Propagation Characteristics of the Rare Endemic Rhododenderon micranthum Turcz.
Abstract
1. Introduction
2. Materials and Methods
2.1. Testing Materials and Environment for Cutting Propagation
2.2. Growth Regulator Treatment
2.3. Plant Growth Traits
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Korea National Arboretum (KNA). Available online: www.nature.go.kr (accessed on 18 November 2022).
- Zhang, M.; Xie, Y.; Zhan, G.; Lei, L.; Shu, P.; Chen, Y.; Xue, Y.; Luo, Z.; Wan, Q.; Yao, G.; et al. Grayanane and leucothane diterpenoids from the leaves of Rhododendron micranthum. Phytochemistry 2015, 117, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.J.; Kim, H.O.; Jung, E.S.; Lee, E.S.; Lee, S.H.; Jeon, G.Y.; Kim, Y.C. Anti-wrinkle and skin-whitening efficacy of Rhododendron micranthum methanol extract. J. Invest. Cosmetol. 2018, 14, 405–411. [Google Scholar]
- Nawrocka-Grześkowiak, U. Effect of growth substances on the rooting of cuttings of rhododendron species. Folia. Horticult. 2004, 16, 115–123. [Google Scholar]
- Hartmann, H.T.; Kester, D.E.; Davies, F.T.; Geneve, R.L. Plant propagation: Principles and Practices, 6th ed.; Prentice-Hall Inc.: Upper Saddle River, New Jersey, USA, 1997. [Google Scholar]
- Spethmann, W. Stem cutting of oaks (Quercus robur L. and Quercus petraea [Matt.] Liebl.). Schriften Forstl. Fak. Uni. Göttingen U.D. Nieders. Forstl. Versuchsanstalt. 1986, 86, 1–99. [Google Scholar]
- Hwang, Y.; Song, C.Y.; Moon, J.Y.; Lee, J.H.; Kim, Y.Y. Effect of cutting time, rooting promoter and light shade on rooting of Rhododendron brachycarpum native and to Korea. Flower Res. J. 2015, 23, 37–42. [Google Scholar] [CrossRef]
