Effects of Hydrogen Peroxide Products on Basil, Lettuce, and Algae in an Ebb and Flow Hydroponic System
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Hydrogen Peroxide and Cultivar Effects on Plant Growth Parameters and Chlorophyll Content
3.2. Hydrogen Peroxide Effects on Algae
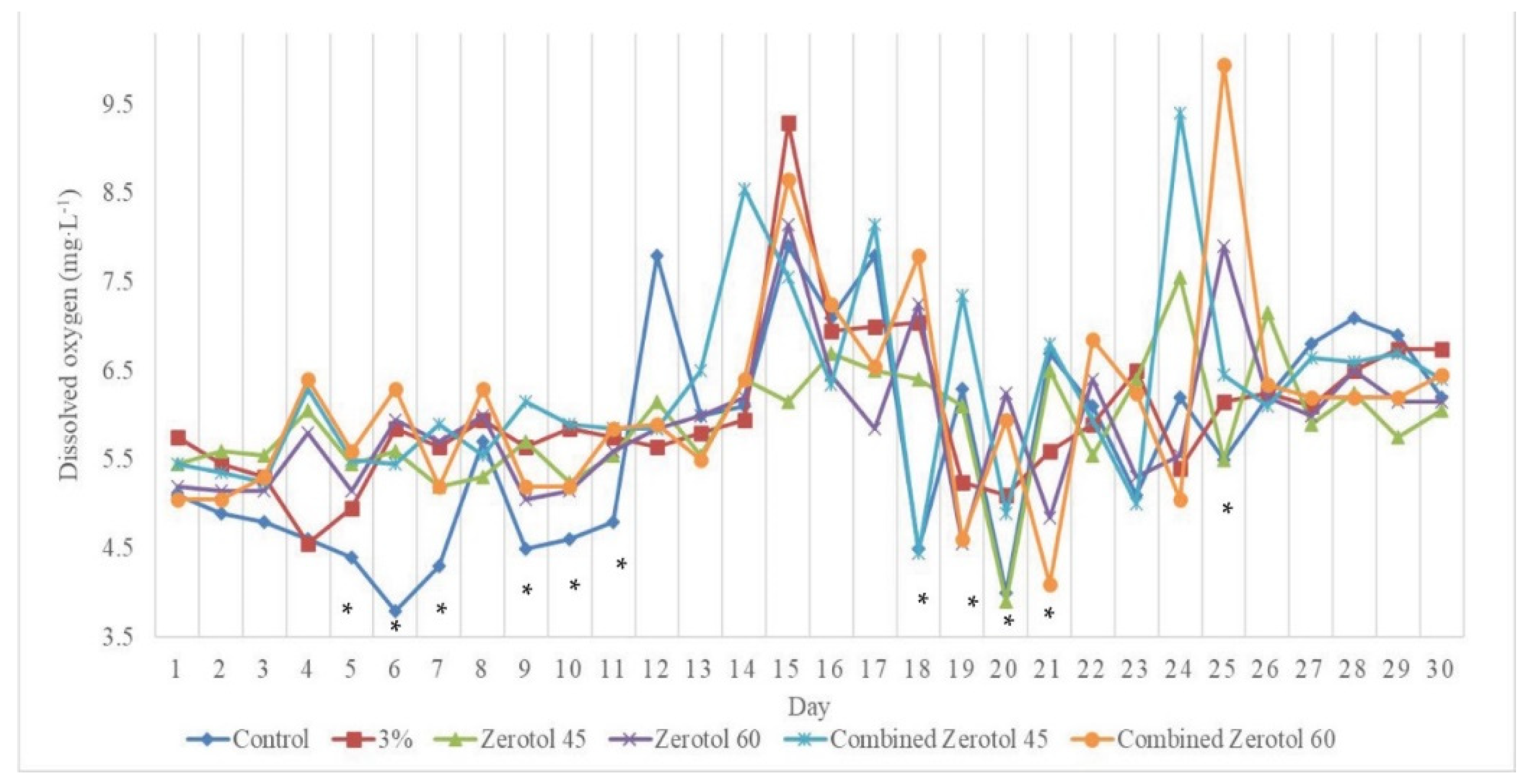
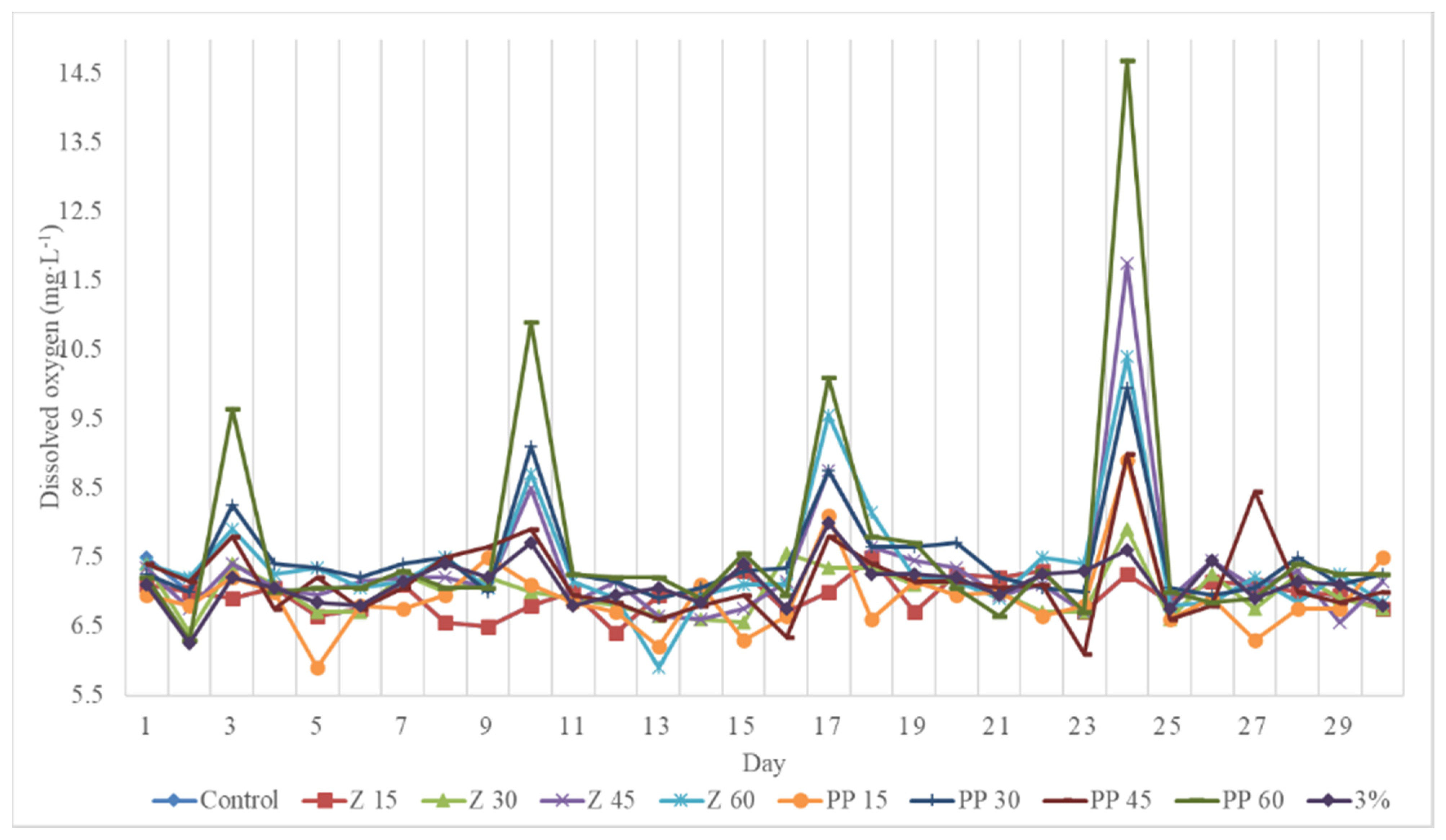
3.3. Effects of Hydrogen Peroxide on Dissolved Oxygen
4. Discussion
4.1. Effects of Hydrogen Peroxide and Cultivar on Lettuce and Basil
4.2. Hydrogen Peroxide Effects on Algae
4.3. Effects of Hydrogen Peroxide on Dissolved Oxygen
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Shrestha, A.; Dunn, B. Hydroponics. Oklahoma Cooperative Extension Service 2016, HLA-6442. Available online: https://shareok.org/bitstream/handle/11244/50283/oksd_hla_6442_2010-03.pdf?sequence=1 (accessed on 21 June 2022).
- Camberato, D.M.; Lopez, R.G. Controlling Algae in Irrigation Ponds; Purdue University: West Lafayette, IN, USA, 2009; Available online: https://www.extension.purdue.edu/extmedia/ho/ho-247-w.pdf (accessed on 21 June 2022).
