Effect of Elevated Temperature and Excess Light on Photosynthetic Efficiency, Pigments, and Proteins in the Field-Grown Sunflower during Afternoon
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Weather Conditions
2.3. Chlorophyll a Fluorescence (ChlF)
2.4. Laboratory Analyses
2.4.1. Photosynthetic Pigment Content Determination
2.4.2. SDS-PAGE and Immunodetection
2.5. Data Analyses
3. Results and Discussion
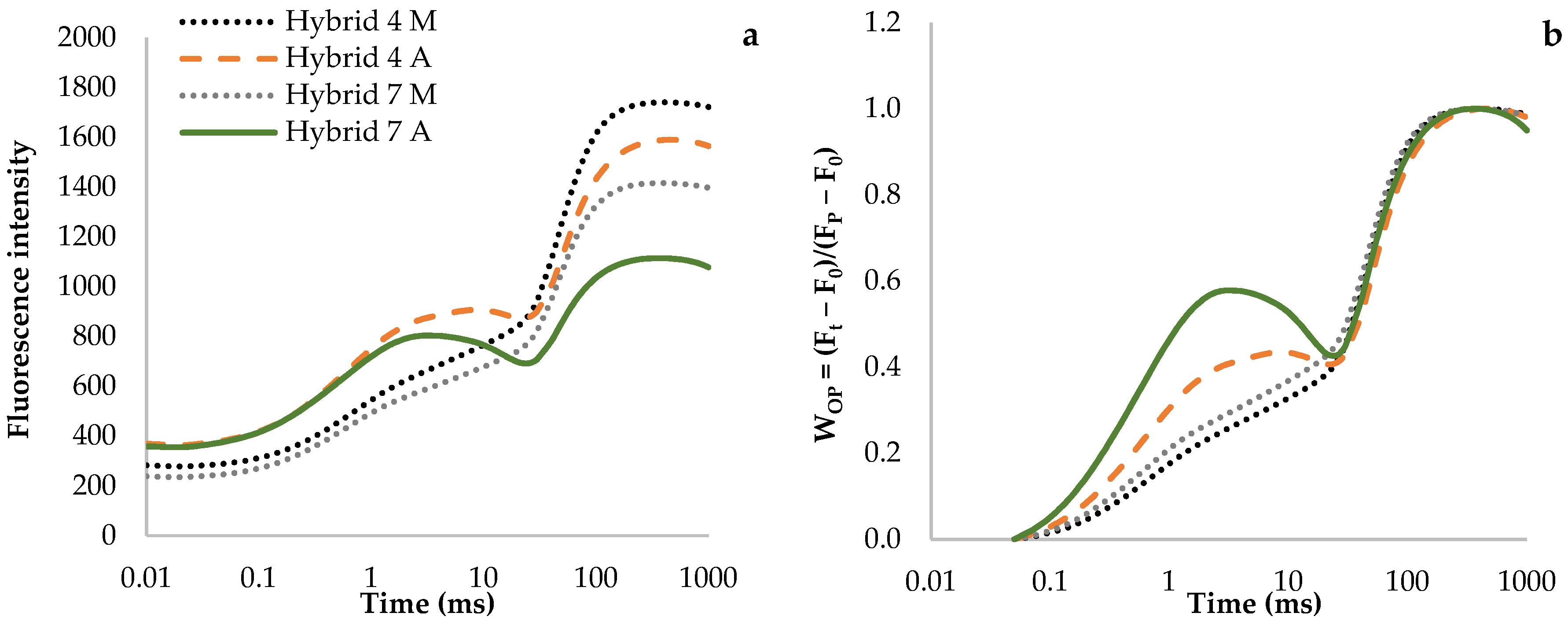
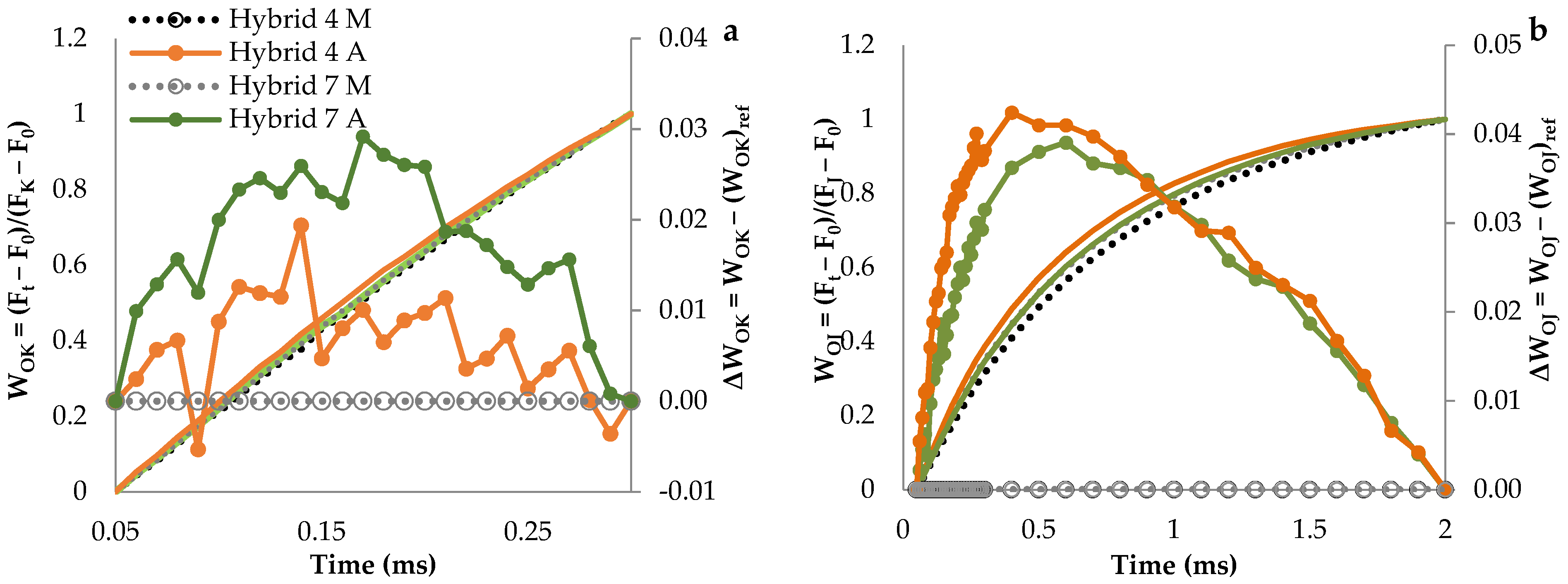
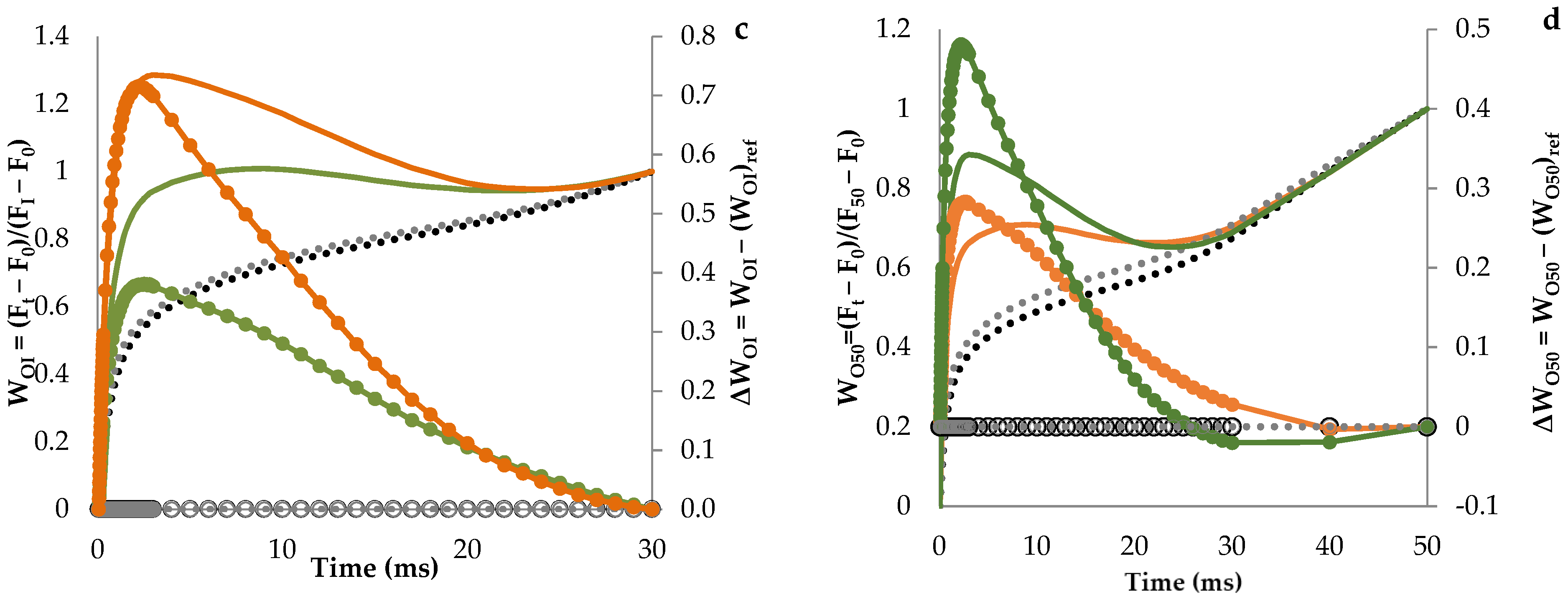
3.1. Fluorescence Transient Curves
3.2. Chlorophyll JIP-Test Parameters
3.3. Photosynthetic Pigment Content
3.4. Photosynthetic Proteins
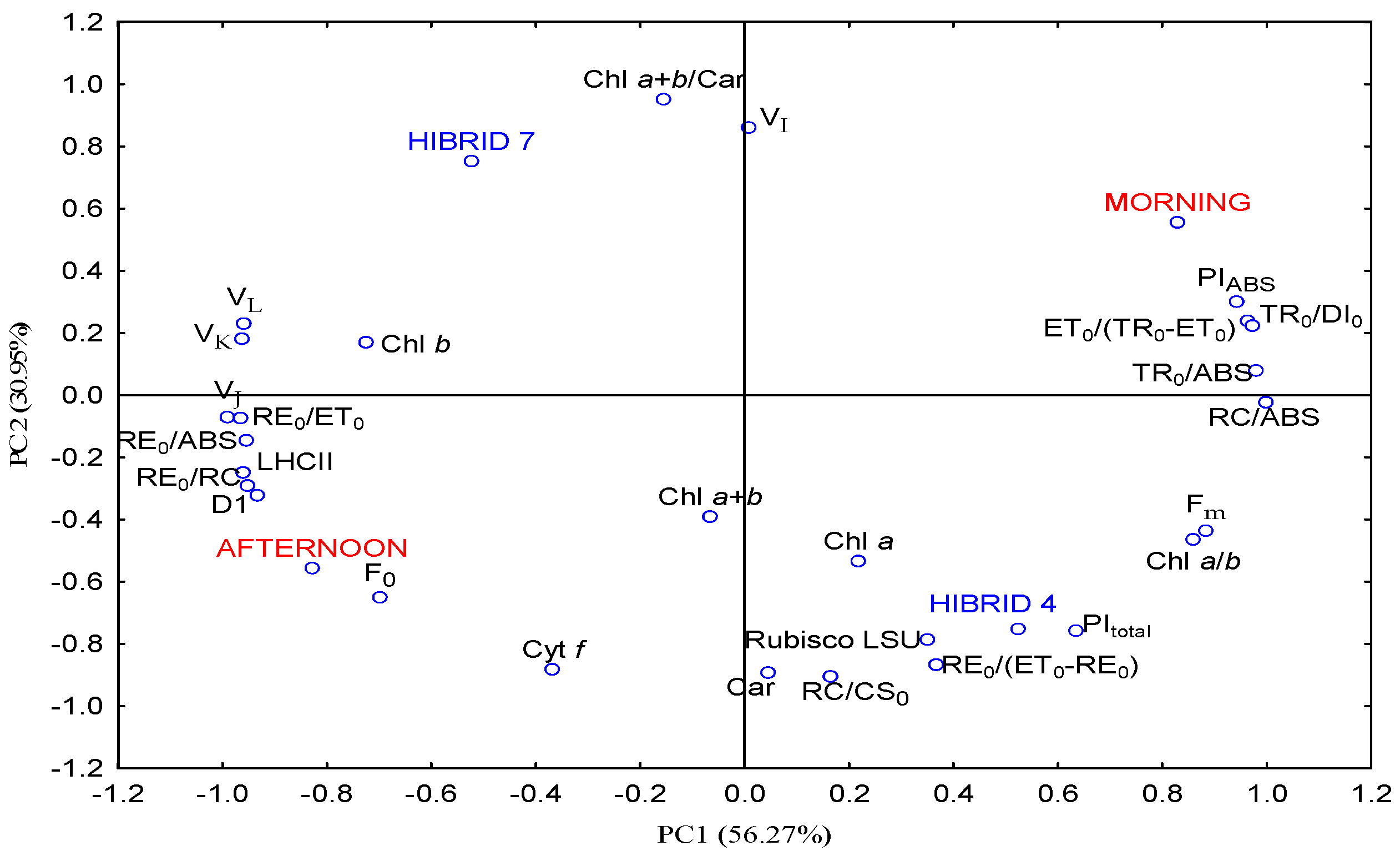
3.5. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jug, D.; Jug, I.; Brozović, B.; Vukadinović, V.; Stipešević, B.; Đurđević, B. The role of conservation agriculture in mitigation and adaptation to climate change. Poljoprivreda 2018, 24, 35–44. [Google Scholar] [CrossRef]
- García-López, J.; Lorite, I.J.; García-Ruiz, R.; Domínguez, J. Evaluation of three simulation approaches for assessing yield of rainfed sunflower in a Mediterranean environment for climate change impact modelling. Clim. Chang. 2014, 124, 147–162. [Google Scholar] [CrossRef]
- De la Haba, P.; De la Mata, L.; Molina, E.; Aguera, E. High temperature promotes early senescence in primary leaves of sunflower (Helianthus annuus L.) plants. Can. J. Plant Sci. 2014, 94, 659–669. [Google Scholar] [CrossRef]
- Greer, D.H. Temperature-dependent responses of the photosynthetic and chlorophyll fluorescence attributes of apple (Malus domestica) leaves during a sustained high temperature event. Plant Physiol. Biochem. 2015, 97, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Mihaljević, I.; Lepeduš, H.; Šimić, D.; Viljevac Vuletić, M.; Tomaš, V.; Vuković, D.; Dugalić, K.; Teklić, T.; Babojelić, M.S.; Zdunić, Z. Photochemical efficiency of photosystem II in two apple cultivars affected by elevated temperature and excess light in vivo. S. Afr. J. Bot. 2020, 130, 316–326. [Google Scholar] [CrossRef]
- Stirbet, A.; Lazár, D.; Guo, Y.; Govindjee, G. Photosynthesis: Basics, history and modelling (Review: Part of a special issue on functional-structural plant growth modelling). Ann. Bot. 2020, 126, 511–537. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the fluorescence transient. In Chlorophyll Fluorescence: A Signature of Photosynthesis; Advances in Photosynthesis and Respiration Series; Govindjee, G., Papageorgiou, G., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Mlinarić, S.; Antunović Dunić, J.; Babojelić, M.S.; Cesar, V. Differential accumulation of photosynthetic proteins regulates diurnal photochemical adjustments of PSII in common fig (Ficus carica L.) leaves. J. Plant Physiol. 2017, 209, 1–10. [Google Scholar] [CrossRef]
