Abstract
The interest in the pre-harvest ultraviolet-B (UV-B) exposure of crops in indoor cultivation has grown consistently, though very little is known about its influence on the nutraceutical quality of microgreens. Flaxseeds constitute a valuable oilseed species, mostly appreciated for their nutritional properties and the presence of health-promoting compounds. Therefore, although scarcely studied, flaxseed sprouts and microgreens might constitute a high-quality food product to be included in a healthy diet. This study aims to unravel the effects of pre-harvest ultraviolet-B irradiation on the nutritional and nutraceutical quality of flaxseed sprouts and microgreens grown under artificial conditions. The UV-B irradiation decreased the biomass and stem length of microgreens. However, the content of total phenolics and flavonoids and the antioxidant capacity were strongly enhanced by the UV-B treatment in both sprouts and microgreens. Among photosynthetic pigments, chlorophyll a, violaxanthin, antheraxanthin, and lutein in sprouts were reduced by the treatment, while chlorophyll b increased in microgreens. In conclusion, our results showed that growing flaxseed sprouts and microgreens in controlled conditions with supplemental UV-B exposure might increase their nutritional and nutraceutical quality, as well as their antioxidant capacity, making them high-quality functional foods.
1. Introduction
Within recent decades, consumers’ awareness towards foods that can provide concrete benefits for human health has constantly grown thanks to a rise in living standards. Fresh vegetables perfectly match this demand thanks to their high content of nutraceuticals, such as polyphenols, vitamins, and carotenoids, which are known to have a preventive effect against several pathologies, e.g., cardiovascular diseases, many types of cancer, and neurodegenerative disorders. Sprouts and microgreens are gaining an ever-increasing interest due to their great nutraceutical quality, commonly in higher concentrations than their adult counterparts [1]. Sprouts are described as “the product obtained from the germination of seeds and their development in water or another medium, harvested before the development of true leaves and which is intended to be eaten whole, including the seed” [2]. While, according to Xiao, Lester, Luo, and Wang (2012) [3], microgreens are “tender immature greens, produced from the seeds of vegetables and herbs, having two fully developed cotyledon leaves with or without the emergence of a rudimentary pair of first true leaves”. Their appeal towards consumers for their health-related properties, together with their constantly growing marketability as novel ingredients in upscale restaurants, have attracted the interest of many greenhouse growers.
Light constitutes a crucial environmental factor for plant growth and development, since it not only provides the energy for photosynthesis, but also plays a key role in a great variety of physiological and biochemical processes. Indeed, light conditions, in terms of spectral quality (wavelength) and light quantity (dose), can influence not only the morphophysiology of sprouts and microgreens, but also the biosynthesis of phytochemicals. With this in mind, several recent studies have investigated the effects of different light treatments on the productivity and accumulation of health-promoting compounds in microgreens of several crop species, e.g., parsley, broccoli, soybean, mustard, pak choi, and beet [4,5,6,7,8]. However, most current literature has investigated the impact of blue, red, and/or far-red light supplementation on the accumulation of bioactive compounds in sprouts and microgreens, but very little is known about the influence of pre-harvest ultraviolet (UV)-B exposure on the nutraceutical quality of microgreens. UV-B radiation (280–315 nm) represents a short fraction of the solar spectrum, with only 0.5% reaching the Earth’s surface. To avoid the potential damage to macromolecules and the photosynthetic apparatus caused by highly energetic UV-B radiation and UV-B-induced reactive oxygen species (ROS), plants have evolved a dedicated intracellular pathway [9,10], leading to the biosynthesis and accumulation of protective metabolites, such as phenolic compounds [11,12,13]. UV-B exposure was also found to impact other classes of phytochemicals, e.g., carotenoids, alkaloids, glucosinolates, and several vitamins [14,15,16,17,18,19]. UV-B irradiation, as an eco-friendly tool to enhance the nutraceutical quality of crops when applied in pre- and post-harvest, has gained great interest among researchers [20,21]. However, the implementation of UV-B LED light in horticulture, and particularly as light supplementation for sprouts and microgreens grown under light-controlled environments, is still in its infancy.
Flaxseed (Linum usitatissimum L., Linaceae family) represents one of the most popular oilseed species, due to its high nutritional and nutraceutical value. With variations depending on the cultivars, flaxseed shows a great content of polyunsaturated fatty acids (mainly α-linolenic and linoleic acids) and a moderate content of monounsaturated fatty acids (especially oleic acid) [22]. In addition, it is characterized by high levels of dietary fibers; phenolic acids (mainly p-hydroxybenzoic acid, chlorogenic acid, ferulic acid, and coumaric acid); lignans; carotenoids (e.g., lutein); and fat-soluble vitamins (mainly vitamin E group derivatives (e.g., tocopherol and tocotrienol) [23,24]. Consistently, the few relevant studies investigating the composition of flaxseed sprouts found a high content of phenolic compounds, high-quality proteins and free amino acids, and a good fatty acid composition, making flaxseed sprouts a valuable plant source of health-promoting compounds [25,26,27]. To date, no studies have studied the effect of UV-B supplementation on the productivity or nutraceutical quality of flaxseed sprouts and microgreens. Indeed, the recent paper of Puccinelli et al. [28] investigated the influence of specific wavelengths of the PAR region on biomass production and phytochemical content in flaxseed sprouts and microgreens; however, the UV-B waveband was not included in the experimental design. Therefore, considering the well-known impact of UV-B radiation on plant bioactive compounds and growth performances, the present study aims to unravel the impact of pre-harvest UV-B exposure on the productivity of flaxseed sprouts and microgreens and to investigate whether controlled UV-B irradiation could increase the concentration of phenolic compounds, flavonoids, and photosynthetic pigments, and improve the antioxidant capacity. Deepening our knowledge of the UV-B-driven responses in flaxseed sprouts and microgreens, especially in terms of health-promoting compound accumulation, might further pave the way for an applicative exploitation of UV-B exposure to increase the nutraceutical value of flaxseed sprouts and microgreens.
2. Materials and Methods
2.1. Plant Material and UV-B Treatment
Flaxseed cultivar ‘Sideral’ (Semfor s.r.l., Verona, Italy) was used in this study. The experimental site was the Department of Agriculture, Food, and Environment of the University of Pisa, Italy. First, the seeds were soaked in distilled water for 2 h in the dark at room temperature (18 °C); then, they were carefully washed with distilled water to remove seed coat mucilage. Next, seeds were sown in plastic trays (25 cm × 40 cm; 2 seeds per cm2) on moistened filter paper over jute felt (air porosity 87.6%, free porosity at pF1 46.5%, water retention capacity 41.1%, Green Felt, http://www.maiano.it/eng/nursery.html (accessed on 19 January 2022) to obtain sprouts, or directly on jute felt to obtain microgreens. Since sprouts are harvested including the roots, the filter paper prevented the roots from penetrating into the jute felt. The trays were placed in climatic chambers and kept in the dark at 20 °C for 72 h. Then, the flaxseeds were irradiated with photosynthetically active radiation (PAR) supplied by blue/red (1:2 ratio) and green (10%) LEDs, with a photosynthetic photon flux density (PPFD) of 228 μmol m−2 s−1 (C-LED, Imola, Italy). While the flaxseed sprouts from three trays were grown under PAR only, another three trays were supplied with UV-B radiation (Philips Ultraviolet-B Narrowband lamps, TL 20W/01–RS, Koninklijke Philips Electronics, Eindhoven, The Netherlands; 1.33 W m−2). Flaxseeds were exposed to a 24 h light (PAR only or PAR+UV-B), 0 h dark photoperiod for 4 days (until sprout harvesting) and 16 h light (PAR only or PAR+UV-B), 8 h dark photoperiod for the following 7 days (until microgreens harvesting).