- Raudales, R. Algae on the nutrient solution and surfaces. E-Grow Edible Alert 2016, 1, 1–4. [Google Scholar]
- Supraja, K.V.; Behera, B.; Balasubramanian, P. Performance evaluation of hydroponic system for co-cultivation of microalgae and tomato plant. J. Clean. Prod. 2020, 272, 122823. [Google Scholar] [CrossRef]
- Huo, S.; Liu, J.; Addy, M.; Chen, P.; Necas, D.; Cheng, P.; Li, K.; Chai, H.; Liu, Y.; Ruan, R. The influence of microalgae on vegetable production and nutrient removal in greenhouse hydroponics. J. Clean. Prod. 2020, 243, 118563. [Google Scholar] [CrossRef]
- Varma, M.M.; DiGiano, F. Kinetics of oxygen uptake by dead algae. Wiley J. Water Pollut. Control Fed. 1968, 40, 613–626. [Google Scholar]
- Lembi, C.A. Identifying and Managing Aquatic Vegetation; Purdue University: West Lafayette, IN, USA, 2009; Available online: https://www.extension.purdue.edu/extmedia/APM/APM_3_W.pdf (accessed on 21 June 2022).
- Chen, Y.; Bai, F.; Li, Z.; Xie, P.; Wang, Z.; Feng, X.; Liu, Z.; Huang, L. UV-assisted chlorination of algae-laden water: Cell lysis and disinfection byproducts formation. Chem. Eng. J. 2020, 383, 123165. [Google Scholar] [CrossRef]
- Dannehl, D.; Schuch, I.; Gao, Y.; Cordiner, S.; Schmidt, U. Hypochlorite application for controlling algae biofilm formation, microorganisms and tomato production in recirculating systems. Gesunde Pflanz. 2015, 67, 191–199. [Google Scholar] [CrossRef]
- Nonomura, T.; Matsuda, Y.; Bingo, M.; Onishi, M.; Matsuda, K.; Harada, S.; Toyoda, H. Algicidal effect of 3-(3-indolyl)butanoic acid, a control agent of the bacterial wilt pathogen, Ralstonia solanacearum. Crop Prot. 2001, 20, 935–939. [Google Scholar] [CrossRef]
- Draäbkova, M.; Admiraal, W.; Marsalek, B. Combined exposure to hydrogen peroxide and light-selective effects on cyanobacteria, green algae and diatoms. Environ. Sci. Technol. 2007, 41, 309–314. [Google Scholar] [CrossRef]
- Kay, S.H.; Quimby, P.C.; Ouzts, J.D. H2O2: A potential algicide for aquaculture. In Proceedings of the 35th Annual Meeting of the Southern Weed Science Society, Atlanta, GA, USA, 19–21 January 1982; pp. 275–289. [Google Scholar]
- Caixeta, V.; Mata, A.; Curvelo, C.; Tavares, W.; Ferreira, L.; Pereira, A. Hydrogen peroxide for insect and algae control in a lettuce hydroponic environment. J. Agric. Sci. 2018, 10, 221. [Google Scholar] [CrossRef]
- Vänninen, I.; Koskula, H. Effect of hydrogen peroxide on algal growth, cucumber seedlings and the reproduction of shore flies (Scatella stagnalis) in rockwool. Crop Prot. 1998, 17, 547–553. [Google Scholar] [CrossRef]
- Ou, H.; Gao, N.; Deng, Y.; Wang, H.; Zhang, H. Inactivation and degradation of Microcystis aeruginosa by UV-C irradiation. Chemisphere 2011, 85, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Yu, S.; Sun, Z.; Xie, X.; Liu, W.; Fu, Z. Effects of copper sulfate, hydrogen peroxide and N-phenyl-2-naphthylamine on oxidative stress and the expression of genes involved photosynthesis and microcystin disposition in Microcystis aeruginosa. Aquat. Toxicol. 2010, 99, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Tesoriero, L.; Jelinek, S.; Forsyth, L. On-Farm Hygiene and Sanitation for Greenhouse Horticulture; Prime Facts 1005. 2020. Available online: https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0003/340284/On-farm-hygiene-and-sanitation-for-greenhouse-horticulture.pdf (accessed on 21 June 2022).
