The Occurrence of Clubroot in Colombia and Its Relationship with Climate and Agronomic Practices
Abstract
:1. Introduction
2. Materials and Methods
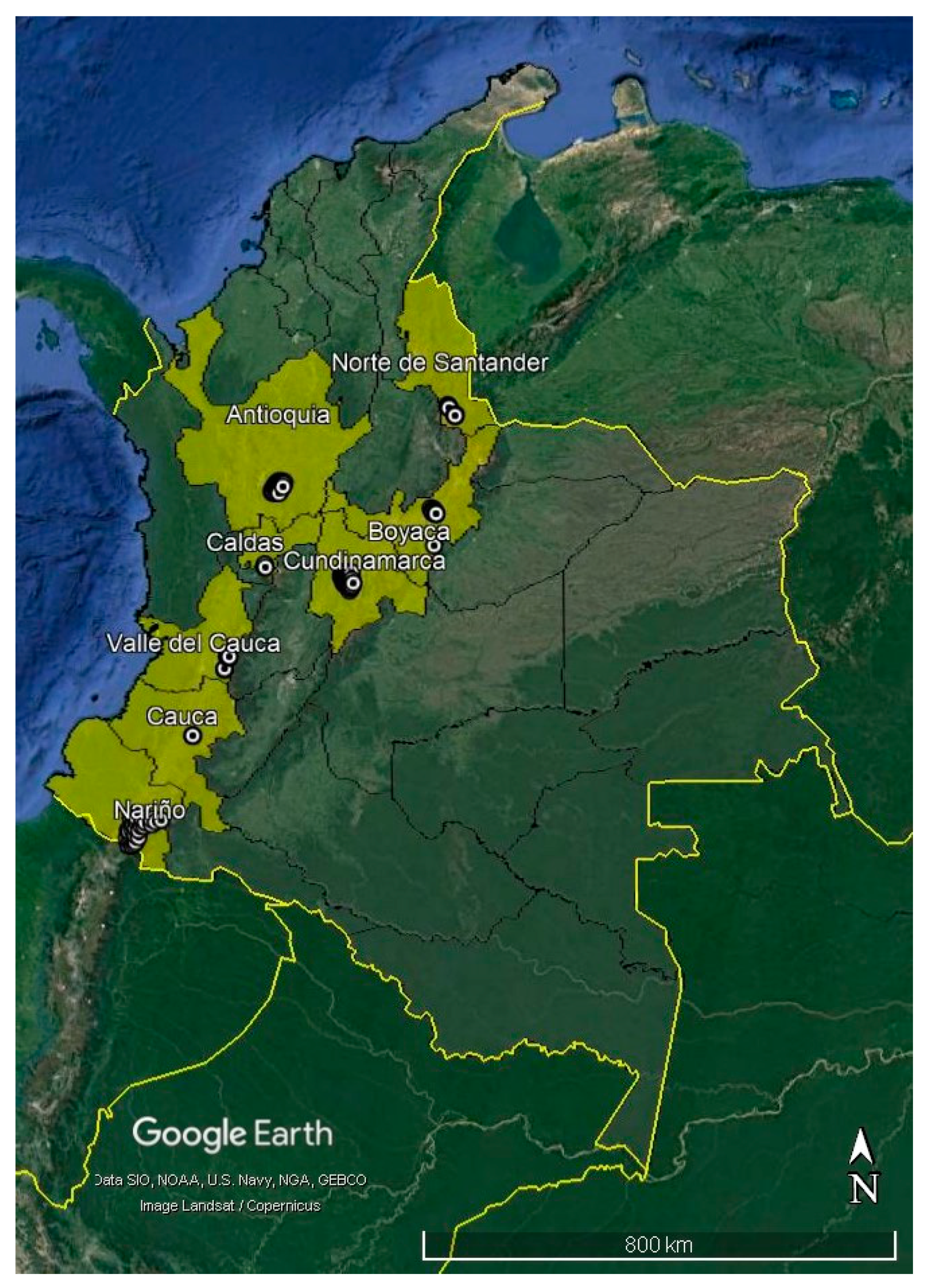
2.1. Sampling
2.2. Clubroot Prevalence
2.3. Soil Samples
2.4. Crop Management Information
2.5. Climatic Information
2.6. DNA Extraction and P. brassicae Quantification
2.7. Statistical Analysis
3. Results
3.1. Clubroot Infestation
3.2. Inoculum Density and Relationship with Clubroot Infestation
3.3. Effect of Management Practices and Weather Conditions on Field Infestation and Inoculum Density
3.4. Climatic Information
3.5. Cruciferous Crops in Colombia and Management Practices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dixon, G.R. The Occurrence and Economic Impact of Plasmodiophora brassicae and Clubroot Disease. J. Plant Growth Regul. 2009, 28, 194–202. [Google Scholar] [CrossRef]
- Kageyama, K.; Asano, T. Life Cycle of Plasmodiophora brassicae. J. Plant Growth Regul. 2009, 28, 203. [Google Scholar] [CrossRef]
- Murakami, H.; Tsushima, S.; Akimoto, T.; Kuroyanagi, Y.; Shishido, Y. Quantitative studies on the relationship between plowing into soil of clubbed roots of preceding crops caused by Plasmodiophora brassicae and disease severity in succeeding crops. Soil Sci. Plant Nutr. 2004, 50, 1307–1311. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.F.; Ahmed, H.U.; Zhou, Q.; Rashid, A.; Strelkov, S.E.; Gossen, B.D.; Peng, G.; Turnbull, G.D. Effect of susceptible and resistant canola plants on Plasmodiophora brassicae resting spore populations in the soil. Plant Pathol. 2013, 62, 404–412. [Google Scholar] [CrossRef]