- Dirr, M.A.; Brinson, B. Magnolia grandiflora: A propagation guide. Am. Nurserym. 1985, 162, 38–51. [Google Scholar]
- Morini, S.; Isoleri, M. Effect of IBA and NAA on rooting of Actinidia chinensis cuttings. Acta Hort. 1986, 179, 885–886. [Google Scholar] [CrossRef]
- Kashyap, U.; Chandel, A.; Sharma, D.; Bhardwaj, S.; Bhargava, B. Propagation of Jasminum parkeri: A critically endangered wild ornamental woody shrub from Western Himalaya. Agronomy 2021, 11, 331. [Google Scholar] [CrossRef]
- Ro, N.Y.; Song, E.Y.; Kim, S.C.; Ko, H.C.; Lee, S.Y. Propagation of a rare variety Daphne kiusiana Miq. in Korea through softwood cuttings. J. Bio-Environ. Control 2010, 19, 246–250. [Google Scholar]
- Lee, J.H.; Song, C.Y.; Woo, H.J.; Kwon, O.W. Effects of plant growth regulators for hard wood and cutting time for soft wood on rooting of Korean native Spiraea spp. Flower Res. J. 2011, 19, 103–109. [Google Scholar]
- Boo, J.Y.; Kim, J.S. A study on the native environment and cutting propagation for the black-berry magnolia vine [Schisandra repanda (Siebold & Zucc.) Radlk] in Halla mountain. Korean J. Med. Crop. Sci. 2020, 28, 354–359. [Google Scholar] [CrossRef]
- Ferus, P.; Konôpková, J.; Bošiaková, D.; Hoťka, P. Effective rhododendron propagation through stem cuttings. J. Appl. Hortic. 2017, 19, 226–229. [Google Scholar]
- Si, G.C.; Zhang, Y.L.; Gu, X.; Wang, Y.Q.; Zhao, B. Study on cutting propagation technology of qinling wild Rhododendron calophytum. J. North. Hortic. 2012, 3, 7. [Google Scholar]
