Evaluation of Host Resistance, Hydrated Lime, and Weed Control to Manage Clubroot in Canola
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Analysis
2.2. PCR Analysis
2.3. Statistical Analysis
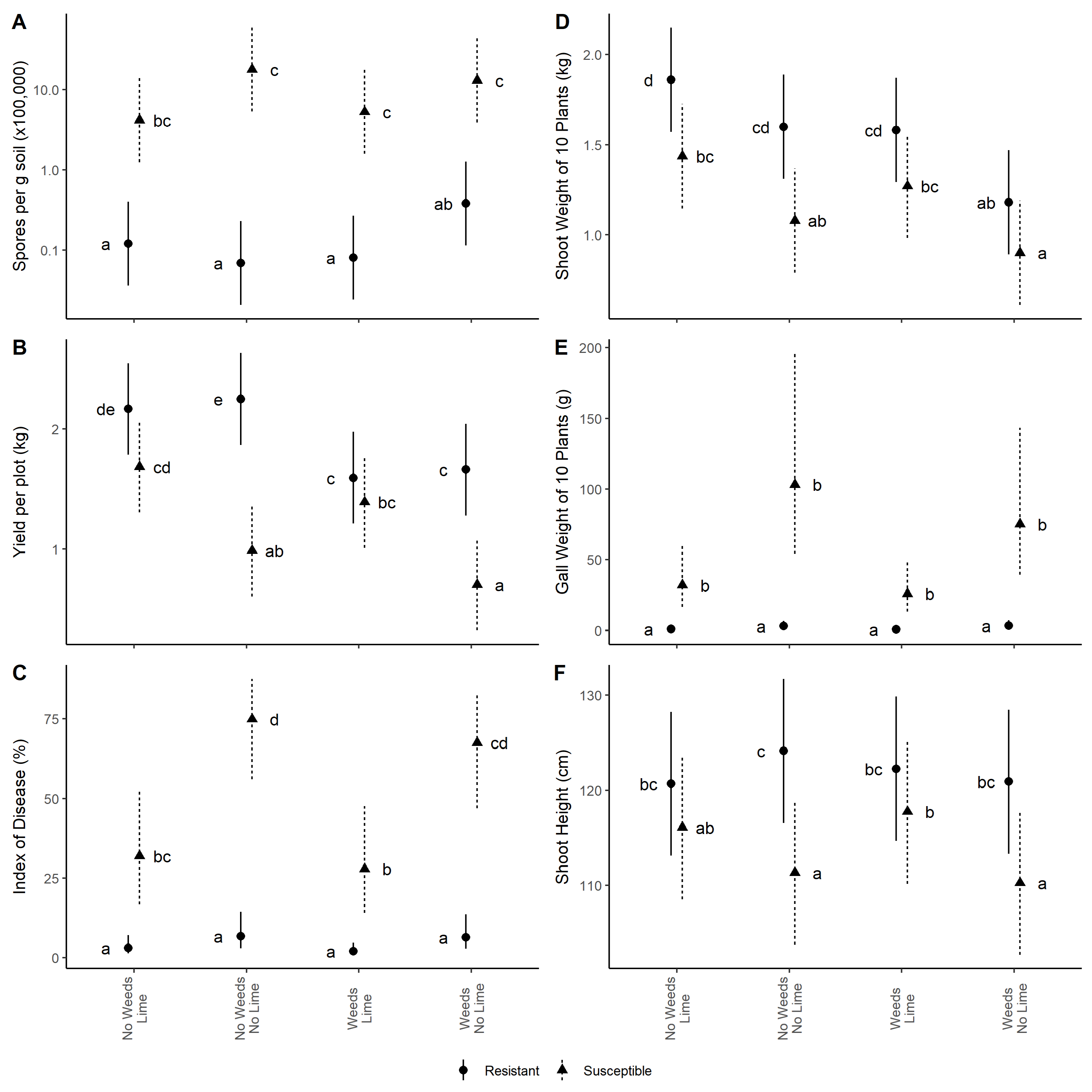
3. Results
3.1. Resting Spore Densities
3.2. Clubroot Severity
3.3. Yield
3.4. Growth Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Gall Weight | Shoot Weight | Shoot Height | |
|---|---|---|---|
| Site-Year | <0.0001 | 201.2408 | 6.1035 |
| Block | <0.0001 | 131.4553 | 2.5843 |
| Residual | 1.1263 | 284.3762 | 4.8779 |
| Index of Disease Rating | Yield | Spore Density | |
| Site-Year | 0.4544 | 268.1289 | 0.3719 |
| Block | 0.0001 | 121.6343 | 0.4428 |
| Residual | 1.2064 | 391.4471 | 1.5088 |
| Treatment | Average Total Weeds per m2 | Proportion of Susceptible Weeds (%) per m2 | ||||
|---|---|---|---|---|---|---|
| Genetics a | Lime b | Weeds c | Site 2 | Site 3 | Site 2 | Site 3 |
| Resistant | Lime | No Weeds | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Resistant | Lime | Weeds | 90.0 ± 35.3 | 93.0 ± 21.1 | 29.4 ± 19.8 | 49.7 ± 27.3 |
| Resistant | No Lime | No Weeds | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Resistant | No Lime | Weeds | 111 ± 14.4 | 145 ± 13.5 | 36.4 ± 7.5 | 46.5 ± 11.0 |
| Susceptible | Lime | No Weeds | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Susceptible | Lime | Weeds | 95.5 ± 36.7 | 119 ± 24.8 | 20.5 ± 8.2 | 33.8 ± 13.5 |
| Susceptible | No Lime | No Weeds | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Susceptible | No Lime | Weeds | 113 ± 27.8 | 109 ± 16.6 | 29.4 ± 15.9 | 46.7 ± 6.2 |
References
- Dixon, G.R. The occurrence and economic impact of Plasmodiophora brassicae and clubroot disease. J. Plant Growth Regul. 2009, 28, 194–202. [Google Scholar] [CrossRef]
- Dixon, G.R. The biology of Plasmodiophora brassicae Wor.—A review of recent advances. In IV International Symposium on Brassicas and XIV Crucifer Genetics Workshop; Lim, Y.P., Ed.; ISHS: Daejeon, Korea, 2006; Volume 1, pp. 271–282. [Google Scholar]
- Howard, R.J.; Strelkov, S.E.; Harding, M.W. Clubroot of cruciferous crops—New perspectives on an old disease. Can. J. Plant Pathol. 2010, 32, 43–57. [Google Scholar] [CrossRef]
