The Inclusion of Green Light in a Red and Blue Light Background Impact the Growth and Functional Quality of Vegetable and Flower Microgreen Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growing Conditions
2.2. Plant Growth Parameters
2.3. Plant Extraction
2.4. Total Phenol Content
2.5. FRAP Antioxidant Capacity
2.6. Chlorophyll and Carotenoid Content
2.7. Chroma Index
2.8. Statistical Analysis
3. Results
3.1. Effects of LED Light Environments on Growth Parameters
3.2. Effects of LED Light Environments on Nutraceutical Properties
4. Discussion
4.1. Microgreen Growth and Yield Is Affected by Irradiance Level
4.2. Microgreen Nutritional Quality Is Affected by Spectral Composition and Irradiance Levels
5. Conclusions
- (i)
- low and middle light intensity levels had a positive effect on antioxidant capacity and total carotenoid content;
- (ii)
- high levels of photosynthetically active photon flux (340 µmol m−2 s−1) increased dry biomass accumulation and enhanced the phenolic contents;
- (iii)
- the inclusion of green light in RB light background at light intensity of 220 and 340 μmol m−2 s−1 caused an increase in plant height compared with plants grown under the RB light combination
- (iv)
- the highest light intensity in combination with green light in the RB light environment was found more effective in increasing phenolic accumulation;
- (v)
- Light intensity and quality had significant interactive effects on elongation, total phenols, and antioxidant capacity.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Di Gioia, F.; Leoni, B.; Santamaria, P. The Selection of the Species to Grow. In Microgreens; Di Gioia, F., Santamaria, P., Eds.; Ecologica: Bari, Italy, 2015; pp. 25–40.kkkk. [Google Scholar]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of Vitamin and Carotenoid Concentrations of Emerging Food Products: Edible Microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, Z.; Ager, E.; Kong, L.; Tan, L. Nutritional quality and health benefits of microgreens, a crop of modern agriculture. J. Future Foods 2021, 1, 58–66. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Edmondson, J.L.; Cunningham, H.; Tingley, D.O.D.; Dobson, M.; Grafius, D.R.; Leake, J.R.; McHugh, N.; Nickles, J.; Phoenix, G.K.; Ryan, A.J.; et al. The hidden potential of urban horticulture. Nat. Food 2020, 1, 155–159. [Google Scholar] [CrossRef]
- Bulgari, R.; Negri, M.; Santoro, P.; Ferrante, A. Quality Evaluation of Indoor-Grown Microgreens Cultivated on Three Different Substrates. Horticulturae 2021, 7, 96. [Google Scholar] [CrossRef]
- Martin, M.; Molin, E. Environmental assessment of an urban vertical hydroponic farming system in Sweden. Sustainability 2019, 11, 4124. [Google Scholar] [CrossRef] [Green Version]
- Romeo, D.; Vea, E.B.; Thomsen, M. Environmental impacts of urban hydroponics in Europe: A case study in Lyon. Procedia CIRP 2018, 69, 540–545. [Google Scholar] [CrossRef]
