Effects of Nonthermal Plasma (NTP) on the Growth and Quality of Baby Leaf Lettuce (Lactuca sativa var. acephala Alef.) Cultivated in an Indoor Hydroponic Growing System
Abstract
:1. Introduction
2. Materials and Methods
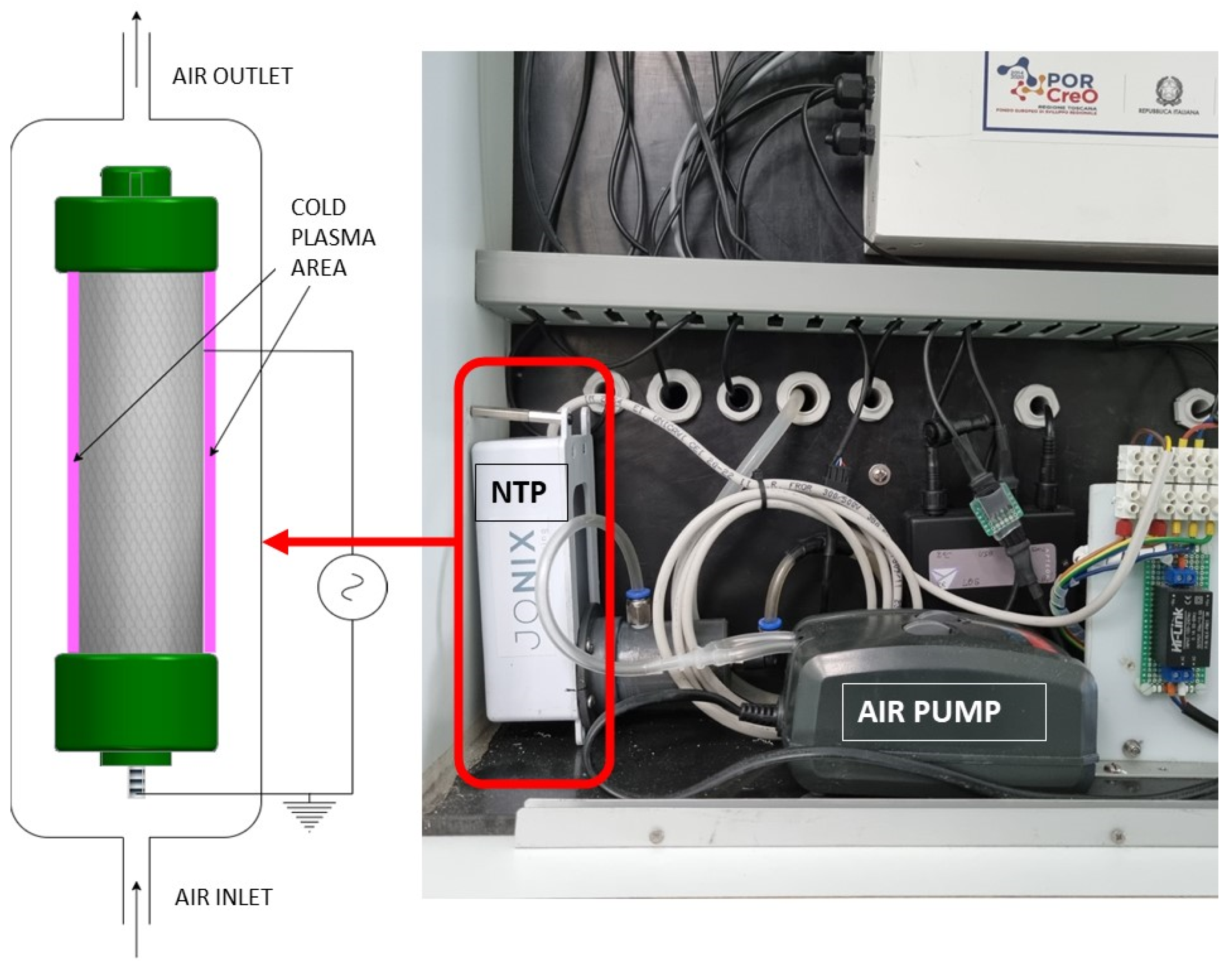
2.1. NTP Generator Description
2.2. Plant Growth Conditions
2.3. Experiments
2.4. Experiments Determining NTP Effects on Lettuce Plants
2.5. Determinations and Measurements
2.5.1. Ion Concentration of Hydroponic Nutrient Solutions
2.5.2. Crop Evapotranspiration and Growth
2.5.3. Leaf Content of Nitrates, Pigments, and Total Phenolics, and Antioxidant Capacity
2.5.4. Chlorophyll a Fluorescence
2.5.5. Statistical Analysis
3. Results
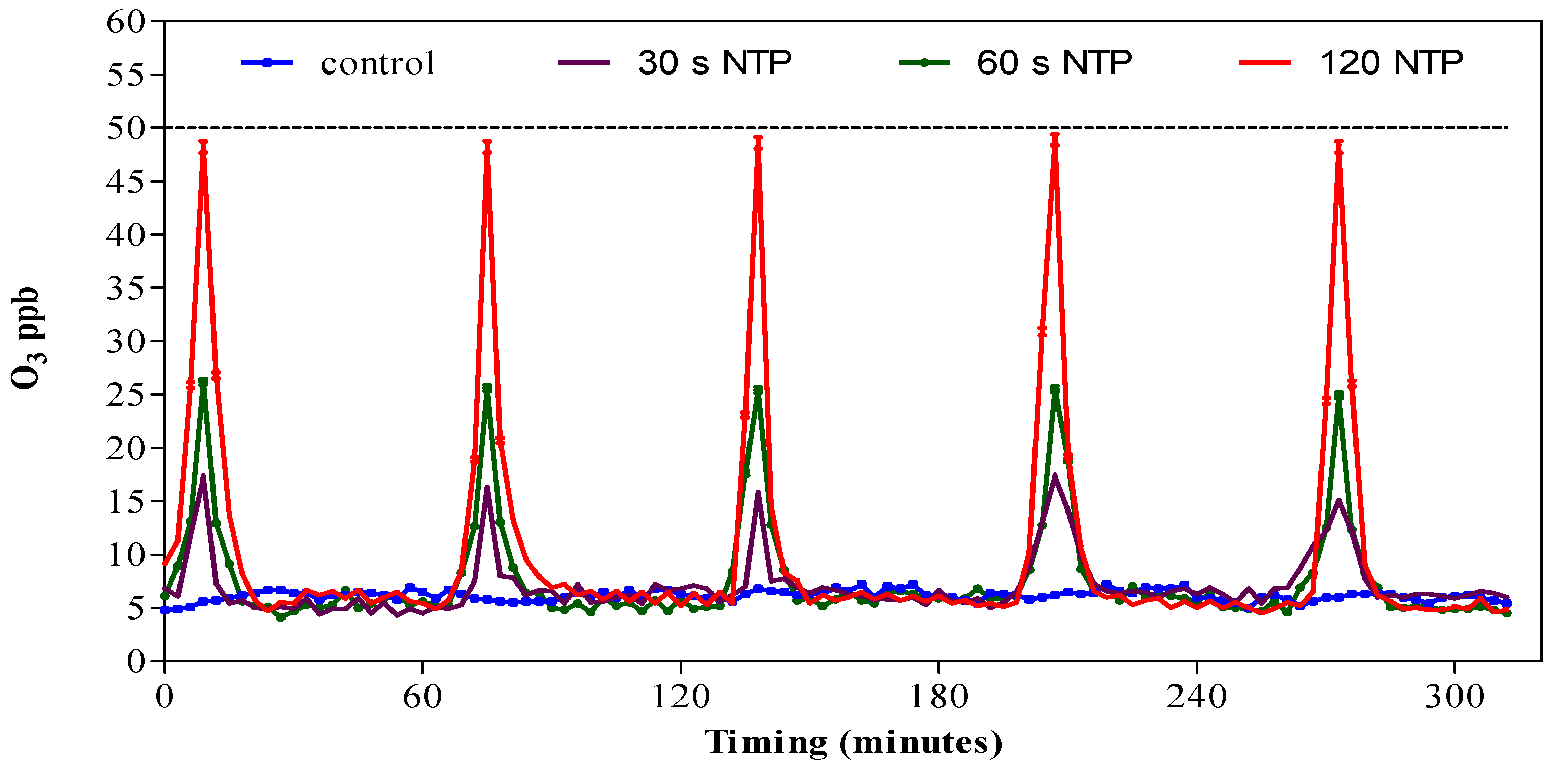
3.1. Ozone Concentration in HGS Produced by NTP Generator with Different Operating Times
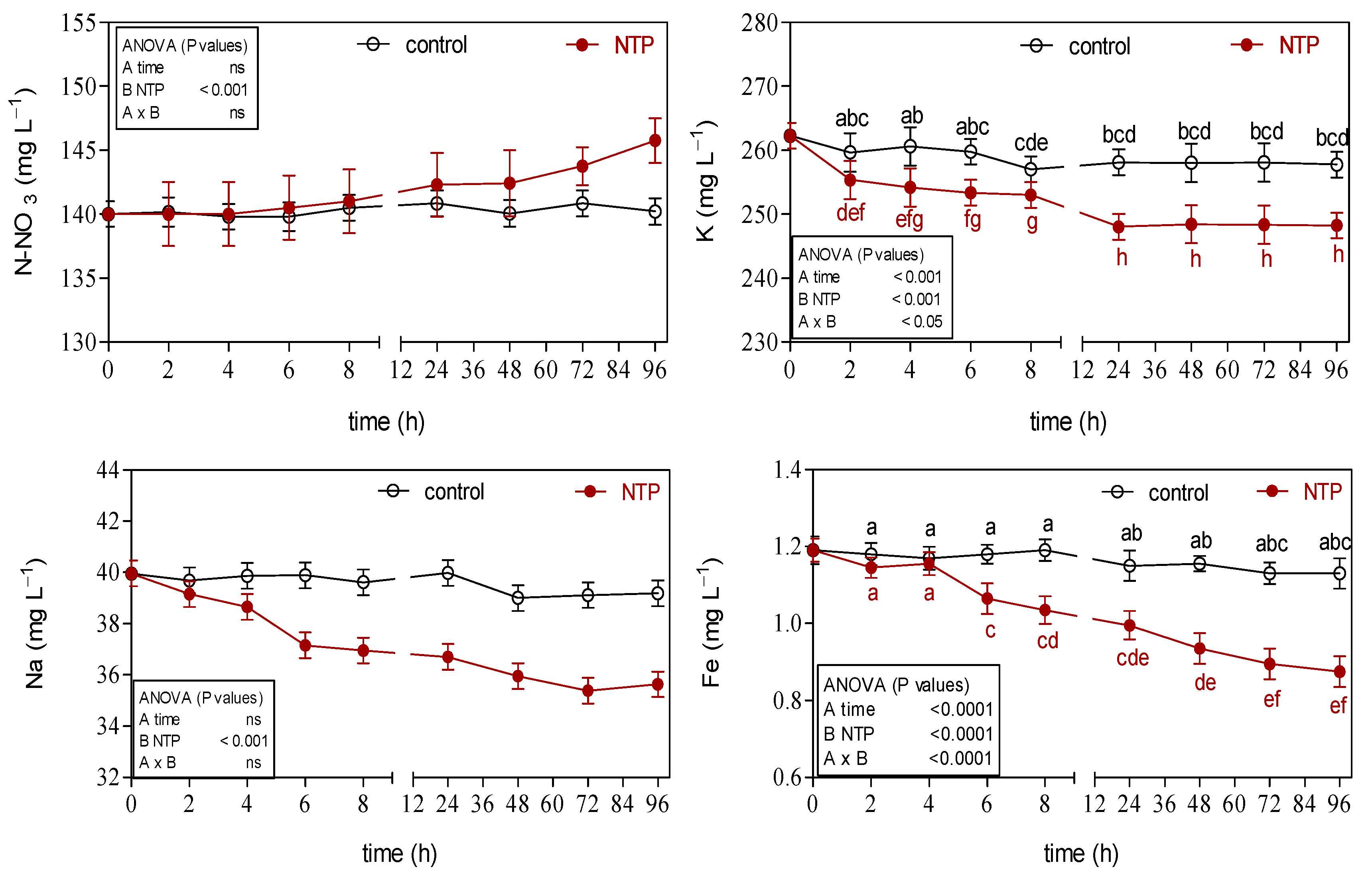
3.2. Effects of NTP on Ion Concentrations of Hydroponic Nutrient Solution
3.3. Effect of NTP on Lettuce Leaf Production and Quality
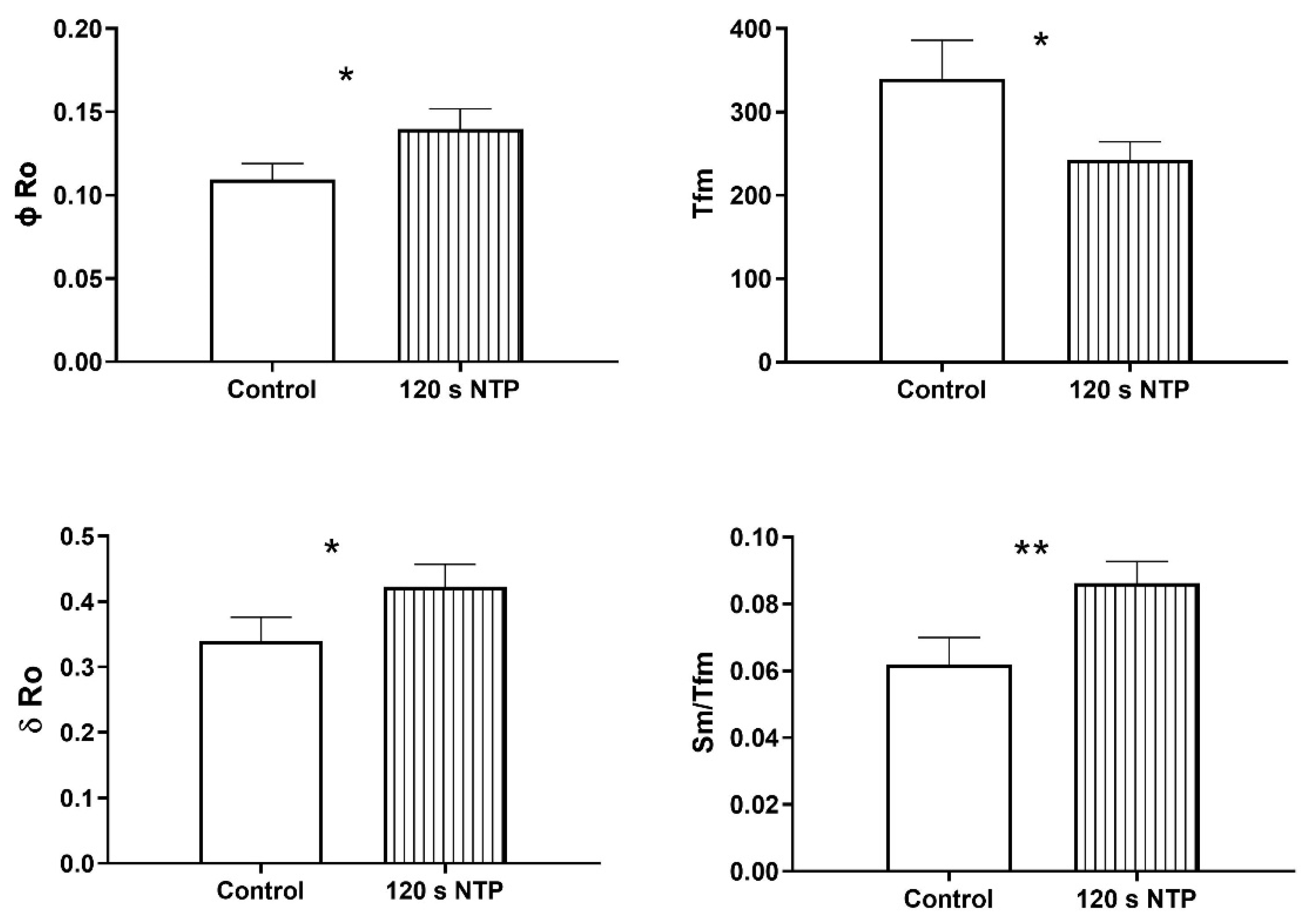
3.4. Chlorophyll a Fluorescence
4. Discussion
4.1. Effects of NTP Treatment on Hydroponic Nutrient Solution
4.2. Effects of NTP Treatment on Plant Growth
Chlorophyll a Fluorescence
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Park, D.P.; Davis, K.; Gilani, S.; Alonzo, C.-A.; Dobrynin, D.; Friedman, G.; Fridman, A.; Rabinovich, A.; Fridman, G. Reactive nitrogen species produced in water by non-equilibrium plasma increase plant growth rate and nutritional yield. Curr. Appl. Phys. 2013, 13, S19–S29. [Google Scholar] [CrossRef]
- Perinban, S.; Orsat, V.; Raghavan, V. Nonthermal Plasma–Liquid Interactions in Food Processing: A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1985–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surowsky, B.; Schlüter, O.; Knorr, D. Interactions of Non-Thermal Atmospheric Pressure Plasma with Solid and Liquid Food Systems: A Review. Food Eng. Rev. 2015, 7, 82–108. [Google Scholar] [CrossRef]
- Holubová, Ľ.; Kyzek, S.; Ďurovcová, I.; Fabová, J.; Horváthová, E.; Ševčovičová, A.; Gálová, E. Non-Thermal Plasma—A New Green Priming Agent for Plants? Int. J. Mol. Sci. 2020, 21, 9466. [Google Scholar] [CrossRef] [PubMed]
