Monitoring Freeze-Damage in Grapefruit by Electric Bioimpedance Spectroscopy and Electric Equivalent Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
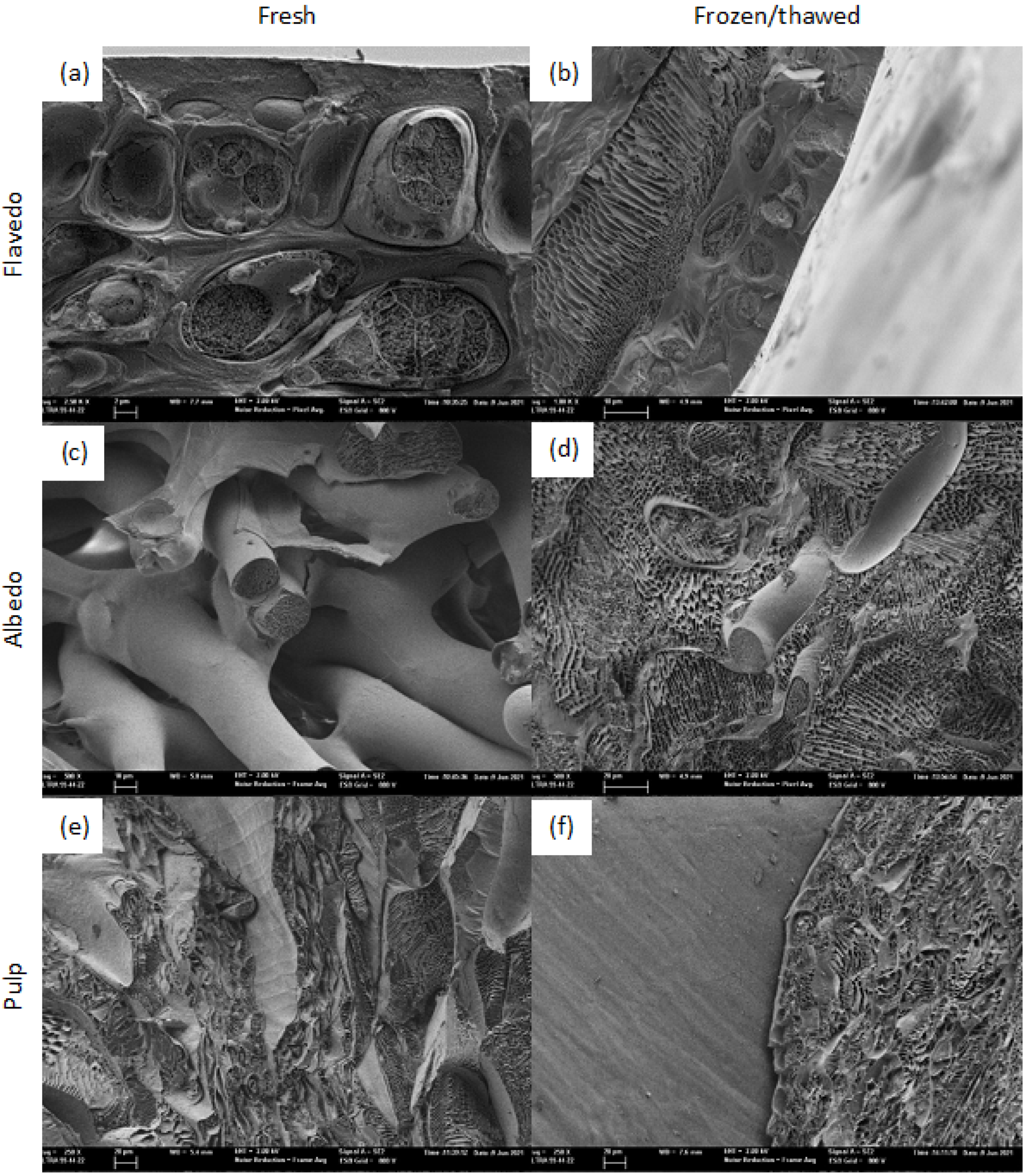
2.2. Cryo-Field Emission Scanning Electron Microscopy (Cryo-FESEM)
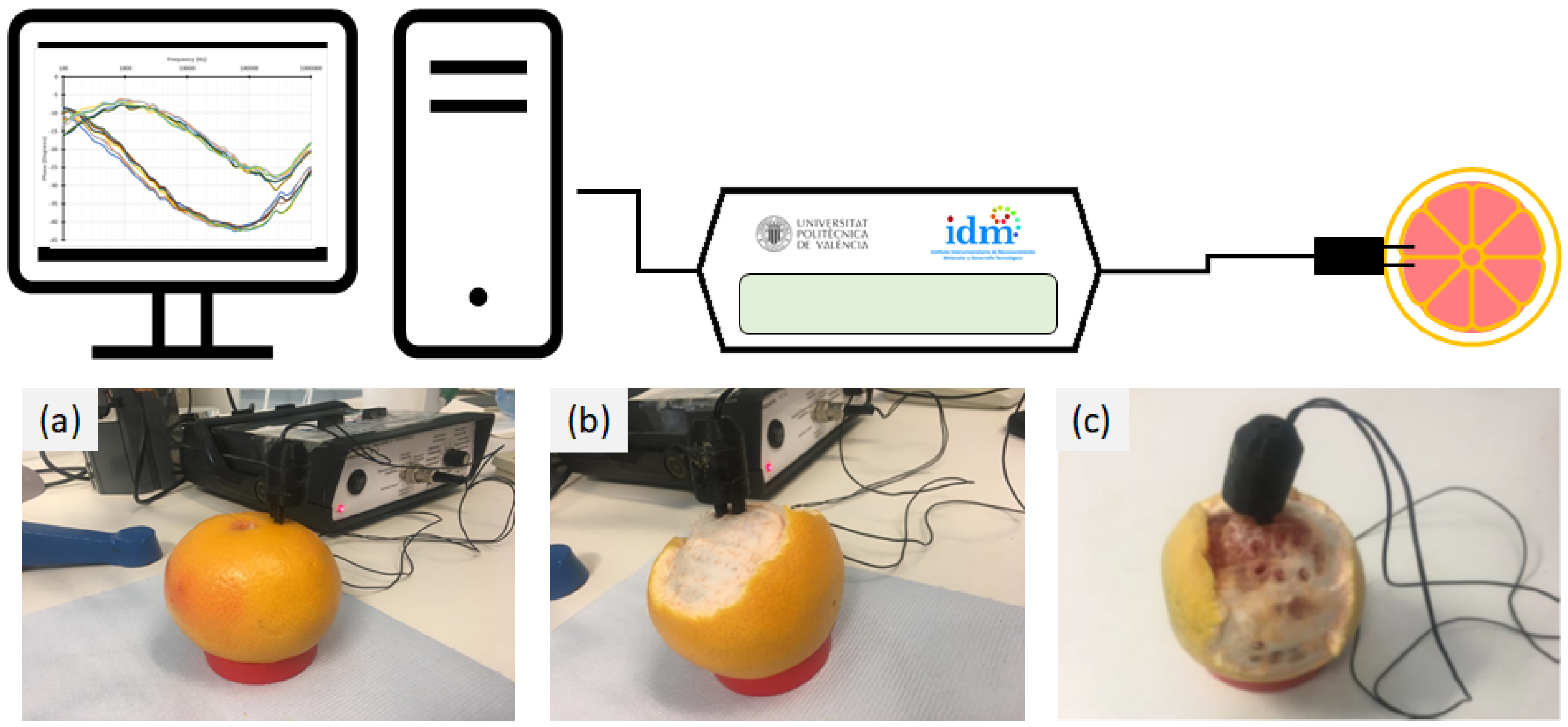
2.3. EIS Measurement System
2.4. EIS Laboratory Analyses and Data Treatment
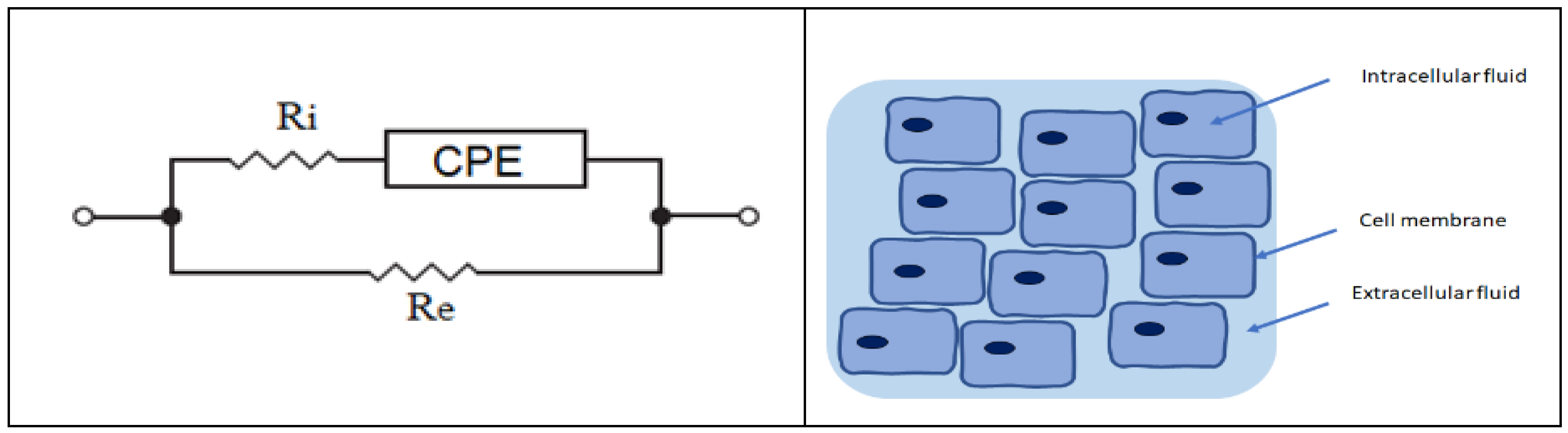
2.5. Electric Equivalent Circuits and Modeling
3. Results
3.1. Cryo-FESEM Observations
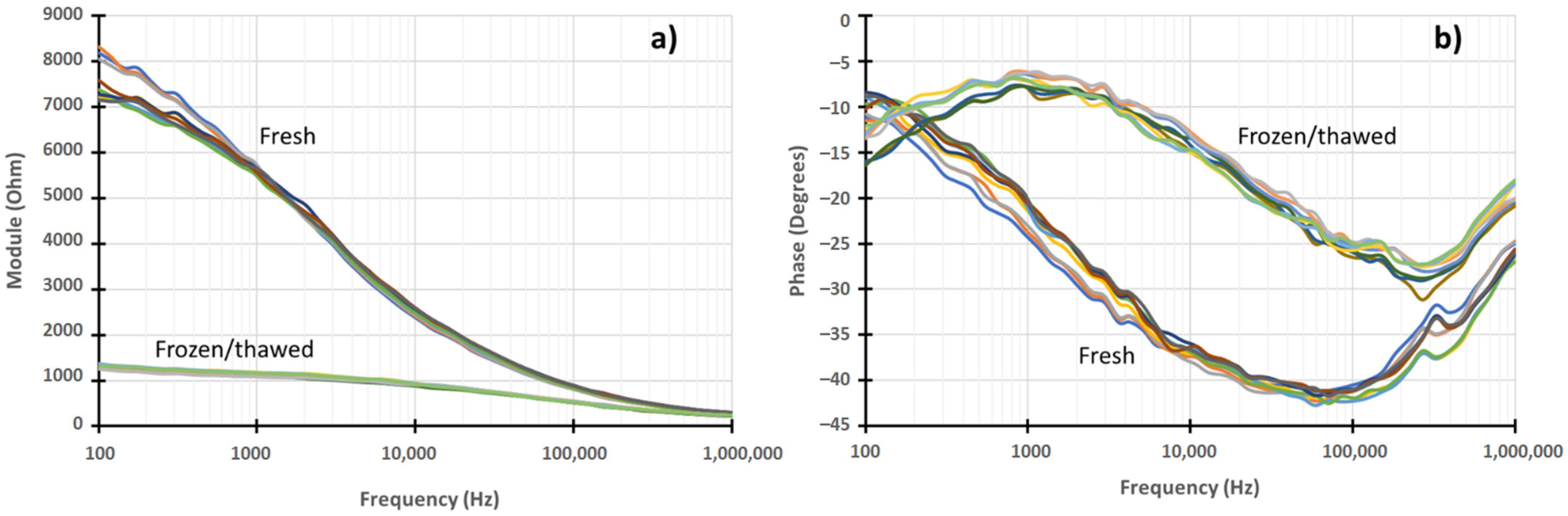
3.2. EIS Laboratory and Data Treatment Results
3.3. Electric Equivalent Circuit Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Igual, M.; García-Martínez, E.; Camacho, M.M.; Navarrete-Camacho, N. Physicochemical and Sensorial Properties of Grapefruit Jams as Affected by Processing. Food Bioprocess Technol. 2013, 6, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Kelebek, H. Sugars, organic acids, phenolic compositions and antioxidant activity of Grapefruit (Citrus paradisi) cultivars grown in Turkey. Ind. Crop Prod. 2010, 32, 269–274. [Google Scholar] [CrossRef]
- Mendes, C.; Rocha, J.; Direito, R.; Fernandes, A.; Sepodes, B.; Figueira, M.E.; Ribeiro, M.H. Anti-inflammatory activity of grapefruit juice in an in vivo model of ulcerative colitis: Comparability studies of unprocessed and bioprocessed juices. J. Funct. Foods 2018, 63, 103564. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Data. Rome, Italy. Available online: http://www.fao.org/faostat/en/#data (accessed on 30 December 2021).