- Aigu, Y.; Laperche, A.; Mendes, J.; Lariagon, C.; Guichard, S.; Gravot, A.; Manzanares-Dauleux, M.J. Nitrogen supply exerts a major/minor switch between two QTLs controlling Plasmodiophora brassicae spore content in rapeseed. Plant Pathol. 2018, 67, 1574–1581. [Google Scholar] [CrossRef]
- Botero-Ramírez, A.; Laperche, A.; Guichard, S.; Jubault, M.; Gravot, A.; Strelkov, S.E.; Manzanares-Dauleux, M.J. Clubroot Symptoms and Resting Spore Production in a Doubled Haploid Population of Oilseed Rape (Brassica napus) Are Controlled by Four Main QTLs. Front. Plant Sci. 2020, 11, 604527. [Google Scholar] [CrossRef]
- Wallenhammar, A.-C. Prevalence of Plasmodiophora brassicae in a spring oilseed rape growing area in central Sweden and factors influencing soil infestation levels. Plant Pathol. 1996, 45, 710–719. [Google Scholar] [CrossRef]
- Strelkov, S.E.; Hwang, S.-F. Clubroot in the Canadian canola crop: 10 years into the outbreak. Can. J. Plant Pathol. 2014, 36, 27–36. [Google Scholar] [CrossRef]
- Agrios, G. Plant Pathology, 5th ed.; Elsevier: Cambridge, MA, USA, 2005. [Google Scholar]
- Scholthof, K.-B.G. The disease triangle: Pathogens, the environment and society. Nat. Rev. Microbiol. 2007, 5, 152–156. [Google Scholar] [CrossRef]
- Diederichsen, E.; Frauen, M.; Linders, E.G.A.; Hatakeyama, K.; Hirai, M. Status and Perspectives of Clubroot Resistance Breeding in Crucifer Crops. J. Plant Growth Regul. 2009, 28, 265–281. [Google Scholar] [CrossRef]
- Syngenta Colombia Clapton. Available online: https://www.syngenta.com.co/clapton (accessed on 6 January 2022).
- Syngenta Colombia Clarify. Available online: https://www.syngenta.com.co/clarify (accessed on 6 January 2022).
