Copper Stress Enhances the Lignification of Axial Organs in Zinnia elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant-Growth Conditions
2.2. Quantification of Copper
2.3. Biochemical Characteristics
2.4. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.5. Biometric and Anatomical Analysis
2.6. Statistical Analysis
3. Results
3.1. Copper Amount
3.2. Concentration of Hydrogen Peroxide and MDA Products
3.3. Concentration of Phenolics and Lignin
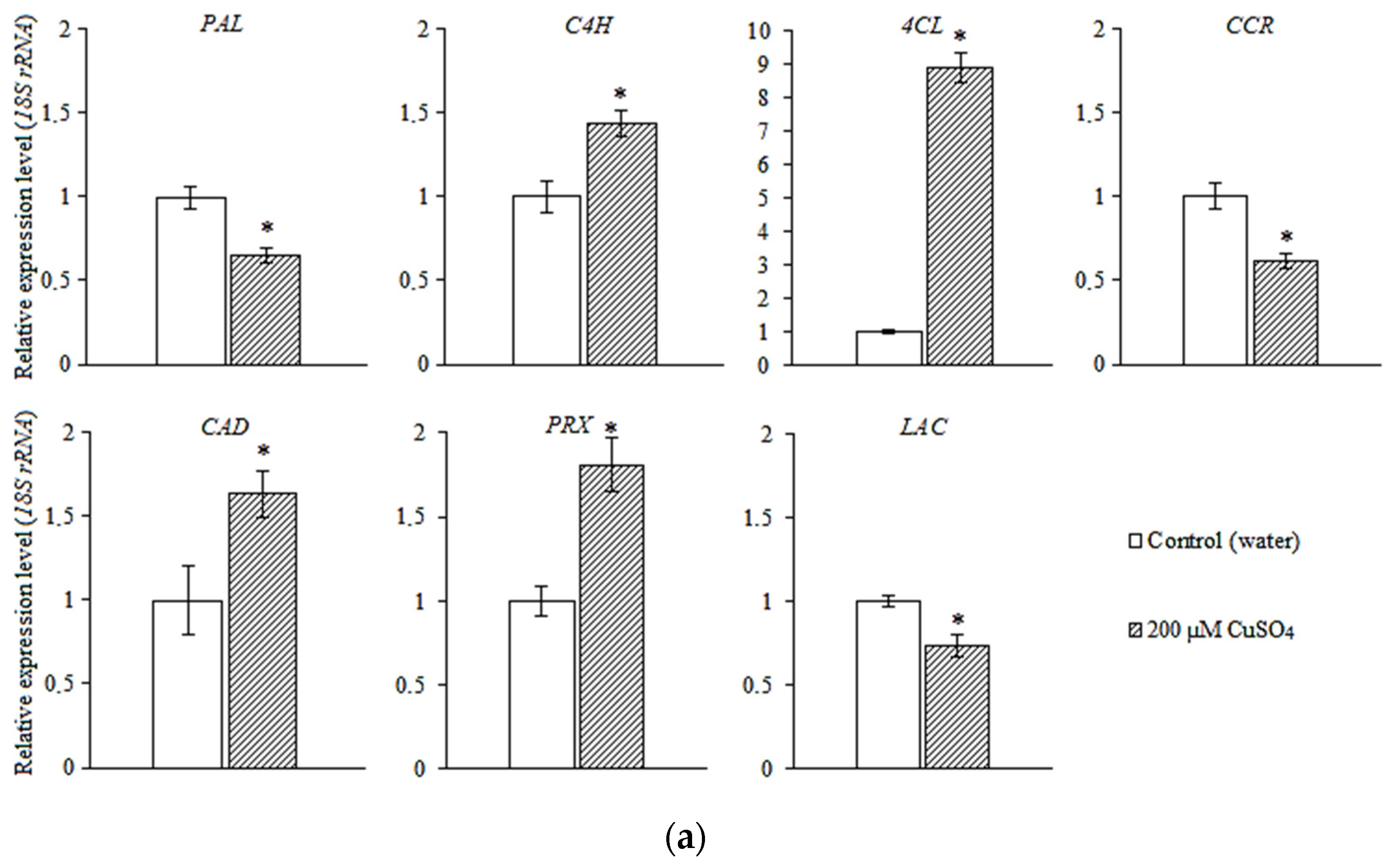
3.4. Expression of the Genes of Phenylpropanoid Metabolic Pathway and Lignin Biosynthesis
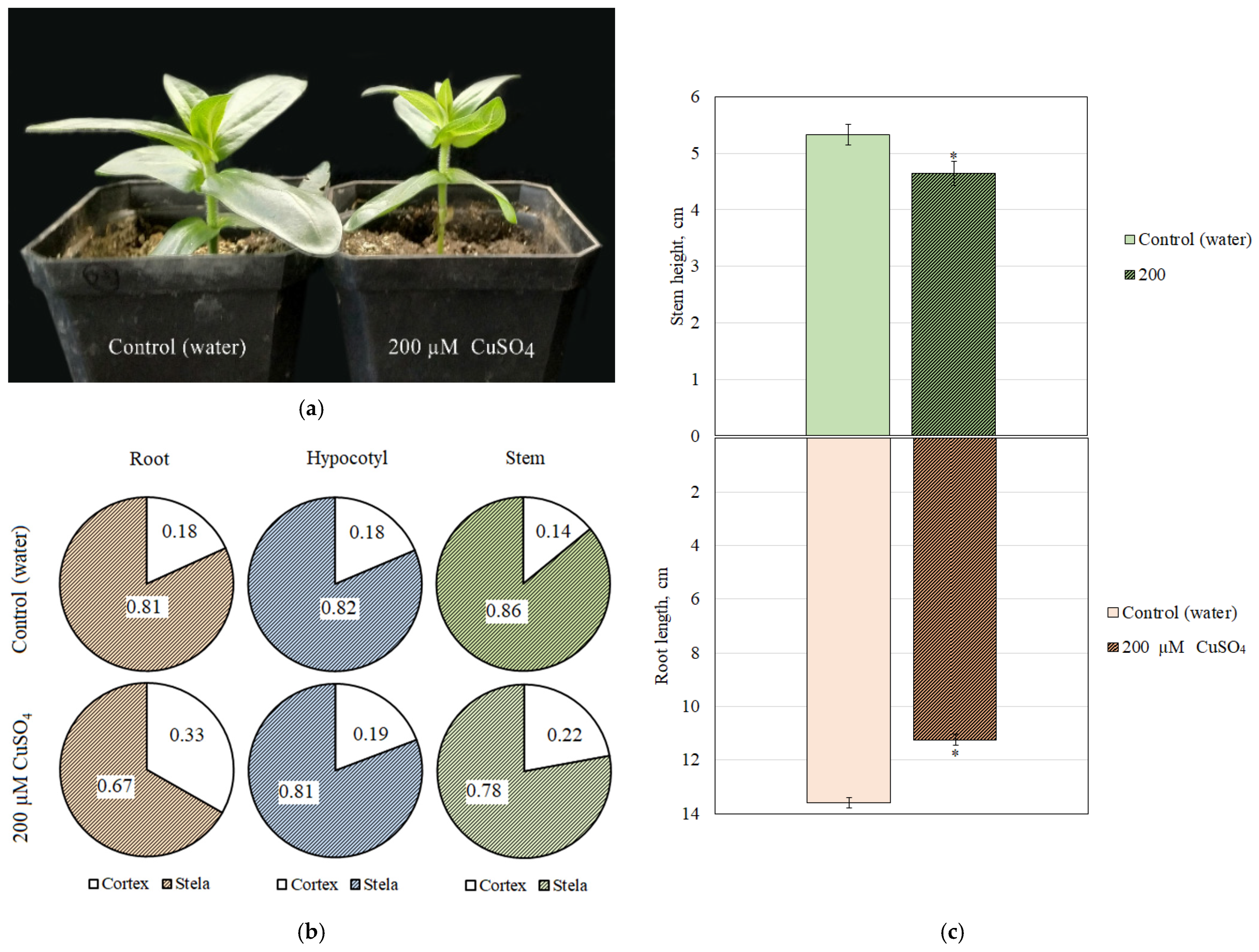
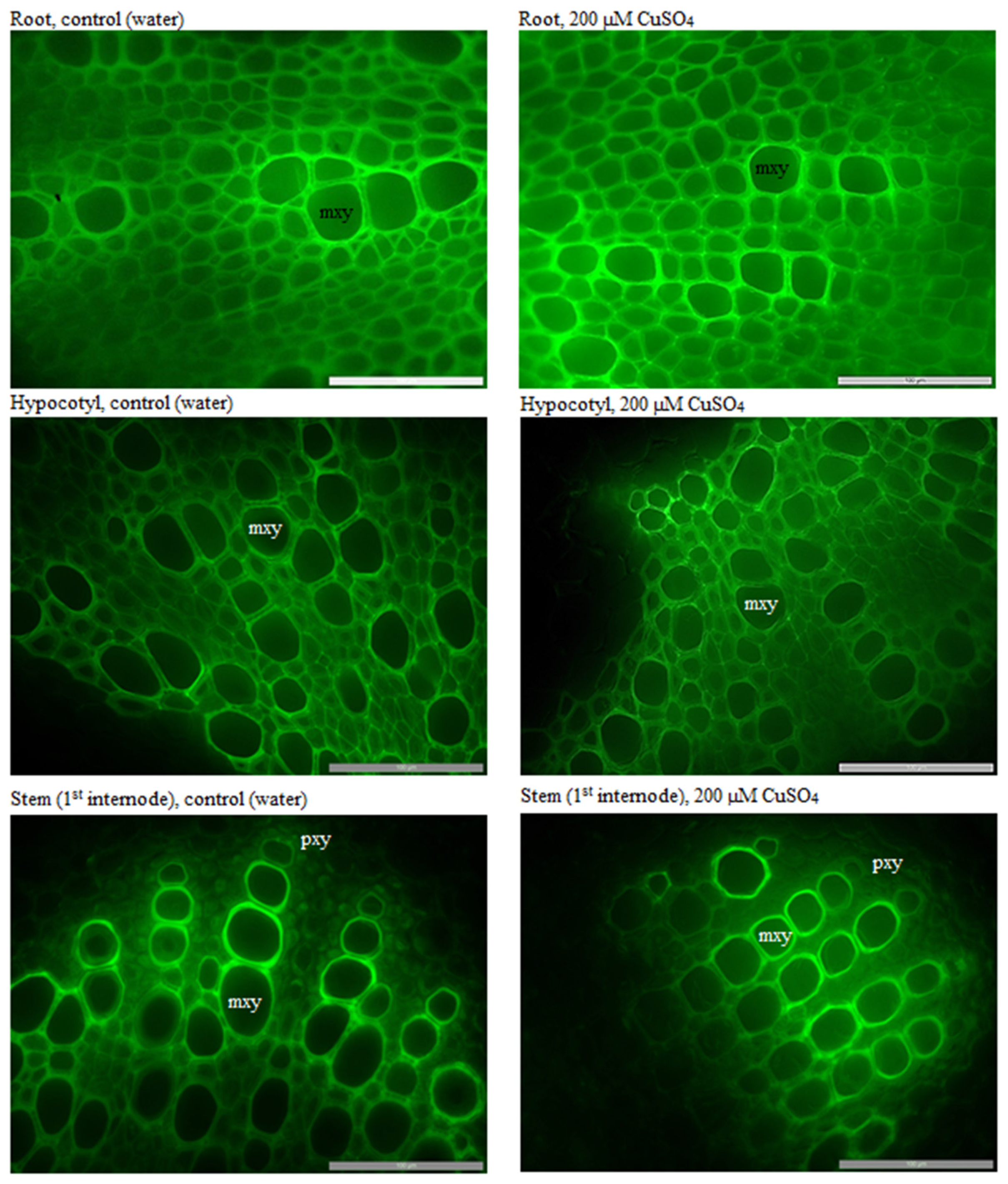
3.5. Anatomical and Morphological Characteristics of Zinnia Plants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ballabio, C.; Panagos, P.; Lugato, E.; Huang, J.-H.; Orgiazzi, A.; Jones, A.; Fernández-Ugalde, O.; Borrelli, P.; Montanarella, L. Copper distribution in European topsoils: An assessment based on LUCAS soil survey. Sci. Total Environ. 2018, 636, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, Z.; Sardar, A.; Shabbir, A.; Abbas, G.; Shamshad, S.; Khalid, S.; Ghulam Murtaza, N.; Dumat, C.; Shahid, M. Copper uptake, essentiality, toxicity, detoxification and risk assessment in soil-plant environment. Chemosphere 2020, 259, 127436. [Google Scholar] [CrossRef]
- Adriano, D.C. Trace Elements in Terrestrial Environments Biogeochemistry, Bioavailability, and Risks of Metals; Springer: New York, NY, USA, 2001; 867p. [Google Scholar] [CrossRef]
- Ali, M.B.; Singh, N.; Shohael, A.M.; Hahn, E.J.; Paek, K.Y. Phenolics metabolism and lignin synthesis in root suspension cultures of Panax ginseng in response to copper stress. Plant Sci. 2006, 171, 147–154. [Google Scholar] [CrossRef]