- FAO. Citrus Fruit Fresh and Processed Statistical Bulletin 2016; Food and Agriculture Organization: Rome, Italy, 2017; p. 66. [Google Scholar]
- Biswas, P.; East, A.; Hewett, E.; Heyes, J.A. Intermittent Warming in Alleviating Chilling Injury—A Potential Technique with Commercial Constraint. Food Bioprocess Technol. 2016, 9, 1–15. [Google Scholar] [CrossRef]
- Martínez, L.; Ibacache, A.; Rojas, L. Daños por heladas en frutales. Tierra Adentro 2008, 80, 32–35. [Google Scholar]
- Snyder, R.L.; Melo-Abreu, J.P.; Villar-Mir, J.M. Protección Contra Las Heladas: Fundamentos, Práctica y Economía; Food and Agriculture Organization: Rome, Italy, 2010; p. 68. [Google Scholar]
- Alférez, F.; Burns, J.K. Postharvest peel pitting at non-chilling temperatures in grapefruit is promoted by changes from low to high relative humidity during storage. Postharvest Biol. Technol. 2004, 32, 79–87. [Google Scholar] [CrossRef]
- Lado, J.; Rodrigo, M.J.; Cronje, P.; Zacarías, L. Involvement of lycopene in the induction of tolerance to chilling injury in grapefruit. Postharvest Biol. Technol. 2015, 100, 176–186. [Google Scholar] [CrossRef]
- Lado, J.; Cronje, P.; Rodrigo, M.J.; Zacarías, L. Citrus. In Postharvest Physiological Disorders of Fruits and Vegetables; de Freitas, K.S.T., Pareek, S., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 321–341. [Google Scholar]
- Lado, J.; Gurrea, A.; Zacarías, L.; Rodrigo, M.J. Influence of the storage temperature on volatile emission, carotenoid content and chilling injury development in Star Ruby red grapefruit. Food Chem. 2019, 295, 72–81. [Google Scholar] [CrossRef]
- Ncama, K.; Tesfay, S.Z.; Fawole, O.A.; Opara, U.L.; Magwaza, L.S. Non-destructive prediction of ‘Marsh’ grapefruit susceptibility to postharvest rind pitting disorder using reflectance Vis/NIR spectroscopy. Sci. Hortic-Amst. 2018, 231, 265–271. [Google Scholar] [CrossRef]
- Biolatto, A.; Vazquez, D.E.; Sancho, A.M.; Carduza, F.J.; Pensel, N.A. Effect of commercial conditioning and cold quarantine storage treatments on fruit quality of “Rouge La Toma” grapefruit (Citrus paradisi Macf.). Postharvest Biol. Technol. 2005, 35, 167–176. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Bodbodak, S. Postharvest Heat Treatment for Mitigation of Chilling Injury in Fruits and Vegetables. Food Bioprocess Technol. 2014, 7, 37–53. [Google Scholar] [CrossRef]
- Maul, P.; McCollum, G.; Guy, C.L.; Porat, R. Temperature conditioning alters transcript abundance of genes related to chilling stress in ‘Marsh’ grapefruit flavedo. Postharvest Biol. Technol. 2011, 60, 177–185. [Google Scholar] [CrossRef]
- Primo-Capella, A.; Martínez-Cuenca, M.-R.; Forner-Giner, M.Á. Cold Stress in Citrus: A Molecular, Physiological and Biochemical Perspective. Horticulturae 2021, 7, 340. [Google Scholar] [CrossRef]