- Henselová, M.; Slováková, Ľ.; Martinka, M.; Zahoranová, A. Growth, anatomy and enzyme activity changes in maize roots induced by treatment of seeds with low-temperature plasma. Biologia 2012, 67, 490–497. [Google Scholar] [CrossRef]
- Randeniya, L.K.; De Groot, G.J.J.B. Non-Thermal Plasma Treatment of Agricultural Seeds for Stimulation of Germination, Removal of Surface Contamination and Other Benefits: A Review. Plasma Process. Polym. 2015, 12, 608–623. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, Y.; Yang, S.; Chen, W. Introduction of a new atmospheric pressure plasma device and application on tomato seeds. Agric. Sci. 2011, 2, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Arc, E.; Galland, M.; Godin, B.; Cueff, G.; Rajjou, L. Nitric oxide implication in the control of seed dormancy and germination. Front. Plant Sci. 2013, 4, 346. [Google Scholar] [CrossRef] [Green Version]
- Sivachandiran, L.; Khacef, A. Enhanced seed germination and plant growth by atmospheric pressure cold air plasma: Combined effect of seed and water treatment. RSC Adv. 2017, 7, 1822–1832. [Google Scholar] [CrossRef] [Green Version]
- ŠERÁ, B.; Šerý, M. Non-thermal plasma treatment as a new biotechnology in relation to seeds, dry fruits, and grains. Plasma Sci. Technol. 2018, 20, 044012. [Google Scholar] [CrossRef] [Green Version]
- Măgureanu, M.; Sîrbu, R.; Dobrin, D.; Gîdea, M. Stimulation of the Germination and Early Growth of Tomato Seeds by Non-thermal Plasma. Plasma Chem. Plasma Process. 2018, 38, 989–1001. [Google Scholar] [CrossRef]
- Gambineri, F.; Bazzichi, A.; Cervelli, F.; Cecchi, A. La Tecnologia NTP Come Sistema Di Sanificazione Microbiologica Potenzialmente Applicabile Ai Substrati Di Coltivazione—Non Thermal Plasma for Sustrate Cultivation of Tomato: Effects on Quality and Productivity. Acta Italus Hortus 2015, 18, 104–106. [Google Scholar]
- Cannazzaro, S.; Traversari, S.; Cacini, S.; Di Lonardo, S.; Pane, C.; Burchi, G.; Massa, D. Non-Thermal Plasma Treatment Influences Shoot Biomass, Flower Production and Nutrition of Gerbera Plants Depending on Substrate Composition and Fertigation Level. Plants 2021, 10, 689. [Google Scholar] [CrossRef] [PubMed]