- Khan, M.M.; Akram, M.T.; Janke, R.; Qadri, R.W.K.; Al-Sadi, A.M.; Farooque, A.A. Urban Horticulture for Food Secure Cities through and beyond COVID-19. Sustainability 2020, 12, 9592. [Google Scholar] [CrossRef]
- Corrado, G.; El-Nakhel, C.; Graziani, G.; Pannico, A.; Zarrelli, A.; Giannini, P.; Ritieni, A.; De Pascale, S.; Kyriacou, M.C.; Rouphael, Y. Productive and Morphometric Traits, Mineral Composition and Secondary Metabolome Components of Borage and Purslane as Underutilized Species for Microgreens Production. Horticulturae 2021, 7, 211. [Google Scholar] [CrossRef]
- Mir, S.A.; Shah, M.A.; Mir, M.M. Microgreens: Production, Shelf Life, and Bioactive Components. Crit. Rev. Food Sci. Nutr. 2016, 57, 2730–2736. [Google Scholar] [CrossRef]
- Butturini, M.; Marcelis, L. Vertical Farming in Europe: Present Status and Outlook. In Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Kozai, T., Niu, G.G., Takagaki, M., Eds.; Elsevier: London, UK, 2020; pp. 77–91. [Google Scholar]
- Di Gioia, F.; Santamaria, P. Microgreens, Agrobiodiversity and Food Security. In Microgreens; Di Gioia, F., Santamaria, P., Eds.; Ecologica: Bari, Italy, 2015; pp. 7–23. [Google Scholar]
- AlShrouf, A. Hydroponics, aeroponic and aquaponic as compared with conventional farming. Am. Sci. Res. J. Eng. Technol. Sci. 2017, 27, 247–255. [Google Scholar]
- Sharma, N.; Acharya, S.; Kumar, K.; Singh, N.; Chaurasia, O. Hydroponics as an advanced technique for vegetable production: An overview. J. Soil Water Conserv. 2018, 17, 364. [Google Scholar] [CrossRef]
- Nicola, S.; Pignata, G.; Ferrante, A.; Bulgari, R.; Cocetta, G.; Ertani, A. Water use efficiency in greenhouse systems and its application in horticulture. AgroLife Sci. J. 2020, 9, 248–262. [Google Scholar]
- Khan, S.; Purohit, A.; Vadsaria, N. Hydroponics: Current and future state of the art in farming. J. Plant Nutr. 2020, 44, 1515–1538. [Google Scholar] [CrossRef]
- Despommier, D. Farming up the city: The rise of urban vertical farms. Trends Biotechnol. 2013, 31, 388–389. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.; Niu, G. Role of the Plant Factory with Artificial Lighting (PFAL) in Urban Areas. In Plant Factory; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: London, UK, 2020; pp. 7–34. [Google Scholar]
- Kraver, J.K.; Gerovac, J.R.; Lopez, R.G.; Kopsell, D.A. Light intensity and light quality from sole-source light-emitting diodes impact phytochemical concentrations within Brassica Microgreens. J. Am. Soc. Hortic. Sci. 2017, 142, 3–12. [Google Scholar] [CrossRef]
- Gerovac, J.R.; Craver, J.K.; Boldt, J.K.; Lopez, R.G. Light intensity and quality from sole-source light-emitting diodes impact growth, morphology, and nutrient content of Brassica microgreens. HortScience 2016, 51, 497–503. [Google Scholar] [CrossRef] [Green Version]
- Devlin, P.F.; Christie, J.M.; Terry, M.J. Many hands make light work. J. Exp. Bot. 2007, 58, 3071–3077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern Led Systems. J. Plant Growth Regul. 2021, 41, 742–780. [Google Scholar] [CrossRef]