- Chaudhary, P.R.; Jayaprakasha, G.K.; Porat, R.; Patil, B.S. Low temperature conditioning reduces chilling injury while maintaining quality and certain bioactive compounds of ‘Star Ruby’ grapefruit. Food Chem. 2014, 153, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Ochandio Fernández, A.; Olguín Pinatti, C.A.; Masot Peris, R.; Laguarda-Miró, N. Freeze-Damage Detection in Lemons Using Electrochemical impedance Spectroscopy. Sensors 2019, 19, 4051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar Jha, P.; Xanthakis, E.; Chevallier, S.; Jury, V.; Le-Bail, A. Assessment of freeze damage in fruits and vegetables. Food Res. Int. 2019, 121, 479–496. [Google Scholar] [CrossRef]
- Cubero, S.; Lee, W.S.; Aleixos, N.; Albert, F.; Blasco, J. Automated Systems Based on Machine Vision for Inspecting Citrus Fruits from the Field to Postharvest—a Review. Food Bioprocess Technol. 2016, 9, 1623–1639. [Google Scholar] [CrossRef] [Green Version]
- Lorente, D.; Aleixos, N.; Gómez-Sanchis, J.; Cubero, S.; García-Navarrete, O.L.; Blasco, J. Recent Advances and Applications of Hyperspectral Imaging for Fruit and Vegetable Quality Assessment. Food Bioprocess Technol. 2012, 5, 1121–1142. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Albelda Aparisi, P.; Fortes Sánchez, E.; Contat Rodrigo, L.; Masot Peris, R.; Laguarda-Miró, N. A Rapid Electrochemical Impedance Spectroscopy and Sensor-Based Method for Monitoring Freeze-Damage in Tangerines. IEEE Sens. J. 2021, 21, 12009–12018. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L.; Nieuwoudt, H.; Cronje, P.J.R.; Saeys, W.; Nicolaï, B. NIR Spectroscopy Applications for Internal and External Quality Analysis of Citrus Fruit—A Review. Food Bioprocess Technol. 2012, 5, 425–444. [Google Scholar] [CrossRef]
- Lasia, A. Electrochemical Impedance Spectroscopy and its Applications; Springer: New York, NY, USA, 2014; p. 367. [Google Scholar] [CrossRef]
- Grossi, M.; Riccò, B. Electrical impedance spectroscopy (EIS) for biological analysis and food characterization: A review. J. Sens. Sens. Syst. 2017, 6, 303–325. [Google Scholar] [CrossRef] [Green Version]
- Ando, Y.; Maeda, Y.; Mizutani, K.; Wakatsuki, N.; Hagiwara, S.; Nabetani, H. Impact of blanching and freeze-thaw pretreatment on drying rate of carrot roots in relation to changes in cell membrane function and cell structure. LWT Food Sci. Technol. 2016, 71, 40–46. [Google Scholar] [CrossRef]
- Fuentes, A.; Vázquez-Gutiérrez, J.L.; Pérez-Gago, M.B.; Vonasek, E.; Nitin, N.; Barret, D.M. Application of nondestructive impedance spectroscopy to determination of the effect of temperature on potato microstructure and texture. J. Food Eng. 2014, 133, 16–22. [Google Scholar] [CrossRef]
- Neto, A.F.; Olivier, N.C.; Cordeiro, E.R.; De Oliveira, H.P. Determination of mango ripening degree by electrical impedance spectroscopy. Comput. Electron. Agr. 2017, 143, 222–226. [Google Scholar] [CrossRef]