- Safari, N.; Iranbakhsh, A.; Ardebili, Z.O. Non-thermal plasma modified growth and differentiation process of Capsicum annuum PP805 Godiva in in vitro conditions. Plasma Sci. Technol. 2017, 19, 055501. [Google Scholar] [CrossRef]
- Świecimska, M.; Tulik, M.; Šerá, B.; Golińska, P.; Tomeková, J.; Medvecká, V.; Bujdáková, H.; Oszako, T.; Zahoranová, A.; Šerý, M. Non-Thermal Plasma Can Be Used in Disinfection of Scots Pine (Pinus sylvestris L.) Seeds Infected with Fusarium oxysporum? Forests 2020, 11, 837. [Google Scholar] [CrossRef]
- Iranbakhsh, A.; Ardebili, N.O.; Ardebili, Z.O.; Shafaati, M.; Ghoranneviss, M. Non-thermal Plasma Induced Expression of Heat Shock Factor A4A and Improved Wheat (Triticum aestivum L.) Growth and Resistance Against Salt Stress. Plasma Chem. Plasma Process. 2017, 38, 29–44. [Google Scholar] [CrossRef]
- Babajani, A.; Iranbakhsh, A.; Oraghi Ardebili, Z.; Eslami, B. Seed Priming with Non-thermal Plasma Modified Plant Reactions to Selenium or Zinc Oxide Nanoparticles: Cold Plasma as a Novel Emerging Tool for Plant Science. Plasma Chem. Plasma Process. 2019, 39, 21–34. [Google Scholar] [CrossRef]
- Lindsay, A.; Byrns, B.; King, W.; Andhvarapou, A.; Fields, J.; Knappe, D.; Fonteno, W.; Shannon, S. Fertilization of Radishes, Tomatoes, and Marigolds Using a Large-Volume Atmospheric Glow Discharge. Plasma Chem. Plasma Process. 2014, 34, 1271–1290. [Google Scholar] [CrossRef]
- Ranieri, P.; Sponsel, N.; Kizer, J.; Rojas-Pierce, M.; Hernández, R.; Gatiboni, L.; Grunden, A.; Stapelmann, K. Plasma agriculture: Review from the perspective of the plant and its ecosystem. Plasma Process. Polym. 2020, 18, 2000162. [Google Scholar] [CrossRef]
- Cocetta, G.; Casciani, D.; Bulgari, R.; Musante, F.; Kołton, A.; Rossi, M.; Ferrante, A. Light use efficiency for vegetables production in protected and indoor environments. Eur. Phys. J. Plus 2017, 132, 132. [Google Scholar] [CrossRef]
- Loconsole, D.; Cocetta, G.; Santoro, P.; Ferrante, A. Optimization of LED Lighting and Quality Evaluation of Romaine Lettuce Grown in An Innovative Indoor Cultivation System. Sustainability 2019, 11, 841. [Google Scholar] [CrossRef] [Green Version]