- Turner, E.R.; Luo, Y.; Buchanan, R.L.A. Microgreen nutrition, food safety, and shelf life: A review. J. Food Sci. 2020, 85, 870–882. [Google Scholar] [CrossRef] [Green Version]
- Brazaitytė, A.; Sakalauskienė, S.; Samuolienė, G.; Jankauskienė, J.; Viršilė, A.; Novičkovas, A.; Sirtautas, R.; Miliauskienė, J.; Vaštakaitė, V.; Dabašinskas, L.; et al. The effects of LED illumination spectra and intensity on carotenoid content in Brassicaceae microgreens. Food Chem. 2015, 173, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Basu, C.; Meinhardt-Wollweber, M.; Roth, B. LEDs for energy efficient greenhouse lighting. Ren. Sustain. Ener. Rev. 2015, 49, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Samuolienė, G.; Brazaitytė, A.; Jankauskienė, J.; Viršilė, A.; Sirtautas, R.; Novičkovas, A.; Sakalauskienė, S.; Sakalauskaitė, J.; Duchovskis, P. LED irradiance level affects growth and nutritional quality of Brassica microgreens. Cent. Eur. J. Biol. 2013, 8, 1241–1249. [Google Scholar] [CrossRef]
- Jones-Baumgardt, C.D.; Lewellyn, L.; Ying, Q.; Zheng, Y. Intensity of sole-source light emitting diodes affects growth, yield, and quality of Brassicaceae microgreens. HortScience 2019, 54, 1168–1174. [Google Scholar] [CrossRef] [Green Version]
- Ying, Q.; Kong, Y.; Jones-baumgardt, C.; Zheng, Y. Responses of yield and appearance quality of four Brassicaceae microgreens to varied blue light proportion in red and blue light-emitting diodes lighting. Sci. Hortic. 2020, 259, 108857. [Google Scholar] [CrossRef]
- Massa, G.D.; Kim, H.H.; Wheeler, R.M.; Mitchell, C.A. Plant productivity in response to LED lighting. HortScience 2008, 43, 1951–1956. [Google Scholar] [CrossRef]
- Goto, E. Plant production in a closed plant factory with artificial lighting. Acta Hortic. 2012, 956, 37–49. [Google Scholar] [CrossRef]
- Mark, G.L.; Dean, A.K.; Carl, E.S. Irradiance from distinct wavelength light-emitting diodes affect secondary metabolites in kale. HortScience 2008, 43, 2243–2244. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.H.; Huang, M.Y.; Huang, W.D.; Hsu, M.-H.; Yang, Z.-W.; Yang, C.-M. The effects of red, blue and white light-emitting diodes (LEDs) on growth, development and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 50, 86–91. [Google Scholar] [CrossRef]
- Alrifai, O.; Hao, X.; Marcone, M.F.; Tsao, R. Current review of the modulatory effects of LED lights on photosynthesis of secondary metabolites and future perspectives of microgreen vegetables. J. Agric. Food Chem. 2019, 67, 6075–6090. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Snowden, M.C.; Cope, K.R.; Bugbee, B. Sensitivity of seven diverse species to blue and green light: Interactions with photon flux. PLoS ONE 2016, 11, e0163121. [Google Scholar] [CrossRef] [PubMed]