Half-strength Hoagland’s solution (N-NO3 7.5 mM, P-H2PO4 0.5 mM, K 3.0 mM, Ca 2.5 mM, Mg 1.0 mM, Fe 25.0 μM, B 23.1 μM, Mn 4.6 μM, Zn 0.39 μM, Cu 0.16 μM, Mo 0.06 μM; pH~5.56; 1.15 mS cm−1 electrical conductivity (EC)) was used for fertigation. Harvesting of sprouts and microgreens was conducted 4 and 7 days after the beginning of the UV-B treatment (corresponding to 7 and 11 days after sowing), respectively. Sprouts were sampled including both shoots and rootlets, while the rootlets were excluded for microgreens.
2.2. Determination of Biomass and Stem Length
Fresh weight (FW) from harvested sprouts and microgreens was measured and used to calculate the fresh biomass (FW m−2). Then, samples were dried at 60 °C till constant weight, and dry weight (DW) was used to calculate the dry production (DW m−2). Percentage DW/FW ratio was also determined. The length of stems of microgreens was also measured.
2.3. Extraction and Determination of Total Phenolics, Flavonoids, and Antioxidant Activity
Fifty mg of fresh sprout and microgreen samples were extracted with 80% methanol (v/v) as reported by Tavarini et al. [29], and extracts were used for the determination of the total phenolic and flavonoid concentration and the antioxidant capacity.
Total phenolic concentration was determined with the Folin–Ciocalteau method [30], and absorbance was recorded at 750 nm using an Ultrospec 2100 pro-UV–vis spectrophotometer (Amersham Biosciences). Total phenolic concentration was expressed as mg of gallic acid equivalents (GAE) g−1 FW.
Total flavonoid concentration was determined using the method by Kim et al. [31]. Absorbance was recorded at 510 nm, and results were expressed as mg of catechin equivalents (CAE) g−1 FW.
Antioxidant activity was determined using both the ABTS (2,2-azinobis (3-ethylbenzothiazoline-6-sulphonic acid)) and the Ferric Reducing Antioxidant Power (FRAP) assays, following the methods described by Pellegrini et al. [32] and Benzie et al. [33], respectively. Absorbance was read at 734 (for ABTS assay) or 593 nm (for FRAP assay). Results from ABTS and FRAP assays were expressed as μmol of Trolox equivalent antioxidant capacity (TEAC) g−1 FW or μmol of Fe (II) g−1 FW, respectively.
The standard curves for the aforementioned determinations were calibrated using the respective commercial standards (Sigma-Aldrich Chemical Co., St. Louis, MO, USA).
2.4. Determination of Chlorophylls and Carotenoids
Extraction and determination of chlorophylls a and b and the carotenoids neoxanthin, lutein, violaxanthin, antheraxanthin, and β-carotene were performed following the method described by Castagna et al. [34]. Briefly, samples were extracted, filtrated using 0.2-μm filters (Sartorius Stedim Biotech, Goettingen, Germany), and run in a Spectra System P4000 HPLC equipped with a UV 6000 LP photodiode array detector (Thermo Fisher Scientific, Waltham, MA, USA) using a Zorbax ODS column (SA, 5-μm particle size, 250 × 4.6 mm; Phenomenex, Castel Maggiore, Italy). Elution of pigments occurred using solvent A (acetonitrile/methanol, 75/25, v/v) and solvent B (methanol/ethyl acetate, 68/32, v/v) with a flow rate of 1 mL min−1 following the gradient reported in Table 1.

Table 1.
Gradient elution used for HPLC analysis of chlorophylls a and b and the carotenoids neoxanthin, lutein, violaxanthin, antheraxanthin, and β-carotene.
Pigment quantification was determined by recording the absorbance at 445 nm, and commercial standards of chlorophylls, lutein, and β-carotene (Sigma-Aldrich, Milan, Italy) were employed to create the respective standard curves. Results were expressed as mg g−1 FW.
2.5. Statystical Analysis
The results reported represent the mean data of three independent groups (trays) of either sprouts or microgreens, control or UV-B-treated, from each of the two experiments conducted (therefore, six replicates per growth stage). Differences between groups considering both the growth stage (sprouts and microgreens) and the treatment (control and UV-B treatment) were evaluated by two-way ANOVA followed by post hoc Tukey–Kramer test (p < 0.05) using JMP software (SAS Institute, Inc., Cary, NC, USA). Data are expressed as mean ± standard error (SE). JMP software was also used to perform multivariate statistics with a supervised approach considering all the biometric and biochemical parameters investigated in this study. In detail, a canonical discriminant analysis (CDA) was used to highlight the differences among groups, while a hierarchical clustering analysis (HCA; Euclidean distance, Ward’s linkage) was employed to emphasize the relatedness across groups and variables considered. Finally, a CDA-based Pearson’s correlation test was performed correlating the variables measured in this study with the scores of the two canonical functions resulting from the CDA, to show the magnitude of the linear association between variables.
3. Results
3.1. Biomass Production
Considering the harvesting stage, microgreens, as expected, had higher fresh biomass content (FW; +194 %) than sprouts, while the dry matter content (DW/FW ratio) was 23% lower as compared to sprouts (Table 2). Similarly, the dried biomass content (DW) was influenced by the harvesting stage, with a 131% higher DW content in microgreens in respect to sprouts. UV-B exposure negatively affected FW and DW (−24 and −21% compared to control, respectively), while the DW/FW ratio was not influenced by the treatment. The interaction between the harvest stage and UV-B radiation significantly affected only FW. Specifically, control microgreens had the highest FW content, and this parameter was about 24% lower in microgreens grown under UV-B radiation. Sprouts, regardless of exposure to UV-B radiation, had the lowest fresh biomass content (Table 2). Additionally, the stem length of microgreens was significantly reduced by the presence of UV-B radiation (−12%) as compared to control.

Table 2.
Fresh (FW) and dry (DW) biomass and dry matter content (DW/FW) of flaxseed sprouts and microgreens grown indoors under controlled conditions under PAR (CTR) or PAR + UV-B radiation (UV-B). Data represent the mean ± SE (n = 6). Means followed by the same letter are not statistically different for p = 0.05 after Tukey–Kramer test. Significance level: *** p ≤ 0.001; ** p ≤ 0.01; * p ≤ 0.05; n.s. = not significant.
3.2. Total Phenolics, Flavonoids, and Antioxidant Activity
The content of phenolics and flavonoids was significantly affected by the growth stage and the UV-B treatment, but not by their interaction. Depending on the harvesting stage, total phenolics were less concentrated in microgreens (−20%) than in sprouts. Conversely, microgreens contained more flavonoids (+24%) as compared to sprouts. However, independently of the growth stage, UV-B exposure always had a positive influence on both total phenolics (+47%) and flavonoids (+35%) content (Figure 1a,b). Similarly, the antioxidant capacity was influenced by both the harvesting stage and the presence of UV-B radiation. Sprouts exhibited a slightly higher antioxidant activity (+22% and +12% for ABTS and FRAP assay, respectively) than microgreens. Considering the UV-B treatment, irrespective of the growth stage, the presence of UV-B radiation in the light spectrum significantly increased the antioxidant capacity measured by both ABTS (+39%) and FRAP (+35%) assay (Figure 1c,d).