- de Neergaard, A.; Drescher, A.W.; Kouamé, C. Urban and Peri-Urban Agriculture in African Cities. In African Indigenous Vegetables in Urban Agriculture; Routledge: London, UK, 2009. [Google Scholar]
- Ronga, D.; Setti, L.; Salvarani, C.; De Leo, R.; Bedin, E.; Pulvirenti, A.; Milc, J.; Pecchioni, N.; Francia, E. Effects of solid and liquid digestate for hydroponic baby leaf lettuce (Lactuca sativa L.) cultivation. Sci. Hortic. 2018, 244, 172–181. [Google Scholar] [CrossRef]
- Kenny, O.; O’Beirne, D. The effects of washing treatment on antioxidant retention in ready-to-use iceberg lettuce. Int. J. Food Sci. Technol. 2009, 44, 1146–1156. [Google Scholar] [CrossRef]
- Mills, G.; Harmens, H. Ozone Pollution: A Hidden Threat to Food Security ICP Vegetation; ICP Veg.: Bangor, UK, 2011. [Google Scholar]
- Page, A.L. Methods of Soil Analysis—Part 2: Chemical and Microbiological Properties, 2nd ed.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; p. 9. [Google Scholar]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Kang, H.-M.; Saltveit, M.E. Antioxidant Capacity of Lettuce Leaf Tissue Increases after Wounding. J. Agric. Food Chem. 2002, 50, 7536–7541. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceppi, M.G.; Oukarroum, A.; Çiçek, N.; Strasser, R.J.; Schansker, G. The IP amplitude of the fluorescence rise OJIP is sensitive to changes in the photosystem I content of leaves: A study on plants exposed to magnesium and sulfate deficiencies, drought stress and salt stress. Physiol. Plant. 2011, 144, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied Plasma Medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Crema, A.P.S.; Borges, L.D.P.; Micke, G.A.; Debacher, N.A. Degradation of indigo carmine in water induced by non-thermal plasma, ozone and hydrogen peroxide: A comparative study and by-product identification. Chemosphere 2019, 244, 125502. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Takahasi, M. Variation of Elements of Nutrient Solution by Ozone Sterilization. Shokubutsu Kojo Gakkaishi 1993, 4, 148–150. [Google Scholar] [CrossRef]