- Jouili, H.; Ferjani, E. Changes in antioxidant and lignifying enzyme activities in sunflower roots (Helianthus annuus L.) stressed with copper excess. Comp. Rend. Biolog. 2003, 326, 639–644. [Google Scholar] [CrossRef]
- Elleuch, A.; Chaâbene, Z.; Grubb, D.C.; Drira, N.; Mejdoub, H.; Khemakhem, B. Morphological and biochemical behavior of fenugreek (Trigonella foenum-graecum) under copper stress. Ecotoxicol. Environ. Saf. 2013, 98, 46–53. [Google Scholar] [CrossRef]
- Aioub, A.A.A.; Zuo, Y.; Aioub, A.A.A.; Hu, Z. Biochemical and phytoremediation of Plantago major L. to protect tomato plants from the contamination of cypermethrin pesticide. Environ. Sci. Pollut. Res. 2021, 28, 43992–44001. [Google Scholar] [CrossRef] [PubMed]
- Loix, C.; Huybrechts, M.; Vangronsveld, J.; Gielen, M.; Keunen, E.; Cuypers, A. Reciprocal interactions between cadmium-induced cell wall responses and oxidative stress in plants. Front. Plant Sci. 2017, 8, 1867. [Google Scholar] [CrossRef] [PubMed]
- Bouazizi, H.; Jouili, H.; Geitmann, A.; Ferjani, E. Cell wall accumulation of Cu ions and modulation of lignifying enzymes in primary leaves of bean seedlings exposed to excess copper. Biol. Trace Elem. Res. 2010, 139, 97–107. [Google Scholar] [CrossRef]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015, 5, 771. [Google Scholar] [CrossRef]
- Vaahtera, L.; Schulz, J.; Hamann, T. Cell wall integrity maintenance during plant development and interaction with the environment. Nat. Plants 2019, 5, 924–932. [Google Scholar] [CrossRef]
- Berthet, S.; Thevenin, J.; Baratiny, D.; Demont-Caulet, N.; Debeaujon, I.; Bidzinski, P.; Leple, J.C.; Huis, R.; Hawkins, S.; Gomez, L.D.; et al. Role of plant laccases in lignin polymerization. Adv. Bot. Res. 2012, 61, 145–172. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Kukavica, B.; Vidovic, M.; Morina, F.; Menckhoff, L. Class III peroxidases: Functions, localization and redox regulation of isoenzymes. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D., Palma, J., Corpas, F., Eds.; Springer: Cham, Switzerland, 2018; pp. 269–300. [Google Scholar] [CrossRef]