- Watanabe, T.A. Bio-Electrochemical Calculation Model for Color Decline Kinetics of Bruised “Shine Muscat” Fruit during Storage. Food Bioprocess Technol. 2020, 13, 727–731. [Google Scholar] [CrossRef]
- Damez, J.L.; Clerjon, S.; Abouelkaram, S.; Lepetit, J. Beef meat electrical impedance spectroscopy and anisotropy sensing for non-invasive early assessment of meat ageing. J. Food Eng. 2008, 85, 116–122. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, R.; Zhang, Y.; Liang, Q.; Li, G.; Yang, N.; Xu, P.; Guo, J. Classifying fish freshness according to the relationship between EIS parameters and spoilage stages. J. Food Eng. 2018, 219, 101–110. [Google Scholar] [CrossRef]
- Zhu, S.; Luo, Y.; Hong, H.; Feng, L.; Shen, H. Correlation Between Electrical Conductivity of the Gutted Fish Body and the Quality of Bighead Carp (Aristichthys nobilis) Heads Stored at 0 and 3 °C. Food Bioprocess Technol. 2013, 6, 3068–3075. [Google Scholar] [CrossRef]
- Huang, T.-K.; Chuang, M.-C.; Kung, Y.; Hsieh, B.-C. Impedimetric sensing of honey adulterated with high fructose corn syrup. Food Control 2021, 130, 108326. [Google Scholar] [CrossRef]
- M’hiri, N.; Veys-Renaux, D.; Rocca, E.; Ioannou, I.; Mihoubi Bourdinova, N.; Ghoul, M. Corrosion inhibition of carbon steel in acidic medium by orange peel extract and its main antioxidant compounds. Corros. Sci. 2016, 102, 55–62. [Google Scholar] [CrossRef]
- Malvano, F.; Albanese, D.; Pilloton, R.; Di Mateo, M.; Crescitelly, A. A New Label-Free Impedimetric Affinity Sensor Based on Cholinesterases for Detection of Organophosphorous and Carbamic Pesticides in Food Samples: Impedimetric Versus Amperometric Detection. Food Bioprocess Technol. 2017, 10, 1834–1843. [Google Scholar] [CrossRef]
- Conesa, C.; Ibáñez, J.; Seguí, L.; Fito, P.; Laguarda-Miro, N. An Electrochemical Impedance Spectroscopy System for Monitoring Pineapple Waste Saccharification. Sensors 2016, 16, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conesa, C.; Laguarda-Miró, N.; Fito, P.; Seguí, L. Evaluation of Persimmon (Diospyros kaki Thunb. cv. Rojo Brillante) Industrial Residue as a Source for Value Added Products. Waste Biomass Valori. 2020, 11, 3749–3760. [Google Scholar] [CrossRef]
- Conesa, C.; Gil, L.; Seguí, L.; Fito, P.; Laguarda-Miro, N. Ethanol quantification in pineapple waste by an electrochemical impedance spectroscopy-based system and artificial neural networks. Chemom. Intell. Lab. Syst. 2017, 161, 1–7. [Google Scholar] [CrossRef]
- MacGregor, J.F.; Bruwer, M.J.; Miletic, I.; Cardin, M.; Liu, Z. Latent Variable Models and Big Data in the Process Industries. IFAC-PapersOnLine 2015, 48, 520–524. [Google Scholar] [CrossRef]