- Lo Porto, C.; Ziuzina, D.; Los, A.; Boehm, D.; Palumbo, F.; Favia, P.; Tiwari, B.K.; Bourke, P.; Cullen, P.J. Plasma activated water and airborne ultrasound treatments for enhanced germination and growth of soybean. Innov. Food Sci. Emerg. Technol. 2018, 49, 13–19. [Google Scholar] [CrossRef]
- Cui, D.J.; Yin, Y.; Wang, J.Q.; Wang, Z.W.; Ding, H.B.; Ma, R.N.; Jiao, Z. Research on the Physio-Biochemical Mechanism of Non-Thermal Plasma-Regulated Seed Germination and Early Seedling Development in Arabidopsis. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pizá, M.C.; Cejas, E.; Zilli, C.; Prevosto, L.; Mancinelli, B.; Santa-Cruz, D.; Yannarelli, G.; Balestrasse, K. Enhancement of soybean nodulation by seed treatment with non–thermal plasmas. Sci. Rep. 2020, 10, 4917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasih, T.P.; Purwondho, R.; Danil, D.; Radjagukguk, R.; Bagaskara, A. Germination enhancement of green bell pepper (Capsicum annuum L.) by using non thermal argon plasma. IOP Conf. Ser. Earth Environ. Sci. 2020, 426, 12131. [Google Scholar] [CrossRef]
- Peethambaran, B.; Han, J.; Kermalli, K.; Jiaxing, J.; Fridman, G.; Balsamo, R.; Fridman, A.A.; Miller, V. Nonthermal Plasma Reduces Water Consumption While Accelerating Arabidopsis thaliana Growth and Fecundity. Plasma Med. 2015, 5, 87–98. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Park, G.; Choi, E.H. Cold Atmospheric Plasma-Activated Water Irrigation Induces Defense Hormone and Gene expression in Tomato seedlings. Sci. Rep. 2019, 9, 16080. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Li, J.; Dong, Y. Effect of cold plasma treatment on seedling growth and nutrient absorption of tomato. Plasma Sci. Technol. 2018, 20, 44007. [Google Scholar] [CrossRef] [Green Version]
- Del Río, L.A. ROS and RNS in plant physiology: An overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef] [Green Version]