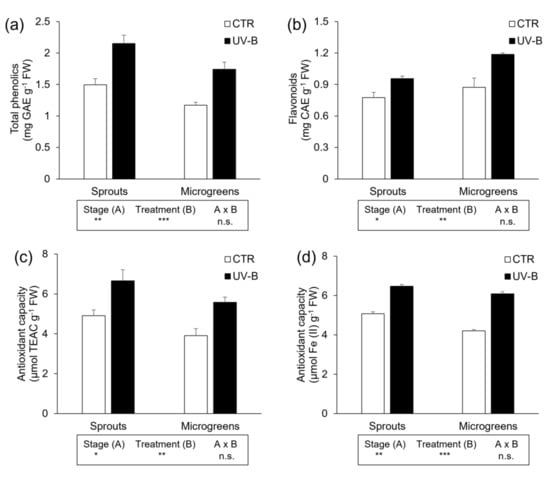
Figure 1.
Determination of (a) total phenolics, (b) flavonoids, and antioxidant capacity measured through (c) ABTS and (d) FRAP assays of flaxseed sprouts and microgreens grown indoors under controlled conditions under PAR (CTR) or PAR + UV-B radiation (UV-B). Different letters indicate statistically significant differences, according to two-way ANOVA, followed by Tukey–Kramer post hoc test (n = 6, p < 0.05). The results of two-way ANOVA are reported in the box below each histogram. Significance level: *** p ≤ 0.001; ** p ≤ 0.01; * p ≤ 0.05; n.s. = not significant.
3.3. Chlorophylls and Carotenoids
The growth stage and its interaction with the UV-B treatment significantly affected the concentration of all photosynthetic pigments (Table 3). All carotenoids and the two chlorophylls were more highly concentrated in microgreens than in sprouts (neoxanthin, +50%; violaxanthin, +73%; antheraxanthin, +33%; lutein, +53%; chlorophyll a, +39%; chlorophyll b, +47%; and β-carotene, +75%).

Table 3.
Concentration (mg g−1 FW) of neoxanthin, violaxanthin, antheraxanthin, lutein, chlorophyll a, chlorophyll b, and β-carotene in flaxseed sprouts and microgreens grown indoors under controlled conditions under PAR (CTR) or PAR + UV-B radiation (UV-B). Data represent the mean ± SE (n = 6). Means followed by the same letter are not statistically different for p = 0.05 after Tukey–Kramer test. Significance level: *** p ≤ 0.001; ** p ≤ 0.01; * p ≤ 0.05; n.s. = not significant.
Generally, sprout pigments were more affected by UV-B exposure than microgreen pigments. Indeed, the sprout concentration of violaxanthin, antheraxanthin, lutein, and chlorophyll a underwent a significant decrease following UV-B treatment as compared to the control sprouts (−38%, −67%, −26%, and −22%, respectively), while in microgreens, only chlorophyll b concentration was influenced by UV-B exposure, which induced a 19% increase in respect to the control (Table 3).
3.4. Multivariate Analyses and Pearson’s Correlation
Two supervised multivariate statistical tools, a canonical discriminant analysis (CDA) and a hierarchical clustering analysis (HCA), were employed to find whether and how the variables measured in this study differentially impacted the different groups considered, and thus if they fitted predetermined groups referring to the parameters analyzed.
The CDA (Figure 2) effectively determined a visible segregation of all four groups considered in this study (sprouts, CTR; sprouts, UV-B; microgreens, CTR; microgreens, UV-B), indicating that both factors, harvesting stage and UV-B treatment, highly influenced the variables investigated in this study. The canonical function 1 (Can 1) explained almost totally the separation among groups, with a coefficient of 99.8%. Based on this canonical function, on the x-axis of the plot, the main separation is strictly correlated with the harvesting stage, regardless the UV-B treatment, since the sprouts are located on the right side of the hyperspace, while the microgreens are on the left side. However, the separation of groups based on the canonical function 2 (Can 2, y-axis), accounting for the 0.2%, is associated with the UV-B treatment. Indeed, the UV-B-treated sprouts and microgreens are both located on the upper part of the plot, while the non-UV-B-irradiated groups are arranged on the lower side.
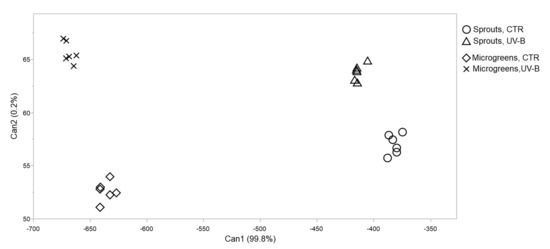
Figure 2.
2D scatterplot of canonical discriminant analysis considering UV-B-treated (UV-B) and untreated (CTR) flaxseed sprouts and microgreens grown indoors under controlled conditions under PAR (CTR) or PAR + UV-B radiation (UV-B). Can 1 and 2 refer to canonical function 1 and 2, which consider all the variables in order to maximize the separation among the groups.
In addition, the CDA-based Pearson’s correlation was also calculated between the canonical scores and the variables measured (Table 4), to determine which parameters are mostly associated with the differences attributable to the harvesting stage (canonical function 1) and/or the UV-B treatment (canonical function 2). The Pearson’ coefficients showed a strong correlation between fresh biomass, dry matter content, and most of the photosynthetic pigments (chlorophyll a, chlorophyll b, β-carotene, lutein, and violaxanthin) and the Can 1 scores (r values of −0.91, 0.73, 0.82, 0.82, 0.86, 0.85, and 0.82, respectively), indicating that the differences in these variables were strongly imputable to the harvesting stage. Contrarily, total phenolics, flavonoids, and antioxidant activity (measured through both ABTS and FRAP assays) were strongly associated with Can 2 scores (r values of 0.79, 0.70, 0.76, and 0.93, respectively), which means that variations in these parameters are mostly influenced by the UV-B treatment.

Table 4.
Pearson’s correlation coefficients (r) between each parameter measured and the canonical scores associated with the canonical discriminant analysis (CDA) reported in Figure 2. * 0.7 ≥ |r| ≥ 1: strong correlation.
In addition, an unsupervised hierarchical clustering (HCA; Euclidean distance, Ward’s linkage) was employed to better visualize the relationships between the groups (Figure 3). The output of this multivariate analysis showed that the main clustering occurred between the sprouts-UV-B group and all the other groups, which are then sub-clustered according to the treatment (sprouts/microgreens-CTR and microgreens-UV-B). It can be observed that the most similar groups are the CTR groups, regardless of harvesting stage, while the UV-B-treated groups are clustered separately and upstream, indicating the effectiveness of the UV-B treatment in modulating the variables analyzed. Furthermore, the heatmap-based clustering of the parameters showed a marked separation between a group consisting of total phenolics, antioxidant activity, DW/FW, and flavonoids and a second group that comprises biomass (FW), chlorophyll a, lutein, chlorophyll b, β-carotene, neoxanthin, violaxanthin, and antheraxanthin, indicating the noticeably different behavior of these two groups of parameters within the groups.
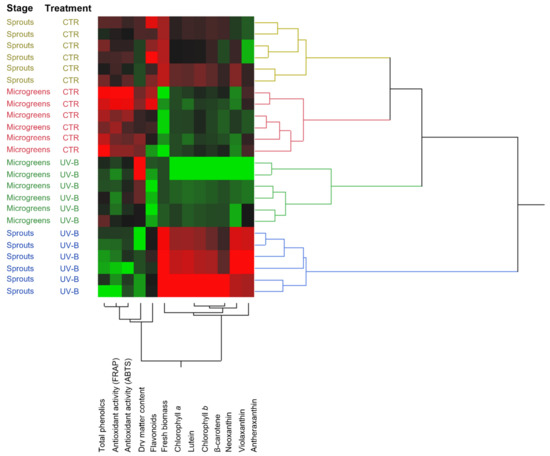
Figure 3.