- Fraser, C.M.; Chapple, C. The phenylpropanoid pathway in Arabidopsis. Arab. Book. 2011, 9, e0152. [Google Scholar] [CrossRef]
- Barros, J.; Serk, H.; Granlund, I.; Pesquet, E. The cell biology of lignification in higher plants. Ann. Bot. 2015, 115, 1053–1074. [Google Scholar] [CrossRef]
- Pesquet, E.; Zhang, B.; Andras Gorzsas, A.; Puhakainen, T.; Serk, H.; Escamez, S.; Barbier, O.; Gerber, L.; Courtois-Moreau, C.; Alatalo, E.; et al. Non-cell-autonomous postmortem lignification of tracheary elements in Zinnia elegans. Plant Cell 2013, 25, 1314–1328. [Google Scholar] [CrossRef]
- Ehsan, N.; Nawaz, R.; Ahmad, S.; Arshad, M.; Umair, M.; Sarmad, M. Remediation of heavy metal-contaminated soil by ornamental plant Zinnia (Zinnia elegance L.). Asian J. Chem. 2016, 28, 1338–1342. [Google Scholar] [CrossRef]
- Plotnikov, D.S.; Tugbaeva, A.S.; Ermoshin, A.A.; Kiseleva, I.S. Response reactions of Zinnia elegans seedlings to the impact of different copper ions concentrations. AIP Conf. Proc. 2021, 2388, 030034. [Google Scholar] [CrossRef]
- Roccotiello, E.; Marescotti, P.; Di Piazza, S.; Cecchi, G.; Mariotti, M.G.; Zotti, M. Biodiversity in Metal-Contaminated Sites—Problem and Perspective. In A Case Study, Biodiversity in Ecosystems—Linking Structure and Function; Blanco, J., Ed.; IntechOpen: London, UK, 2015; 1865p. [Google Scholar] [CrossRef]
- Bellincampi, D.; Dipierro, N.; Salvi, G.; Cervone, F.; De Lorenzo, G. Extracellular H2O2 induced by oligogalacturonics is not involved in the inhibition of the auxin-regulated rolB gene expression in tobacco leaf explants. Plant Physiol. 2000, 122, 1379–1385. [Google Scholar] [CrossRef]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Bioch. 1978, 86, 287–297. [Google Scholar] [CrossRef]