- Hernandez, J.C.; Tornos, P.; Cuervo, U.Y.; Furet, N.R.; Orihuela, D.L. Influence of hydrogen peroxide (H2O2) in the fruit of pepper (Capsicum annuum L.). In Proceedings of the 48th Annual Meeting of Caribbean Food Crops Society, Playa del Carmen, Mexico, 20–26 May 2012. [Google Scholar]
- Thakulla, D.; Dunn, B.; Hu, B.; Goad, C.; Maness, N. Nutrient solution temperature affects growth and °Brix parameters of seventeen lettuce cultivars grown in an NFT hydroponic system. Horticulturae 2021, 7, 321. [Google Scholar] [CrossRef]
- Mejía-Teniente, L.; Durán-Flores, B.; Torres-Pacheco, I.; González-Chavira, M.M. Hydrogen peroxide protects pepper (Capsicum annuum L.) against pepper golden mosaic geminivirus (PepGMV) infections. Physiol. Mol. Plant Pathol. 2019, 106, 23–29. [Google Scholar] [CrossRef]
- Niu, L.; Liao, W. Hydrogen peroxide signaling in plant development and abiotic responses: Crosstalk with nitric oxide and calcium. Front. Plant Sci. 2016, 7, 230. [Google Scholar] [CrossRef] [Green Version]
- Slesak, I.; Libik, M.; Karpinska, B.; Karpinski, S.; Miszalski, Z. The role of hydrogen peroxide in regulation of plant metabolism and cellular signaling in response to environmental stresses. Acta Biochim. Pol. 2007, 54, 39–50. [Google Scholar] [CrossRef]
- Barroin, G.; Feuillade, M. Hydrogen peroxide as a potential algaecide for Oscillatoria rubescens D.C. Water Res. 1986, 20, 619–623. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, L.; Huang, L.; Guo, L.; Song, L. Combining hydrogen peroxide with sunlight regulation to control algal blooms. Environ. Sci. Pollut. Res. 2018, 25, 2239–2247. [Google Scholar] [CrossRef]
- Sarathy, S.; Mohseni, M. Effects of UV/H2O2 advanced oxidation on chemical characteristics and chlorine reactivity of surface water natural organic matter. Water Res. 2010, 44, 4087–4096. [Google Scholar] [CrossRef]
- Wan, Y.; Xie, P.; Wang, Z.; Ding, J.; Wang, J.; Wang, S.; Wiesnec, M.R. Comparative study on the pretreatment of algae-laden water by UV/persulfate, UV/chlorine, and UV/H2O2: Variation of characteristics and alleviation of ultrafiltration membrane fouling. Water Res. 2019, 158, 213–226. [Google Scholar] [CrossRef]
- Coosemans, J. Control of algae in hydroponic systems. Acta Hortic. 1995, 382, 263–268. [Google Scholar] [CrossRef]
- Currey, C.J. Managing Basil Production throughout the Year. Produce Grower. 2020. Available online: https://www.producegrower.com/article/hydroponic-production-primer-managing-basil-production-throughout-the-year (accessed on 21 June 2022).
- Saha, S.; Monroe, A.; Day, M.R. Growth, yield, plant quality and nutrition of basil (Ocimum basilicum L.) under soilless agricultural systems. Ann. Agric. Sci. 2016, 61, 181–186. [Google Scholar] [CrossRef]
- University of Illinois. Lettuce. In Watch Your Garden Grow; University of Illinois: Champaign, IL, USA, 2022; Available online: https://web.extension.illinois.edu/veggies/lettuce.cfm (accessed on 21 June 2022).
- Edler, L.; Elbrachter, M. The Utermohl Method for Quantitative Phytoplankton Analysis; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 2010. [Google Scholar]
- Charles, D.F.; Knowles, C.; Davis, R.S. Protocols for the Analysis of Algal Samples Collected As Part of the U.S. Geological Survey National Water-Quality Assessment Program; Report No. 02-06; The Academy of Natural Sciences: Philadelphia, PA, USA, 2002. [Google Scholar]