- Cope, K.R.; Bugbee, B. Spectral effects of three types of white light-emitting diodes on plant growth and development: Absolute versus relative amounts of blue light. HortScience 2013, 48, 504–509. [Google Scholar] [CrossRef]
- Bantis, F. Light Spectrum Differentially Affects the Yield and Phytochemical Content of Microgreen Vegetables in a Plant Factory. Plants 2021, 10, 2182. [Google Scholar] [CrossRef]
- Jones, M.A. Using light to improve commercial value. Hortic Res. 2018, 5, 47. [Google Scholar] [CrossRef] [Green Version]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Effects of light quality on growth and phytonutrient accumulation of herbs under controlled environments. Horticulturae 2017, 3, 36. [Google Scholar] [CrossRef] [Green Version]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: A review. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef]
- Ying, Q.; Jones-Baumgardt, C.; Zheng, Y.; Bozzo, G. The Proportion of blue light from light-emitting diodes (RB light environment) alters microgreen phytochemical profiles in a species-specific manner. HortScience 2021, 56, 13–20. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Jurga, M.; Viktorija, V.-K.; Rūta, S.; Kristina, L.; Pavelas, D.; Stanisław, M. Effect of Different Ratios of Blue and Red LED Light on Brassicaceae Microgreens under a Controlled Environment. Plants 2021, 10, 801. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Zarrelli, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Genotype-specific modulatory effects of select spectral bandwidths on the nutritive and phytochemical composition of microgreens. Front. Plant Sci. 2019, 10, 1501. [Google Scholar] [CrossRef]
- Samuoliene, G.A.; Virisile, A.; Brazaityte, J.; Jankauskiee, S.; Sakalauskiene, V.; Va stakaite, A.; Novickovas, A.; Viskeliene, A.; Sasnauskas, A.; Duchovskis, P. Blue light dosage affects carotenoids and tocopherols in microgreens. Food Chem. 2017, 228, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Ouzounis, T.; Parjikolaei, B.R.; Frette, X.; Rosenqvist, E.; Ottosen, C.-O. Predawn and high intensity application of supplemental blue light decreases the quantum yield of PSII and enhances the amount of phenolic acids, flavonoids, and pigments in Lactuca sativa. Front. Plant Sci. 2015, 6, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Nishio, J.N.; Vogelmann, T.C. Green light drives CO2 fixation deep within leaves. Plant Cell Physiol. 1998, 39, 1020–1026. [Google Scholar] [CrossRef]
- Zhang, T.; Folta, K.M. Green light signaling and adaptive response. Plant Signal. Behav. 2012, 7, 267–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J. Morphological and Physiological Responses in Basil and Brassica Species to Different Proportions of Red, Blue, and Green Wavelengths in Indoor Vertical Farming. J. Am. Soc. Hortic. Sci. 2020, 145, 267–278. [Google Scholar] [CrossRef]