- Ermoshin, A.A.; Kiseleva, I.S.; Nsengiyumva, I.V.; Nsengiumva, D.S.; Duan, S.; Ma, C.; Kurchenko, V.P. Antioxidant activity and chemical composition of extracts from fruiting bodies of xylotrophic fungi growing on birch. J. Sib. Fed. Univ. Biol. 2021, 14, 339–353. [Google Scholar] [CrossRef]
- Bajpai, P. Pulp Bleaching. In Biermann’s Handbook of Pulp and Paper; Bajpai, P., Ed.; Elsevier: New York, NY, USA, 2018; pp. 465–491. [Google Scholar] [CrossRef]
- Kuluev, B.; Mikhaylova, E.; Ermoshin, A.; Veselova, S.; Tugbaeva, A.; Gumerova, G.; Gainullina, K.; Zaikina, E. The ARGOS-LIKE genes of Arabidopsis and tobacco as targets for improving plant productivity and stress tolerance. J. Plant Physiol. 2019, 242, 153033. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Berlin, G.P.; Miksche, J.P. Botanical Microtechnique and Cytochemistry; Iowa State University Press: Ames, IA, USA, 1976; 321p. [Google Scholar]
- Bizzo, A.L.; Intorne, A.C.; Gomes, P.H.; Susuki, M.S.; Esteves, B.D. Short-term physiological responses to copper stress in Salvinia auriculata Aubl. Acta Limnol. Bras. 2014, 26, 268–277. [Google Scholar] [CrossRef][Green Version]
- Thounaojam, T.C.; Panda, P.; Choudhury, S.; Patra, H.K.; Panda, S.K. Zinc ameliorates copper-induced oxidative stress in developing rice (Oryza sativa L.) seedlings. Protoplasma 2014, 251, 61–69. [Google Scholar] [CrossRef]
- Lequeux, H.; Hermans, C.; Lutts, S.; Verbruggen, N. Response to copper excess in Arabidopsis thaliana: Impact on the root system architecture, hormone distribution, lignin accumulation and mineral profile. Plant Physiol. Biochem. 2010, 48, 673–682. [Google Scholar] [CrossRef]
