Valorization of Hazelnut Shells as Growing Substrate for Edible and Medicinal Mushrooms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates
2.2. Mushrooms Cultures and Mycelial Growth Rate Evaluation
2.3. Mushrooms Cultivation Trials
2.4. Micro ATR-FTIR Spectroscopy Analysis
2.5. Statistical Analyses
3. Results
3.1. In Vitro Mycelial Growth and ATR-FTIR of Substrates
3.2. Fruiting Body Production
3.3. Qualitative Evaluation of Mushrooms by ATR-FTIR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agricultural Organization of the United Nations. FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 15 December 2021).
- Król, K.; Gantner, M. Morphological traits and chemical composition of hazelnut from different geographical origins: A review. Agriculture 2020, 10, 375. [Google Scholar] [CrossRef]
- Silvestri, C.; Bacchetta, L.; Bellincontro, A.; Cristofori, V. Advances in cultivar choice, hazelnut orchard management, and nut storage to enhance product quality and safety: An overview. J. Sci. Food Agric. 2021, 101, 27–43. [Google Scholar] [CrossRef]
- Król, K.; Gantner, M.; Piotrowska, A. The quality characteristic and fatty acid profile of cold-pressed hazelnut oils during nine months of storage. Agronomy 2021, 11, 2045. [Google Scholar] [CrossRef]
- Rivas, S.; Moure, A.; Parajó, J.C. Pretreatment of hazelnut shells as a key strategy for the solubilization and valorization of hemicelluloses into bioactive compounds. Agronomy 2020, 10, 760. [Google Scholar] [CrossRef]
- Pérez-Armada, L.; Rivas, S.; González, B.; Moure, A. Extraction of phenolic compounds from hazelnut shells by green processes. J. Food Eng. 2019, 255, 1–8. [Google Scholar] [CrossRef]
- Hoşgün, E.Z.; Bozan, B. Effect of different types of thermochemical pretreatment on the enzymatic hydrolysis and the composition of hazelnut shells. Waste Biomass Valorization 2020, 11, 3739–3748. [Google Scholar] [CrossRef]
- Guney, M.S. Utilization of hazelnut husk as biomass. Sustain. Energy Technol. Assess. 2013, 4, 72–77. [Google Scholar] [CrossRef]
- Arslan, Y.; Takaç, S.; Eken-Saraçoĝlu, N. Kinetic study of hemicellulosic sugar production from hazelnut shells. Chem. Eng. J. 2012, 185–186, 23–28. [Google Scholar] [CrossRef]
- Özçelik, E.; Pekşen, A. Hazelnut husk as a substrate for the cultivation of shiitake mushroom (Lentinula edodes). Bioresour. Technol. 2007, 98, 2652–2658. [Google Scholar] [CrossRef]
- Uzuner, S.; Sharma Shivappa, R.R.; Cekmecelioglu, D. Bioconversion of alkali pretreated hazelnut shells to fermentable sugars for generation of high value products. Waste Biomass Valorization 2017, 8, 407–416. [Google Scholar] [CrossRef]
- Çöpür, Y.; Güler, C.; Taşçioǧlu, C.; Tozluoǧlu, A. Incorporation of hazelnut shell and husk in MDF production. Bioresour. Technol. 2008, 99, 7402–7406. [Google Scholar] [CrossRef] [PubMed]
- Barbu, M.C.; Sepperer, T.; Tudor, E.M.; Petutschnigg, A. Walnut and hazelnut shells: Untapped industrial resources and their suitability in lignocellulosic composites. Appl. Sci. 2020, 10, 6340. [Google Scholar] [CrossRef]
- Şencan, A.; Karaboyacı, M.; Kılıç, M. Determination of lead(II) sorption capacity of hazelnut shell and activated carbon obtained from hazelnut shell activated with ZnCl2. Environ. Sci. Pollut. Res. 2015, 22, 3238–3248. [Google Scholar] [CrossRef] [PubMed]
- Cimino, G.; Passerini, A.; Toscano, G. Removal of toxic cations and Cr(VI) from aqueous solution by hazelnut shell. Water Res. 2000, 34, 2955–2962. [Google Scholar] [CrossRef]
- Kazemipour, M.; Ansari, M.; Tajrobehkar, S.; Majdzadeh, M.; Kermani, H.R. Removal of lead, cadmium, zinc, and copper from industrial wastewater by carbon developed from walnut, hazelnut, almond, pistachio shell, and apricot stone. J. Hazard. Mater. 2008, 150, 322–327. [Google Scholar] [CrossRef]