- Folta, K.M.; Maruhnich, S.A. Green light: A signal to slow down or stop. J. Exp. Bot. 2007, 58, 3099–3111. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M. Photosynthesis, morphology, yield, and phytochemical accumulation in basil plants influenced by substituting green light for partial red and/or blue light. HortScience 2019, 54, 1769–1776. [Google Scholar] [CrossRef] [Green Version]
- Ying, Q.; Kong, Y.; Zheng, Y. Growth and appearance quality of four microgreen species under light-emitting diode lights with different spectral combinations. HortScience 2020, 55, 1399–1405. [Google Scholar] [CrossRef]
- Meng, Q.; Kelly, N.; Runkle, E.S. Substituting green or far-red radiation for blue radiation induces shade avoidance and promotes growth in lettuce and kale. Environ. Exp. Bot. 2019, 162, 383–391. [Google Scholar] [CrossRef]
- Orlando, M.; Trivellini, A.; Puccinelli, M.; Ferrante, A.; Incrocci, L.; Mensuali, A. Increasing the functional quality of Crocus sativus L. by-product (tepals) by controlling spectral composition. Hortic. Environ. Biotechnol. 2022. [Google Scholar] [CrossRef]
- Bian, Z.H.; Cheng, R.F.; Yang, Q.C.; Wang, J. Continuous Light from Red, Blue, and Green Light-emitting Diodes Reduces Nitrate Content and Enhances Phytochemical Concentrations and Antioxidant Capacity in Lettuce. J. Am. Soc. Hortic. Sci. 2016, 141, 186–195. [Google Scholar] [CrossRef] [Green Version]
- Maggini, R.; Galluzzo, F.; Pardossi, A. Effect of Nitrogen Nutrition on Growth and Accumulation of Caffeic Acid Deriva-tives in Hydroponically-Grown Echinacea Angustifolia DC. Var Angustifolia. Agrochimica 2013, 57, 22–30. [Google Scholar]
- Kang, H.-M.; Saltveit, M.E. Antioxidant Capacity of Lettuce Leaf Tissue Increases after Wounding. J. Agric. Food Chem. 2002, 50, 7536–7541. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments Ofphotosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Yeh, D.M.; Hsu, P.Y. Differential Growth and Photosynthetic Response of Selected Cultivars of English Ivy to Irradiance. J. Hortic. Sci. Biotechnol. 2004, 79, 633–637. [Google Scholar] [CrossRef]
- Zervoudakis, G.; Salahas, G.; Kaspiris, G.; Konstantopoulou, E. Influence of Light Intensity on Growth and Physiological Characteristics of Common Sage (Salvia Officinalis L.). Braz. Arch. Biol. Technol. 2012, 55, 89–95. [Google Scholar] [CrossRef]
- Potter, T.I.; Rood, S.B.; Zanewich, K.P. Light Intensity, Gibberellin Content and the Resolution of Shoot Growth in Brassica. Planta 1999, 207, 505–511. [Google Scholar] [CrossRef]
- Samuolienė, G.; Sirtautas, R.; Brazaitytė, A.; Sakalauskaite, J.; Sakalauskienė, S.; Duchovskis, P. The Impact of Red and Blue Light-Emitting Diode Illumination on Radish Physiological Indices. Cent. Eur. J. Biol. 2011, 6, 821–828. [Google Scholar] [CrossRef]
- Heins, R.D.; Liu, B.; Runkle, E.S. Regulation of Crop Growth and Development Based on Environmental Factors. Acta Hortic. 2000, 511, 13–22. [Google Scholar] [CrossRef]