- Yoo, B.A.; Lee, K.S.; Kim, W.H.; Lee, D.W.; Oh, Y.N.; Lee, J.S. Effect of cutting dates, rooting medium, and rooting promoters for rooted cutting production of Rhododendron mucronulatum T., R. Knap Hill hyb. ‘Golden Sunset’. Korean J. Hortic. Sci. Technol. 2002, 20, 102. [Google Scholar]
- Huh, Y.J.; Pak, C.H.; Kwack, B.H. Effect of ABA and NAA on rooting of Rhododendron mucronulatum and Rhododendron yedoense var. poukhanense cuttings. J. Korean Soc. Hortic. Sci. 1997, 21, 13–17. [Google Scholar]
- Lee, J.S.; Chang, Y.J.; Moon, C.Y.; Lee, S.C.; Kim, G.S.; Kim, Y.S. Effects of rooting promoters on plug cutting of new cultivar of pot azalea. Korean Hortic. Sci. 2006, 27, 313. [Google Scholar]
- Song, J.H.; Jang, K.H.; Hur, S.D. Propagation of cutting method of a rare endemic Juniperus chinensis var. sargentii Henry in Korea. Korean J. Plant. Res. 2010, 23, 368–373. [Google Scholar]
- Spethmann, W. The Vegetative Propagation of Trees; Gerd Krussmann Nursery, Parey Buchverlag: Berlin, Germany, 1997; pp. 382–438. [Google Scholar]
- Park, J.B.; Kim, J.S.; Kim, D.G. The change of somatic cell embryogenesis in Kalanchoe pinnata because of agar concentration in stimulating root stress. J. Plant Biotechnol. 2017, 44, 320–324. [Google Scholar] [CrossRef]
- Medrano, H.; Parry, M.A.J.; Socias, X.; Lawlor, D.W. Long term water stress inactivates Rubisco in subterranean clover. Ann. Appl. Biol. 1997, 131, 494–501. [Google Scholar] [CrossRef]
- Isebrands, J.G.; Crow, T.R. Techniques for Rooting juvenile Softwood Cutting of Northern Red Oak. In Proceedings of the 5th Central Hardwood Forest Conference, Urbana-Champaign, IL, USA, 15–17 April 1985; pp. 228–233. [Google Scholar]