- Pavlović, I.; Mlinarić, S.; Tarkowská, D.; Oklestkova, J.; Novák, O.; Lepeduš, H.; Bok, V.V.; Brkanac, S.R.; Strnad, M.; Salopek-Sondi, B. Early Brassica Crops Responses to Salinity Stress: A Comparative Analysis Between Chinese Cabbage, White Cabbage, and Kale. Front. Plant Sci. 2019, 10, 450. [Google Scholar] [CrossRef]
- Markulj Kulundžić, A.; Viljevac Vuletić, M.; Matoša Kočar, M.; Mijić, A.; Varga, I.; Sudarić, A.; Cesar, V.; Lepeduš, H. The Combination of Increased Temperatures and High Irradiation Causes Changes in Photosynthetic Efficiency. Plants 2021, 10, 2076. [Google Scholar] [CrossRef]
- Misra, A.N.; Misra, M.; Singh, R. Chlorophyll fluorescence in plant biology. In Biophysics; Misra, A.N., Ed.; Intech Open: London, UK, 2012; pp. 171–192. Available online: http://www.intechopen.com (accessed on 22 April 2022).
- Morales, A.; Kaiser, E. Photosynthetic Acclimation to Fluctuating Irradiance in Plants. Front. Plant Sci. 2020, 11, 268. [Google Scholar] [CrossRef]
- Scheurwater, I.; Dunnebacke, M.; Eising, R.; Lambers, H. Respiratory costs and rate of protein turnover in the roots of a fast-growing (Dactylis glomerata L.) and a slow-growing (Festuca ovina L.) grass species. J. Exp. Bot. 2000, 51, 1089–1097. [Google Scholar]
- Mlinarić, S.; Antunović Dunić, J.; Štolfa, I.; Cesar, V.; Lepeduš, H. High irradiation and increased temperature induce different strategies for competent photosynthesis in young and mature fig leaves. S. Afr. J. Bot. 2016, 103, 25–31. [Google Scholar] [CrossRef]
- Yanhui, C.; Hongrui, W.; Beining, Z.; Shixing, G.; Zihan, W.; Yue, W.; Huihui, Z.; Guangyu, S. Elevated air temperature damage to photosynthetic apparatus alleviated by enhanced cyclic electron flow around photosystem I in tobacco leaves. Ecotoxicol. Environ. Saf. 2020, 204, 111136. [Google Scholar] [CrossRef] [PubMed]
- Pospíšil, P. Production of Reactive Oxygen Species by Photosystem II as a Response to Light and Temperature Stress. Front. Plant Sci. 2016, 7, 1950. [Google Scholar] [CrossRef]
- Busheva, M.; Garab, G.; Liker, E.; Tóth, Z.; Szèll, M.; Nagy, F. Diurnal fluctuations in the content and functional properties of the light harvesting chlorophyll a/b complex in thylakoid membranes. Plant Physiol. 1991, 95, 997–1003. [Google Scholar] [CrossRef][Green Version]