- Kobya, M.; Demirbas, E.; Öncel, M.S.; Sencan, S. Adsorption kinetic models applied to nickel ions on hazelnut shell activated carbons. Adsorpt. Sci. Technol. 2002, 20, 179–188. [Google Scholar] [CrossRef]
- Demirbas, E.; Dizge, N.; Sulak, M.T.; Kobya, M. Adsorption kinetics and equilibrium of copper from aqueous solutions using hazelnut shell activated carbon. Chem. Eng. J. 2009, 148, 480–487. [Google Scholar] [CrossRef]
- Sert, S.; Çelik, A.; Tirtom, V.N. Removal of arsenic(III) ions from aqueous solutions by modified hazelnut shell. Desalin. Water Treat. 2017, 75, 115–123. [Google Scholar] [CrossRef]
- Pehlivan, E.; Altun, T.; Cetin, S.; Iqbal Bhanger, M. Lead sorption by waste biomass of hazelnut and almond shell. J. Hazard. Mater. 2009, 167, 1203–1208. [Google Scholar] [CrossRef]
- Demirbaş, E. Adsorption of cobalt(II) ions from aqueous solution onto activated carbon prepared from hazelnut shells. Adsorpt. Sci. Technol. 2003, 21, 951–963. [Google Scholar] [CrossRef]
- Lewicka, K. Activated carbons prepared from hazelnut shells, walnut shells and peanut shells for high CO2 adsorption. Polish J. Chem. Technol. 2017, 19, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Contini, M.; Baccelloni, S.; Massantini, R.; Anelli, G. Extraction of natural antioxidants from hazelnut (Corylus avellana L.) shell and skin wastes by long maceration at room temperature. Food Chem. 2008, 110, 659–669. [Google Scholar] [CrossRef]
- Yuan, B.; Lu, M.; Eskridge, K.M.; Isom, L.D.; Hanna, M.A. Extraction, identification, and quantification of antioxidant phenolics from hazelnut (Corylus avellana L.) shells. Food Chem. 2018, 244, 7–15. [Google Scholar] [CrossRef]
- Yuan, B.; Lu, M.; Eskridge, K.M.; Hanna, M.A. Valorization of hazelnut shells into natural antioxidants by ultrasound-assisted extraction: Process optimization and phenolic composition identification. J. Food Process Eng. 2018, 41, 1–13. [Google Scholar] [CrossRef]
- Esposito, T.; Sansone, F.; Franceschelli, S.; Del Gaudio, P.; Picerno, P.; Aquino, R.P.; Mencherini, T. Hazelnut (Corylus avellana L.) shells extract: Phenolic composition, antioxidant effect and cytotoxic activity on human cancer cell lines. Int. J. Mol. Sci. 2017, 18, 392. [Google Scholar] [CrossRef] [PubMed]
- Surek, E.; Buyukkileci, A.O. Production of xylooligosaccharides by autohydrolysis of hazelnut (Corylus avellana L.) shell. Carbohydr. Polym. 2017, 174, 565–571. [Google Scholar] [CrossRef]
- Midilli, A.; Rzayev, P.; Olgun, H.; Ayhan, T. Solar hydrogen production from hazelnut shells. Int. J. Hydrogen Energy 2000, 25, 723–732. [Google Scholar] [CrossRef]
- Midilli, A.; Dogru, M.; Howarth, C.R.; Ayhan, T. Hydrogen production from hazelnut shell by applying air-blown downdraft gasification technique. Int. J. Hydrogen Energy 2001, 26, 29–37. [Google Scholar] [CrossRef]
- Hoşgün, E.Z.; Berikten, D.; Kıvanç, M.; Bozan, B. Ethanol production from hazelnut shells through enzymatic saccharification and fermentation by low-temperature alkali pretreatment. Fuel 2017, 196, 280–287. [Google Scholar] [CrossRef]
- Fuso, A.; Risso, D.; Rosso, G.; Rosso, F.; Manini, F.; Manera, I.; Caligiani, A. Potential valorization of hazelnut shells through extraction, purification and structural characterization of prebiotic compounds: A critical review. Foods 2021, 10, 1197. [Google Scholar] [CrossRef]
- Badalyan, S.; Zambonelli, A. The potential of mushrooms to develop healthy food and biotech products. In Fungi and Fungal Products in Human Welfare and Biotechnology; Satyanarayana, T., Deshmukh, S.K., Eds.; Springer Nature: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Royse, D.J.; Baars, J.; Tan, Q. Current overview of mushroom production in the world. In Edible and Medicinal Mushrooms: Technology and Applications; Zied, D.C., Pardo-Giménez, A., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2017; pp. 5–13. [Google Scholar]