- Yang, F.; Wang, X.; Liao, D.; Lu, F.; Gao, R.; Liu, W.; Yong, T.; Wu, X.; Du, J.; Liu, J.; et al. Yield Response to Different Planting Geometries in Maize-Soybean Relay Strip Intercropping Systems. Agron. J. 2015, 107, 296–304. [Google Scholar] [CrossRef]
- Wu, Y.; Gong, W.; Yang, F.; Wang, X.; Yong, T.; Yang, W. Responses to Shade and Subsequent Recovery of Soya Bean in Maize-Soya Bean Relay Strip Intercropping. Plant Prod. Sci. 2016, 19, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Alrifai, O.; Mats, L.; Liu, R.; Zhu, H.; Hao, X.; Marcone, M.F.; Tsao, R. Characterization and Profiling of Polyphenolics of Brassica Microgreens by LC-HRMS/MS and the Effect under LED Light. J. Food Bioact. 2021, 14. [Google Scholar] [CrossRef]
- Heo, J.W.; Kang, D.H.; Bang, H.S.; Hong, S.G.; Chun, C.; Kang, K.K. Early growth, pigmentation, protein content, and phenylalanine ammonia-lyase activity of red curled lettuces grown under different lighting conditions. Korean J. Hortic. Sci. Technol. 2012, 30, 6–12. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Gu, M.; Cui, J.; Shi, K.; Zhou, T.; Yu, J. Effects of light quality on CO2 assimilation, chlorophyll-fluorescence quenching, expression of Calvin cycle genes and carbohydrate accumulation in Cucumis sativus. J. Photochem. Photobiol. B 2009, 96, 30–37. [Google Scholar] [CrossRef]
- Wu, M.C.; Hou, C.Y.; Jiang, C.M.; Wang, Y.T.; Wang, C.Y.; Chen, H.H.; Chang, H.M. A novel approach of LED light radiation improves the antioxidant activity of pea seedlings. Food Chem. 2007, 101, 1753–1758. [Google Scholar] [CrossRef]
- Son, K.H.; Oh, M.M. Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. HortScience 2013, 48, 988–995. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef] [Green Version]
- Ohashi-Kaneko, K.; Takase, N.; Kon, N.; Fujiwara, K.; Kurata, K. Effects of light quality on growth and vegetable quality in leaf lettuce, spinach and komatsuna. Environ. Control Biol. 2007, 45, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Lobiuc, A.; Vasilache, V.; Oroian, M.; Stoleru, T.; Burducea, M.; Pintilie, O.; Zamfirache, M.M. Blue and red LED illumination improves growth and bioactive compounds contents in acyanic and cyanic Ocimum basilicum L. microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, H.; Liu, T.; Deng, M.; Miao, H.; Cai, C.; Shen, W.; Wang, Q. Effects of light quality on main health-promoting compounds and antioxidant capacity of chinese kale sprouts. Food Chem. 2016, 196, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Xing, T.; Wang, X. The role of light in the regulation of anthocyanin accumulation in Gerbera hybrida. Plant Growth Regulat. 2004, 44, 267–278. [Google Scholar] [CrossRef]