- Ayers, G.W. Studies on the Life History of the Club Root Organism, Plasmodiophora brassicae. Can. J. Res. 1944, 22, 143–149. [Google Scholar] [CrossRef]
- Thuma, B.A.; Rowe, R.C.; Madden, L.V. Relationships of Soil Temperature and Moisture to Clubroot (Plasmodiophora brassicae) Severity on Radish in Organic Soil. Plant Dis. 1983, 67, 758–762. [Google Scholar] [CrossRef]
- Sharma, K.; Gossen, B.D.; McDonald, M.R. Effect of temperature on primary infection by Plasmodiophora brassicae and initiation of clubroot symptoms. Plant Pathol. 2011, 60, 830–838. [Google Scholar] [CrossRef]
- Sharma, K.; Gossen, B.D.; McDonald, M.R. Effect of temperature on cortical infection by Plasmodiophora brassicae and clubroot severity. Phytopathology 2011, 101, 1424–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gossen, B.D.; Adhikari, K.K.C.; McDonald, M.R. Effects of temperature on infection and subsequent development of clubroot under controlled conditions. Plant Pathol. 2012, 61, 593–599. [Google Scholar] [CrossRef]
- Gossen, B.D.; Kasinathan, H.; Cao, T.; Manolii, V.P.; Strelkov, S.E.; Hwang, S.-F.; McDonald, M.R. Interaction of pH and temperature affect infection and symptom development of Plasmodiophora brassicae in canola. Can. J. Plant Pathol. 2013, 35, 294–303. [Google Scholar] [CrossRef]
- Luo, H.; Chen, G.; Liu, C.; Huang, Y.; Xiao, C. An improved culture solution technique for Plasmodiophora brassicae infection and the dynamic infection in the root hair. Australas. Plant Pathol. 2014, 43, 53–60. [Google Scholar] [CrossRef]
- Samuel, G.; Garrett, S.D. The infected root-hair count for estimating the activity of Plasmodiophora brassicae Woron. in the soil. Ann. Appl. Biol. 1945, 32, 96–101. [Google Scholar] [CrossRef]
- Hamilton, H.; Crête, R. Influence of soil moisture, soil pH, and liming sources on the incidence of clubroot, germination and growth of cabbage produced in mineral and organic soils under controlled conditions. Can. J. Plant Sci. 1978, 58, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Dobson, R.; Gabrielson, R.L.; Baker, A.S. Soil Water Matric Potential Requirements for Root-Hair and Cortical Infection of Chinese Cabbage by Plasmodiophora brassicae. Phytopathology 1982, 72, 1598–1600. [Google Scholar] [CrossRef]
- Narisawa, K.; Shimura, M.; Usuki, F.; Fukuhara, S.; Hashiba, T. Effects of Pathogen Density, Soil Moisture, and Soil pH on Biological Control of Clubroot in Chinese Cabbage by Heteroconium chaetospira. Plant Dis. 2005, 89, 285–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, D.F.; Campell, R.N.; Greathead, A.S. Thermal inactivation of Plasmodiophora brassicae Woron. and its attempted control by solarization in the Salinas Valley of California. Crop Prot. 1983, 2, 325–333. [Google Scholar] [CrossRef]
- Webster, M.A.; Dixon, G.R. Calcium, pH and inoculum concentration influencing colonization by Plasmodiophora brassicae. Mycol. Res. 1991, 95, 64–73. [Google Scholar] [CrossRef]
- Webster, M.A.; Dixon, G.R. Boron, pH and inoculum concentration influencing colonization by Plasmodiophora brassicae. Mycol. Res. 1991, 95, 74–79. [Google Scholar] [CrossRef]
- Donald, E.C.; Porter, I.J. A sand—Solution culture technique used to observe the effect of calcium and pH on root hair and cortical stages of infection by Plasmodiophora brassicae. Australas. Plant Pathol. 2004, 33, 585–589. [Google Scholar] [CrossRef]
- Colhoun, J. Observations on the Incidence of Club-Root Disease of Brassicae in Limed Soils in Relation to Temperature. Ann. Appl. Biol. 1953, 40, 639–644. [Google Scholar] [CrossRef]