- Creelman, D.W. Summary of the prevalence of plant diseases in Canada in 1965. A compilation. Can. Plant Dis. Surv. 1967, 47, 31–71. [Google Scholar]
- Morasse, P.I.; Lafond, J. Attention à la hernie des cruifères dans le canola. Gd. Cult. 1997, 7, 22–23. [Google Scholar]
- Tewari, J.P.; Strelkov, S.E.; Orchard, D.; Hartman, M.; Lange, R.M.; Turkington, T.K. Identification of clubroot ofcrucifers on canola (Brassica napus) in Alberta. Can. J. Plant Pathol. 2005, 27, 143–144. [Google Scholar] [CrossRef]
- Cao, T.; Manolii, V.P.; Strelkov, S.E.; Hwang, S.F.; Howard, R.J. Virulence and spread of Plasmodiophora brassicae [clubroot] in Alberta, Canada. Can. J. Plant Pathol. 2009, 31, 321–329. [Google Scholar] [CrossRef]
- Strelkov, S.E.; Hwang, S.F. Clubroot in the Canadian canola crop: 10 years into the outbreak. Can. J. Plant Pathol. 2014, 36, 27–36. [Google Scholar] [CrossRef]
- Froese, R.D.; Derksen, H.; Guo, X.; McLaren, D.L. Monitoring and occurrence of clubroot in Manitoba in 2018. The Canadian Phytopathological Society Canadian Plant Disease Survey: Disease Highlights. Can. J. Plant Pathol. 2019, 41, 179. [Google Scholar]
- Ziesman, B.; Avila, R.; Ursu, K.; Kwasnicki, J.; Roszell, L.; Baraniecki, C.; Johnson, B.; Fennig, C.; Makohoniuk, K.; Junek, C.; et al. 2018 Clubroot survey and known occurrence of clubroot in Saskatchewan. The Canadian Phytopathological Society Canadian Plant Disease Survey: Disease Highlights. Can. J. Plant Pathol. 2019, 41, 159–161. [Google Scholar]
- Pageau, D.; Lajeunesse, J.; Lafond, J. Impact of clubroot [Plasmodiophora brassicae] on the yield and quality of canola. Can. J. Plant Pathol. 2006, 28, 137–143. [Google Scholar] [CrossRef]
- Strelkov, S.E.; Manolii, V.P.; Cao, T.; Xue, S.; Hwang, S.F. Pathotype classification of Plasmodiophora brassicae and its occurrence in Brassica napus in Alberta, Canada. J. Phytopathol. 2007, 155, 706–712. [Google Scholar] [CrossRef]
- The Economic Impact of Canola on the Canadian Economy by LMC International. Available online: https://www.canolacouncil.org/download/215/pages/5255/lmc_canola_10-year_impact_study_-_canada_final_dec_2016 (accessed on 5 September 2019).