- Sami, F.; Faizan, M.; Faraz, A.; Siddiqui, H.; Yusuf, M.; Hayat, S. Nitric oxide-mediated integrative alterations in plant metabolism to confer abiotic stress tolerance, NO crosstalk with phytohormones and NO-mediated post translational modifications in modulating diverse plant stress. Nitric Oxide 2018, 73, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Cannazzaro, S.; Di Lonardo, S.; Cacini, S.; Traversari, S.; Burchi, G.; Pane, C.; Gambineri, F.; Cursi, L.; Massa, D. Opportunities and challenges of using non-thermal plasma treatments in soilless cultures: Experience from greenhouse experiments. Acta Hortic. 2021, 259–266. [Google Scholar] [CrossRef]
- Oh, M.-M.; Carey, E.; Rajashekar, C. Environmental stresses induce health-promoting phytochemicals in lettuce. Plant Physiol. Biochem. 2009, 47, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Pangomm, K.; Veerana, M.; Mitra, S.; Park, G. Plant Disease Control by Non-Thermal Atmospheric-Pressure Plasma. Front. Plant Sci. 2020, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Hmelak Gorenjak, A.; Cencič, A. Nitrate in Vegetables and Their Impact on Human Health. A Review. Acta Alimentaria 2013, 42, 158–172. [Google Scholar] [CrossRef]
- Desotgiu, R.; Pollastrini, M.; Cascio, C.; Gerosa, G.; Marzuoli, R.; Bussotti, F. Chlorophyll a fluorescence analysis along a vertical gradient of the crown in a poplar (Oxford clone) subjected to ozone and water stress. Tree Physiol. 2012, 32, 976–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar] [CrossRef]
- Schansker, G.; Srivastava, A.; Govindjee; Strasser, R.J. Characterization of the 820-nm transmission signal paralleling the chlorophyll a fluorescence rise (OJIP) in pea leaves. Funct. Plant Biol. 2003, 30, 785–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cascio, C.; Schaub, M.; Novak, K.; Desotgiu, R.; Bussotti, F.; Strasser, R.J. Foliar responses to ozone of Fagus sylvatica L. seedlings grown in shaded and in full sunlight conditions. Environ. Exp. Bot. 2010, 68, 188–197. [Google Scholar] [CrossRef]
- Lin, Z.-H.; Chen, L.-S.; Chen, R.-B.; Zhang, F.-Z.; Jiang, H.-X.; Tang, N. CO2assimilation, ribulose-1,5-bisphosphate carboxylase/oxygenase, carbohydrates and photosynthetic electron transport probed by the JIP-test, of tea leaves in response to phosphorus supply. BMC Plant Biol. 2009, 9, 43. [Google Scholar] [CrossRef] [Green Version]