- Martinez Gil, P.; Laguarda-Miró, N.; Soto Camino, J.; Masot Peris, R. Glyphosate detection with ammonium nitrate and humic acids as potential interfering substances by pulsed voltammetry technique. Talanta 2013, 115, 702–705. [Google Scholar] [CrossRef]
- Ulrich, C.; Petersson, H.; Sundgren, H.; Björefors, F.; Krantz-Rülcker, C. Simultaneous estimation of soot and diesel contamination in engine oil using electrochemical impedance spectroscopy. Sens. Actuators B Chem. 2007, 127, 613–618. [Google Scholar] [CrossRef]
- Olivati, C.A.; Riul, A.; Balogh, D.T.; Oliveira, O.N.; Ferreira, M. Detection of phenolic compounds using impedance spectroscopy measurements. Bioprocess Biosyst. Eng. 2009, 32, 41–46. [Google Scholar] [CrossRef]
- Górski, Ł.; Sordoń, W.; Ciepiela, F.; Kubiak, W.W.; Jakubowska, M. Voltammetric classifcation of ciders with PLS-DA. Talanta 2016, 146, 231–236. [Google Scholar] [CrossRef]
- Garcia-Breijo, E.; Garrigues, J.; Gil Sanchez, L.; Laguarda-Miró, N. An Embedded Simplified Fuzzy ARTMAP Implemented a Microcontroller for Food Classification. Sensors 2013, 13, 10418–10429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conesa, C.; Seguí, L.; Laguarda-Miró, N.; Fito, P. Microwaves as a pretreatment for enhancing enzymatic hydrolysis of pineapple industrial waste for bioethanol production. Food. Bioprod. Process. 2016, 100, 203–213. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Vijayalakshmi Pai, G.A. Neural Networks, Fuzzy Logic and Genetic Algorithms: Synthesis and Applications; Prentice Hall: New Delhi, India, 2004; p. 456. [Google Scholar]
- Kasuba, T. Simplified fuzzy ARTMAP. AI Expert 1993, 8, 18–25. [Google Scholar]
- Garcia-Breijo, E.; Atkinson, J.; Gil-Sanchez, L.; Masot, R.; Ibañez, J.; Garrigues, J.; Glanc, M.; Laguarda-Miro, N.; Olguin, C. A comparison study of pattern recognition algorithms implemented on a microcontroller for use in an electronic tongue for monitoring drinking waters. Sens. Actuators A Phys. 2011, 2, 570–582. [Google Scholar] [CrossRef]
- Kumar, G.; Buchheit, R.G. Use of Artificial Neural Network Models to Predict Coated Component Life from Short-Term Electrochemical Impedance Spectroscopy Measurements. Corrosion 2008, 64, 241–254. [Google Scholar] [CrossRef]
- Eddahech, A.; Briat, O.; Bertrand, N.; Delétage, J.Y.; Vinassa, J.M. Behavior and state-of-health monitoring of Li-ion batteries using impedance spectroscopy and recurrent neural networks. Int. J. Electron. Power Energy Syst. 2012, 42, 487–494. [Google Scholar] [CrossRef]
- Garcia Breijo, E.; Olguin Pinatti, C.; Masot Peris, R.; Alcañiz Fillol, M.; Martínez-Máñez, R.; Soto Camino, J. TNT detection using a voltammetric electronic tongue based on neural networks. Sensor. Actuat. A-Phys 2013, 192, 1–8. [Google Scholar] [CrossRef]