Unsupervised hierarchical cluster analysis of the variables investigated in this study clustered by harvesting stage (sprouts and microgreens) and UV-B treatment (UV-B and CTR). Clustering and dendrograms were produced by choosing the Euclidean distance and Ward’s linkage rule.
4. Discussion
This manuscript aims to shed light on the effects of UV-B irradiation on the productivity and quality of flaxseed sprouts and microgreens grown in a controlled environment.
4.1. The Growth Stage of Sprouts and Microgreens Influences Biomass Production and Phytochemical Content
The biomass of microgreens was significantly higher than that of sprouts, considering both fresh (+194%) and dry (+131%) weight. This result was due to the advanced developmental stage of microgreens compared to sprouts, which determined the greater biomass, although, in contrast to sprouts, the roots of microgreens were not harvested. Indeed, the fully expanded cotyledons, as well as the longer and thicker stems, of microgreens contributed to the greater fresh weight. A similar result was observed by Puccinelli et al. [28] who detected a higher fresh biomass in flaxseed microgreens than in sprouts, irrespective of the different light spectrum compositions. The expected higher biomass of microgreens was also found in three Brassica oleracea L. cultivars, namely ’Broccolo Nero’, ‘Cavolo Lacinato Nero di Toscana’, and ‘Cavolo Broccolo Ramoso Calabrese’, whose microgreens had weights on average +240, +125, and +100% compared to the sprouts’ weights, respectively [35]. A more recent study from the same research group on the same cultivars [36] confirmed this trend, observing a 211, 119, and 133% increase in the microgreen biomass of ‘Broccolo Nero’, ‘Cavolo Lacinato Nero di Toscana’, and ‘Cavolo Broccolo Ramoso Calabrese’ cultivars compared to the sprouts’ biomass, respectively.
The significantly higher dry matter content detected in flaxseed sprouts (+30%) compared to microgreens is in accordance with the recent report of Puccinelli et al. [28] on the same species and cultivar, and it was likely due to the progressively higher water absorption during the growth of the seedlings. An increase in the moisture content of flaxseed sprouts following germination was observed also by Wang et al. [26] and, in several amaranth cultivars, by Ebert et al. [37]. Compared to other studies in the literature, the dry matter content of sprouts in our work was higher than that reported by Wang et al. [26,38] and Wu et al. [39], who found a ~10% dry matter content regardless of the flaxseed cultivars. Contrarily, Narina et al. [25] found a slightly lower moisture content in 4-day-old sprouts of ‘Rahab-94’, ‘Pembina’, and ‘Linott’ flaxseed cultivars (76.58% average content) compared to our results, probably due to the older developmental stage of our flaxseed sprouts. Moving to other sprout species, the dry matter content can vary from what was observed in flaxseed sprouts. Indeed, while Brassica juncea and wheat sprouts of the same age showed a similar moisture content [40,41], corn and broccoli sprouts exhibited a lower and higher water content [41,42] compared to what measured in flaxseed sprouts, respectively. Moving to microgreens, Puccinelli et al. [28] found a 36% lower dry matter content compared to our observations. However, although the harvesting stage was the same, the light conditions were different. Indeed, the authors reported that flaxseed microgreens were grown under red:green:blue LEDs (1:1:1), while in our study the PAR was supplied by blue:red (1:2 ratio) and green (10%). Although no other studies reported the dry matter content in flaxseed microgreens, microgreens of most plant species showed a lower dry matter content compared to our observations [3,5,37,43].
Flaxseed microgreens showed a lower total phenolic content (−20.1%), which likely explains the lower antioxidant capacity (−18.1 and −10.7% through ABTS and FRAP assays, respectively) compared to sprouts. Similar to our results, Di Bella et al. [35] showed a significantly lower total phenolic concentration and a lower antioxidant capacity in Brassica oleracea L. microgreens than what was detected in sprouts. Additionally, Ebert et al. [37] found a lower antioxidant activity in microgreens of several amaranth cultivars compared to the correspondent sprouts. Conversely, flavonoids were more concentrated in microgreens than in sprouts, which might be due to the older developmental stage and consequently the longer light exposure prior to harvesting. Indeed, it is well known that flavonoid biosynthesis and accumulation are strongly influenced by light exposure, both in terms of quality and quantity [16,44,45]. The influence of light in phenolic biosynthesis and accumulation is well visible when comparing our results with those obtained by Puccinelli et al. [28]. Indeed, they detected a higher phenolic content in flaxseed microgreens of the same age, most likely due to the different light conditions as indicated above.
The phenolic concentration in sprouts reported in this study was similar to that observed by Wang et al. [38], but only if we consider the 10-day-old flaxseed sprouts. The discrepancy might be due to the different light conditions during sprout germination, since the cited authors kept the sprouts in the dark until harvesting; therefore, in our study, the light applied soon after the germination might have stimulated phenolic biosynthesis. Another possibility is the different flaxseed genotypes used, since the phenolic content is strictly dependent on the cultivars considered [46,47,48]. The flavonoid content, however, was in between the concentration detected in 4-day-old and in the 6-day-old flaxseed sprouts [38].
All the photosynthetic pigments considered in this study were more concentrated in microgreens compared to sprouts, with an increase ranging from 33 to 75%. The higher abundance of pigments in microgreens is likely due to the longer light exposure than in sprouts, since pigment biosynthesis is strictly dependent on light [49,50,51]. Furthermore, the superiority of microgreens in terms of pigment concentration can be explained by considering also that microgreens have fully developed cotyledons with the first pair of true leaves starting to grow. Moreover, conversely to sprouts, microgreens are harvested without roots, which have a much lower content of photosynthetic pigments than shoots. In line with our findings, average increases of 63% and 24% were observed for the total chlorophyll and total carotenoid concentration of flaxseed microgreens as compared with sprouts [28]. Moreover, a study on amaranth [37] reported that microgreens had a 5–10-times higher concentration of pigments (violaxanthin, neoxanthin, lutein, α-carotene, and β-carotene) than sprouts.
4.2. UV-B Treatment Negatively Impacts the Productivity and the Photosynthetic Pigments of Sprouts, but Enhances the Phenolic Content and the Antioxidant Activity of Both Sprouts and Microgreens
UV-B radiation is well-known to impact the germination, growth, development, and biosynthesis of secondary metabolites in plant organisms [52,53,54,55]. Indeed, plants have evolved several morphological and biochemical adaptations to this radiation (e.g., decrease in leaf area, increase in leaf thickness, reduction in height growth, and enhanced biosynthesis and accumulation of ROS-scavenging compounds) [11,52,56,57,58,59]. In the present study, UV-B treatment resulted in a decrease in stem length and fresh biomass in microgreens by 12.3 and 23.6%, respectively. Although not in flaxseed, reduced stem elongation was also observed in many plant species [60,61,62]. The reduction in hypocotyl length has been partially explained by the alteration and cessation of the cell cycle due to UV-B-induced DNA damages [63]. Biomass reduction following UV-B exposure has been observed in other crop species [64,65,66,67,68], mainly due to plants’ sensitivity to UV-B radiation, likely causing impairments in many physiological functions [69]. The total phenolic and flavonoid concentration, as well as the antioxidant activity, was positively influenced by the UV-B treatment regardless of the harvesting stage. No previous studies have reported the influence of UV-B exposure on such health-promoting compounds specifically in flaxseed sprouts or microgreens. However, many manuscripts have investigated UV-B-induced effects in several crops, mostly agreeing on the general positive impact of UV-B irradiation on the total phenolic content [16]. In accordance with our results, a pre-harvest UV-B supplementation increased the total phenolic and flavonoid concentration in basil [70,71] and lettuce [72]. UV-B was also effective in enhancing phenolic and flavonoid content, depending on the UV-B dose applied, when exposing plants soon after germination, as observed in rice and wheat seedlings [73,74], and in mung bean and broccoli sprouts [17,18]. Evidence of the positive impact of UV on the accumulation of such health-promoting compounds in sprouts and microgreens was also observed for UV-A exposure. Indeed, UV-A irradiation enhanced the accumulation of phenolic compounds in broccoli sprouts [18], as well as in turnip and tomato seedlings [75,76]. The observed increase in phenolic (especially flavonoid) content following UV-B exposure is likely due to the perception of the UV-B radiation by the UV-B photoreceptor UV RESISTANCE LOCUS 8 (UVR8) [10,77,78]. Indeed, the downstream signal transduction pathway leads to several acclimation responses to UV-B conditions, including the overexpression of biosynthetic and regulatory genes involved in the phenylpropanoid pathway [10,11]. Due to their high antioxidant capacity, plants benefit from the accumulation of phenolics (especially flavonoids), since they effectively counteract the likely UV-B-triggered ROS overproduction [79]. As a consequence of the accumulation of phenolic and flavonoid compounds, UV-B-exposed plants show a higher antioxidant activity, which in turn determines stronger beneficial properties for human health.
