; Jang, J.-Y.; Jeong, M.-J.; Lee, S.I. The Importance of the Circadian Clock in Regulating Plant Metabolism. Int. J. Mol. Sci. 2017, 18, 2680. [Google Scholar] [CrossRef]
- Cho, J.Y.; Yoo, K.S.; Kim, J.; Choi, B.J.; Oh, W. Growth and bioactive compounds of lettuce as affected by light intensity and photoperiod in a plant factory using external electrode fluorescent lamps. Hortic. Sci. Technol. 2020, 38, 645–659. [Google Scholar] [CrossRef]
- Ji, F.; Wei, S.; Liu, N.; Xu, L.; Yang, P. Growth of cucumber seedlings in different varieties as affected by light environment. Int. J. Agric. Biol. Eng. 2020, 13, 73–78. [Google Scholar] [CrossRef]
- Lam, V.P.; Choi, J.; Park, J. Enhancing growth and glucosinolate accumulation in watercress (Nasturtium officinale L.) by regulating light intensity and photoperiod in plant factories. Agriculture 2021, 11, 723. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Responses of sweet basil to different daily light integrals in photosynthesis, morphology, yield, and nutritional quality. HortScience 2018, 53, 496–503. [Google Scholar] [CrossRef]
- Yan, Z.; He, D.; Niu, G.; Zhou, Q.; Qu, Y. Growth, nutritional quality, and energy use efficiency of hydroponic lettuce as influenced by daily light integrals exposed to white versus white plus red light-emitting diodes. HortScience 2019, 54, 1737–1744. [Google Scholar] [CrossRef]
- Chen, D.; Mei, Y.; Liu, Q.; Wu, Y.; Yang, Z. Carbon dioxide enrichment promoted the growth, yield, and light-use efficiency of lettuce in a plant factory with artificial lighting. Agron. J. 2021, 113, 5196–5206. [Google Scholar] [CrossRef]
- Gillani, S.A.; Abbasi, R.; Martinez, P.; Ahmad, R. Review on Energy Efficient Artificial Illumination in Aquaponics. Clean. Circ. Bioecon. 2022, 2, 100015. [Google Scholar] [CrossRef]
- Bantis, F. Light spectrum differentially affects the yield and phytochemical content of microgreen vegetables in a plant factory. Plants 2021, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Rihan, H.Z.; Aldarkazali, M.; Mohamed, S.J.; McMulkin, N.B.; Jbara, M.H.; Fuller, M.P. A novel new light recipe significantly increases the growth and yield of sweet basil (Ocimum basilicum) grown in a plant factory system. Agronomy 2020, 10, 934. [Google Scholar] [CrossRef]
- Gómez, C.; Poudel, M.; Yegros, M.; Fisher, P.R. Radiation intensity and quality affect indoor acclimation of blueberry transplants. HortScience 2021, 56, 1521–1530. [Google Scholar] [CrossRef]
- Gao, M.; He, R.; Shi, R.; Li, Y.; Song, S.; Zhang, Y.; Su, W.; Liu, H. Combination of selenium and UVA radiation affects growth and phytochemicals of broccoli microgreens. Molecules 2021, 26, 4646. [Google Scholar] [CrossRef]
- Gao, M.; He, R.; Shi, R.; Zhang, Y.; Song, S.; Su, W.; Liu, H. Differential effects of low light intensity on broccoli microgreens growth and phytochemicals. Agronomy 2021, 11, 537. [Google Scholar] [CrossRef]
- Saito, K.; Ishigami, Y.; Goto, E. Evaluation of the light environment of a plant factory with artificial light by using an optical simulation. Agronomy 2020, 10, 1663. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, E.-P.; Han, Y.-S.; Lee, S.-I.; Cho, K.-T.; Hong, Y.-S.; You, Y.-H. The effects of LEDs and duty ratio on the growth and physiological responses of Silene capitata Kom., endangered plant, in a plant factory. J. Ecol. Environ. 2018, 42, 21. [Google Scholar] [CrossRef]
- Huang, J.J.; D’Souza, C.; Zhou, W. Light-time-biomass response model for predicting the growth of choy sum (Brassica rapa var. parachinensis) in soil-based LED-constructed indoor plant factory for efficient seedling production. Front. Plant Sci. 2021, 12, 623682. [Google Scholar] [CrossRef]
- Park, Y.G.; Jeong, B.R. How supplementary or night-interrupting low-intensity blue light affects the flower induction in chrysanthemum, a qualitative short-day plant. Plants 2020, 9, 1694. [Google Scholar] [CrossRef] [PubMed]