- Fletcher, J.T.; Hims, M.J.; Archer, F.C.; Brown, A. Effects of adding calcium and sodium salts to field soils on the incidence of clubroot. Ann. Appl. Biol. 1982, 100, 245–251. [Google Scholar] [CrossRef]
- Myers, D.F.; Campbell, R.N. Lime and the Control of Clubroot of Crucifers: Effects of pH, Calcium, Magnesium, and Their Interactions. Phytopathology 1985, 75, 670. [Google Scholar] [CrossRef]
- Baker, R. Analyses Involving Inoculum Density of Soil-Borne Plant Pathogens in Epidemiology. Phytopathology 1971, 61, 1280. [Google Scholar] [CrossRef]
- Voorrips, R.E. Production, characterization and interaction of single-spore isolates of Plasmodiophora brassicae. Eur. J. Plant Pathol. 1996, 102, 377–383. [Google Scholar] [CrossRef]
- Murakami, H.; Tsushima, S.; Shishido, Y. Factors affecting the pattern of the dose response curve of clubroot disease caused by Plasmodiophora brassicae. Soil Sci. Plant Nutr. 2002, 48, 421–427. [Google Scholar] [CrossRef]
- Botero-Ramírez, A.; Hwang, S.-F.; Strelkov, S.E. Effect of clubroot (Plasmodiophora brassicae) on yield of canola (Brassica napus). Can. J. Plant Pathol. 2022, 44, 372–385. [Google Scholar] [CrossRef]
- Hwang, S.F.; Strelkov, S.E.; Ahmed, H.U.; Manolii, V.P.; Zhou, Q.; Fu, H.; Turnbull, G.; Fredua-Agyeman, R.; Feindel, D. Virulence and inoculum density-dependent interactions between clubroot resistant canola (Brassica napus) and Plasmodiophora brassicae. Plant Pathol. 2017, 66, 1318–1328. [Google Scholar] [CrossRef]
- Faggian, R.; Strelkov, S.E. Detection and Measurement of Plasmodiophora brassicae. J. Plant Growth Regul. 2009, 28, 282–288. [Google Scholar] [CrossRef]
- Howard, R.J.; Strelkov, S.E.; Harding, M.W. Clubroot of cruciferous crops—New perspectives on an old disease. Can. J. Plant Pathol. 2010, 32, 43–57. [Google Scholar] [CrossRef]
- Cao, T.; Tewari, J.; Strelkov, S.E. Molecular Detection of Plasmodiophora brassicae, Causal Agent of Clubroot of Crucifers, in Plant and Soil. Plant Dis. 2007, 91, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Rennie, D.C.; Manolii, V.P.; Cao, T.; Hwang, S.F.; Howard, R.J.; Strelkov, S.E. Direct evidence of surface infestation of seeds and tubers by Plasmodiophora brassicae and quantification of spore loads. Plant Pathol. 2011, 60, 811–819. [Google Scholar] [CrossRef]
- Wallenhammar, A.-C.; Almquist, C.; Söderström, M.; Jonsson, A. In-field distribution of Plasmodiophora brassicae measured using quantitative real-time PCR. Plant Pathol. 2012, 61, 16–28. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Shi, Y.; Xie, X.; A-li, C.; Li, B. Development of A Real-Time PCR Assay for Plasmodiophora brassicae and Its Detection in Soil Samples. J. Integr. Agric. 2013, 12, 1799–1806. [Google Scholar] [CrossRef]
- Deora, A.; Gossen, B.D.; Amirsadeghi, S.; McDonald, M.R. A Multiplex qPCR Assay for Detection and Quantification of Plasmodiophora brassicae in Soil. Plant Dis. 2015, 99, 1002–1009. [Google Scholar] [CrossRef] [Green Version]
- Gossen, B.D.; Al-Daoud, F.; Dumonceaux, T.; Dalton, J.A.; Peng, G.; Pageau, D.; McDonald, M.R. Comparison of techniques for estimation of resting spores of Plasmodiophora brassicae in soil. Plant Pathol. 2019, 68, 954–961. [Google Scholar] [CrossRef]
- Botero, A.; García, C.; Gossen, B.D.; Strelkov, S.E.; Todd, C.D.; Bonham-Smith, P.C.; Pérez-López, E. Clubroot disease in Latin America: Distribution and management strategies. Plant Pathol. 2019, 68, 827–833. [Google Scholar] [CrossRef] [Green Version]
- Ministerio de Agricultura y Desarrollo Rural Estadísticas Agrícolas: Área, Producción, Rendimiento y Participación. Available online: http://www.agronet.gov.co/estadistica/Paginas/default.aspx (accessed on 20 August 2018).