- Kim, J.Y.; Kim, C.S.; You, D.H.; Kim, D.W.; Choi, D.C.; Kim, J.M.; Oh, N.K.; Park, C.G.; Ahn, Y.S.; Lee, K.S. Cuttings for mass propagation affecting the impact of increasing reproductive efficiency of Schisandra chinensis. Korean J. Med. Crop. Sci. 2014, 22, 231–236. [Google Scholar] [CrossRef]
- Christopher, J.F. Rooting of Rhododendron ‘Anna Rose Whitney’ cutting as related to stem carbohydrate concentration. Hort. Sci. 1990, 25, 409–411. [Google Scholar] [CrossRef]
- Kim, H.K.; Nam, Y.K.; Lee, J.S. Effect of cutting media and growth regulators on the cutting of Dendrobium nobile. Flower Res. J. 2010, 18, 23–28. [Google Scholar]
- Thimann, K.V.; Koepfli, J.B. Identity of the growth-promoting and root-forming substances of plants. Nature 1935, 135, 101–102. [Google Scholar] [CrossRef]
- Phillips, J.B. Effect of cutting techniques on coppice regrowth. Q. J. For. 1971, 65, 220–223. [Google Scholar]
- Hitchcock, A.E.; Zimmerman, P.W. Effects obtained with mixtures of root-inducing and other substances. Contrib. Boyce Thompson Inst. Plant Res. 1940, 11, 143–160. [Google Scholar]
- Kaushik, S.; Shukla, N. A review on effect of IBA and NAA and their combination on the rooting of stem cuttings of different ornamental crops. J. Pharmacogn. Phytochem. 2020, 9, 1881–1885. [Google Scholar]
- Yoo, B.S.; Park, K.Y.; Kwon, O.H.; Junh, H.H. Effect of periodic cutting, media and auxin treatments for rooting percentage and growth of rooted cutting of R. mucronulatum by cutting. Hortic. Sci. Technol. 2018, 36, 157. [Google Scholar]