- Michaud, J.P. Measuring total suspended solids and turbidity in lakes and streams. In A Citizen’s Guide to Understanding and Monitoring Lakes and Streams; Washington State Dept. of Ecology, Publications Office: Olympia, WA, USA, 1994. [Google Scholar]
- LeGresley, M.; McDermott, G. Counting Chamber Methods for Quantitative Phytoplankton Analysis—Haemocytometer, Palmer-Maloney Cell and Sedgewick-Rafter Cell; IOC Manuals and Guides; UNESCO: Paris, France, 2020; pp. 25–30. [Google Scholar]
- Kumar, S.S.; Saramma, A.V. A revised method for pigment extraction from marine nannoplanktonic algal cultures. J. Algal Biomass Util. 2013, 4, 47–52. [Google Scholar]
- Lau, V.; Mattson, N. Effects of hydrogen peroxide on organically fertilized hydroponic lettuce (Lactuca sativa L.). Horticulturae 2021, 7, 106. [Google Scholar] [CrossRef]
- Kučerová, K.; Henselová, M.; Slováková, L.; Bačovčinová, M.; Hensel, K. Effect of plasma activated water, hydrogen peroxide, and nitrates on lettuce growth and its physiological parameters. Appl. Sci. 2021, 11, 1985. [Google Scholar] [CrossRef]
- Raudales, R.E.; Parke, J.L.; Guy, C.L.; Fisher, P.R. Control of waterborne microbes in irrigation: A review. Agric. Water Manag. 2014, 143, 9–28. [Google Scholar] [CrossRef]
- Thakulla, D.; Dunn, B.; Hu, B.; Goad, C. Timing and rates of two products using hydrogen peroxide (H2O2) to control algae in ebb and flow hydroponic systems. HortScience 2022, 57, 32–39. [Google Scholar] [CrossRef]
- Deng, X.; Cheng, Y.; Wu, X.; Kwak, S.; Chen, W.; Eneji, A.E. Exogenous hydrogen peroxide positively influences root growth and exogenous hydrogen peroxide positively influences root growth and metabolism in leaves of sweet potato seedlings. Aust. J. Crop Sci. 2012, 6, 1572–1578. [Google Scholar]
- Popescu, E.M.; Pantea, O.; Gologan, D.; Doukeh, R. Hydrogen peroxide and peracetic acid oxidizing potential in the treatment of water. Rev. Chim. 2019, 70, 2036–2039. [Google Scholar] [CrossRef]
- Breithaupt, J. Summary Review of Available Literature for Hydrogen Peroxide and Peroxyacetic Acid for New Use to Treat Wastewater; U.S. Environmental Protection Agency: Washington, DC, USA, 2007; pp. 1–35.
- Schwarz, D.; Krienitz, L. Do algae cause growth-promoting effects on vegetables grown hydroponically? In Proceedings of the IPI–NATESC-CAU-CAAS International Symposium on Fertirrigation, Beijing, China, 20–24 September 2005. [Google Scholar]
- Southard, G.M. Chapter 7: Use of hydrogen peroxide as an algaecide for Prymnesium parvum. In Management of Prymnesium Parvum at Texas State Fish Hatcheries; Texas Parks and Wildlife Department: Austin, TX, USA, 2005. [Google Scholar]
- Yang, T.; Kim, H. Characterizing nutrient composition and concentration in tomato-, basil-, and lettuce-based aquaponic and hydroponic systems. Water 2020, 12, 1259. [Google Scholar] [CrossRef]
- Liu, M.; Shi, X.; Chen, C.; Yu, L.; Sun, C. Responses of Microcystis colonies of different sizes to hydrogen peroxide stress. Toxins 2017, 9, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weenink, E.F.J.; Matthijs, H.C.P.; Schuurmans, J.M.; Piel, T.; van Herk, M.J.; Sigon, C.A.M.; Visser, P.M.; Huisman, J. Interspecific protection against oxidative stress: Green algae protect harmful cyanobacteria against hydrogen peroxide. Environ. Microbiol. 2021, 23, 2404–2419. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ghaly, M.; Hobson, P.; Chow, C.W.K. Innovative method of utilizing hydrogen peroxide for source water management of cyanobacteria. Environ. Sci. Pollut. Res. 2021, 29, 22651–22660. [Google Scholar] [CrossRef] [PubMed]