- Ghassemi-Golezani, K.; Lotfi, R. The impact of salicylic acid and silicon on chlorophyll a fluorescence in mung bean under salt stress. Russ. J. Plant Physiol. 2015, 62, 611–616. [Google Scholar] [CrossRef]
- Chen, L.-S.; Cheng, L. The acceptor side of photosystem II is damaged more severely than the donor side of photosystem II in ‘Honeycrisp’ apple leaves with zonal chlorosis. Acta Physiol. Plant. 2009, 32, 253–261. [Google Scholar] [CrossRef]
- Agathokleous, E. The rise and fall of photosynthesis: Hormetic dose response in plants. J. For. Res. 2020, 32, 889–898. [Google Scholar] [CrossRef]
- Agathokleous, E.; Araminiene, V.; Belz, R.G.; Calatayud, V.; De Marco, A.; Domingos, M.; Feng, Z.; Hoshika, Y.; Kitao, M.; Koike, T.; et al. A quantitative assessment of hormetic responses of plants to ozone. Environ. Res. 2019, 176, 108527. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Hernandez, M.; Macias-Bobadilla, I.; Guevara-Gonzalez, R.G.; Romero-Gomez, S.D.J.; Rico-Garcia, E.; Ocampo-Velazquez, R.V.; Alvarez-Arquieta, L.D.L.; Torres-Pacheco, I. Plant Hormesis Management with Biostimulants of Biotic Origin in Agriculture. Front. Plant Sci. 2017, 8, 1762. [Google Scholar] [CrossRef] [PubMed]




| Treatments | Shoot FW (g Plant−1) | Root FW (g Plant−1) | Leaf Number (Plant−1) | Leaf Area (cm2 Plant−1) | Root Length (cm) | Leaf DW/FW (%) | Root DW/FW (% FW) |
|---|---|---|---|---|---|---|---|
| Control | 3.10 ± 0.07 Z b | 0.33 ± 0.00 b | 8.33 ± 0.47 c | 46.80 ± 0.92 b | 8.94 ± 0.28 c | 3.34 ± 0.07 a | 2.47 ± 0.26 a |
| NTP–30 s | 3.06 ± 0.04 b | 0.34 ± 0.02 b | 9.67 ± 0.47 bc | 47.04 ± 0.09 b | 11.83 ± 0.36 b | 3.79 ± 0.23 a | 2.39 ± 0.25 a |
| NTP–60 s | 3.55 ± 0.10 a | 0.34 ± 0.03 b | 11.00 ± 0.82 ab | 48.38 ± 0.43 b | 11.17 ± 0.0.36 b | 2.40 ± 0.23 b | 1.53 ± 0.28 b |
| NTP–120 s | 3.61 ± 0.06 a | 0.39 ± 0.02 a | 11.67 ± 1.25 a | 51.53 ± 0.31 a | 13.78 ± 1.10 a | 2.54 ± 0.34 b | 2.05 ± 0.56 ab |
| Significance y | ** | * | ** | * | *** | ** | NS |
| Treatments | Shoot FW (g Plant−1) | Roots FW (g Plant−1) | Leaf Number (n Plant −1) | Leaf Area (cm2 Plant−1) | Root Length (cm) | Leaf DW/FW (%) | Root DW/FW (% FW) |
|---|---|---|---|---|---|---|---|