- Michoux, F.; Ahmad, N.; Wei, Z.Y.; Belgio, E.; Ruban, A.V.; Nixon, P.J. Testing the role of the N-terminal tail of D1 in the maintenance of photosystem II in tobacco chloroplasts. Front. Plant Sci. 2016, 7, 844. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jia, H.; Tian, Q.; Du, L.; Gao, Y.; Miao, X.; Liu, Y. Protecting effect of phosphorylation on oxidative damage of D1 protein by down-regulating the production of superoxide anion in photosystem II membranes under high light. Photosynth. Res. 2012, 112, 141–148. [Google Scholar] [CrossRef]
- Cramer, W.A.; Baniulus, D.; Yamashita, E.; Zhang, H.; Zatsman, A.I.; Hendrich, M.P. Cytochrome b6f complex, colon structure, spectroscopy, and function of heme cn: N-side electron and proton transfer reactions. In Photosynthetic Protein Complexes; Fromme, P., Ed.; Weily-Blackwell Verlag GmbH&Co.: Weinheim, Germany, 2008; pp. 155–179. [Google Scholar]
- Helbling, E.W.; Buma, A.G.J.; Boelen, P.; Strate, H.J.; Giordanino, M.V.F.; Villafane, V.E. Increase in Rubisco activity and gene expression due to elevated temperature partially counteracts ultraviolet radiation-induced photoinhibition in the marine diatom Thalassiosira weissflogii. Limnol. Oceanogr. 2011, 56, 1330–1342. [Google Scholar] [CrossRef]
- Markulj Kulundžić, A.; Kovačević, J.; Viljevac Vuletić, M.; Josipović, A.; Liović, I.; Mijić, A.; Lepeduš, H.; Matoša Kočar, M. Impact of abiotic stress on photosynthetic efficiency and leaf temperature in sunflower. Poljoprivreda 2016, 22, 17–22. [Google Scholar] [CrossRef]
- Markulj Kulundžić, A.; Kovačević, J.; Viljevac Vuletić, M.; Jocić, S.; Cvejić, S.; Matoša Kočar, M.; Mijić, A.; Liović, I.; Sudarić, A.; Lepeduš, H.; et al. Effect of different soil water content effect on genotype expession in photosynthetic efficiency and leaf temperature in sunflower. Genet.-Belgrade 2016, 48, 971–982. [Google Scholar] [CrossRef][Green Version]
- Schneiter, A.A.; Miller, J.F. Description of sunflower growth stages. Crop Sci. 1981, 21, 901–903. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and sensitive method for quantitation of microgram quantities of protein utilising principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during assembly of head of Bacteriophage-T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef]
- Martinazzo, E.G.; Ramm, A.; Bacarin, M.A. The chlorophyll a fluorescence as an indicator of the temperature stress in the leaves of Prunus persica. Braz. J. Plant Physil. 2012, 24, 237–246. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M.; Strasser, R. The energy flux theory 35 years later: Formulations and applications. Photosynth. Res. 2013, 117, 289–320. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samoborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Yusuf, M.A.