- Panou-Filotheou, H.; Bosabalidis, A.M. Root structural aspects associated with copper toxicity in oregano (Origanum vulgare subsp. hirtum). Plant Sci. 2004, 166, 1497–1504. [Google Scholar] [CrossRef]
- Chen, E.L.; Chen, Y.A.; Chen, L.M.; Liu, Z.H. Effect of copper on peroxidase activity and lignin content in Raphanus sativus. Plant Physiol. Biochem. 2002, 40, 439–444. [Google Scholar] [CrossRef]
- Lin, C.-C.; Chen, L.-M.; Liu, Z.-H. Rapid effect of copper on lignin biosynthesis in soybean roots. Plant Sci. 2005, 168, 855–861. [Google Scholar] [CrossRef]
- Yasuda, S.; Fukushima, K.; Kakehi, A. Formation and chemical structures of acid-soluble lignin I: Sulfuric acid treatment time and acid-soluble lignin content of hardwood. J. Wood Sci. 2001, 47, 69–72. [Google Scholar] [CrossRef]
- Michalak, M. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Kısa, D.; Öztürk, L.; Doker, S.; Gökçe, İ. Expression analysis of metallothioneins and mineral contents in tomato (Lycopersicon esculentum) under heavy metal stress. J. Sci. Food Agric. 2017, 97, 1916–1923. [Google Scholar] [CrossRef]
- Lee, D.; Meyer, K.; Chapple, C.; Douglas, C.J. Antisense suppression of 4-Coumarate: Coenzyme A Ligase activity in Arabidopsis leads to altered lignin subunit composition. Plant Cell 1997, 9, 1985–1998. [Google Scholar] [CrossRef]
- Mao, C.; Yi, K.; Yang, L.; Zheng, B.; Wu, Y.; Liu, F.; Wu, P. Identification of aluminium-regulated genes by cDNA-AFLP in rice (Oryza sativa L.): Aluminium-regulated genes for the metabolism of cell wall components. J. Exp. Bot. 2004, 55, 137–143. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Ma, X.; Guo, L.; He, Y.; Ren, Z.; Kuang, Z.; Zhang, X.; Zhang, Z. Analysis of potential strategies for cadmium stress tolerance revealed by transcriptome analysis of upland cotton. Sci. Rep. 2019, 9, 86. [Google Scholar] [CrossRef]