- Nishio, J. Why are higher plants green? Evolution of the higher plant photosynthetic pigment complement. Plant Cell Environ. 2000, 23, 539–548. [Google Scholar] [CrossRef]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, Z.; Wang, Y.; Zhang, X.; Grundy, S.; Hardy, K.; Yanh, Q.; Lu, C. A Transcriptome Analysis Revealing the New Insight of Green Light on Tomato Plant Growth and Drought Stress Tolerance. Front. Plant Sci. 2021, 12, 2325. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [Green Version]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid Metabolism in Plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef] [Green Version]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Harish; Marwal, A. Vital Roles of Carotenoids in Plants and Humans to Deteriorate Stress with Its Structure, Biosynthesis, Metabolic Engineering and Functional Aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- Tuan, P.A.; Thwe, A.A.; Kim, Y.B.; Kim, J.K.; Kim, S.-J.; Lee, S.; Chung, S.-O.; Park, S.U. Effects of White, Blue, and Red Light-Emitting Diodes on Carotenoid Biosynthetic Gene Expression Levels and Carotenoid Accumulation in Sprouts of Tartary Buckwheat (Fagopyrum Tataricum Gaertn.). J. Agric. Food Chem. 2013, 61, 12356–12361. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Pantanizopoulos, N.I.; Sams, C.E.; Kopsell, D.E. Shoot tissue pigment levels increase in ‘Florida Broadleaf’ mustard (Brassica juncea L.) microgreens following high light treatment. Sci. Hortic. 2012, 140, 96–99. [Google Scholar] [CrossRef]
- Samuoliene, G.; Sirtautas, R.; Brazaityte, A.; Duchovskis, P. LED lighting and seasonality affects antioxidant properties of baby leaf lettuce. Food Chem. 2012, 134, 1494–1499. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Gilmore, A.M.; Adams, W.W. In vivo functions of carotenoids in higher plants. FASEB J. 1996, 10, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Lefsrud, M.G.; Kopsell, D.A.; Kopsell, D.E.; Curran-Celentano, J. Irradiance Levels Affect Growth Parameters and Carotenoid Pigments in Kale and Spinach Grown in a Controlled Environment. Physiol. Plant. 2006, 127, 624–631. [Google Scholar] [CrossRef]

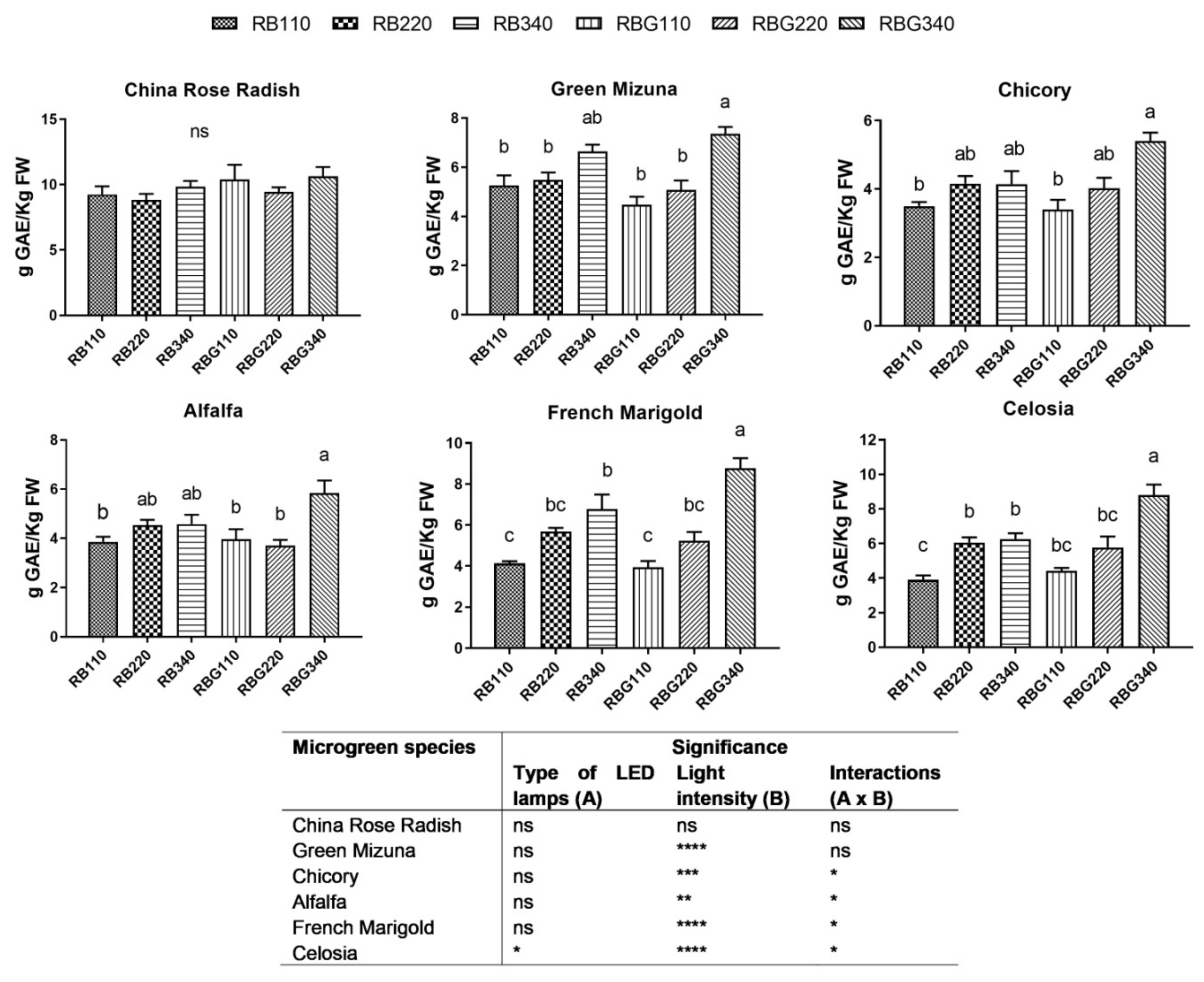



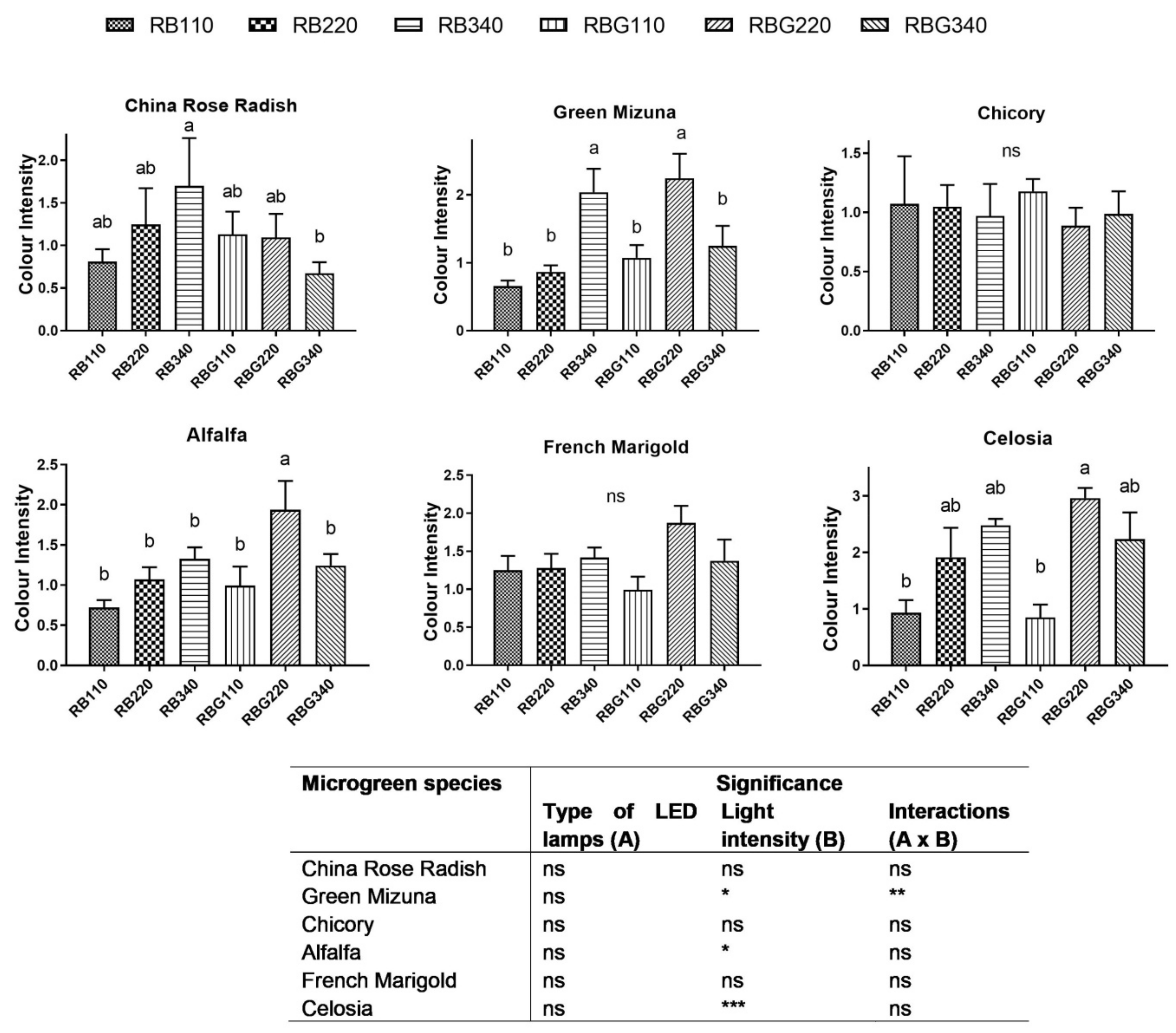
| H (cm) PPFD (µmol m−2 s−1) | RB | RBG | Significance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 110 | 220 | 340 | 110 | 220 | 340 | Light Spectra (A) | Light Intensity (B) | Interaction (A × B) | |
| China Rose Radish | 5.22 ± 0.05 a | 3.77 ± 0.04 c | 3.89 ± 0.03 c | 5.15 ± 0.03 a | 4.43 ± 0.06 b | 4.42 ± 0.05 b | **** | **** | **** |
| Green Mizuna | 1.99 ± 0.04 b | 1.48 ± 0.02 d | 1.28 ± 0.02 e | 2.47 ± 0.03 a | 1.54 ± 0.03 d | 1.79 ± 0.02 c | **** | **** | **** |
| Chicory | 1.01 ± 0.02 c | 0.78 ± 0.01 d | 1.35 ± 0.02 b | 1.86 ± 0.04 a | 1.31 ± 0.01 b | 1.24 ± 0.01 b | **** | **** | **** |
| Alfalfa | 2.19 ± 0.05 a | 1.63 ± 0.02 d | 1.67 ± 0.02 d | 2.24 ± 0.04 a | 2.02 ± 0.01 b | 1.87 ± 0.02 c | **** | **** | **** |
| French Marigold | 2.04 ± 0.02 a | 1.55 ± 0.01 c | 2.06 ± 0.03 a | 2.12 ± 0.02 a | 2.03 ± 0.03 a | 1.93 ± 0.02 b | **** | **** | **** |
| Celosia | 1.91 ± 0.01 b | 1.44 ± 0.01 e | 1.71 ± 0.02 c | 2.04 ± 0.03 a | 1.73 ± 0.01 c | 1.60 ± 0.02 d | **** | **** | **** |
| FW (g) PPFD (µmol m−2 s−1) | RB | RBG | |||||||