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli-Michael, M.; Govindjee; Sarin, N.B. Overexpression of g-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: Physiological and chlorophyll a fluorescence measurements. Biochim. Biophys. Acta 2010, 1797, 1428–1438. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M.; Kalaji, H.M.; Carpentier, R.; Allakhverdiev, S.I. Photosystem II thermostability in situ: Environmentally induced acclimation and genotype-specific reactions in Triticum aestivum L. Plant Physiol. Biochem. 2012, 57, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, G.C.; Govindjee. The Non-Photochemical Quenching of the Electronically Excited State of Chlorophyll a in Plants: Definitions, Timelines, Viewpoints, Open Questions. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Demmig-Adams, B., Garab, G., Adams, W., III, Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2014; p. 44. [Google Scholar] [CrossRef]
- Paunov, M.; Koleva, L.; Vassilev, A.; Vangronsveld, J.; Golstev, V. Effects Different Metals on Photosynthesis: Cadmium and Zink Affect Chlorophyll Fluorescence in Durum Wheat. Int. J. Mol. Sci. 2018, 19, 787. [Google Scholar] [CrossRef] [PubMed]
- Desotgiu, R.; Pollastrini, M.; Cascio, C.; Gerosa, G.; Marzuoli, R.; Bussotti, F. Responses to ozone on Populus Oxford clone in an open top chamber experiment assessed before sunrise and in full sunlight. Photosynthetica 2013, 51, 267–280. [Google Scholar] [CrossRef]
- Schansker, G.; Tóth, S.Z.; Holzwarth, A.R.; Garab, G. Chlorophyll a fluorescence: Beyond the limits of the QA model. Photosyn. Res. 2014, 120, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, M.H.; Goltsev, V.N.; Żuk-Gołaszewska, K.; Zivcak, M.; Brestic, M. Chlorophyll Fluorescence Understanding Crop Performance—Basics and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Misra, A.N. Chlorophyll fluorescence: A practical approach to study ecophysiology of green plants. In Advances in Plant Ecophysiology Techniques; Sánchez-Moreiras, A.M., Reigosa, M.J., Eds.; Springer: Cham, Switzerland, 2018; Chapter 5; pp. 77–97. [Google Scholar]
- Feng, B.; Liu, P.; Li, G.; Dong, S.T.; Wang, F.H.; Kong, L.A.; Zhang, J.W. Effect of Heat Stress on the Photosynthetic Characteristics in Flag Leaves at the Grain-Filling Stage of Different Heat-Resistant Winter Wheat Varieties. J. Agro. Crop Sci. 2014, 200, 143–155. [Google Scholar] [CrossRef]
- Fghire, R.; Anaya, F.; Ali, O.I.; Benlhabib, O.; Ragab, R.; Wahbi, S. Physiological and photosynthetic response of quinoa to drought stress. ChileanJAR 2015, 75, 174–183. [Google Scholar] [CrossRef]