- Weber, M.; Trampczynska, A.; Clemens, S. Comparative transcriptome analysis of toxic metal responses in Arabidopsis thaliana and the Cd2+-hypertolerant facultative metallophyte Arabidopsis halleri. Plant Cell Environ. 2006, 29, 950–963. [Google Scholar] [CrossRef]
- Tamas, L.; Durcekova, K.; Huttova, J.; Mistrik, I. Expression of defence-related peroxidases Prx7 and Prx8 during abiotic stresses in barley roots. Acta Physiol. Plant. 2009, 31, 139. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, C.; Xue, Z.; Jin, Q.; Xu, Y. De novo transcriptome sequencing and discovery of genes related to copper tolerance in Paeonia ostii. Gene 2016, 576, 126–135. [Google Scholar] [CrossRef]
- Li, G.-Z.; Zheng, Y.-X.; Chen, S.-J.; Liu, J.; Wang, P.-F.; Wang, Y.-H.; Guo, T.-C.; Kang, G.-Z. TaWRKY74 participates copper tolerance through regulation of TaGST1 expression and GSH content in wheat. Ecotoxicol. Environ. Saf. 2021, 221, 112469. [Google Scholar] [CrossRef]
- Gómez-Ros, L.V.; Gabaldón, C.; Núñez-Flores, M.J.L.; Gutiérrez, J.; Herrero, J.; Zapata, J.M.; Sottomayor, M.; Juan Cuello, J.; Barceló, A.R. The promoter region of the Zinnia elegans basic peroxidase isoenzyme gene contains cis-elements responsive to nitric oxide and hydrogen peroxide. Planta 2012, 236, 327–342. [Google Scholar] [CrossRef]
- Maksimovic, I.; Kastori, R.; Krstic, L.; Lukovic, J. Steady presence of cadmium and nickel affects root anatomy, accumulation and distribution of essential ions in maize seedlings. Biol. Plant. 2007, 51, 589–592. [Google Scholar] [CrossRef]
- Yadav, V.; Arif, N.; Kováč, J.; Singh, V.P.; Tripathi, D.K.; Chauhan, D.K.; Vaculík, M. Structural modifications of plant organs and tissues by metals and metalloids in the environment: A review. Plant Physiol. Biochem. 2021, 159, 100–112. [Google Scholar] [CrossRef]