- Amagai, Y.; Lu, N.; Hayashi, E.; Takagaki, M.; Kikuchi, M.; Ibaraki, Y.; Kozai, T. External green light as a new tool to change colors and nutritional components of inner leaves of head cabbages. J. Food Meas. Charact. 2022, 16, 269–280. [Google Scholar] [CrossRef]
- McAusland, L.; Lim, M.-T.; Morris, D.E.; Smith-Herman, H.L.; Mohammed, U.; Hayes-Gill, B.R.; Crowe, J.A.; Fisk, I.D.; Murchie, E.H. growth spectrum complexity dictates aromatic intensity in coriander (Coriandrum sativum L.). Front. Plant Sci. 2020, 11, 462. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.P.; Lu, N.; Kagawa, N.; Kitayama, M.; Takagaki, M. Short-term root-zone temperature treatment enhanced the accumulation of secondary metabolites of hydroponic coriander (Coriandrum sativum L.) grown in a plant factory. Agronomy 2020, 10, 413. [Google Scholar] [CrossRef]
- An, S.; Hwang, H.; Chun, C.; Jang, Y.; Lee, H.J.; Wi, S.H.; Yeo, K.-H.; Yu, I.-H.; Kwack, Y. Evaluation of air temperature, photoperiod, and light intensity conditions to produce cucumber scions and rootstocks in a plant factory with artificial lighting. Horticulturae 2021, 7, 102. [Google Scholar] [CrossRef]
- Hwang, H.; An, S.; Lee, B.; Chun, C. Improvement of growth and morphology of vegetable seedlings with supplemental far-red enriched led lights in a plant factory. Horticulturae 2020, 6, 109. [Google Scholar] [CrossRef]
- Kang, D.I.; Jeong, H.K.; Park, Y.G.; Jeong, B.R. Flowering and morphogenesis of kalanchoe in response to quality and intensity of night interruption light. Plants 2019, 8, 90. [Google Scholar] [CrossRef]
- Carotti, L.; Graamans, L.; Puksic, F.; Butturini, M.; Meinen, E.; Heuvelink, E.; Stanghellini, C. Plant factories are heating up: Hunting for the best combination of light intensity, air temperature and root-zone temperature in lettuce production. Front. Plant Sci. 2021, 11, 592171. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Cho, K.M.; Lee, H.Y.; Cho, D.Y.; Lee, G.O.; Jang, S.N.; Lee, Y.; Kim, D.; Son, K.-H. Effects of white LED lighting with specific shorter blue and/or green wavelength on the growth and quality of two lettuce cultivars in a vertical farming system. Agronomy 2021, 11, 2111. [Google Scholar] [CrossRef]
- Xu, W.; Nguyen, D.T.P.; Sakaguchi, S.; Akiyama, T.; Tsukagoshi, S.; Feldman, A.; Lu, N. Relation between relative growth rate and tipburn occurrence of romaine lettuce under different light regulations in a plant factory with LED lighting. Eur. J. Hortic. Sci. 2020, 85, 354–361. [Google Scholar] [CrossRef]
- Ohtake, N.; Ju, Y.; Ishikura, M.; Suzuki, H.; Adachi, S.; Yamori, W. Alternating red/blue light increases leaf thickness and mesophyll cell density in the early growth stage, improving photosynthesis and plant growth in lettuce. Environ. Control Biol. 2021, 59, 59–67. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Y.; Liu, W.; Zha, L.; Shao, M.; Li, B. Light quality affected the growth and root organic carbon and autotoxin secretions of hydroponic Lettuce. Plants 2020, 9, 1542. [Google Scholar] [CrossRef]
- Li, L.; Tong, Y.; Lu, J.; Li, Y.; Yang, Q. Lettuce growth, nutritional quality, and energy use efficiency as affected by red–blue light combined with different monochromatic wavelengths. HortScience 2020, 55, 613–620. [Google Scholar] [CrossRef]
- Wittmann, S.; Jüttner, I.; Mempel, H. Indoor farming marjoram production—Quality, resource efficiency, and potential of application. Agronomy 2020, 10, 1769. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, S.B.; Lee, E.P.; Lee, S.Y.; Kim, E.J.; Lee, J.M.; Park, J.H.; Cho, K.T.; Jeong, H.M.; Choi, S.S.; et al. Study on the photosynthetic characteristics of Eutrema japonica (Siebold) Koidz. under the pulsed LEDs for simulated sunflecks. J. Ecol. Environ. 2021, 45, 6. [Google Scholar] [CrossRef]
- Palmer, S.; van Iersel, M.W. Increasing growth of lettuce and mizuna under sole-source LED lighting using longer photoperiods with the same daily light integral. Agronomy 2020, 10, 1659. [Google Scholar] [CrossRef]