| 110 | 220 | 340 | 110 | 220 | 340 | ||||
| China Rose Radish | 15.00 ± 1.10 | 11.00 ± 0.57 | 13.50 ± 0.50 | 10.53 ± 1.05 | 12.03 ± 1.05 | 14.43 ± 1.41 | ns | ns | * |
| Green Mizuna | 4.56 ± 0.52 a | 3.87 ± 0.23 b | 3.63 ± 0.24 a | 4.63 ± 0.26 b | 3.90 ± 0.17 b | 4.70 ± 0.31 a | ns | ns | ns |
| Chicory | 3.00 ± 0.05 a | 1.90 ± 0.20 b | 3.13 ± 0.32 a | 2.67 ± 0.18 b | 2.57 ± 0.07 b | 3.33 ± 0.12 a | ns | *** | ns |
| Alfalfa | 5.07 ± 0.34 ab | 4.00 ± 0.20 b | 6.10 ± 0.06 a | 3.20 ± 0.25 b | 5.27 ± 0.21 ab | 5.73 ± 0.43 a | ns | **** | *** |
| French Marigold | 2.40 ± 0.46 | 3.10 ± 0.15 | 3.27 ± 0.88 | 1.83 ± 0.26 b | 2.83 ± 0.35 ab | 4.20 ± 0.67 a | ns | * | ns |
| Celosia * | 1.10 ± 0.12 | 0.80 ± 0.06 | 0.87 ± 0.18 | 1.07 ± 0.03 | 0.83 ± 0.12 | 1.43 ± 0.30 | ns | ns | ns |
| DW (g) PPFD (µmol m−2 s−1) | RB | RBG | |||||||
| 110 | 220 | 340 | 110 | 220 | 340 | ||||
| China Rose Radish | 1.12 ± 0.01 c | 1.17 ± 0.06 b | 1.43 ± 0.05 a | 0.85 ± 0.07 c | 1.23 ± 0.001 b | 1.45 ± 0.12 a | ns | **** | ns |
| Green Mizuna | 0.32 ± 0.06 b | 0.47 ± 0.04 a | 0.51 ± 0.05 a | 0.37 ± 0.02 b | 0.47 ± 0.01 ab | 0.58 ± 0.08 a | ns | ** | ns |
| Chicory | 0.18 ± 0.01 c | 0.23 ± 0.003 b | 0.31 ± 0.02 a | 0.17 ± 0.02 c | 0.25 ± 0.01 b | 0.35 ± 0.04 a | ns | **** | ns |
| Alfalfa | 0.41 ± 0.01 b | 0.44 ± 0.007 b | 0.66 ± 0.02 a | 0.26 ± 0.01 c | 0.53 ± 0.02 ab | 0.59 ± 0.03 a | * | **** | **** |
| French Marigold | 0.18 ± 0.03 b | 0.31 ± 0.01 a | 0.35 ± 0.08 a | 0.11 ± 0.02 c | 0.27 ± 0.02 b | 0.41 ± 0.05 a | ns | *** | ns |
| Celosia | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.09 ± 0.01 | 0.06 ± 0.01 b | 0.07 ± 0.006 b | 0.13 ± 0.02 a | ns | ** | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlando, M.; Trivellini, A.; Incrocci, L.; Ferrante, A.; Mensuali, A. The Inclusion of Green Light in a Red and Blue Light Background Impact the Growth and Functional Quality of Vegetable and Flower Microgreen Species. Horticulturae 2022, 8, 217. https://doi.org/10.3390/horticulturae8030217
Orlando M, Trivellini A, Incrocci L, Ferrante A, Mensuali A. The Inclusion of Green Light in a Red and Blue Light Background Impact the Growth and Functional Quality of Vegetable and Flower Microgreen Species. Horticulturae. 2022; 8(3):217. https://doi.org/10.3390/horticulturae8030217
Chicago/Turabian StyleOrlando, Matteo, Alice Trivellini, Luca Incrocci, Antonio Ferrante, and Anna Mensuali. 2022. "The Inclusion of Green Light in a Red and Blue Light Background Impact the Growth and Functional Quality of Vegetable and Flower Microgreen Species" Horticulturae 8, no. 3: 217. https://doi.org/10.3390/horticulturae8030217
APA StyleOrlando, M., Trivellini, A., Incrocci, L., Ferrante, A., & Mensuali, A. (2022). The Inclusion of Green Light in a Red and Blue Light Background Impact the Growth and Functional Quality of Vegetable and Flower Microgreen Species. Horticulturae, 8(3), 217. https://doi.org/10.3390/horticulturae8030217