- Tewari, R.K.; Yadav, N.; Gupta, R.; Kumar, R. Oxidative stress under macronutrient deficiency. J. Soil Sci. Plant Nutr. 2021, 21, 832–859. [Google Scholar] [CrossRef]
- Rucińska-Sobkowiak, R. Water relations in plants subjected to heavy metal stresses. Acta Physiol. Plant. 2016, 38, 257. [Google Scholar] [CrossRef]

| Gene, GenBank Access No. | Forward Primer Sequence (5′ → 3′) | Reverse Primer Sequence (5′ → 3′) |
|---|---|---|
| PAL FM879196 | GTCACCAGGCGAAGAGTTTG | CGGAACACCATCCCATCCTT |
| C4H FM880082 | GAACTTTGAGCTGTTGCCGC | TGAAAAACCCACAAACAACAATCC |
| 4CL AU294519 | ACGTCACCTTCCGTTACACC | CGTCAGCGATTATCGACGGT |
| CCR FM881365 | CCTCGGCTTCTGGTCGATAC | TGTATGGCTTTGCTCGTGGT |
| CAD FM881026 | CCGTAAACCATCCTCTTGCG | CAAGCTTCCTCCCCACAATC |
| PRX AB023959 | TCGCAGCTTCAATGGTCAAAC | TCTCTTCTTCTTTCATACTTCCCTT |
| LAC AU286008 | AATAAGGACGGGTTGGGCTG | AGGGTAAGGGATACCACGCT |
| 18S rRNA AB089282 | ATGTGGTAGCCGTTTCTCAGG | TGCCCGTTGCTGCGAT |
| Treatment | Copper Amount, µg g−1 Dry Substrate | Copper Amount, µg g−1 DW | BCF | TF | |||
|---|---|---|---|---|---|---|---|
| Available | Total | Root | Stem | Root | Stem | ||
| Control (water) | n.d. | 7.75 ± 0.65 | 9.80 ± 0.52 | 11.36 ± 0.70 | n.d. | n.d. | 1.16 ± 0.07 |
| 200 µM CuSO4 | 1.12 ± 0.17 * 1 | 167.63 ± 1.87 * | 26.32 ± 1.32 * | 12.04 ± 0.69 | 23.5 ± 1.2 * | 10.75 ± 0.65 * | 0.46 ± 0.02 * |
| Treatment | H2O2, µmol g−1 FW | MDA, µmol g−1 FW | ||
|---|---|---|---|---|
| Root | Stem | Root | Stem | |
| Control (water) | 29.5 ± 3.6 1 | 120.5 ± 9.1 | 0.56 ± 0.03 | 0.31 ± 0.02 |
| 200 µM CuSO4 | 159.61 ± 5.88 * | 61.50 ± 3.8 * | 0.67 ± 0.02 * | 0.48 ± 0.02 * |
| Treatment | Phenolics, mg g−1 FW | KL, % | ASL, % | Total Lignin, % | ||||
|---|---|---|---|---|---|---|---|---|
| Root | Stem | Root | Stem | Root | Stem | Root | Stem | |
| Control (water) | 0.43 ± 0.01 1 | 0.61 ± 0.03 | 9.29 ± 0.08 | 8.02 ± 0.44 | 4.90 ± 0.23 | 6.43 ± 0.11 | 14.19 ± 0.31 | 14.19 ± 0.31 |