- Çiçek, N.; Pekcan, V.; Arslan, Ö.; Erdal, S.E.; Balkan Nalçaiyi, A.S.; Çil, A.N.; Şahin, V.; Kaya, Y.; Ekmekçi, Y. Assessing drought tolerance in field-grown sunflower hybrids by chlorophyll fluorescence kinetics. Braz. J. Bot. 2019, 42, 249–260. [Google Scholar] [CrossRef]
- Viljevac Vuletić, M.; Španić, V. Special issue in honour of Prof. Reto, J. Strasser–Characterisation of photosynthetic performance during natural leaf senescence in winter wheat: Multivariate analysis as a tool for phenotypic characterisation. Photosynthetica 2020, 58, 301–313. [Google Scholar] [CrossRef]
- Gururani, M.A.; Venkatesh, J.; Ganesan, M.; Strasser, R.J.; Han, Y.; Kim, J.-I.; Lee, H.-Y.; Song, P.-S. In Vivo Assessment of Cold Tolerance through Chlorophyll-a Fluorescence in Transgenic Zoysiagrass Expressing Mutant Phytochrome A. PLoS ONE 2015, 10, e0127200. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef]
- Yan, K.; Shao, H.; Shao, C.; Zhao, S.; Brestic, M. Dissection of photosynthetic electron transport process in sweet sorghum under heat stress. PLoS ONE 2013, 8, e62100. [Google Scholar] [CrossRef]
- Arslan, Ö.; Balkan Nalçaiyi, A.S.; Çulha Erdal, S.; Pekcan, V.; Kaya, Y.; Çiçek, N.; Ekmekçi, Y. Special issue in honour of Prof. Reto J. Strasser–Analysis of drought response of sunflower inbred lines by chlorophyll a fluorescence induction kinetics. Photosynthetica 2020, 58, 348–357. [Google Scholar] [CrossRef]
- Gupta, N.K.; Shubhi Agarwal, S.; Agarwal, V.P.; Nathawat, N.S.; Gupta, S.; Singh, G. Effect of short-term heat stress on growth, physiology and antioxidative defence system in wheat seedlings. Acta Physiol. Plant 2013, 35, 1837–1842. [Google Scholar] [CrossRef]
- Prasad, S.M.; Dwivedi, R.; Zeeshan, M. Growth, photosynthetic electron transport, and antioxidant responses of young soybean seedlings to simultaneous exposure of nickel and UV-B stress. Photosynthetica 2005, 43, 177–185. [Google Scholar] [CrossRef]
- Chen, Y.E.; Zhang, C.M.; Su, Y.Q.; Ma, J.; Zhang, Z.W.; Yuan, M.; Zhang, H.U.; Yuan, S. Responses of photosystem II and antioxidative systems to high light and high temperature co-stress in wheat. Environ. Exp. Bot. 2017, 135, 45–55. [Google Scholar] [CrossRef]
- Oguchi, R.; Hikosaka, K.; Hirose, T. Does the photosynthetic light-acclimation need change in leaf anatomy? Plant Cell Environ. 2003, 26, 505–512. [Google Scholar] [CrossRef]
- Tanaka, R.; Tanaka, A. Chlorophyll cycle regulates the construction and destruction of the light-harvesting complexes. Biochim. Biophys. Acta 2011, 1807, 968–976. [Google Scholar] [CrossRef]
- Li, H.; Xu, H.; Zhang, P.; Gao, M.; Wang, D.; Zhao, H. High temperature effects on D1 protein turnover in three wheat varieties with different heat susceptibility. Plant Growth Regul. 2017, 81, 385–397. [Google Scholar] [CrossRef]