- Gómez, C. Estimación de las Pérdidas en Rendimiento Ocasionadas por Plasmodiophora brassicae Woron en Cultivos de Repollo, Brócoli y Coliflor; Universidad Nacional de Colombia: Bogotá, Colombia, 2017. [Google Scholar]
- Torres, E. Brote epidémico de la hernia del repollo. El Espectador, 1969; 10D. [Google Scholar]
- Mapit GIS Ltd. Map It Spatial; Android App; Mapit GIS Ltd.: Wishaw, UK, 2017. [Google Scholar]
- IDEAM SERVICIOS—IDEAM. Available online: http://www.ideam.gov.co/web/atencion-y-participacion-ciudadana/tramites-servicios (accessed on 12 January 2022).
- RStudio Team. RStudio: Integrated Development Rnvironment for R; RStudio Team: Boston, MA, USA, 2018. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Lenth, R.V. Least-Squares Means: The R Package lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Jaramillo, J.; Díaz, C. El Cultivo de las Crucíferas: Brócoli, Coliflor, Repollo, Col China; Corporación Colombiana de Investigación Agropecuaria, CORPOICA: Rionegro, Antioquia, Colombia, 2006. [Google Scholar]
- Hwang, S.F.; Ahmed, H.U.; Zhou, Q.; Strelkov, S.E.; Gossen, B.D.; Peng, G.; Turnbull, G.D. Influence of cultivar resistance and inoculum density on root hair infection of canola (Brassica napus) by Plasmodiophora brassicae. Plant Pathol. 2011, 60, 820–829. [Google Scholar] [CrossRef]
- Peng, G.; Lahlali, R.; Hwang, S.-F.; Pageau, D.; Hynes, R.K.; McDonald, M.R.; Gossen, B.D.; Strelkov, S.E. Crop rotation, cultivar resistance, and fungicides/biofungicides for managing clubroot (Plasmodiophora brassicae) on canola. Can. J. Plant Pathol. 2014, 36, 99–112. [Google Scholar] [CrossRef]
- Peng, G.; Pageau, D.; Strelkov, S.E.; Gossen, B.D.; Hwang, S.-F.; Lahlali, R. A >2-year crop rotation reduces resting spores of Plasmodiophora brassicae in soil and the impact of clubroot on canola. Eur. J. Agron. 2015, 70, 78–84. [Google Scholar] [CrossRef]
- Hwang, S.F.; Ahmed, H.U.; Zhou, Q.; Fu, H.; Turnbull, G.D.; Fredua-Agyeman, R.; Strelkov, S.E.; Gossen, B.D.; Peng, G. Influence of resistant cultivars and crop intervals on clubroot of canola. Can. J. Plant Sci. 2019, 99, 862–872. [Google Scholar] [CrossRef]
- Yang, X.; Huang, X.; Wu, W.; Xiang, Y.; Du, L.; Zhang, L.; Liu, Y. Effects of different rotation patterns on the occurrence of clubroot disease and diversity of rhizosphere microbes. J. Integr. Agric. 2020, 19, 2265–2273. [Google Scholar] [CrossRef]
- Ernst, T.W.; Kher, S.; Stanton, D.; Rennie, D.C.; Hwang, S.F.; Strelkov, S.E. Plasmodiophora brassicae resting spore dynamics in clubroot resistant canola (Brassica napus) cropping systems. Plant Pathol. 2019, 68, 399–408. [Google Scholar] [CrossRef]
- Rauschert, E. Survivorship Curves. Nat. Educ. Knowl. Proj. 2010, 3, 18. [Google Scholar]
- Botero-Ramirez, A.; Hwang, S.-F.; Strelkov, S.E. Plasmodiophora brassicae Inoculum Density and Spatial Patterns at the Field Level and Relation to Soil Characteristics. Pathogens 2021, 10, 499. [Google Scholar] [CrossRef] [PubMed]