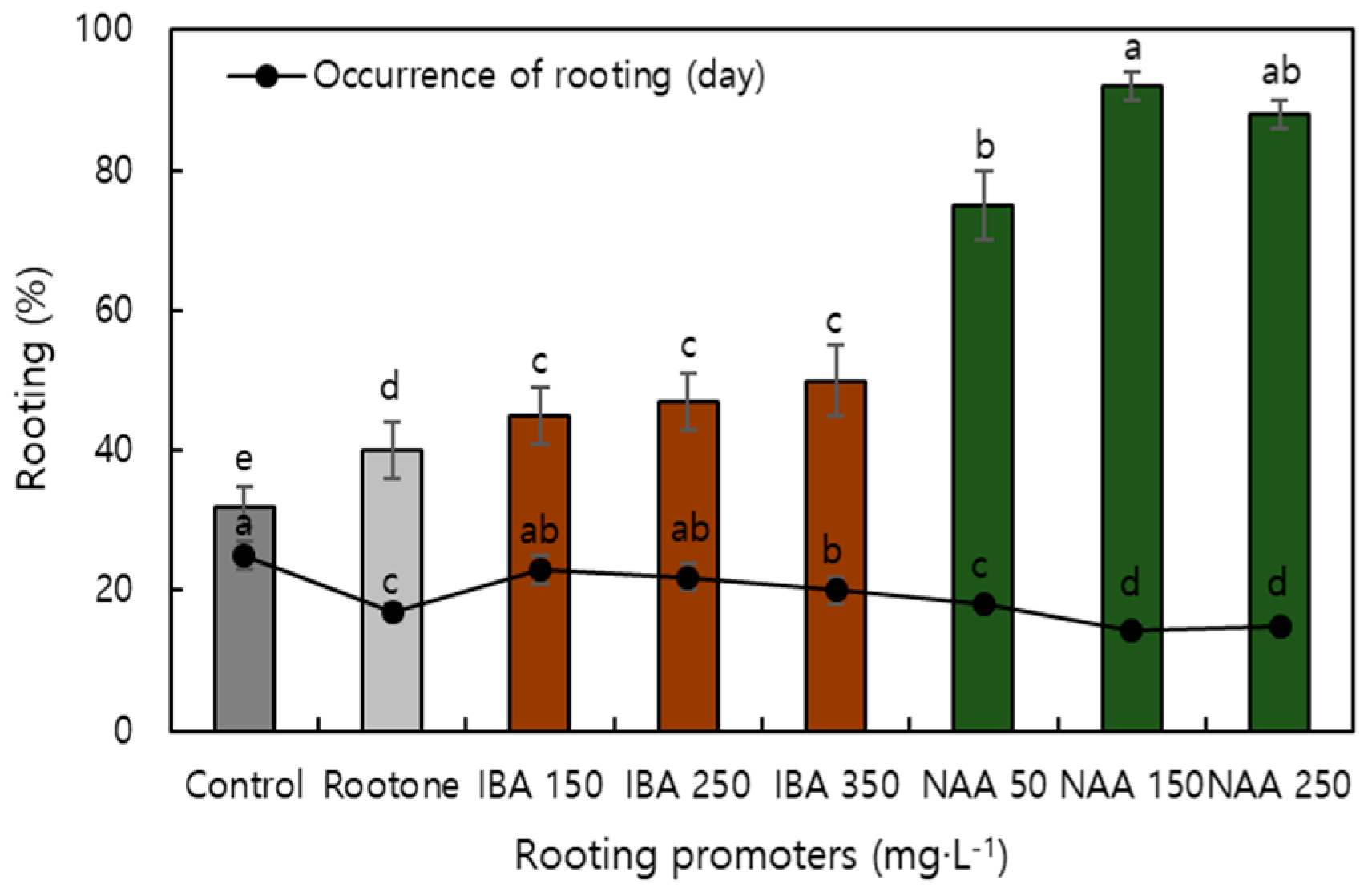
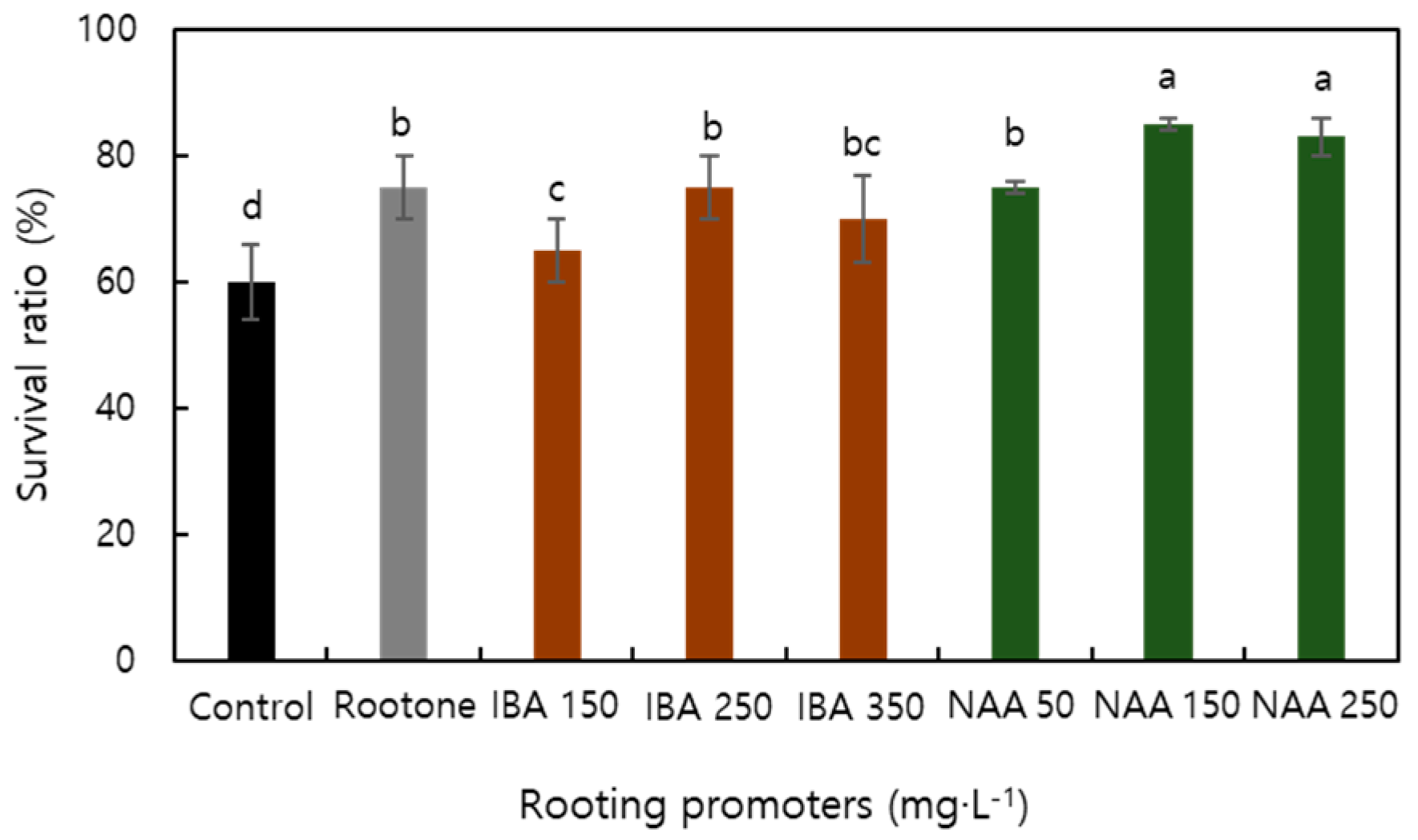
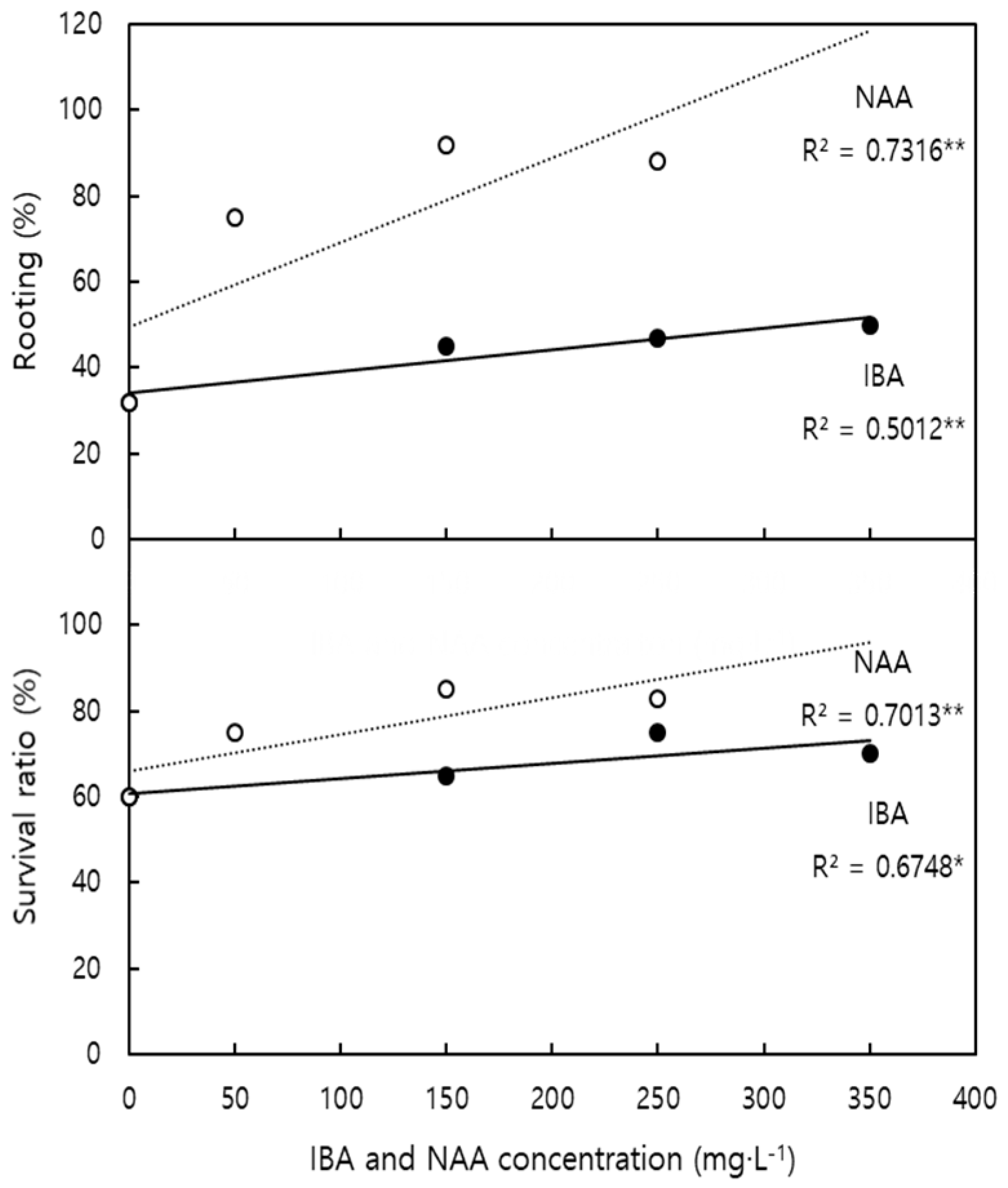
| Factor | Characteristics of Root | Characteristics of Shoot | Survival Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Root | Root Length | Fresh Weight | Dry Weight | Plant Height | No. of Leaf | Fresh Weight | Dry Weight | Chlorophyll Contents | ||
| Rooting | 0.740 **z | 0.844 ** | 0.470 * | 0.454 * | 0.337 NS | 0.649 ** | 0.597 NS | 0.444 NS | 0.695 * | 0.794 ** |
| Survival ratio | 0.646 ** | 0.673 ** | 0.738 * | 0.562 NS | 0.454 NS | 0.738 ** | 0.750 * | 0.746 * | 0.710 ** | 1 |
| Rooting Promoters | No. of Roots (ea) | Root Length (cm) | Fresh Weight (g) | Dry Weight (g) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Control | 3.2 | dz | 2.4 | d | 0.118 | d | 0.037 | c | |
| Rootone | 5.0 | c | 4.8 | b | 0.260 | b | 0.080 | ab | |
| IBA | 150 mg·L−1 | 4.6 | c | 3.7 | c | 0.149 | c | 0.074 | b |
| 250 mg·L−1 | 4.1 | c | 3.3 | c | 0.128 | c | 0.062 | b | |
| 350 mg·L−1 | 4.2 | c | 3.2 | c | 0.142 | c | 0.065 | b | |
| NAA | 50 mg·L−1 | 6.3 | b | 4.8 | b | 0.252 | b | 0.094 | a |
| 150 mg·L−1 | 7.0 | a | 6.2 | a | 0.333 | a | 0.084 | a | |
| 250 mg·L−1 | 7.2 | a | 5.3 | a | 0.326 | a | 0.099 | a | |
| Significant | **y | * | ** | * | |||||
| Rooting Promoters | Plant Height (cm) | No. of Leaf (ea) | Fresh Weight (g) | Dry weight (g) | Chlorophyll Contents (SPAD Value) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 2.2 | cz | 2.8 | c | 0.201 | d | 0.025 | d | 28.6 | d | |