Considering the photosynthetic pigments, UV-B irradiation alone did not significantly affect their concentration. However, if the growth stage is included in the statistics, UV-B exposure decreased violaxanthin, antheraxanthin, lutein, and chlorophyll a concentrations in sprouts, while only chlorophyll b concentration was decreased in microgreens. The negative influence of UV-B radiation on pigment content, particularly lutein, neoxanthin, and β-carotene, was also reported by Caldwell et al. [80] in several red leaf lettuce cultivars, although the green leaf cultivars showed a general accumulation. Contrary to our observation, UV-B treatment induced an increase in lutein and neoxanthin in broccoli sprouts, although, in another study with results similar to ours, β-carotene was unchanged after UV-B irradiation [81,82]. The current relevant literature has not exhaustively deepened the understanding of a UV-B induced reduction in photosynthetic pigment concentration, since this is not a univocal response and strictly depends on the UV-B dose applied and the species/cultivars irradiated. Under high-energetic light conditions, such as UV-B, plants might address their biosynthetic machinery towards the production and accumulation of more effective UV-screening and ROS-scavenging compounds, e.g., flavonoids and other phenolic compounds, to better acclimate to UV conditions. In this case, it might be that the biosynthesis of photosynthetic pigments is slowed down when compared to control (no UV-B) conditions. Secondarily, it might be that the UV-B-induced ROS have degraded or partially damaged the photosynthetic pigments, as observed for carotenoids in UV-B-exposed peach fruits by Santin et al. [14]. However, it is interesting to remark that such a negative effect of UV-B radiation on pigments was almost exclusively evident in sprouts and that, with the exception of chlorophyll b, all the other pigments were numerically, though not statistically, more concentrated in UV-B-exposed microgreens.
5. Conclusions
Several studies have investigated the effects of pre-harvest UV-B treatment on the productivity and content of several secondary metabolites on plants of food interest. However, to date, the impact of UV-B irradiation on flaxseed sprouts and microgreens has not been investigated. The present study showed that the addition of UV-B radiation into the growth light spectrum, though it induced a reduction in the fresh biomass in microgreens (−24%), effectively increased the content of health-promoting compounds, such as total phenolics and particularly flavonoids, boosting the antioxidant capacity and therefore the beneficial properties for human health. In particular, the total phenolic and flavonoid concentrations were increased by 47% and 35%, respectively, while antioxidant capacity was increased by 39% or 35%, according to the ABTS or the FRAP assay, respectively. Photosynthetic pigments were generally unaffected by the UV-B treatment, or underwent a slight decrease, e.g., for violaxanthin (−38%), lutein (−67%), antheraxanthin (−26%), and chlorophyll a (−22%) in sprouts, or chlorophyll b (−19%) in microgreens. Thus, the application of pre-harvest UV-B irradiation in horticulture and particularly in greenhouse cultivation, as well as the consumption of flaxseed sprouts and microgreens as a valid source of bioactive compounds, is highly encouraged.
Author Contributions
Conceptualization, M.S., M.P., L.G.A., A.C., A.R. and L.I.; methodology, M.S.; formal analysis, M.S., S.T., M.P., M.C.S. and A.M.; investigation, M.S., M.P., L.G.A., A.C., S.T. and L.I.; resources, L.I., A.C., A.R. and L.G.A.; data curation, M.S., M.P. and M.C.S.; writing—original draft preparation, M.S., M.C.S., A.M. and A.C.; writing—review and editing, M.S., M.C.S., A.M., M.P., A.C., A.R., L.G.A., S.T. and M.A.; supervision, M.S., A.C. and A.R.; project administration, L.G.A., A.C. and L.I.; funding acquisition, L.G.A., A.C. and L.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Pisa, “Progetti di Ricerca di Ateneo—PRA 2018” (“Valorizzazione agronomica e nutraceutica del lino da olio—Linum usitatissimum L.”), grant number PRA_2018_25.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Acknowledgments
We thank C-Led S.r.l. company for kindly providing the LEDs used for sprouts and microgreens growth.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kyriacou, M.C.; Rouphael, Y.; di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; de Pascale, S.; Santamaria, P. Micro-Scale Vegetable Production and the Rise of Microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- di Gioia, F.; Renna, M.; Santamaria, P. Sprouts, Microgreens and “Baby Leaf” Vegetables. In Minimally Processed Refrigerated Fruits and Vegetables; Yildiz, F., Wiley, R.C., Eds.; Springer US: Boston, MA, USA, 2017; pp. 403–432. ISBN 978-1-4939-7018-6. [Google Scholar]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of Vitamin and Carotenoid Concentrations of Emerging Food Products: Edible Microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef]
- Samuolienė, G.; Viršilė, A.; Brazaitytė, A.; Jankauskienė, J.; Sakalauskienė, S.; Vaštakaitė, V.; Novičkovas, A.; Viškelienė, A.; Sasnauskas, A.; Duchovskis, P. Blue Light Dosage Affects Carotenoids and Tocopherols in Microgreens. Food Chem. 2017, 228, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Samuoliene, G.; Brazaityte, A.; Jankauskiene, J.; Viršile, A.; Sirtautas, R.; Novičkovas, A.; Sakalauskiene, S.; Sakalauskaite, J.; Duchovskis, P. LED Irradiance Level Affects Growth and Nutritional Quality of Brassica Microgreens. Cent. Eur. J. Biol. 2013, 8, 1241–1249. [Google Scholar] [CrossRef]
- Samuoliene, G.; Brazaityte, A.; Viršile, A.; Jankauskiene, J.; Sakalauskiene, S.; Duchovskis, P. Red Light-Dose or Wavelength-Dependent Photoresponse of Antioxidants in Herb Microgreens. PLoS ONE 2016, 11, e0163405. [Google Scholar] [CrossRef]
- Brazaityte, A.; Sakalauskiene, S.; Samuoliene, G.; Jankauskiene, J.; Viršile, A.; Novičkovas, A.; Sirtautas, R.; Miliauskiene, J.; Vaštakaite, V.; Dabašinskas, L.; et al. The Effects of LED Illumination Spectra and Intensity on Carotenoid Content in Brassicaceae Microgreens. Food Chem. 2015, 173, 600–606. [Google Scholar] [CrossRef]