- Vedder, J.C.; Van den Munckhof-Vedder, M. Modern Mushroom Growing 2020 Harvesting; Vedder, P.J.C., Ed.; Harvest House: Irvine, CA, USA, 2020. [Google Scholar]
- Wang, L.; Li, J.Q.; Zhang, J.; Li, Z.M.; Liu, H.G.; Wang, Y.Z. Traditional uses, chemical components and pharmacological activities of the genus: Ganoderma P. Karst.: A review. RSC Adv. 2020, 10, 42084–42097. [Google Scholar] [CrossRef]
- Di Piazza, S.; Benvenuti, M.; Damonte, G.; Cecchi, G.; Mariotti, M.G.; Zotti, M. Fungi and circular economy: Pleurotus ostreatus grown on a substrate with agricultural waste of lavender, and its promising biochemical profile. Recycling 2021, 6, 40. [Google Scholar] [CrossRef]
- Pekşen, A.; Küçükomuzlu, B. Yield potential and quality of some Pleurotus species grown in substrates containing hazelnut husk. Pak. J. Biol. Sci. 2004, 7, 768–771. [Google Scholar] [CrossRef] [Green Version]
- Yildiz, S.; Yildiz, Ü.C.; Gezer, E.D.; Temiz, A. Some lignocellulosic wastes used as raw material in cultivation of the Pleurotus ostreatus culture mushroom. Process Biochem. 2002, 38, 301–306. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2006; pp. 10815–10837. [Google Scholar]
- Li, X.P.; Li, J.; Liu, H.; Wang, Y.Z. A new analytical method for discrimination of species in Ganodermataceae mushrooms. Int. J. Food Prop. 2020, 23, 227–240. [Google Scholar] [CrossRef] [Green Version]
- Pin, M.W.; Park, E.J.; Choi, S.; Kim, Y.I.; Jeon, C.H.; Ha, T.H.; Kim, Y.H. Atomistic evolution during the phase transition on a metastable single NaYF4:Yb,Er upconversion nanoparticle. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bekiaris, G.; Tagkouli, D.; Koutrotsios, G.; Kalogeropoulos, N.; Zervakis, G.I. Pleurotus mushrooms content in glucans and ergosterol assessed by ATR-FTIR spectroscopy and multivariate analysis. Foods 2020, 9, 535. [Google Scholar] [CrossRef]
- O’Gorman, A.; Downey, G.; Gowen, A.A.; Barry-Ryan, C.; Frias, J.M. Use of fourier transform infrared spectroscopy and chemometric data analysis to evaluate damage and age in mushrooms (Agaricus bisporus) grown in Ireland. J. Agric. Food Chem. 2010, 58, 7770–7776. [Google Scholar] [CrossRef] [Green Version]
- Fornito, S.; Puliga, F.; Leonardi, P.; Di Foggia, M.; Zambonelli, A.; Francioso, O. Degradative ability of mushrooms cultivated on corn silage digestate. Molecules 2020, 25, 3020. [Google Scholar] [CrossRef]
- Bekiaris, G.; Koutrotsios, G.; Tarantilis, P.A.; Pappas, C.S.; Zervakis, G.I. FTIR assessment of compositional changes in lignocellulosic wastes during cultivation of Cyclocybe cylindracea mushrooms and use of chemometric models to predict production performance. J. Mater. Cycles Waste Manag. 2020, 22, 1027–1035. [Google Scholar] [CrossRef]
- Tryfinopoulou, P.; Chourdaki, A.; Nychas, G.J.E.; Panagou, E.Z. Competitive yeast action against Aspergillus carbonarius growth and ochratoxin A production. Int. J. Food Microbiol. 2020, 317, 108460. [Google Scholar] [CrossRef]
- Sinclair, C.G.; Cantero, D. Fermentation modelling. In Fermentation a Practical Approach; McNeil, B.L., Harvey, M., Eds.; IRL Press: New York, NY, USA, 1989; pp. 65–112. [Google Scholar]
- Oei, P. Mushroom Cultivation IV: Appropriate Technology for Mushroom Growers; ECO Consult Foundation: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Zhang, H.; Peng, J.; Zhang, Y.R.; Liu, Q.; Pan, L.Q.; Tu, K. Discrimination of volatiles of shiitakes (Lentinula edodes) produced during drying process by electronic nose. Int. J. Food Eng. 2020, 16, 1–13. [Google Scholar] [CrossRef]
- Chang, S.T.; Lau, O.W.; Cho, K.Y. The cultivation and nutritional value of Pleurotus sajor-caju. Eur. J. Appl. Microbiol. Biotechnol. 1981, 12, 58–62. [Google Scholar] [CrossRef]
- R Studio. Available online: https://www.rstudio.com (accessed on 6 February 2022).