| 200 µM CuSO4 | 0.27 ± 0.01 * | 0.50 ± 0.01 * | 12.27 ± 0.20 * | 10.58 ± 0.56 * | 4.35 ± 0.42 | 4.47 ± 0.42 * | 16.62 ± 0.76 * | 15.05 ± 0.98 |
| Treatment | Cross-Sectional Diameter of Organ, mm | Metaxylem Cell-Wall Thickness, µm | Cross-Sectional Area of Metaxylem Vessels, µm2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Root | Hypocotyl | Stem (1st Internode) | Root | Hypocotyl | Stem (1st Internode) | Root | Hypocotyl | Stem (1st Internode) | |
| Control (water) | 1.74 ± 0.06 1 | 2.44 ± 0.05 | 2.57 ± 0.08 | 2.90 ± 0.06 | 2.62 ± 0.05 | 2.94 ± 0.08 | 51.34 ± 1.43 | 42.19 ± 2.17 | 42.97 ± 1.59 |
| 200 µM CuSO4 | 2.24 ± 0.06 * | 2.23 ± 0.05 * | 1.98 ± 0.13 * | 3.11 ± 0.06 * | 2.60 ± 0.06 | 2.90 ± 0.07 | 45.06 ± 1.88 * | 34.35 ± 1.41 * | 30.55 ± 0.59 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tugbaeva, A.; Ermoshin, A.; Wuriyanghan, H.; Maleva, M.; Borisova, G.; Kiseleva, I. Copper Stress Enhances the Lignification of Axial Organs in Zinnia elegans. Horticulturae 2022, 8, 558. https://doi.org/10.3390/horticulturae8060558
Tugbaeva A, Ermoshin A, Wuriyanghan H, Maleva M, Borisova G, Kiseleva I. Copper Stress Enhances the Lignification of Axial Organs in Zinnia elegans. Horticulturae. 2022; 8(6):558. https://doi.org/10.3390/horticulturae8060558
Chicago/Turabian StyleTugbaeva, Anastasia, Alexander Ermoshin, Hada Wuriyanghan, Maria Maleva, Galina Borisova, and Irina Kiseleva. 2022. "Copper Stress Enhances the Lignification of Axial Organs in Zinnia elegans" Horticulturae 8, no. 6: 558. https://doi.org/10.3390/horticulturae8060558
APA StyleTugbaeva, A., Ermoshin, A., Wuriyanghan, H., Maleva, M., Borisova, G., & Kiseleva, I. (2022). Copper Stress Enhances the Lignification of Axial Organs in Zinnia elegans. Horticulturae, 8(6), 558. https://doi.org/10.3390/horticulturae8060558