- Samuoliene, G.; Brazaityte, A.; Sirtautas, R.; Sakalauskiene, S.; Jankauskiene, J.; Duchovskis, P.; Novičkovas, A. The Impact of Supplementary Short-Term Red LED Lighting on the Antioxidant Properties of Microgreens. Acta Hortic. 2012, 956, 649–655. [Google Scholar] [CrossRef]
- Kliebenstein, D.J.; Lim, J.E.; Landry, L.G.; Last, R.L. Arabidopsis UVR8 Regulates Ultraviolet-B Signal Transduction and Tolerance and Contains Sequence Similarity to Human Regulator of Chromatin Condensation 1. Plant Physiol. 2002, 130, 234–243. [Google Scholar] [CrossRef]
- Rizzini, L.; Favory, J.J.; Cloix, C.; Faggionato, D.; O’Hara, A.; Kaiserli, E.; Baumeister, R.; Schäfer, E.; Nagy, F.; Jenkins, G.I.; et al. Perception of UV-B by the Arabidopsis UVR8 Protein. Science 2011, 332, 103–106. [Google Scholar] [CrossRef]
- Brown, B.A.; Cloix, C.; Jiang, G.H.; Kaiserli, E.; Herzyk, P.; Kliebenstein, D.J.; Jenkins, G.I. A UV-B-Specific Signaling Component Orchestrates Plant UV Protection. Proc. Natl. Acad. Sci. USA 2005, 102, 18225–18230. [Google Scholar] [CrossRef]
- Favory, J.J.; Stec, A.; Gruber, H.; Rizzini, L.; Oravecz, A.; Funk, M.; Albert, A.; Cloix, C.; Jenkins, G.I.; Oakeley, E.J.; et al. Interaction of COP1 and UVR8 Regulates UV-B-Induced Photomorphogenesis and Stress Acclimation in Arabidopsis. EMBO J. 2009, 28, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Santin, M.; Lucini, L.; Castagna, A.; Rocchetti, G.; Hauser, M.T.; Ranieri, A. Comparative “Phenol-Omics” and Gene Expression Analyses in Peach (Prunus Persica) Skin in Response to Different Postharvest UV-B Treatments. Plant Physiol. Biochem. 2019, 135, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Santin, M.; Lucini, L.; Castagna, A.; Chiodelli, G.; Hauser, M.-T.; Ranieri, A. Post-Harvest UV-B Radiation Modulates Metabolite Profile in Peach Fruit. Postharvest Biol. Technol. 2018, 139, 127–134. [Google Scholar] [CrossRef]
- Santin, M.; Ranieri, A.; Hauser, M.-T.; Miras-Moreno, B.; Rocchetti, G.; Lucini, L.; Strid, Å.; Castagna, A. The Outer Influences the Inner: Postharvest UV-B Irradiation Modulates Peach Flesh Metabolome Although Shielded by the Skin. Food Chem. 2021, 338, 127782. [Google Scholar] [CrossRef]
- Santin, M.; Ranieri, A.; Castagna, A. Anything New under the Sun? An Update on Modulation of Bioactive Compounds by Different Wavelengths in Agricultural Plants. Plants 2021, 10, 1485. [Google Scholar] [CrossRef]
- Wang, H.; Gui, M.; Tian, X.; Xin, X.; Wang, T.; Li, J. Effects of UV-B on Vitamin C, Phenolics, Flavonoids and Their Related Enzyme Activities in Mung Bean Sprouts (Vigna Radiata). Int. J. Food Sci. Technol. 2017, 52, 827–833. [Google Scholar] [CrossRef]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB Light Doses and Harvesting Time Differentially Tailor Glucosinolate and Phenolic Profiles in Broccoli Sprouts. Molecules 2017, 22, 1065. [Google Scholar] [CrossRef]
- Carletti, P.; Masi, A.; Wonisch, A.; Grill, D.; Tausz, M.; Ferretti, M. Changes in Antioxidant and Pigment Pool Dimensions in UV-B Irradiated Maize Seedlings. Environ. Exp. Bot. 2003, 50, 149–157. [Google Scholar] [CrossRef]
- Schreiner, M.; Mewis, I.; Huyskens-Keil, S.; Jansen, M.A.K.; Zrenner, R.; Winkler, J.B.; O’Brien, N.; Krumbein, A. UV-B-Induced Secondary Plant Metabolites—Potential Benefits for Plant and Human Health. Crit. Rev. Plant Sci. 2012, 31, 229–240. [Google Scholar] [CrossRef]
- Neugart, S.; Schreiner, M. UVB and UVA as Eustressors in Horticultural and Agricultural Crops. Sci. Hortic. 2018, 234, 370–381. [Google Scholar] [CrossRef]
- Carter, J.F. Potential of Flaxseed and Flaxseed Oil in Baked Goods and Other Products in Human Nutrition. Cereal Foods World 1993, 38, 753–759. [Google Scholar]
- Daun, J.K.; Barthet, V.J.; Chornick, T.L.; Duguid, S. Structure, Composition, and Variety Development of Flaxseed. In Flaxseed in Human Nutrition; AOCS Press: Champaign, IL, USA, 2003; pp. 1–40. ISBN 1893997383. [Google Scholar]
- Bekhit, A.E.D.A.; Shavandi, A.; Jodjaja, T.; Birch, J.; Teh, S.; Mohamed Ahmed, I.A.; Al-Juhaimi, F.Y.; Saeedi, P.; Bekhit, A.A. Flaxseed: Composition, Detoxification, Utilization, and Opportunities. Biocatal. Agric. Biotechnol. 2018, 13, 129–152. [Google Scholar] [CrossRef]
- Narina, S.S.; Hamama, A.A.; Bhardwaj, H.L. Nutritional and Mineral Composition of Flax Sprouts. J. Agric. Sci. 2012, 4, 60–65. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Qiu, C.; Abbasi, A.M.; Chen, G.; You, L.; Li, T.; Fu, X.; Wang, Y.; Guo, X.; Liu, R.H. Effect of Germination on Vitamin C, Phenolic Compounds and Antioxidant Activity in Flaxseed (Linum Usitatissimum L.). Int. J. Food Sci. Technol. 2015, 50, 2545–2553. [Google Scholar] [CrossRef]
- Wanasundara, P.K.J.P.D.; Wanasundara, U.N.; Shahidi, F. Changes in Flax (Linum Usitatissimum L.) Seed Lipids During Germination. J. Am. Oil Chem. Soc. 1999, 76, 41–48. [Google Scholar] [CrossRef]
- Puccinelli, M.; Maggini, R.; Angelini, L.G.; Santin, M.; Landi, M.; Tavarini, S.; Castagna, A.; Incrocci, L. Can Light Spectrum Composition Increase Growth and Nutritional Quality of Linum Usitatissimum L. Sprouts and Microgreens? Horticulturae 2022, 8, 98. [Google Scholar] [CrossRef]
- Tavarini, S.; Castagna, A.; Conte, G.; Foschi, L.; Sanmartin, C.; Incrocci, L.; Ranieri, A.; Serra, A.; Angelini, L.G. Evaluation of Chemical Composition of Two Linseed Varieties as Sources of Health-Beneficial Substances. Molecules 2019, 24, 729. [Google Scholar] [CrossRef]
- Alonso Borbalán, Á.M.; Zorro, L.; Guillén, D.A.; García Barroso, C. Study of the Polyphenol Content of Red and White Grape Varieties by Liquid Chromatography-Mass Spectrometry and Its Relationship to Antioxidant Power. J. Chromatogr. A 2003, 1012, 31–38. [Google Scholar] [CrossRef]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of Polyphenolics and Their Antioxidant Capacity in Fresh Plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved Abts Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “‘Antioxidant Power’”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Castagna, A.; di Baccio, D.; Tognetti, R.; Ranieri, A.; Sebastiani, L. Differential Ozone Sensitivity Interferes with Cadmium Stress in Poplar Clones. Biol. Plant. 2013, 57, 313–324. [Google Scholar] [CrossRef]