- Rahman, H.; Peng, G.; Yu, F.; Falk, K.C.; Kulkarni, M.; Selvaraj, G. Genetics and breeding for clubroot resistance in Canadian spring canola (Brassica napus L.). Can. J. Plant Pathol. 2014, 36, 122–134. [Google Scholar] [CrossRef]
- Hwang, S.-F.; Strelkov, S.E.; Feng, J.; Gossen, B.D.; Howard, R.J. Plasmodiophora brassicae: A review of an emerging pathogen of the Canadian canola (Brassica napus) crop. Mol. Plant Pathol. 2012, 13, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Strelkov, S.E.; Hwang, S.-F.; Manolii, V.P.; Cao, T.; Feindel, D. Emergence of new virulence phenotypes of Plasmodiophora brassicae on canola (Brassica napus) in Alberta, Canada. Eur. J. Plant Pathol. 2016, 145, 517–529. [Google Scholar] [CrossRef]
- Strelkov, S.E.; Hwang, S.-F.; Manolii, V.P.; Cao, T.; Fredua-Agyeman, R.; Harding, M.W.; Peng, G.; Gossen, B.D.; Mcdonald, M.R.; Feindel, D. Virulence and pathotype classification of Plasmodiophora brassicae populations collected from clubroot resistant canola (Brassica napus) in Canada. Can. J. Plant Pathol. 2018, 40, 284–298. [Google Scholar] [CrossRef]
- Hollman, K.B.; Hwang, S.-F.; Manolii, V.P.; Strelkov, S.E. Pathotypes of Plasmodiophora brassicae collected from clubroot resistant canola (Brassica napus L.) cultivars in western Canada in 2017–2018. Can. J. Plant Pathol. 2021, 43, 622–630. [Google Scholar] [CrossRef]
- Fox, N.M.; Hwang, S.-F.; Manolii, V.P.; Turnbull, G.; Strelkov, S.E. Evaluation of Lime Products for Clubroot (Plasmodiophora brassicae) Management in Canola (Brassica napus) Cropping Systems. Can. J. Plant Pathol. 2021, 44, 21–38. [Google Scholar] [CrossRef]
- Karling, J.S. The Plasmodiophorales, 1st ed.; John, S. Karling: New York, NY, USA, 1942; pp. 110–114. [Google Scholar]
- Zamani-Noor, N.; Rodemann, B. Reducing the build-up of Plasmodiophora brassicae inoculum by early management of oilseed rape volunteers. Plant Pathol. 2018, 67, 426–432. [Google Scholar] [CrossRef]
- Alberta Agriculture, Food and Rural Development, Agri-Facts Liming. Available online: https://open.alberta.ca/dataset/8a143d84-86a5-45b0-a432-d35f269de006/resource/d75bfaef-e123-4bfe-bea4-500e1e70e46e/download/1996-534-1.pdf (accessed on 1 December 2020).
- Penney, D. Crop Response to Liming Acid Soils of Alberta and Northeastern British Columbia. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 1973. [Google Scholar]
- Kuginuki, Y.; Hiroaki, Y.; Hirai, M. Variation in virulence of Plasmodiophora brassicae in Japan tested with clubroot-resistant cultivars of Chinese cabbage (Brassica rapa L. ssp. pekinensis). Eur. J. Plant Pathol. 1999, 105, 327–332. [Google Scholar] [CrossRef]
- Horiuchi, S.; Hori, M. A simple greenhouse technique for obtaining high levels of clubroot incidence. Bull. Chugoku Natl. Agric. Exp. Stn. Ser. E 1980, 17, 33–55. [Google Scholar]
- Strelkov, S.E.; Tewari, J.P.; Smith-Degenhardt, E. Characterization of Plasmodiophora brassicae populations from Alberta, Canada. Can. J. Plant Pathol. 2006, 28, 467–474. [Google Scholar] [CrossRef]
- Cao, T.; Tewari, J.; Strelkov, S.E. Molecular detection of Plasmodiophora brassicae, causal agent of clubroot of crucifers, in plant and soil. Plant Dis. 2007, 91, 80–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rennie, D.C.; Manolii, V.P.; Cao, T.; Hwang, S.F.; Howard, R.J.; Strelkov, S.E. Direct evidence of surface infestation of seeds and tubers by Plasmodiophora brassicae and quantification of spore loads. Plant Pathol. 2011, 60, 811–819. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/.