- Rahman, M.R.; Hamdan, S.; Lai, J.C.H.; Jawaid, M.; Yusof, F.A.; Bin, M. Physico-mechanical, thermal and morphological properties of furfuryl alcohol/2-ethylhexyl methacrylate/halloysite nanoclay wood polymer nanocomposites (WPNCs). Heliyon 2017, 3, e00342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohebby, B. Attenuated total reflection infrared spectroscopy of white-rot decayed beech wood. Int. Biodeterior. Biodegrad. 2005, 55, 247–251. [Google Scholar] [CrossRef]
- Popescu, C.M.; Popescu, M.C.; Vasile, C. Structural changes in biodegraded lime wood. Carbohydr. Polym. 2010, 79, 362–372. [Google Scholar] [CrossRef]
- Singh, S.; Harms, H.; Schlosser, D. Screening of ecologically diverse fungi for their potential to pretreat lignocellulosic bioenergy feedstock. Appl. Microbiol. Biotechnol. 2014, 98, 3355–3370. [Google Scholar] [CrossRef]
- Huang, X.; Kocaefe, D.; Kocaefe, Y.; Boluk, Y.; Pichette, A. Study of the degradation behavior of heat-treated jack pine (Pinus banksiana) under artificial sunlight irradiation. Polym. Degrad. Stab. 2012, 97, 1197–1214. [Google Scholar] [CrossRef]
- Bekiaris, G.; Lindedam, J.; Peltre, C.; Decker, S.R.; Turner, G.B.; Magid, J.; Bruun, S. Rapid estimation of sugar release from winter wheat straw during bioethanol production using FTIR-photoacoustic spectroscopy. Biotechnol. Biofuels 2015, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Fan, M.; Dai, D.; Huang, B. Fourier transform infrared spectroscopy for natural fibres. Fourier Transform-Mater. Anal. 2012, 3, 45–68. [Google Scholar]
- De Rosa, I.M.; Kenny, J.M.; Puglia, D.; Santulli, C.; Sarasini, F. Morphological, thermal and mechanical characterization of okra (Abelmoschus esculentus) fibres as potential reinforcement in polymer composites. Compos. Sci. Technol. 2010, 70, 116–122. [Google Scholar] [CrossRef]
- Pandey, K.K.; Pitman, A.J. FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int. Biodeterior. Biodegrad. 2003, 52, 151–160. [Google Scholar] [CrossRef]
- Zervakis, G.I.; Bekiaris, G.; Tarantilis, P.A.; Pappas, C.S. Rapid strain classification and taxa delimitation within the edible mushroom genus Pleurotus through the use of diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy. Fungal Biol. 2012, 116, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Choong, Y.K.; Sun, S.Q.; Zhou, Q.; Ismail, Z.; Rashid, B.A.A.; Tao, J.X. Determination of storage stability of the crude extracts of Ganoderma lucidum using FTIR and 2D-IR spectroscopy. Vib. Spectrosc. 2011, 57, 87–96. [Google Scholar] [CrossRef]
- Mohaček-Grošev, V.; Božac, R.; Puppels, G.J. Vibrational spectroscopic characterization of wild growing mushrooms and toadstools. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 2815–2829. [Google Scholar] [CrossRef]
- Das, B.; Rajkonwar, J.; Jagannath, A.; Raul, P.K.; Deb, U. Infra-red spectra of different species of cultivated oyster mushrooms: Possible tool for identifying bioactive compounds and establishing taxonomic linkage. Def. Life Sci. J. 2020, 5, 118–124. [Google Scholar] [CrossRef]
- Melanouri, E.M.; Dedousi, M.; Diamantopoulou, P. Cultivating Pleurotus ostreatus and Pleurotus eryngii mushroom strains on agro-industrial residues in solid-state fermentation. Part II: Effect on productivity and quality of carposomes. Carbon Resour. Convers. 2022, 5, 52–60. [Google Scholar] [CrossRef]
- Şenol, H. Biogas potential of hazelnut shells and hazelnut wastes in Giresun City. Biotechnol. Rep. 2019, 24, e00361. [Google Scholar] [CrossRef]