- Su, X.Y.; Wu, S.; Yang, L.; Xue, R.L.; Li, H.; Wang, Y.X.; Zhao, H.J. Exogenous progesterone alleviates heat and high light stress-induced inactivation of photosystem II in wheat by enhancing antioxidant defense and D1 protein stability. Plant Growth Regul. 2014, 74, 311–318. [Google Scholar] [CrossRef]
- Chan, T.; Shimizu, Y.; Pospisil, P.; Nijo, N.; Fujiwara, A.; Taninaka, Y.; Ishikawa, T.; Hori, H.; Nanba, D.; Imai, A.; et al. Quality Control of Photosystem II: Lipid Peroxidation Accelerates Photoinhibition under Excessive Illumination. PLoS ONE 2013, 8, e52100. [Google Scholar] [CrossRef]
- Guo, W.-D.; Guo, Y.-P.; Liu, J.-R.; Mattson, N. Midday depression of photosynthesis is related with carboxylation efficiency decrease and D1 degradation in bayberry (Myrica rubra) plants. Sci. Hortic. 2009, 123, 188–196. [Google Scholar] [CrossRef]
- Järvi, S.; Suorsa, M.; Aro, E.-M. Photosystem II repair in plant chloroplasts-Regulation, assisting proteins and shared components with photosystem II biogenesis. Biochim. Biophys. Acta 2015, 1847, 900–909. [Google Scholar] [CrossRef]
- Vojta, L.; Carić, D.; Cesar, V.; Antunović Dunić, J.; Lepeduš, H.; Kveder, M.; Fulgosi, H. TROL-FNR interaction reveals alternative parthways of electron partitioning in photosynthesis. Sci. Rep. 2015, 5, 10085. [Google Scholar] [CrossRef]
- Björkman, O. Response to different quantum flux densities. In Physiological Plant Ecology I. Responses to the Physical Environment; Lange, O.D., Nobel, P.S., Osmond, C.B., Ziegler, H., Eds.; Springer: Berlin, Germany, 1981; pp. 57–107. [Google Scholar]
- Hojka, M.; Thiele, W.; Tóth, S.Z.; Lein, W.; Bock, R.; Schöttler, M.A. Inducible Repression of Nuclear-Encoded Subunits of the Cytochrome b6f Complex in Tobacco Reveals an Extraordinarily Long Lifetime of the Complex. Plant Physiol. 2014, 165, 1632–1646. [Google Scholar] [CrossRef]
- Yamori, W.; Evans, J.R.; von Caemmerer, S. Effects of growth and measurement light intensities on temperature dependence of CO2 assimilation rate in tobacco leaves. Plant Cell Environ. 2010, 33, 332–343. [Google Scholar] [CrossRef]
- Pavlovič, A.; Stolárik, T.; Nosek, L.; Kouřil, R.; Ilík, P. Light-induced gradual activation of photosystem II in dark-grown Norway spruce seedlings. Biochim. Biophys. Acta 2016, 1857, 799–809. [Google Scholar] [CrossRef]
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature response of photosynthesis in C3, C4, and CAM plants: Temperature acclimation and temperature adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef]