- Raffellini, S.; Guerrero, S.; Alzamora, S.M. Effect of hydrogen peroxide concentration and pH on inactivation of kinetics of Escherichia coli. J. Food Safety 2008, 28, 514–533. [Google Scholar] [CrossRef]
- Marker, A.F.H.; Bolas, P.M. Sampling of nonplanktonic algae (benthic algae or periphyton). In Methods for the Examination of Waters and Associated Materials; Pittwell, L.R., Ed.; Department of the Environment: London, UK, 1982. [Google Scholar]
- Francoeur, S.N.; Rier, S.T.; Whorley, S.B. Methods for sampling and analyzing wetland algae. In Wetland Techniques, 1st ed.; Anderson, J., Davis, C., Eds.; Springer: Dordrecht, Switzerland, 2013; Volume 2, pp. 1–87. [Google Scholar]
- Douglas, B. The ecology of the attached diatoms and other algae in a small stony stream. J. Ecol. 1958, 46, 295–322. [Google Scholar] [CrossRef]
- Peniuk, G.T.; Schnurr, P.J.; Allen, D.G. Identification and quantification of suspended algae and bacteria populations using flow cytometry: Applications for algae biofuel and biochemical growth systems. J. Appl. Phycol. 2016, 28, 95–104. [Google Scholar] [CrossRef]
- Simon, D.; Helliwell, S. Extraction and quantification of chlorophyll a from freshwater green algae. Water Res. 1998, 32, 2220–2223. [Google Scholar] [CrossRef]
- Schumann, R.; Häubner, N.; Klausch, S.; Karsten, U. Chlorophyll extraction methods for the quantification of green microalgae colonizing building facades. Int. Biodeterior. Biodegrad. 2005, 55, 213–222. [Google Scholar] [CrossRef]
- Schwarz, D.; Grosch, R.; Gross, W.; Hoffmann-Hergarten, S. Water quality assessment of different reservoir types in relation to nutrient solution use in hydroponics. Agric. Water Manag. 2005, 71, 145–166. [Google Scholar] [CrossRef]
- Hinchee, R.E.; Downey, D.C.; Aggarwal, P.K. Use of hydrogen peroxide as an oxygen source for in situ biodegradation: Part I. field studies. J. Hazard. Mater. 1991, 27, 287–299. [Google Scholar] [CrossRef]
- Tusseau-Vuillemin, M.H.; Lagarde, F.; Chauviere, C.; Heduit, A. Hydrogen peroxide (H2O2) as a source of dissolved oxygen in COD-degradation respirometric experiments. Water Res. 2002, 36, 793–798. [Google Scholar] [CrossRef]
- Taylor, N.I.; Ross, L.G. The use of hydrogen peroxide as a source of oxygen for the transport of live fish. Aquaculture 1988, 70, 183–192. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Silva, C.N.; Yoshida, M.I.; Lago, R.M. The effect of H2 treatment on the activity of activated carbon for the oxidation of organic contaminants in water and the H2O2 decomposition. Carbon 2004, 42, 2279–2284. [Google Scholar] [CrossRef]
- Soffer, H.; Burger, D.W.; Lieth, J.H. Plant growth and development of Chrysanthemum and Ficus in aero-hydroponics: Response to low dissolved oxygen concentrations. Sci. Hortic. 1991, 45, 287–294. [Google Scholar] [CrossRef]
- Ruso, T.; Cowden, R.J.; Moraru, P.I.; Maxim, M.A.; Ghaley, B.B. Overview of multiple applications of basil species and cultivars and the effects of production environmental parameters on yields and secondary metabolites in hydroponic systems. Sustainability 2021, 13, 11332. [Google Scholar] [CrossRef]
- Sikawa, D.C.; Yakupitiyage, A. The hydroponic production of lettuce (Lactuca sativa L.) by using hybrid catfish (Clarias macrocephalus × C. gariepinus) pond water: Potentials and constraints. Agric. Water Manag. 2010, 97, 1317–1325. [Google Scholar] [CrossRef]

| Type | Cultivar | Chemical Treatment | Cultivar × Chemical Treatment | |
|---|---|---|---|---|