- Kaya, N.; Yıldız, Z.; Ceylan, S. Preparation and characterisation of biochar from hazelnut shell and its adsorption properties for methylene blue dye. J. Polytech. 2018, 21, 765–776. [Google Scholar] [CrossRef] [Green Version]
- Kumla, J.; Suwannarach, N.; Sujarit, K.; Penkhrue, W.; Kakumyan, P.; Jatuwong, K.; Vadthanarat, S.; Lumyong, S. Cultivation of mushrooms and their lignocellulolytic enzyme production through the utilization of agro-industrial waste. Molecules 2020, 25, 2811. [Google Scholar] [CrossRef]
- Stamets, P. Growing Gourmet and Medicinal Mushrooms; Ten Speed Press: Berkeley, CA, USA, 2000. [Google Scholar]
- Tamaki, Y.; Mazza, G. Rapid determination of lignin content of straw using Fourier transform mid-infrared spectroscopy. J. Agric. Food Chem. 2011, 59, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Del Río, J.C.; Rencoret, J.; Prinsen, P.; Martínez, Á.T.; Gutiérrez, A.; Ralph, J. Structural characterization of wheat straw lignin. Evidence for a novel monomer in grasses. Cons. Super. Investig. Científicas 2013. Available online: https://digital.csic.es/bitstream/10261/86427/1/JCdelRio-17ISWFPC-proceeding.pdf. (accessed on 16 December 2021).
- Pettersen, R.C. The chemical composition of wood. In The Chemistry of Solid Wood; American Chemical Society: New York, NY, USA, 1984; pp. 57–126. [Google Scholar]
- Le Floch, A.; Jourdes, M.; Teissedre, P.L. Polysaccharides and lignin from oak wood used in cooperage: Composition, interest, assays: A review. Carbohydr. Res. 2015, 417, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, V.; Spina, S.; Luna, M.; Petrucci, G.; Romagnoli, M. Structural analysis of lignin in chestnut wood by pyrolysis-gas chromatography/mass spectrometry. J. Anal. Appl. Pyrolysis 2011, 92, 273–279. [Google Scholar] [CrossRef]
- Madeira, J.V.; Contesini, F.J.; Calzado, F.; Rubio, M.V.; Zubieta, M.P.; Lopes, D.B.; de Melo, R.R. Agro-industrial residues and microbial enzymes: An Overview on the eco-friendly bioconversion into high value-added products. In Biotechnology of Microbial Enzymes: Production, Biocatalysis and Industrial Applications; Elsevier, Inc.: London, UK, 2017; pp. 475–511. ISBN 9780128037461. [Google Scholar]
- Jurak, E.; Patyshakuliyeva, A.; De Vries, R.P.; Gruppen, H.; Kabel, M.A. Compost grown Agaricus bisporus lacks the ability to degrade and consume highly substituted xylan fragments. PLoS ONE 2015, 10, e0134169. [Google Scholar] [CrossRef]
- Nandni, S.; Mishra, S. Crop room studies in relation to yield potential of Lentinula edodes strains on wheat straw. J. Hill Agric. 2018, 9, 124. [Google Scholar] [CrossRef]
- Kalmiş, E.; Sargin, S. Cultivation of two Pleurotus species on wheat straw substrates containing olive mill waste water. Int. Biodeterior. Biodegrad. 2004, 53, 43–47. [Google Scholar] [CrossRef]
- Ćilerdžić, J.L.; Vukojević, J.B.; Klaus, A.S.; Ivanović, Ž.S.; Blagojević, J.D.; Stajić, M.M. Wheat straw—A promissing substrate for Ganoderma lucidum cultivation. Acta Sci. Pol. Hortorum Cultus 2018, 17, 13–22. [Google Scholar] [CrossRef]
- Ritz, K.; Crawford, J.W. Colony development in nutritionally heterogeneous environments. In The Fungal Colony; Gow, N.A.R., Robson, G.D., Gadd, G.M., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 49–74. [Google Scholar]
- Bruhn, J.; Hall, M. Growing Shiitake Mushrooms in an Agroforestry Practice. Available online: https://centerforagroforestry.org/wp-content/uploads/2021/05/af1010 (accessed on 16 December 2021).