| Rootone | 2.8 | bc | 3.8 | bc | 0.356 | bc | 0.036 | bc | 42.2 | b | |
| IBA | 150 mg·L−1 | 4.2 | b | 4.8 | b | 0.248 | c | 0.028 | c | 32.6 | c |
| 250 mg·L−1 | 4.2 | b | 4.4 | b | 0.226 | c | 0.032 | c | 31.7 | c | |
| 350 mg·L−1 | 4.1 | b | 4.7 | b | 0.271 | c | 0.033 | c | 33.5 | c | |
| NAA | 50 mg·L−1 | 3.3 | bc | 5.2 | ab | 0.389 | b | 0.045 | b | 38.9 | b |
| 150 mg·L−1 | 5.2 | a | 6.2 | a | 0.485 | a | 0.063 | a | 48.5 | a | |
| 250 mg·L−1 | 5.8 | a | 6.9 | a | 0.490 | a | 0.057 | a | 49.0 | a | |
| Significant | *y | ** | ** | * | * | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.I.; Park, E.H.; Koh, H.M.; Gil, M.; Yoon, M.J.; Bae, J.K.; Ko, C.H. Propagation Characteristics of the Rare Endemic Rhododenderon micranthum Turcz. Horticulturae 2023, 9, 101. https://doi.org/10.3390/horticulturae9010101
Oh SI, Park EH, Koh HM, Gil M, Yoon MJ, Bae JK, Ko CH. Propagation Characteristics of the Rare Endemic Rhododenderon micranthum Turcz. Horticulturae. 2023; 9(1):101. https://doi.org/10.3390/horticulturae9010101
Chicago/Turabian StyleOh, Sang Im, Eun Hye Park, Hong Min Koh, Min Gil, Mi Jeong Yoon, Jun Kyu Bae, and Chung Ho Ko. 2023. "Propagation Characteristics of the Rare Endemic Rhododenderon micranthum Turcz." Horticulturae 9, no. 1: 101. https://doi.org/10.3390/horticulturae9010101
APA StyleOh, S. I., Park, E. H., Koh, H. M., Gil, M., Yoon, M. J., Bae, J. K., & Ko, C. H. (2023). Propagation Characteristics of the Rare Endemic Rhododenderon micranthum Turcz. Horticulturae, 9(1), 101. https://doi.org/10.3390/horticulturae9010101