| Control | 3.99 ± 0.09 Z b | 0.40 ± 0.1 b | 9.0 ± 0.3 b | 61.17 ± 6.28 b | 13.00 ± 0.11 b | 3.28 ± 0.10 a | 3.03 ± 0.04 a |
| NTP–12 s | 4.48 ± 0.03 a | 0.46 ± 0.1 a | 11.5 ± 0.5 a | 73.59 ± 3.35 a | 16.20 ± 0.50 a | 2.87 ± 0.14 a | 2.92 ± 0.26 a |
| Significance y | * | * | * | * | ** | NS | NS |
| Treatments | Total Carotenoids (µg gFW−1) | Total Chlorophylls (µg gFW−1) | Antioxidant Capacity (µmol Fe(II)g FW−1) | Total Phenols (mg GAE gFW−1) |
|---|---|---|---|---|
| Control | 94.60 ± 10.18 Z b | 485.99 ± 34.28 b | 14.23 ± 0.46 b | 2.68 ± 0.05 b |
| NTP-120 s | 135.70 ± 10.49 a | 616.01 ± 10.13 a | 19.95 ± 0.25 a | 3.67 ± 0.09 a |
| Significance y | * | * | ** | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmassi, G.; Cela, F.; Trivellini, A.; Gambineri, F.; Cursi, L.; Cecchi, A.; Pardossi, A.; Incrocci, L. Effects of Nonthermal Plasma (NTP) on the Growth and Quality of Baby Leaf Lettuce (Lactuca sativa var. acephala Alef.) Cultivated in an Indoor Hydroponic Growing System. Horticulturae 2022, 8, 251. https://doi.org/10.3390/horticulturae8030251
Carmassi G, Cela F, Trivellini A, Gambineri F, Cursi L, Cecchi A, Pardossi A, Incrocci L. Effects of Nonthermal Plasma (NTP) on the Growth and Quality of Baby Leaf Lettuce (Lactuca sativa var. acephala Alef.) Cultivated in an Indoor Hydroponic Growing System. Horticulturae. 2022; 8(3):251. https://doi.org/10.3390/horticulturae8030251
Chicago/Turabian StyleCarmassi, Giulia, Fatjon Cela, Alice Trivellini, Francesca Gambineri, Lamberto Cursi, Antonio Cecchi, Alberto Pardossi, and Luca Incrocci. 2022. "Effects of Nonthermal Plasma (NTP) on the Growth and Quality of Baby Leaf Lettuce (Lactuca sativa var. acephala Alef.) Cultivated in an Indoor Hydroponic Growing System" Horticulturae 8, no. 3: 251. https://doi.org/10.3390/horticulturae8030251
APA StyleCarmassi, G., Cela, F., Trivellini, A., Gambineri, F., Cursi, L., Cecchi, A., Pardossi, A., & Incrocci, L. (2022). Effects of Nonthermal Plasma (NTP) on the Growth and Quality of Baby Leaf Lettuce (Lactuca sativa var. acephala Alef.) Cultivated in an Indoor Hydroponic Growing System. Horticulturae, 8(3), 251. https://doi.org/10.3390/horticulturae8030251