| Basil | SPAD | * z | NS | NS |
| Plant height | NS | NS | NS | |
| Plant width | * | NS | NS | |
| Number of leaves | NS | NS | NS | |
| Shoot FW | NS | NS | NS | |
| Shoot DW | NS | NS | NS | |
| Root DW | NS | NS | NS | |
| Lettuce | SPAD | *** | NS | NS |
| Plant height | * | ** | NS | |
| Plant width | * | NS | NS | |
| Number of leaves | NS | * | NS | |
| Shoot FW | * | *** | * | |
| Shoot DW | NS | ** | NS | |
| Root DW | NS | * | NS |
| Cultivar | Chemical Treatment | Shoot FW (g Plant−1) |
|---|---|---|
| Green Forest | Control | 271.12a z |
| 3% H2O2 (50 mL) | 298.80a | |
| ZeroTol (45 mL) | 159.26bc | |
| ZeroTol (60 mL) | 114.60bc | |
| ZeroTol (45 ppm) and 3% H2O2 (50 mL) | 108.40c | |
| ZeroTol (60 ppm) and) | 154.79bc | |
| Tropicana | Control | 223.18ab |
| 3% H2O2 (50 mL) | 209.43abc | |
| ZeroTol (45 mL) | 148.85bc | |
| ZeroTol (60 mL) | 135.15bc | |
| ZeroTol (45 ppm) and 3% H2O2 (50 mL) | 148.09bc | |
| ZeroTol (60 ppm) and 3% H2O2 (50 mL) | 134.40bc |
| Chemical | Plant Height (cm) | Number of Leaves | Shoot DW (g Plant−1) | Root DW (g Plant−1) |
|---|---|---|---|---|
| Control | 28.34a z | 12.94ab | 10.04a | 1.27a |
| 3% H2O2 (50 mL) | 26.45ab | 15.35a | 9.81a | 1.10ab |
| ZeroTol (45 mL) | 22.80b | 12.85ab | 6.50ab | 1.07ab |
| ZeroTol (60 mL) | 29.40bc | 11.79b | 5.59b | 0.95ab |
| ZeroTol (45 mL) and 3% H2O2 (50 mL) | 17.19c | 12.54ab | 6.54ab | 0.80b |
| ZeroTol (60 mL) and 3% H2O2 (50 mL) | 22.35bc | 13.10ab | 6.67ab | 0.93ab |
| Type | Cultivar | SPAD Index (Unitless) | Plant Height (cm) | Plant Width (cm) |
|---|---|---|---|---|
| Basil | Aroma II | 33.99a z | 35.53a | 17.35a |
| Genovese | 32.38b | 33.53a | 15.34a | |
| Lettuce | Green Forest | 40.57a | 23.96a | 27.59b |
| Tropicana | 35.24b | 21.86b | 28.83a |
| Type | Cultivar | Chemical Treatment | Cultivar × H2O2 | |
|---|---|---|---|---|
| Basil | SPAD | NS z | NS | NS |
| Plant height | NS | NS | NS | |
| Plant width | NS | NS | NS | |
| Number of leaves | NS | NS | NS | |
| Shoot (FW). | NS | NS | NS | |
| Shoot (DW) | NS | * | NS | |
| Root (DW) | NS | NS | NS | |
| Lettuce | SPAD | *** z | NS | NS |
| Plant height | * | NS | NS | |
| Number of leaves | NS | NS | NS | |
| Shoot (FW) | NS | NS | NS | |
| Shoot (DW) | NS | *** | NS | |
| Root (DW) | NS | NS | NS |
| Type | Chemical Treatment | Shoot DW (g) |
|---|---|---|
| Basil | Control | 7.98ab z |
| 3% H2O2 (70 mL) | 7.79ab | |
| ZeroTol (15 mL) | 7.82ab | |
| ZeroTol (30 mL) | 7.78ab | |
| ZeroTol (45 mL) | 8.35a | |
| ZeroTol (60 mL) | 7.27ab | |
| PERpose Plus (15 mL) | 7.99ab | |
| PERpose Plus (30 mL) | 7.29ab | |
| PERpose Plus (45 mL) | 8.16ab | |
| PERpose Plus (60 mL) | 6.58b | |
| Lettuce | Control | 14.01abc |
| 3% H2O2 (70 mL) | 14.38abc | |
| ZeroTol (15 mL) | 15.16ab | |
| ZeroTol (30 mL) | 13.04abc | |
| ZeroTol (45 mL) | 14.89ab | |
| ZeroTol (60 mL) | 11.17bc | |
| PERpose Plus (15 mL) | 15.24a | |
| PERpose Plus (30 mL) | 15.07ab | |
| PERpose Plus (45 mL) | 13.83abc | |
| PERpose Plus (60 mL) | 10.51c |
| Type | Cultivar | SPAD Index (Unitless) | Plant Height (cm) |
|---|---|---|---|
| Lettuce | Green Forest | 40.47a | 20.12a z |
| Tropicana | 33.38b | 14.62b |
| Experiment | Chemical Treatment | Dry Weight (mg L−1) | Algal Cells (105) | Chl a (µg L−1) | Visual Scale z |
|---|---|---|---|---|---|
| 1 | Control | 0.66a y | 13.66a | 740.53a | 3 |
| 3% H2O2 (50 mL) | 0.61a | 13.38a | 801.59a | 3 | |
| ZeroTol (45 mL) | 0.71a | 12.98a | 708.35a | 2 | |
| ZeroTol (60 mL) | 0.86a | 13.26a | 755.07a | 1 | |