- Cheng, L.; Fuchigami, L.H.; Breen, P.J. The relationship between photosystem II efficiency and quantum yield for CO2 assimilation is not affected by nitrogen content in apple leaves. J. Exp. Bot. 2001, 52, 1865–1872. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Kunderlikova, K.; Sytar, O.; Allakhverdiev, S.I. Repetitive light pulse-induced photoinhibition of photosystem I severely affects CO2 assimilation and photoprotection in wheat leaves. Photosynth. Res. 2015, 126, 449–463. [Google Scholar] [CrossRef]
- Lu, T.; Meng, Z.; Zhang, G.; Qi, M.; Sun, Z.; Liu, Y.; Li, T. Sub-high Temperature and High Light Intensity Induced Irreversible Inhibition on Photosynthesis System of Tomato Plant (Solanum lycopersicum L.). Front. Plant Sci. 2017, 8, 365. [Google Scholar] [CrossRef]

| Hybrid 4 | Hybrid 7 | |||
|---|---|---|---|---|
| Parameters | Morning | Afternoon | Morning | Afternoon |
| Minimum fluorescence intensity (F0) | 267.50 ± 8.51 b | 348.75 ± 25.09 a | 223.75 ± 7.31 c | 341.83 ± 28.87 a |
| Maximum fluorescence intensity (Fm) | 1740.92 ± 67.50 a | 1591.83 ± 104.15 b | 1416.75 ± 99.07 c | 1115.58 ± 92.91 d |
| Relative variable fluorescence at 150 µs (VL step) | 0.54 ± 0.01 b | 0.54 ± 0.02 b | 0.54 ± 0.01 b | 0.56 ± 0.02 a |
| Relative variable fluorescence at 300 µs (VK step) | 0.36 ± 0.02 c | 0.39 ± 0.03 b | 0.40 ± 0.02 b | 0.44 ± 0.03 a |
| Relative variable fluorescence at 3 ms (VJ step) | 0.24 ± 0.02 d | 0.40 ± 0.03 b | 0.28 ± 0.02 c | 0.58 ± 0.03 a |
| Relative variable fluorescence at 30 ms (VI step) | 0.46 ± 0.05 ab | 0.45 ± 0.03 b | 0.50 ± 0.05 a | 0.47 ± 0.03 ab |
| Maximum quantum yield of PSII (TR0/ABS) | 0.85 ± 0.01 a | 0.78 ± 0.01 b | 0.84 ± 0.01 a | 0.69 ± 0.03 c |
| Density of active PSII reaction centres (RCs) per cross section (RC/CS0) | 157.84 ± 7.44 b | 173.32 ± 12.43 a | 119.35 ± 6.53 d | 135.53 ± 10.54 c |
| Density of RC on chlorophyll a basis (RC/ABS) | 0.59 ± 0.03 a | 0.50 ± 0.04 b | 0.53 ± 0.03 b | 0.40 ± 0.04 c |
| Flux ratio trapping per dissipation (TR0/DI0) | 5.51 ± 0.19 a | 3.58 ± 0.30 b | 5.34 ± 0.45 a | 2.28 ± 0.31 c |
| Electron transport further than primary acceptor QA (ET0/(TR0-ET0)) | 3.16 ± 0.26 a | 1.53 ± 0.20 c | 2.61 ± 0.26 b | 0.73 ± 0.10 d |
| Performance index (PIABS) | 10.26 ± 0.66 a | 2.73 ± 0.48 c | 7.44 ± 1.20 b | 0.67 ± 0.17 d |
| Quantum yield for reduction in end electron acceptors at the PSI acceptor side (RE0/ABS) | 0.60 ± 0.04 c | 0.72 ± 0.03 b | 0.58 ± 0.04 c | 0.87 ± 0.07 a |
| Probability that an electron from the electron transport chain is transferred to reduce end electron acceptors at the PSI acceptor side (RE0/ET0) | 0.71 ± 0.05 c | 0.92 ± 0.04 b | 0.69 ± 0.06 c | 1.26 ± 0.11 a |