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. R Core Team. Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-153. 2021. Available online: https://CRAN.R-project.org/package=nlme/.
- Russell, V. Lenth. Emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.7.2. 2022. Available online: https://CRAN.R-project.org/package=emmeans.
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Lüdecke, D. ggeffects: Tidy Data Frames of Marginal Effects from Regression Models. J. Open Source Softw. 2018, 3, 772. [Google Scholar] [CrossRef] [Green Version]
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. R Package Version 0.4.0. 2020. Available online: https://CRAN.R-project.org/package=ggpubr.
- Hwang, S.-F.; Ahmed, H.U.; Strelkov, S.E.; Gossen, B.D.; Turnbull, G.D.; Peng, G.; Howard, R.J. Seedling age and inoculum density affect clubroot severity and seed yield in canola. Can. J. Plant Sci. 2011, 91, 183–190. [Google Scholar] [CrossRef]
- Peng, G.; Lahlali, R.; Hwang, S.-F.; Pageau, D.; Hynes, R.K.; McDonald, M.-R.; Gossen, B.D.; Strelkov, S.E. Special Issue: Crop rotation, cultivar resistance, and fungicides/biofungicides for managing clubroot (Plasmodiophora brassicae) on canola. Can. J. Plant Pathol. 2014, 36, 99–112. [Google Scholar] [CrossRef]
- Xue, S.; Cao, T.; Howard, R.J.; Hwang, S.-F.; Strelkov, S.E. Isolation and variation in virulence of single-spore isolates of Plasmodiophora brassicae from Canada. Plant Dis. 2008, 92, 456–462. [Google Scholar] [CrossRef] [Green Version]
- Strelkov, S.E.; Manolii, V.P.; Harding, M.W.; Daniels, G.C.; Nuffer, P.; Aigu, Y.; Hwang, S.-F. The Occurrence and spread of clubroot on canola in Alberta in 2019. Can. J. Plant Pathol. 2020, 42, 117–120. [Google Scholar]
- Fredua-Agyeman, R.; Hwang, S.F.; Strelkov, S.E.; Zhou, Q.; Feindel, D. Potential loss of clubroot resistance genes from donor parent Brassica rapa subsp. rapifera (ECD 04) during doubled haploid production. Plant Pathol. 2018, 67, 892–901. [Google Scholar] [CrossRef]
- Canola Council of Canada. Clubroot. Available online: https://www.canolacouncil.org/canola-encyclopedia/diseases/clubroot/ (accessed on 10 December 2020).
- Hwang, S.-F.; Strelkov, S.E.; Ahmed, H.U.; Manolii, V.P.; Zhou, Q.; Fu, H.; Turnbull, G.; Freuda-Agyeman, R.; Feindel, D. Virulence and inoculum density-dependent interactions between clubroot resistant canola (Brassica napus) and Plasmodiophora brassicae. Plant Pathol. 2017, 66, 1318–1328. [Google Scholar] [CrossRef]
- Ernst, T.W.; Kher, S.; Stanton, D.; Rennie, D.C.; Hwang, S.-F.; Strelkov, S.E. Plasmodiophora brassicae resting spore dynamics in clubroot resistant canola (Brassica napus) cropping systems. Plant Pathol. 2019, 68, 399–408. [Google Scholar] [CrossRef]
- Tremblay, N.; Bélec, C.; Coulombe, J.; Godin, C. Evaluation of calcium cyanamide and liming for control of clubroot disease in cauliflower. Crop Prot. 2005, 24, 798–803. [Google Scholar] [CrossRef]
- Binkley, D.; Vitousek, P. Soil Nutrient Availability. In Plant Physiological Ecology; Springer: Dordrecht, The Netherlands, 1991; pp. 75–96. [Google Scholar]
- Cornell University, Agronomy Fact Sheet #5: Soil pH for Field Crops. Available online: http://nmsp.cals.cornell.edu/guidelines/factsheets.html (accessed on 11 October 2020).