- Xu, W.; Lu, N.; Kikuchi, M.; Takagaki, M. Continuous lighting and high daily light integral enhance yield and quality of mass-produced nasturtium (Tropaeolum majus L.) in plant factories. Plants 2021, 10, 1203. [Google Scholar] [CrossRef]
- Zhou, C.; Cui, W.; Yuan, T.; Cheng, H.; Su, Q.; Guo, P. Water content, carbohydrate accumulation, and secondary metabolites in Allium victorialis sprouts exposed to shoot cutting in varied irradiations. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12524. [Google Scholar] [CrossRef]
- An, S.; Park, S.W.; Kwack, Y. Growth of cucumber scions, rootstocks, and grafted seedlings as affected by different irrigation regimes during cultivation of ‘Joenbaekdadagi’ and ‘Heukjong’ seedlings in a plant factory with artificial lighting. Agronomy 2020, 10, 1943. [Google Scholar] [CrossRef]
- Date, S.; Ogawa, T.; Matsuura, K.; Hata, N.; Terabayashi, S. Effects of day length and air temperature fluctuation on the occurrence of leaf browning in sesame seedlings cultured in a plant factory under artificial light. Environ. Control Biol. 2020, 58, 37–42. [Google Scholar] [CrossRef]
- Fernández-Cabanás, V.M.; Pérez-Urrestarazu, L.; Juárez, A.; Kaufman, N.T.; Gross, J.A. Comparative analysis of horizontal and vertical decoupled aquaponic systems for basil production and effect of light supplementation by LED. Agronomy 2020, 10, 1414. [Google Scholar] [CrossRef]
- Bantis, F.; Fotelli, M.; Ilić, Z.S.; Koukounaras, A. Physiological and phytochemical responses of spinach baby leaves grown in a PFAL system with LEDs and saline nutrient solution. Agriculture 2020, 10, 574. [Google Scholar] [CrossRef]
- Noh, K.; Jeong, B.R. Increased carbon dioxide by occupants promotes growth of leafy vegetables grown in indoor cultivation system. Sustainability 2021, 13, 13288. [Google Scholar] [CrossRef]
- Azad, M.O.K.; Kjaer, K.H.; Adnan, M.; Naznin, M.T.; Lim, J.D.; Sung, I.J.; Park, C.H.; Lim, Y.S. The evaluation of growth performance, photosynthetic capacity, and primary and secondary metabolite content of leaf lettuce grown under limited irradiation of Blue and Red LED light in an urban plant factory. Agriculture 2020, 10, 28. [Google Scholar] [CrossRef]
- Zheng, J.; Gan, P.; Ji, F.; He, D.; Yang, P. Growth and energy use efficiency of grafted tomato transplants as affected by led light quality and photon flux density. Agriculture 2021, 11, 816. [Google Scholar] [CrossRef]
- Dieleman, J.A.; De Visser, P.H.B.; Meinen, E.; Grit, J.G.; Dueck, T.A. Integrating morphological and physiological responses of tomato plants to light quality to the crop level by 3D modeling. Front. Plant Sci. 2019, 10, 839. [Google Scholar] [CrossRef]





| Parameter | Description |
|---|---|
| Electromagnetic spectrum | It is the wavelength that determines the distribution of energy, including visible and non-visible wavelengths. In the case of the spectra emitted by lamps, a range of electromagnetic radiation detectable by the human eye is considered, from 380 nm to 780 nm, within the portion of the spectrum between ultraviolet and infrared, which are components of solar radiation. It has been demonstrated than approximately 95% of solar UV radiation at sea level is absorbed by plant photoreceptors, which are largely responsible for plant growth and development [25]. Some lamps include the above-mentioned entire range, such as fluorescent lamps, and others, such as LED luminaries, have more specific spectrum absorption peaks. |
| Photosyntenthically active radiation (PAR) | It consists of elementary, particles/wavelets, or photons and is the part of the radiation spectrum that plants use for photosynthesis corresponding to the range from 400 to 700 nm [26,27,28]. The measurement is done with PAR sensors, expressed as μmol∙m−2·s−1. |