- di Bella, M.C.; Niklas, A.; Toscano, S.; Picchi, V.; Romano, D.; lo Scalzo, R.; Branca, F. Morphometric Characteristics, Polyphenols and Ascorbic Acid Variation in Brassica Oleracea L. Novel Foods: Sprouts, Microgreens and Baby Leaves. Agronomy 2020, 10, 782. [Google Scholar] [CrossRef]
- di Bella, M.C.; Toscano, S.; Arena, D.; Moreno, D.A.; Romano, D.; Branca, F. Effects of Growing Cycle and Genotype on the Morphometric Properties and Glucosinolates Amount and Profile of Sprouts, Microgreens and Baby Leaves of Broccoli (Brassica Oleracea L. Var. Italica Plenck) and Kale (B. Oleracea L. Var. Acephala Dc.). Agronomy 2021, 11, 1685. [Google Scholar] [CrossRef]
- Ebert, A.W.; Wu, T.H.; Yang, R.Y. Amaranth Sprouts and Microgreens-a Homestead Vegetable Production Option to Enhance Food and Nutrition Security in the Rural-Urban Continuum. In Proceedings of the Regional Symposium on Sustaining Small-Scale Vegetable Production and Marketing Systems for Food and Nutrition Security (SEAVEG 2014), Bangkok, Thailand, 25–27 February 2014. [Google Scholar]
- Wang, H.; Wang, J.; Guo, X.; Brennan, C.S.; Li, T.; Fu, X.; Chen, G.; Liu, R.H. Effect of Germination on Lignan Biosynthesis, and Antioxidant and Antiproliferative Activities in Flaxseed (Linum Usitatissimum L.). Food Chem. 2016, 205, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, H.; Wang, Y.; Brennan, C.S.; Anne Brennan, M.; Qiu, C.; Guo, X. Comparison of Lignans and Phenolic Acids in Different Varieties of Germinated Flaxseed (Linum Usitatissimum L.). Int. J. Food Sci. Technol. 2021, 56, 196–204. [Google Scholar] [CrossRef]
- Park, C.H.; Park, Y.E.; Yeo, H.J.; Kim, J.K.; Park, S.U. Effects of Light-Emitting Diodes on the Accumulation of Phenolic Compounds and Glucosinolates in Brassica Juncea Sprouts. Horticulturae 2020, 6, 77. [Google Scholar] [CrossRef]
- Niroula, A.; Khatri, S.; Khadka, D.; Timilsina, R. Total Phenolic Contents and Antioxidant Activity Profile of Selected Cereal Sprouts and Grasses. Int. J. Food Prop. 2019, 22, 427–437. [Google Scholar] [CrossRef]
- Yang, L.; Fanourakis, D.; Tsaniklidis, G.; Li, K.; Yang, Q.; Li, T. Contrary to Red, Blue Monochromatic Light Improves the Bioactive Compound Content in Broccoli Sprouts. Agronomy 2021, 11, 2139. [Google Scholar] [CrossRef]
- Puccinelli, M.; Pezzarossa, B.; Pintimalli, L.; Malorgio, F.; Colla, G.; de Pascale, S. Selenium Biofortification of Three Wild Species, Rumex Acetosa L., Plantago Coronopus L., and Portulaca Oleracea L., Grown as Microgreens. Agronomy 2021, 11, 1155. [Google Scholar] [CrossRef]
- Nam, T.G.; Kim, D.O.; Eom, S.H. Effects of Light Sources on Major Flavonoids and Antioxidant Activity in Common Buckwheat Sprouts. Food Sci. Biotechnol. 2018, 27, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; He, H.; Song, W. Application of Light-Emitting Diodes and the Effect of Light Quality on Horticultural Crops: A Review. HortScience 2019, 54, 1656–1661. [Google Scholar] [CrossRef]
- Vagiri, M.; Ekholm, A.; Öberg, E.; Johansson, E.; Andersson, S.C.; Rumpunen, K. Phenols and Ascorbic Acid in Black Currants (Ribes Nigrum L.): Variation Due to Genotype, Location, and Year. J. Agric. Food Chem. 2013, 61, 9298–9306. [Google Scholar] [CrossRef]
- Connor, A.M.; Luby, J.J.; Tong, C.B.S.; Finn, C.E.; Hancock, J.F. Genotypic and Environmental Variation in Antioxidant Activity, Total Phenolic Content, and Anthocyanin Content among Blueberry Cultivars. J. Am. Soc. Hortic. Sci. 2002, 127, 89–97. [Google Scholar] [CrossRef]
- Scalzo, J.; Politi, A.; Pellegrini, N.; Mezzetti, B.; Battino, M. Plant Genotype Affects Total Antioxidant Capacity and Phenolic Contents in Fruit. Nutrition 2005, 21, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Hoober, J.K.; Eggink, L.L. Assembly of Light-Harvesting Complex II and Biogenesis of Thylakoid Membranes in Chloroplasts. Photosynth. Res. 1999, 61, 197–215. [Google Scholar] [CrossRef]
- Jilani, A.; Kar, S.; Bose, S.; Jilani, B.C.T.; Kar, A.; Bose, S.; Tripathy, S.; Jilani, N.A.; Kar, S.; Tripathy, B.C. Regulation of the Carotenoid Content and Chloroplast Development by Levulioic Acid. Physiol. Plant. 1996, 96, 139–145. [Google Scholar] [CrossRef]
- Nishio, J.N. Why Are Higher Plants Green Evolution of the Higher Plant Photosynthetic. Plant Cell Environ. 2000, 23, 539–548. [Google Scholar] [CrossRef]
- Robson, T.M.; Klem, K.; Urban, O.; Jansen, M.A.K. Re-Interpreting Plant Morphological Responses to UV-B Radiation. Plant Cell Environ. 2015, 38, 856–866. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Changing scenario in plant UV-B research: UV-B from a generic stressor to a specific regulator. J. Photochem. Photobiol. B Biol. 2015, 153, 334–343. [Google Scholar] [CrossRef]
- Hernandez-Aguilar, C.; Dominguez-Pacheco, A.; Tenango, M.P.; Valderrama-Bravo, C.; Hernández, M.S.; Cruz-Orea, A.; Ordonez-Miranda, J. Characterization of Bean Seeds, Germination, and Phenolic Compounds of Seedlings by UV-C Radiation. J. Plant Growth Regul. 2021, 40, 642–655. [Google Scholar] [CrossRef]
- Dotto, M.; Casati, P. Developmental Reprogramming by UV-B Radiation in Plants. Plant Sci. 2017, 264, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Robson, T.M.; Aphalo, P.J. Species-Specific Effect of UV-B Radiation on the Temporal Pattern of Leaf Growth. Physiol. Plant. 2012, 144, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Klem, K.; Ač, A.; Holub, P.; Kováč, D.; Špunda, V.; Robson, T.M.; Urban, O. Interactive Effects of PAR and UV Radiation on the Physiology, Morphology and Leaf Optical Properties of Two Barley Varieties. Environ. Exp. Bot. 2012, 75, 52–64. [Google Scholar] [CrossRef]
- Hectors, K.; Prinsen, E.; de Coen, W.; Jansen, M.A.K.; Guisez, Y. Arabidopsis Thaliana Plants Acclimated to Low Dose Rates of Ultraviolet B Radiation Show Specific Changes in Morphology and Gene Expression in the Absence of Stress Symptoms. New Phytol. 2007, 175, 255–270. [Google Scholar] [CrossRef]