- Oerke, E.C. Crop Losses to Pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Niwa, R.; Nomura, Y.; Osaki, M.; Ezawa, T. Suppression of clubroot disease under neutral pH caused by inhibition of spore germination of Plasmodiophora brassicae in the rhizosphere. Plant Pathol. 2008, 57, 445–452. [Google Scholar] [CrossRef]
- Donald, C.; Porter, I. Integrated control of clubroot. J. Plant Growth Regul. 2009, 28, 289. [Google Scholar] [CrossRef]
- Dixon, G.R. Clubroot (Plasmodiophora brassicae Woronin)—An agricultural and biological challenge worldwide. Can. J. Plant Pathol. 2014, 36, 5–18. [Google Scholar] [CrossRef]
- Hwang, S.-F.; Howard, R.J.; Strelkov, S.E.; Gossen, B.D.; Peng, G. Management of clubroot (Plasmodiophora brassicae) on canola (Brassica napus) in western Canada. Can. J. Plant Pathol. 2014, 36, 49–65. [Google Scholar] [CrossRef]
| Gall Weight | Shoot Weight | Shoot Height | |||||||
| DF | F-Value | p-Value | DF | F-Value | p-Value | DF | F-Value | p-Value | |
| Genetics a | 1,77 | 162.77 | <0.0001 | 1,77 | 43.49 | <0.0001 | 1,77 | 66.72 | <0.0001 |
| Lime b | 1,77 | 17.65 | 0.0001 | 1,77 | 35.68 | <0.0001 | 1,77 | 6.47 | 0.0130 |
| Weeds c | 1,77 | 0.54 | 0.4654 | 1,77 | 20.10 | <0.0001 | 1,77 | 0.07 | 0.7863 |
| Genetics:Lime | 1,77 | 0.31 | 0.5772 | 1,77 | 0.08 | 0.7838 | 1,77 | 12.98 | 0.0006 |
| Genetics:Weeds | 1,77 | 0.15 | 0.6971 | 1,77 | 2.31 | 0.1323 | 1,77 | 0.31 | 0.5766 |
| Lime:Weeds | 1,77 | 0.04 | 0.8409 | 1,77 | 0.43 | 0.5144 | 1,77 | 3.58 | 0.0623 |
| Genetics:Lime:Weeds | 1,77 | 0.19 | 0.6632 | 1,77 | 0.30 | 0.5882 | 1,77 | 0.27 | 0.6024 |
| Index of Disease Rating | Yield | Spore Density (Sites 2 & 3 only) | |||||||
| DF | F-value | p-value | DF | F-value | p-value | DF | F-value | p-value | |
| Genetics a | 1,77 | 167.96 | <0.0001 | 1,77 | 82.90 | <0.0001 | 1,49 | 124.27 | <0.0001 |
| Lime b | 1,77 | 31.28 | <0.0001 | 1,77 | 15.00 | 0.0002 | 1,49 | 4.98 | 0.0302 |
| Weeds c | 1,77 | 1.13 | 0.2916 | 1,77 | 29.70 | <0.0001 | 1,49 | 0.66 | 0.4202 |
| Genetics:Lime | 1,77 | 2.49 | 0.1187 | 1,77 | 23.12 | <0.0001 | 1,49 | 0.80 | 0.3743 |
| Genetics:Weeds | 1,77 | 0.01 | 0.9432 | 1,77 | 3.33 | 0.0718 | 1,49 | 0.84 | 0.3642 |
| Lime:Weeds | 1,77 | 0.04 | 0.8417 | 1,77 | <0.01 | 0.9847 | 1,49 | 1.09 | 0.3011 |
| Genetics:Lime:Weeds | 1,77 | 0.30 | 0.5874 | 1,77 | 0.01 | 0.9408 | 1,49 | 3.14 | 0.0825 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hennig, B.C.; Hwang, S.-F.; Manolii, V.P.; Turnbull, G.; Robinson, S.V.J.; Strelkov, S.E. Evaluation of Host Resistance, Hydrated Lime, and Weed Control to Manage Clubroot in Canola. Horticulturae 2022, 8, 215. https://doi.org/10.3390/horticulturae8030215
Hennig BC, Hwang S-F, Manolii VP, Turnbull G, Robinson SVJ, Strelkov SE. Evaluation of Host Resistance, Hydrated Lime, and Weed Control to Manage Clubroot in Canola. Horticulturae. 2022; 8(3):215. https://doi.org/10.3390/horticulturae8030215
Chicago/Turabian StyleHennig, Brittany C., Sheau-Fang Hwang, Victor P. Manolii, George Turnbull, Samuel V. J. Robinson, and Stephen E. Strelkov. 2022. "Evaluation of Host Resistance, Hydrated Lime, and Weed Control to Manage Clubroot in Canola" Horticulturae 8, no. 3: 215. https://doi.org/10.3390/horticulturae8030215
APA StyleHennig, B. C., Hwang, S.-F., Manolii, V. P., Turnbull, G., Robinson, S. V. J., & Strelkov, S. E. (2022). Evaluation of Host Resistance, Hydrated Lime, and Weed Control to Manage Clubroot in Canola. Horticulturae, 8(3), 215. https://doi.org/10.3390/horticulturae8030215