- Itakura, K.; Saito, Y.; Suzuki, T.; Kondo, N.; Hosoi, F. Estimation of Citrus Maturity with Fluorescence Spectroscopy Using Deep Learning. Horticulturae 2019, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Imaizumi, T.; Tanaka, F.; Hamanaka, D.; Sato, Y.; Uchino, T. Effects of hot water treatment on electrical properties, cell membrane structure and texture of potato tubers. J. Food Eng. 2015, 162, 56–62. [Google Scholar] [CrossRef]
- Ando, Y.; Maeda, Y.; Mizutani, K.; Wakatsuki, N.; Hagiwara, S.; Nabetani, H. Effect of air-dehydration pretreatment before freezing on the electrical impedance characteristics and texture of carrots. J. Food Eng. 2016, 169, 114–121. [Google Scholar] [CrossRef]
- Cole, K.S. Permeability and impermeability of cell membranes for ions. Cold Spring Harb. Symp. Quant. Biol. 1940, 8, 110–122. [Google Scholar] [CrossRef]
- Ibba, P.; Falco, A.; Demelash Abera, B.; Cantarella, G.; Petti, L.; Lugli, P. Bio-impedance and circuit parameters: An analysis for tracking fruit ripening. Postharvest Biol. Technol. 2020, 159, 110978. [Google Scholar] [CrossRef]
- Chowdhury, A.; Bera, T.K.; Ghoshal, D.; Chakraborty, B. Electrical Impedance Variations in Banana Ripening: An Analytical Study with Electrical Impedance Spectroscopy. J. Food Process Eng. 2017, 40, 1–14. [Google Scholar] [CrossRef]
- Watanabe, T.; Ando, Y. Evaluation of heating uniformity and quality attributes during vacuum microwave thawing of frozen apples. LWT 2021, 150, 111997. [Google Scholar] [CrossRef]
- Paredes, A.M. MICROSCOPY|Scanning Electron Microscopy. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 693–701. [Google Scholar] [CrossRef]
- Macdonald, J.R.; Barsoukov, E. Impedance Spectroscopy: Theory, Experiment and Applications, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; p. 595. [Google Scholar]
- Masot, R.; Alcañiz, M.; Fuentes, A.; Schmidt, F.C.; Barat, J.M.; Gil, L.; Baigts, D.; Martínez-Máñez, R.; Soto, J. Design of a low-cost non-destructive system for punctual measurements of salt levels in food products using impedance spectroscopy. Sens. Actuators A Phys. 2010, 158, 217–223. [Google Scholar] [CrossRef]
- Mills, T.C. Seasonal and exponential smoothing. In Applied Time Series Analysis; Mills, T.C., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2019; p. 354. [Google Scholar] [CrossRef]
- Wold, S.; Sjostrom, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Borràs, E.; Ferré, J.; Boqué, R.; Mestres, M.; Aceña, L.; Calvo, A.; Busto, O. Olive oil sensory defects classification with data fusion of instrumental techniques and multivariate analysis (PLS-DA). Food Chem. 2016, 203, 314–322. [Google Scholar] [CrossRef]
- Serrano-Pallicer, E.; Muñoz-Albero, M.; Pérez-Fuster, C.; Masot Peris, R.; Laguarda-Miró, N. Early Detection of Freeze Damage in Navelate Oranges with Electrochemical Impedance Spectroscopy. Sensors 2018, 18, 4503. [Google Scholar] [CrossRef] [Green Version]