| ZeroTol (45 mL) and 3% H2O2 (50 mL) | 1.00a | 13.28a | 856.90a | 2 | |
| ZeroTol (60 ppm) and 3% H2O2 (50 mL) | 0.64a | 12.57a | 875.28a | 2 | |
| 2 | Control | 0.47a z | 6.50a | 945.57a | 3 |
| 3% H2O2 (50 mL) | 0.26a | 5.42a | 1209.09a | 3 | |
| ZeroTol (15 mL) | 0.37a | 6.42a | 616.90a | 2 | |
| ZeroTol (30 mL) | 0.37a | 6.75a | 637.19a | 2 | |
| ZeroTol (45 mL) | 0.43a | 5.97a | 716.51a | 2 | |
| ZeroTol (60 mL) | 0.23a | 6.24a | 509.46a | 1 | |
| PERpose Plus (15 mL) | 0.21a | 6.15a | 573.41a | 2 | |
| PERpose Plus (30 mL) | 0.15a | 6.01a | 597.24a | 2 | |
| PERpose Plus (45 mL) | 0.21a | 5.93a | 607.17a | 2 | |
| PERpose Plus (60 mL) | 0.14a | 5.76a | 690.83a | 1 |
| Chemical Treatment | Scientific Name | Average Cells/mL z | Average Natural Units/mL z |
|---|---|---|---|
| Control | Microspora tumidula | 478,071 | 976 |
| Leptolyngbya spp | 48,771 | 2342 | |
| Scenedesmus acuminatus | 92 | 92 | |
| Scenedesmus acutus | 46 | 11 | |
| Pennate Diatom spp. Live | 11 | 11 | |
| Centric Diatom spp. Live | 11 | 11 | |
| Chlamydomonas spp. | 11 | 11 | |
| 3% H2O2 (70 mL) | Microspora tumidula | 10,035 | 1476 |
| Gloeocystis vesiculosa | 9888 | 325 | |
| Chlamydomonas spp. | 6316 | 6316 | |
| Tetraspora cylindrica | 1476 | 30 | |
| Centric Diatom spp. Live | 472 | 472 | |
| Scenedesmus acuminatus | 236 | 148 | |
| Pennate Diatom spp. Live | 118 | 118 | |
| ZeroTol (15 mL) | Gloeocystis vesiculosa | 29,494 | 1756 |
| Microspora tumidula | 18,956 | 568 | |
| Chlamydomonas spp. | 12,913 | 12,913 | |
| Scenedesmus acutus | 930 | 258 | |
| Scenedesmus quadricauda | 207 | 52 | |
| ZeroTol (60 mL) | Pennate Diatom spp. Live | 4527 | 4527 |
| Microspora tumidula | 3587 | 244 | |
| Chlamydomonas spp. | 451 | 451 | |
| Centric Diatom spp. Live | 394 | 394 | |
| Gloeocystis vesiculosa | 225 | 19 | |
| PERpose Plus (15 mL) | Microspora tumidula | 28,620 | 942 |
| Gloeocystis vesiculosa | 9818 | 355 | |
| Microspora pachyderma | 826 | 8 | |
| Chlamydomonas spp. | 496 | 496 | |
| Scenedesmus acutus | 314 | 99 | |
| Scenedesmus quadricauda | 83 | 25 | |
| Centric Diatom spp. Live | 83 | 83 | |
| PERpose Plus (60 mL) | Gloeocystis vesiculosa | 4846 | 1183 |
| Sphaerocystis planktonica | 1165 | 949 | |
| Scenedesmus acutus | 301 | 103 | |
| Pennate Diatom spp. Live | 272 | 272 | |
| Scenedesmus acuminatus | 213 | 188 | |
| Centric Diatom spp. Live | 150 | 150 | |
| Chlamydomonas spp. | 9 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendrickson, T.D.; Dunn, B.L.; Goad, C.; Hu, B.; Singh, H. Effects of Hydrogen Peroxide Products on Basil, Lettuce, and Algae in an Ebb and Flow Hydroponic System. Horticulturae 2022, 8, 569. https://doi.org/10.3390/horticulturae8070569
Hendrickson TD, Dunn BL, Goad C, Hu B, Singh H. Effects of Hydrogen Peroxide Products on Basil, Lettuce, and Algae in an Ebb and Flow Hydroponic System. Horticulturae. 2022; 8(7):569. https://doi.org/10.3390/horticulturae8070569
Chicago/Turabian StyleHendrickson, Teal D., Bruce L. Dunn, Carla Goad, Bizhen Hu, and Hardeep Singh. 2022. "Effects of Hydrogen Peroxide Products on Basil, Lettuce, and Algae in an Ebb and Flow Hydroponic System" Horticulturae 8, no. 7: 569. https://doi.org/10.3390/horticulturae8070569
APA StyleHendrickson, T. D., Dunn, B. L., Goad, C., Hu, B., & Singh, H. (2022). Effects of Hydrogen Peroxide Products on Basil, Lettuce, and Algae in an Ebb and Flow Hydroponic System. Horticulturae, 8(7), 569. https://doi.org/10.3390/horticulturae8070569