- Mannucci, A.; Mariotti, L.; Castagna, A.; Santin, M.; Trivellini, A.; Reyes, T.H.; Mensuali-Sodi, A.; Ranieri, A.; Quartacci, M.F. Hormone Profile Changes Occur in Roots and Leaves of Micro-Tom Tomato Plants When Exposing the Aerial Part to Low Doses of UV-B Radiation. Plant Physiol. Biochem. 2020, 148, 291–301. [Google Scholar] [CrossRef]
- Hofmann, R.W.; Campbell, B.D. Response of Trifolium Repens to UV-B Radiation: Morphological Links to Plant Productivity and Water Availability. Plant Biol. 2011, 13, 896–901. [Google Scholar] [CrossRef]
- Germ, M.; Breznik, B.; Dolinar, N.; Kreft, I.; Gaberšcik, A. The Combined Effect of Water Limitation and UV-B Radiation on Common and Tartary Buckwheat. Cereal Res. Commun. 2012, 41, 97–110. [Google Scholar] [CrossRef]
- Furness, N.H.; Jolliffe, P.A.; Upadhyaya, M.K. Competitive Interactions in Mixtures of Broccoli and Chenopodium Album Grown at Two UV-B Radiation Levels under Glasshouse Conditions. Weed Res. 2005, 45, 449–459. [Google Scholar] [CrossRef]
- Biever, J.J.; Brinkman, D.; Gardner, G. UV-B Inhibition of Hypocotyl Growth in Etiolated Arabidopsis Thaliana Seedlings Is a Consequence of Cell Cycle Arrest Initiated by Photodimer Accumulation. J. Exp. Bot. 2014, 65, 2949–2961. [Google Scholar] [CrossRef]
- Rajabbeigi, E.; Eichholz, I.; Beesk, N.; Ulrichs, C.; Kroh, L.W.; Rohn, S.; Huyskens-Keil, S. Interaction of Drought Stress and UV-B Radiation—Impact on Biomass Production and Flavonoid Metabolism in Lettuce (Lactuca Sativa L.). J. Appl. Bot. Food Qual. 2013, 86, 190–197. [Google Scholar] [CrossRef]
- Chen, M.; Huang, Y.; Liu, G.; Qin, F.; Yang, S.; Xu, X. Effects of Enhanced UV-B Radiation on Morphology, Physiology, Biomass, Leaf Anatomy and Ultrastructure in Male and Female Mulberry (Morus Alba) Saplings. Environ. Exp. Bot. 2016, 129, 85–93. [Google Scholar] [CrossRef]
- Singh, S.; Kumari, R.; Agrawal, M.; Agrawal, S.B. Modification in Growth, Biomass and Yield of Radish under Supplemental UV-B at Different NPK Levels. Ecotoxicol. Environ. Saf. 2011, 74, 897–903. [Google Scholar] [CrossRef]
- Teramura, A.H.; Ziska, L.H.; Sztein Teramura, A.E.; Ziska, A.H.; Sztein, L.H. Changes in Growth and Photosynthetic Capacity of Rice with Increased UV-B Radiation. Physiol. Plant. 1991, 83, 373–380. [Google Scholar] [CrossRef]
- Allen, D.J.; Nogués, S.; Morison, J.I.L.; Greenslade, P.D.; McLeod, A.R.; Baker, N.R. A Thirty Percent Increase in UV-B Has No Impact on Photosynthesis in Well-Watered and Droughted Pea Plants in the Field. Glob. Chang. Biol. 1999, 5, 235–244. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, D.; Lingwan, M.; Yadukrishnan, P.; Masakapalli, S.K.; Datta, S. Light Signaling and UV-B-Mediated Plant Growth Regulation. J. Integr. Plant Biol. 2020, 62, 1270–1292. [Google Scholar] [CrossRef]
- Mosadegh, H.; Trivellini, A.; Ferrante, A.; Lucchesini, M.; Vernieri, P.; Mensuali, A. Applications of UV-B Lighting to Enhance Phenolic Accumulation of Sweet Basil. Sci. Hortic. 2018, 229, 107–116. [Google Scholar] [CrossRef]
- Nascimento, L.B.D.S.; Brunetti, C.; Agati, G.; lo Iacono, C.; Detti, C.; Giordani, E.; Ferrini, F.; Gori, A. Short-Term Pre-Harvest Uv-b Supplement Enhances the Polyphenol Content and Antioxidant Capacity of Ocimum Basilicum Leaves during Storage. Plants 2020, 9, 797. [Google Scholar] [CrossRef]
- Assumpção, C.F.; Assis, R.Q.; Hermes Poletto, V.S.; Castagna, A.; Ranieri, A.; Neugart, S.; Flôres, S.H.; de Oliveira Rios, A. Application of Supplemental UV-B Radiation in Pre-Harvest to Enhance Health-Promoting Compounds Accumulation in Green and Red Lettuce. J. Food Processing Preserv. 2019, 43, e14213. [Google Scholar] [CrossRef]
- Faseela, P.; Puthur, J.T. The Imprints of the High Light and UV-B Stresses in Oryza Sativa L. ‘Kanchana’ Seedlings Are Differentially Modulated. J. Photochem. Photobiol. B Biol. 2018, 178, 551–559. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Y.; Weng, Y.; Yang, R.; Gu, Z.; Wang, P. Effects of UV-B Radiation on Phenolic Accumulation, Antioxidant Activity and Physiological Changes in Wheat (Triticum Aestivum L.) Seedlings. Food Biosci. 2019, 30, 100409. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, B.; Sun, M.; Li, Y.; Kawabata, S. UV-A Light Induces Anthocyanin Biosynthesis in a Manner Distinct from Synergistic Blue + UV-B Light and UV-A/Blue Light Responses in Different Parts of the Hypocotyls in Turnip Seedlings. Plant Cell Physiol. 2012, 53, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Mariz-Ponte, N.; Mendes, R.J.; Sario, S.; Melo, P.; Santos, C. Moderate UV-A Supplementation Benefits Tomato Seed and Seedling Invigoration: A Contribution to the Use of UV in Seed Technology. Sci. Hortic. 2018, 235, 357–366. [Google Scholar] [CrossRef]
- Tilbrook, K.; Arongaus, A.B.; Binkert, M.; Heijde, M.; Yin, R.; Ulm, R. The UVR8 UV-B Photoreceptor: Perception, Signaling and Response. Arab. Book/Am. Soc. Plant Biol. 2013, 11, e0164. [Google Scholar] [CrossRef]
- Jenkins, G.I. The UV-B Photoreceptor UVR8: From Structure to Physiology. Plant Cell 2014, 26, 21–37. [Google Scholar] [CrossRef]
- Hideg, É.; Jansen, M.A.K.; Strid, Å. UV-B Exposure, ROS, and Stress: Inseparable Companions or Loosely Linked Associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef]
- Caldwell, C.R.; Britz, S.J. Effect of Supplemental Ultraviolet Radiation on the Carotenoid and Chlorophyll Composition of Green House-Grown Leaf Lettuce (Lactuca Sativa L.) Cultivars. J. Food Compos. Anal. 2006, 19, 637–644. [Google Scholar] [CrossRef]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB Light, and Methyl Jasmonate, Alone or Combined, Redirect the Biosynthesis of Glucosinolates, Phenolics, Carotenoids, and Chlorophylls in Broccoli Sprouts. Int. J. Mol. Sci. 2017, 18, 2330. [Google Scholar] [CrossRef]
- Mewis, I.; Schreiner, M.; Nguyen, C.N.; Krumbein, A.; Ulrichs, C.; Lohse, M.; Zrenner, R. UV-B Irradiation Changes Specifically the Secondary Metabolite Profile in Broccoli Sprouts: Induced Signaling Overlaps with Defense Response to Biotic Stressors. Plant Cell Physiol. 2012, 53, 1546–1560. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).