- Del Brío, B.M.; Molina, A.S. Redes Neuronales y Sistemas Borrosos, 2nd ed.; Ra-Ma: Madrid, Spain, 2001; p. 399. (In Spanish) [Google Scholar]
- Martínez-Alcántara, B.; Tadeo, F.; Mesejo, C.; Martínez-Cuenca, M.R.; Ruiz, M.; Reig, C.; Former-Giner, M.A.; Iglesias, D.J.; Agustí, M.; Primo-Millo, E. Anatomía de los Cítricos; Universitat Politècnica de València: Valencia, Spain, 2015; p. 173. [Google Scholar]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]
- Zhao, X.; Zhuang, H.; Yoon, S.-C.; Dong, Y.; Wang, W.; Zhao, W. Electrical impedance spectroscopy for quality assessment of meat and fish: A review on basic principles measurement methods, and recent advances. J. Food Qual. 2017, 2017, 6370739. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-W.; Moon, T.; Son, J.-E. Development of Growth Estimation Algorithms for Hydroponic Bell Peppers Using Recurrent Neural Networks. Horticulturae 2021, 7, 284. [Google Scholar] [CrossRef]
- Duarte-Carvajalino, J.M.; Silva-Arero, E.A.; Góez-Vinasco, G.A.; Torres-Delgado, L.M.; Ocampo-Paez, O.D.; Castaño-Marín, A.M. Estimation of Water Stress in Potato Plants Using Hyperspectral Imagery and Machine Learning Algorithms. Horticulturae 2021, 7, 176. [Google Scholar] [CrossRef]
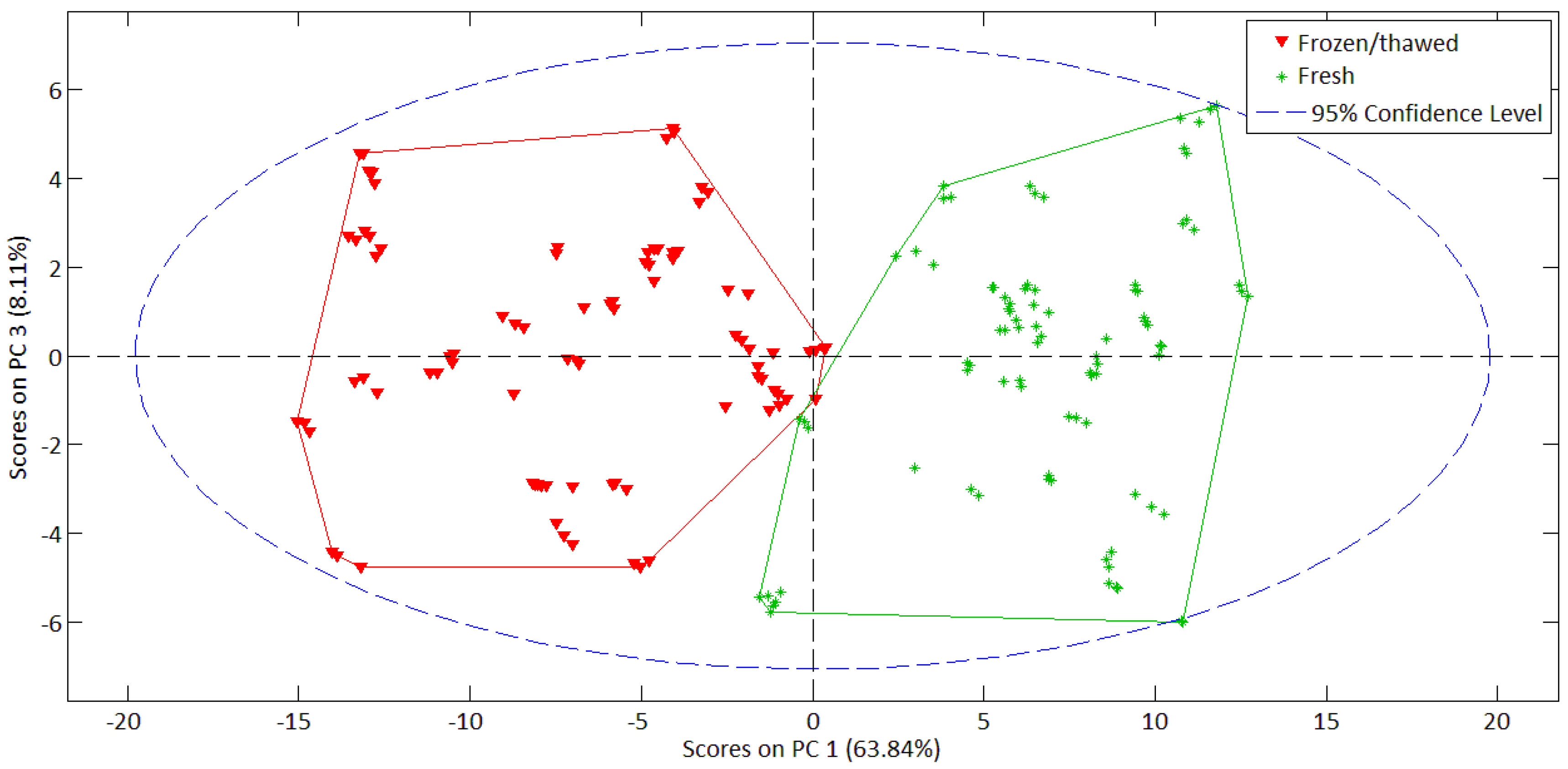
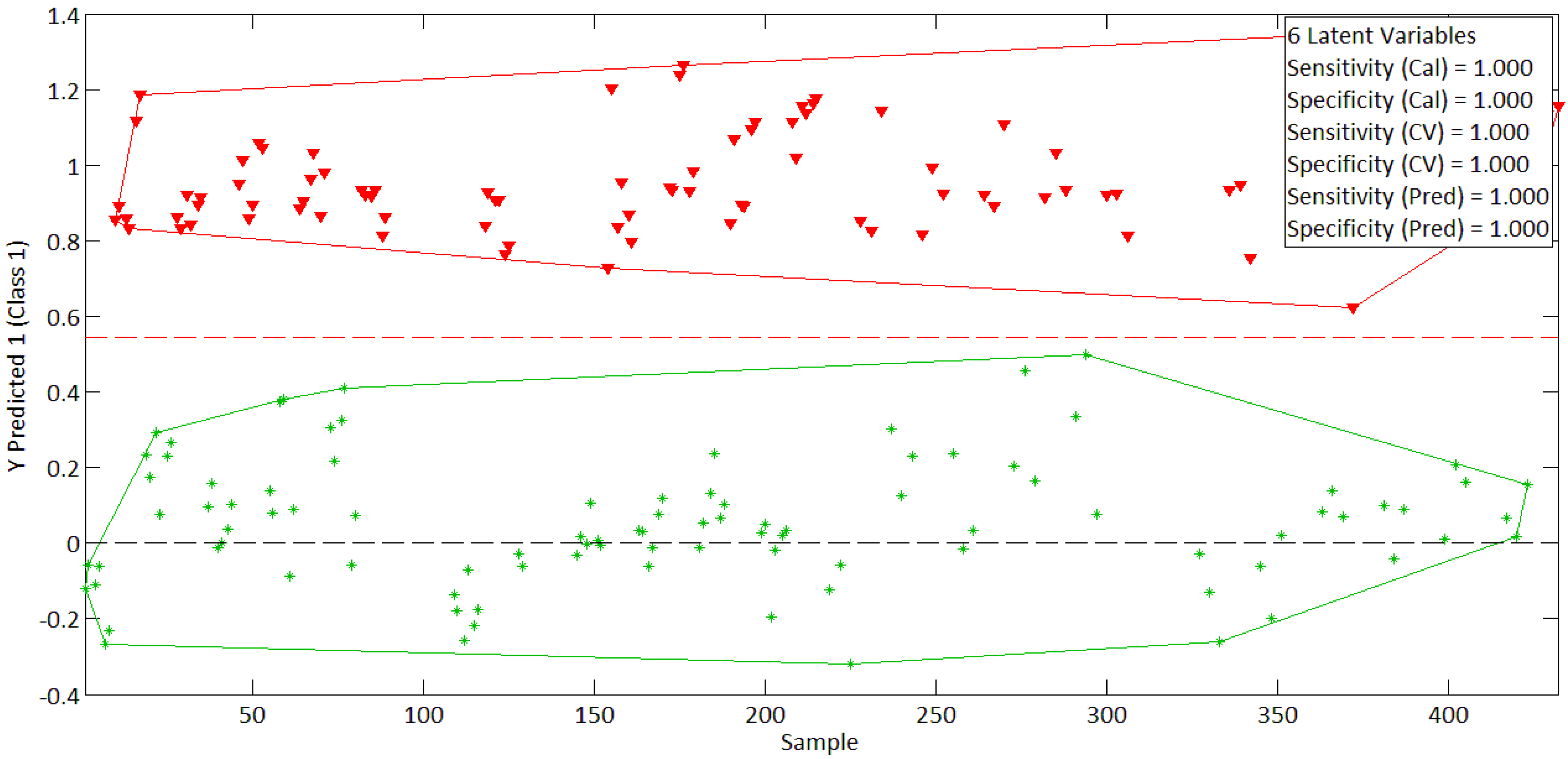
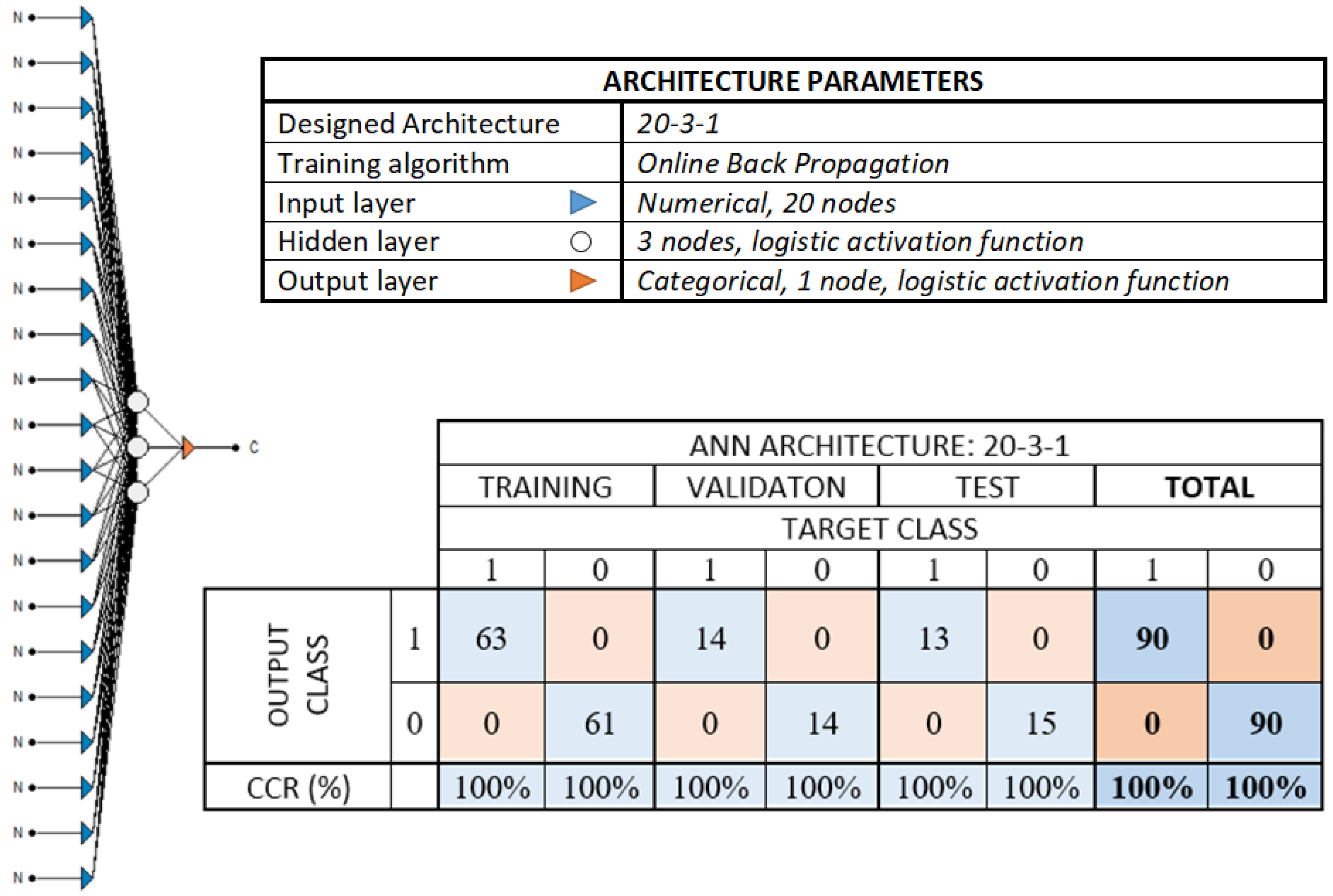
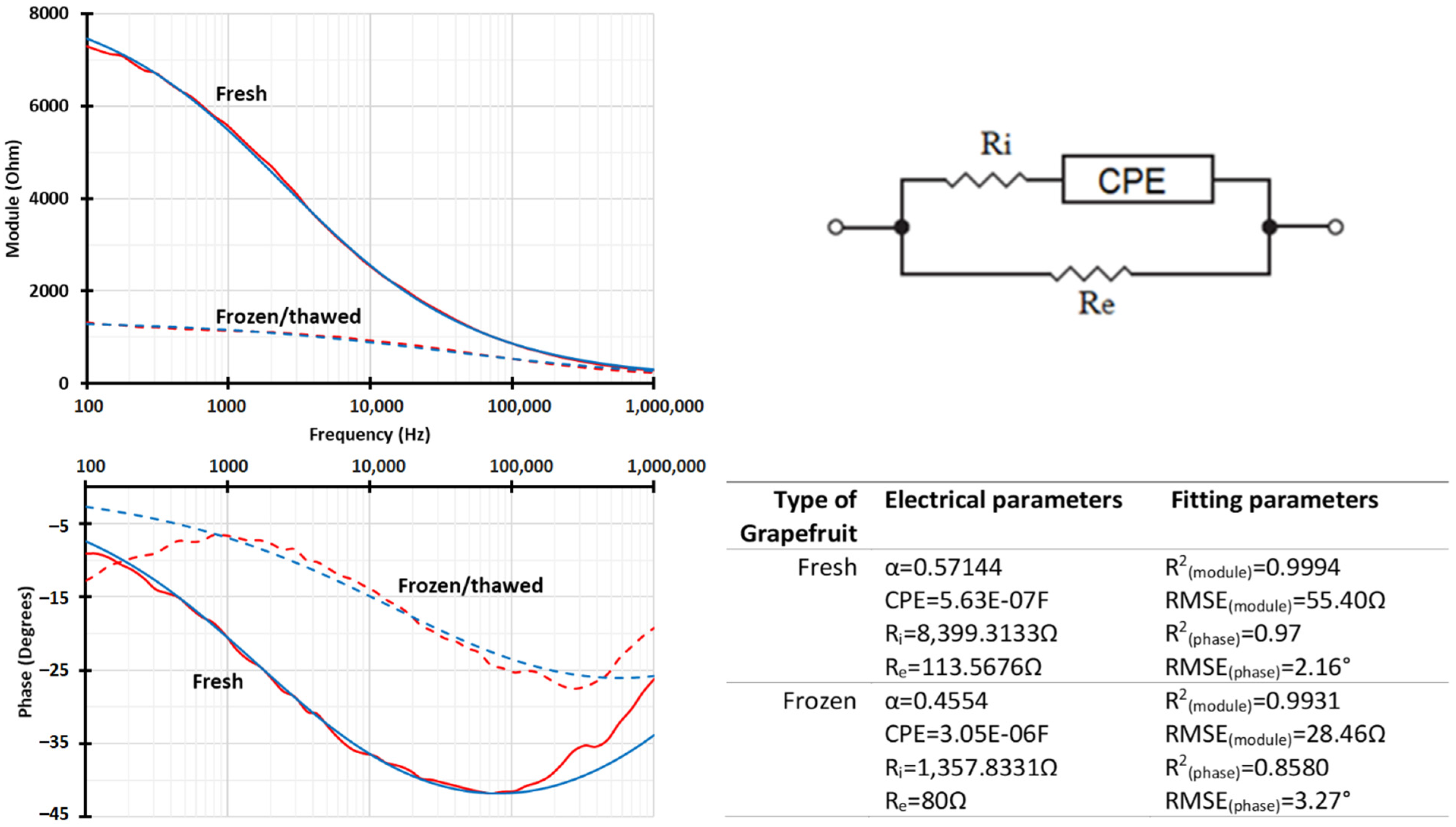
| PCA Analysis | ||||
|---|---|---|---|---|
| N° of PC | 1 | 2 | 3 | 4 |
| Variance (%) | 63.84% | 22.83% | 8.11% | 3.20% |
| Σ of variance (%) | 63.84% | 86.67% | 94.78% | 97.98% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero Fogué, D.; Masot Peris, R.; Ibáñez Civera, J.; Contat Rodrigo, L.; Laguarda-Miro, N. Monitoring Freeze-Damage in Grapefruit by Electric Bioimpedance Spectroscopy and Electric Equivalent Models. Horticulturae 2022, 8, 218. https://doi.org/10.3390/horticulturae8030218
Romero Fogué D, Masot Peris R, Ibáñez Civera J, Contat Rodrigo L, Laguarda-Miro N. Monitoring Freeze-Damage in Grapefruit by Electric Bioimpedance Spectroscopy and Electric Equivalent Models. Horticulturae. 2022; 8(3):218. https://doi.org/10.3390/horticulturae8030218
Chicago/Turabian StyleRomero Fogué, David, Rafael Masot Peris, Javier Ibáñez Civera, Laura Contat Rodrigo, and Nicolas Laguarda-Miro. 2022. "Monitoring Freeze-Damage in Grapefruit by Electric Bioimpedance Spectroscopy and Electric Equivalent Models" Horticulturae 8, no. 3: 218. https://doi.org/10.3390/horticulturae8030218
APA StyleRomero Fogué, D., Masot Peris, R., Ibáñez Civera, J., Contat Rodrigo, L., & Laguarda-Miro, N. (2022). Monitoring Freeze-Damage in Grapefruit by Electric Bioimpedance Spectroscopy and Electric Equivalent Models. Horticulturae, 8(3), 218. https://doi.org/10.3390/horticulturae8030218