- Czubatka-Bieńkowska, A.; Kaczmarek, J.; Marzec-Schmidt, K.; Nieróbca, A.; Czajka, A.; Jędryczka, M. Country-Wide qPCR Based Assessment of Plasmodiophora brassicae Spread in Agricultural Soils and Recommendations for the Cultivation of Brassicaceae Crops in Poland. Pathogens 2020, 9, 1070. [Google Scholar] [CrossRef] [PubMed]
- Ophel-Keller, K.; McKay, A.; Hartley, D.; Herdina; Curran, J. Development of a routine DNA-based testing service for soilborne diseases in Australia. Australas. Plant Pathol. 2008, 37, 243–253. [Google Scholar] [CrossRef]

| Department | Cabbage Area (ha) | % National Area | Broccoli Area (ha) | % National Area | Cauliflower Area (ha) | % National Area | Total Area Cruciferous Vegetables (ha) | % National Area | Number of Samples |
|---|---|---|---|---|---|---|---|---|---|
| Antioquia | 551.70 | 38 | 197.00 | 45 | 81.8 | 17 | 831.33 | 35 | 29 |
| Cundinamarca | 318.50 | 22 | 311.36 | 71 | 100.91 | 21 | 731.70 | 31 | 35 |
| Nariño | 218.70 | 15 | 100.80 | 23 | 202.5 | 42 | 522.38 | 22 | 28 |
| Norte de Santander | 90.20 | 6 | 73.00 | 17 | 70 | 15 | 233.43 | 10 | 9 |
| Valle del Cauca | 221.76 | 15 | 0 | 0 | 0 | 0 | 221.91 | 9 | 10 |
| Boyacá | 87.49 | 6 | 27.10 | 6 | 2.5 | 1 | 117.21 | 5 | 10 |
| Caldas | 51.90 | 4 | 0.00 | 0 | 0 | 0 | 51.94 | 2 | 3 |
| Cauca | 4.00 | 0 | 23.00 | 5 | 10.5 | 2 | 37.56 | 2 | 3 |
| Colombia total | 1445.4 | 100 | 436.00 | 100 | 479.8 | 100 | 2363.16 | 100 | 127 |
| Department | Number of Surveyed Fields | Fields with Cruciferous Crops at the Time of Visit | Fields Where Resistant Hybrid * Cultivars Were Grown | Fields Where Clubroot Symptoms Were Observed | Fields Where Plasmodiophora brassicae DNA Was Detected | ||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Fields | % | Number of Fields | % | Number of Fields | % ** | Number of Fields | % | ||
| Antioquia | 29 | 17 | 58.6 | 10 | 34.5 | 6 | 20.7 | 25 | 86.2 |
| Cundinamarca | 35 | 19 | 54.3 | 1 | 2.9 | 10 | 28.6 | 31 | 91.9 |
| Nariño | 28 | 12 | 42.9 | 0 | 0.0 | 0 | 0.0 | 25 | 89.3 |
| Norte de Santander | 9 | 9 | 100.0 | 0 | 0.0 | 8 | 88.9 | 9 | 100.0 |
| Valle del Cauca | 10 | 10 | 100.0 | 0 | 0.0 | 7 | 70.0 | 10 | 100.0 |
| Boyacá | 10 | 9 | 90.0 | 0 | 0.0 | 5 | 50.0 | 10 | 100.0 |
| Caldas | 3 | 3 | 100.0 | 0 | 0.0 | 2 | 66.7 | 3 | 100.0 |
| Cauca | 3 | 3 | 100.0 | 0 | 0.0 | 2 | 66.7 | 3 | 100.0 |
| Total | 127 | 82 | 64.6 | 11 | 8.7 | 40 | 48.8 | 116 | 91.3 |
| Department | Average (Resting Spores g−1 of Soil) | Minimum Inoculum Density in Positive Samples (Resting Spores g−1 of Soil) | Maximum Inoculum Density in Positive Samples (Resting Spores g−1 of Soil) | Number of Samples Negative for Plasmodiophora brassicae |
|---|---|---|---|---|
| Boyacá | 3.4 × 103 | 8.4 × 102 | 1.0 × 104 | 0 |
| Caldas | 4.0 × 103 | 2.2 × 103 | 5.8 × 103 | 0 |