| Photosynthetic Photon Flux Density (PPFD) | Light intensity falling onto a surface measured as photosynthetic photon flux density (PPFD) with units of μmol∙m−2·s−1. PPFD comprises about half the energy of solar radiation and scales well with the time at which photosynthesis responds to changes in light intensity [29]. Direct PPFD measurement, using PAR sensors. |
| Photoperiod | All living beings need a period of light and darkness to regulate their processes. These periods are regulated in a daily cycle of 24 h that results from the rotation of the Earth around its axis, where the greater or lesser perpendicular of the sun’s rays varies depending on the latitude of the place, time of year and time of day [30]. Photoperiod plays an important role on plant growth and development, where being deficient in plants with high light requirements can induce stress and growth imbalance and can also significantly influence energy and economic efficiency in a VF system [31]. |
| Daily Light Integral (DLI) | DLI is a measure of the total photosynthetic photon flux density (PPFD) delivered over the course of one day. It is a useful tool for assessing the irradiance delivered to various horticultural crops, therefore, it is of great importance in VF systems [32]. |
| Radiation use efficiency (RUE) and Light use efficiency (LUE) | RUE is the efficiency with which a crop uses absorbed light energy for biomass production and is determined as the ratio of biomass accumulation per unit of photosynthetically active radiation (PAR) absorbed [33]. Unlike RUE, LUE, is the net CO2 assimilation, which is the efficiency of plants to convert absorbed light into CO2 absorption, LUE can be measured in a short time or quantified daily at the leaf level [34]. |
| Parameter | Description |
|---|---|
| Photosynthetic parameters | It is one of the most widely used physiological parameters in crops and is non-destructive in nature, being used to accurately understand plant ecophysiology. It is the process where plants produce organic compounds by CO2 and H2O [35,36]. |
| Leaf Area (LA) | It is a parameter that determines the amount of photoassimilates produced and affects who is growth, development, and productivity [37]. In general, it is assumed that LA and biomass accumulation in crops may be affected by some factors, such as light quality and radiation intensity, plant nutrition, substrate type, and container design and volume [38,39,40,41]. |
| Plant productivity | It is determined by fresh weight, being of commercial interest in the market by edible fresh weight. However, every organ (stem, leaf, root, or fruit) is of research interest by dry weight, since biomass accumulation is the result of photosynthetic activity, CO2 concentration [26] and is an indicator of long-term plant survival. |
| Total Dry Matter (TDM) | It represents the net gain in dry matter, and it is considered one of the best parameters for indicating plant quality [42]. Moreover, plants with a high TDM content show high growth potential and field yield. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nájera, C.; Gallegos-Cedillo, V.M.; Ros, M.; Pascual, J.A. Role of Spectrum-Light on Productivity, and Plant Quality over Vertical Farming Systems: Bibliometric Analysis. Horticulturae 2023, 9, 63. https://doi.org/10.3390/horticulturae9010063
Nájera C, Gallegos-Cedillo VM, Ros M, Pascual JA. Role of Spectrum-Light on Productivity, and Plant Quality over Vertical Farming Systems: Bibliometric Analysis. Horticulturae. 2023; 9(1):63. https://doi.org/10.3390/horticulturae9010063
Chicago/Turabian StyleNájera, Cinthia, Victor M. Gallegos-Cedillo, Margarita Ros, and José Antonio Pascual. 2023. "Role of Spectrum-Light on Productivity, and Plant Quality over Vertical Farming Systems: Bibliometric Analysis" Horticulturae 9, no. 1: 63. https://doi.org/10.3390/horticulturae9010063
APA StyleNájera, C., Gallegos-Cedillo, V. M., Ros, M., & Pascual, J. A. (2023). Role of Spectrum-Light on Productivity, and Plant Quality over Vertical Farming Systems: Bibliometric Analysis. Horticulturae, 9(1), 63. https://doi.org/10.3390/horticulturae9010063