| Electron-flux-reducing end electron acceptors at the PSI acceptor side per RC (RE0/RC) | 0.78 ± 0.09 b | 0.87 ± 0.10 ab | 0.79 ± 0.10 b | 0.92 ± 0.12 a |
| Electron transport from PQH2 to final PSI acceptors (RE0/(ET0-RE0)) | 2.54 ± 0.51 b | 15.95 ± 11.20 a | 2.33 ± 0.60 b | −5.83 ± 2.98 c |
| Performance index for energy conservation from exciton to the reduction in PSI end acceptors (PItotal) | 26.09 ± 5.89 b | 40.21 ± 22.01 a | 16.97 ± 3.80 b | −4.22 ± 3.26 c |
| Chlorophyll a (Chl a) | 1.38 ± 0.02 b | 1.49 ± 0.03 a | 1.46 ± 0.02 a | 1.37 ± 0.01 b |
| Chlorophyll b (Chl b) | 0.32 ± 0.01 c | 0.35 ± 0.01 b | 0.37 ± 0.00 ab | 0.37 ± 0.01 a |
| Total chlorophyll a + b (Chl a + b) | 1.70 ± 0.02 c | 1.85 ± 0.03 a | 1.83 ± 0.02 a | 1.74 ± 0.01 b |
| Total carotenoids (Car) | 0.40 ± 0.00 bc | 0.45 ± 0.01 a | 0.41 ± 0.01 b | 0.40 ± 0.00 c |
| Ratio of chlorophyll a and b (Chl a/b) | 4.37 ± 0.20 a | 4.21 ± 0.04 a | 3.99 ± 0.06 b | 3.70 ± 0.06 c |
| Ratio of total chlorophyll content and carotenoids (Chl a + b/Car) | 4.24 ± 0.01 c | 4.09 ± 0.03 d | 4.47 ± 0.05 a | 4.39 ± 0.03 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markulj Kulundžić, A.; Viljevac Vuletić, M.; Matoša Kočar, M.; Antunović Dunić, J.; Varga, I.; Zdunić, Z.; Sudarić, A.; Cesar, V.; Lepeduš, H. Effect of Elevated Temperature and Excess Light on Photosynthetic Efficiency, Pigments, and Proteins in the Field-Grown Sunflower during Afternoon. Horticulturae 2022, 8, 392. https://doi.org/10.3390/horticulturae8050392
Markulj Kulundžić A, Viljevac Vuletić M, Matoša Kočar M, Antunović Dunić J, Varga I, Zdunić Z, Sudarić A, Cesar V, Lepeduš H. Effect of Elevated Temperature and Excess Light on Photosynthetic Efficiency, Pigments, and Proteins in the Field-Grown Sunflower during Afternoon. Horticulturae. 2022; 8(5):392. https://doi.org/10.3390/horticulturae8050392
Chicago/Turabian StyleMarkulj Kulundžić, Antonela, Marija Viljevac Vuletić, Maja Matoša Kočar, Jasenka Antunović Dunić, Ivana Varga, Zvonimir Zdunić, Aleksandra Sudarić, Vera Cesar, and Hrvoje Lepeduš. 2022. "Effect of Elevated Temperature and Excess Light on Photosynthetic Efficiency, Pigments, and Proteins in the Field-Grown Sunflower during Afternoon" Horticulturae 8, no. 5: 392. https://doi.org/10.3390/horticulturae8050392
APA StyleMarkulj Kulundžić, A., Viljevac Vuletić, M., Matoša Kočar, M., Antunović Dunić, J., Varga, I., Zdunić, Z., Sudarić, A., Cesar, V., & Lepeduš, H. (2022). Effect of Elevated Temperature and Excess Light on Photosynthetic Efficiency, Pigments, and Proteins in the Field-Grown Sunflower during Afternoon. Horticulturae, 8(5), 392. https://doi.org/10.3390/horticulturae8050392