| Antioquia | 4.1 × 103 | 1.5 × 103 | 3.6 × 104 | 4 |
| Cundinamarca | 4.9 × 103 | 3.0 × 102 | 1.3 × 105 | 4 |
| Cauca | 5.0 × 103 | 2.9 × 103 | 6.2 × 103 | 0 |
| Nariño | 7.0 × 103 | 2.0 × 103 | 2.1 × 104 | 3 |
| Valle del Cauca | 7.3 × 103 | 4.0 × 103 | 2.2 × 104 | 0 |
| Norte de Santander | 1.6 × 105 | 1.6 × 103 | 1.1 × 106 | 0 |
| Annual Precipitation (mm) | Rainy Days per Year (Days) | Average Temperature (°C) | Minimum Temperature (°C) | Maximum Temperature (°C) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Department | Mean | Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | Min | Max | |||||
| Antioquia | 775.5 | 567.5 | 880.9 | a | 151 | 115 | 174 | ab | 13.2 | 11.2 | 14.4 | a | 9.9 | 6.5 | 16.8 | abc | 20.7 | 16.0 | 25.2 | b |
| Boyacá | 793.2 | 548.6 | 972.4 | a | 146 | 92 | 176 | ab | 13.3 | 11.7 | 15.6 | ab | 7.4 | 6.8 | 8.9 | a | 19.1 | 16.1 | 22.1 | ab |
| Cundinamarca | 1394.0 | 728.0 | 2111.0 | bc | 172 | 88 | 236 | bc | 15.8 | 11.0 | 23.6 | cb | 11.2 | 5.8 | 19.1 | bc | 20.5 | 16.0 | 29.4 | b |
| Caldas | 765.7 | 728.0 | 784.5 | ab | 116 | 88 | 130 | a | 14.2 | 14.2 | 14.2 | abc | 6.0 | 6.0 | 6.0 | ab | 21.3 | 21.3 | 21.3 | abc |
| Cauca | 807.7 | 784.5 | 819.3 | ab | 145 | 130 | 152 | abc | 15.1 | 14.2 | 15.6 | abcd | 7.9 | 6.0 | 8.9 | abc | 21.8 | 21.3 | 22.1 | abc |
| Nariño | 1650.4 | 826.4 | 2699.6 | c | 206 | 154 | 279 | d | 13.6 | 11.0 | 17.0 | a | 10.0 | 7.1 | 13.5 | abc | 19.9 | 15.5 | 21.3 | a |
| Norte de Santander | 2273.0 | 2178.0 | 2606.0 | d | 204 | 194 | 238 | cd | 18.2 | 18.2 | 18.2 | cd | 12.9 | 12.9 | 12.9 | cd | 23.3 | 23.3 | 23.3 | bc |
| Valle del Cauca | 2524.0 | 1964.0 | 2606.0 | d | 239 | 238 | 241 | d | 20.4 | 17.0 | 24.1 | d | 15.2 | 12.5 | 18.8 | d | 25.7 | 22.2 | 29.5 | c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botero-Ramírez, A.; Padilla-Huertas, F.L.; Strelkov, S.E.; García-Dominguez, C. The Occurrence of Clubroot in Colombia and Its Relationship with Climate and Agronomic Practices. Horticulturae 2022, 8, 711. https://doi.org/10.3390/horticulturae8080711
Botero-Ramírez A, Padilla-Huertas FL, Strelkov SE, García-Dominguez C. The Occurrence of Clubroot in Colombia and Its Relationship with Climate and Agronomic Practices. Horticulturae. 2022; 8(8):711. https://doi.org/10.3390/horticulturae8080711
Chicago/Turabian StyleBotero-Ramírez, Andrea, Fabián Leonardo Padilla-Huertas, Stephen E. Strelkov, and Celsa García-Dominguez. 2022. "The Occurrence of Clubroot in Colombia and Its Relationship with Climate and Agronomic Practices" Horticulturae 8, no. 8: 711. https://doi.org/10.3390/horticulturae8080711
APA StyleBotero-Ramírez, A., Padilla-Huertas, F. L., Strelkov, S. E., & García-Dominguez, C. (2022). The Occurrence of Clubroot in Colombia and Its Relationship with Climate and Agronomic Practices. Horticulturae, 8(8), 711. https://doi.org/10.3390/horticulturae8080711