- Zivanovic, S.; Busher, R.W.; Kim, K.S. Textural changes in mushrooms (Agaricus bisporus) associated with tissue ultrastructure and composition. J. Food Sci. 2000, 65, 1404–1408. [Google Scholar] [CrossRef]
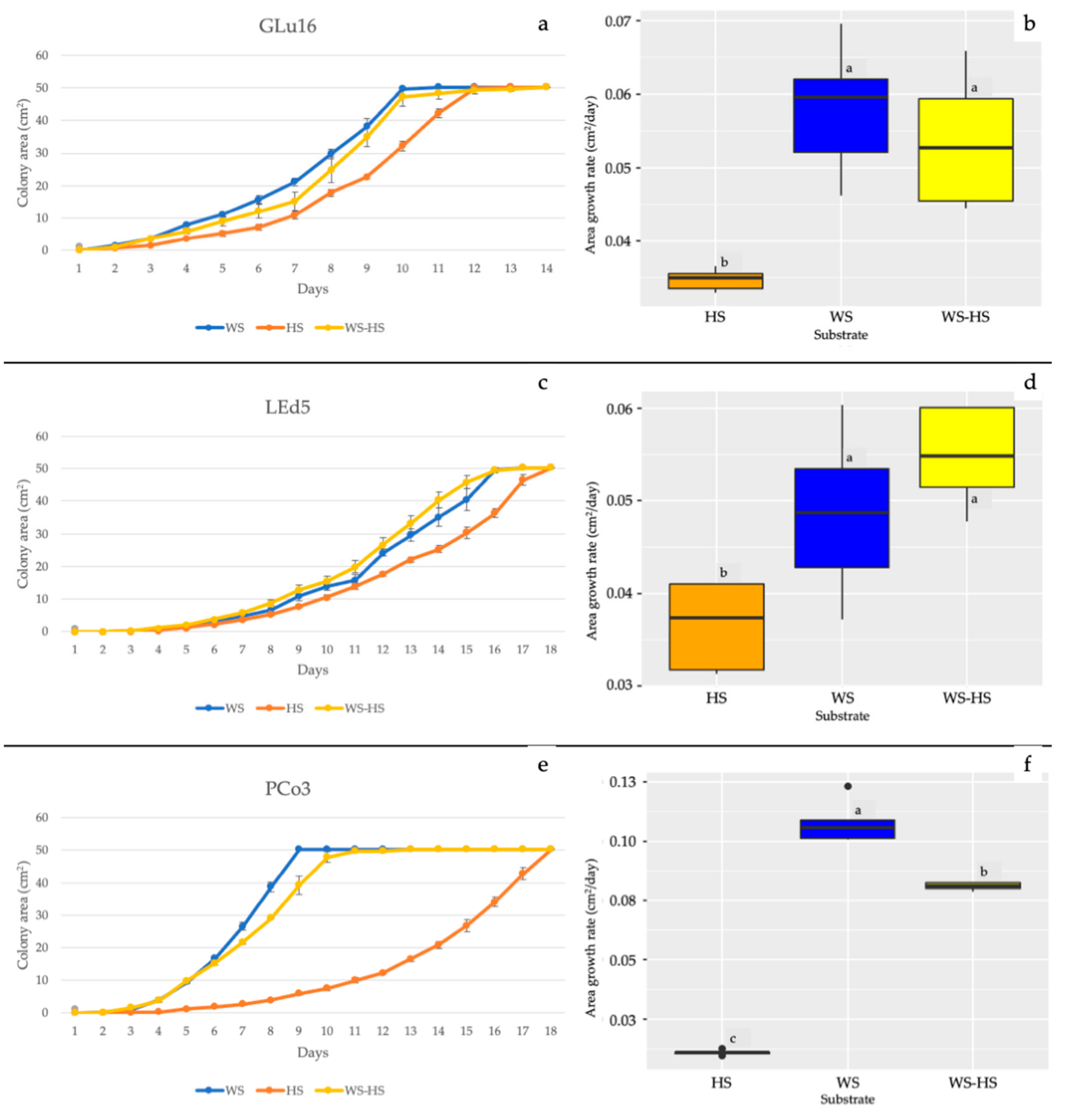

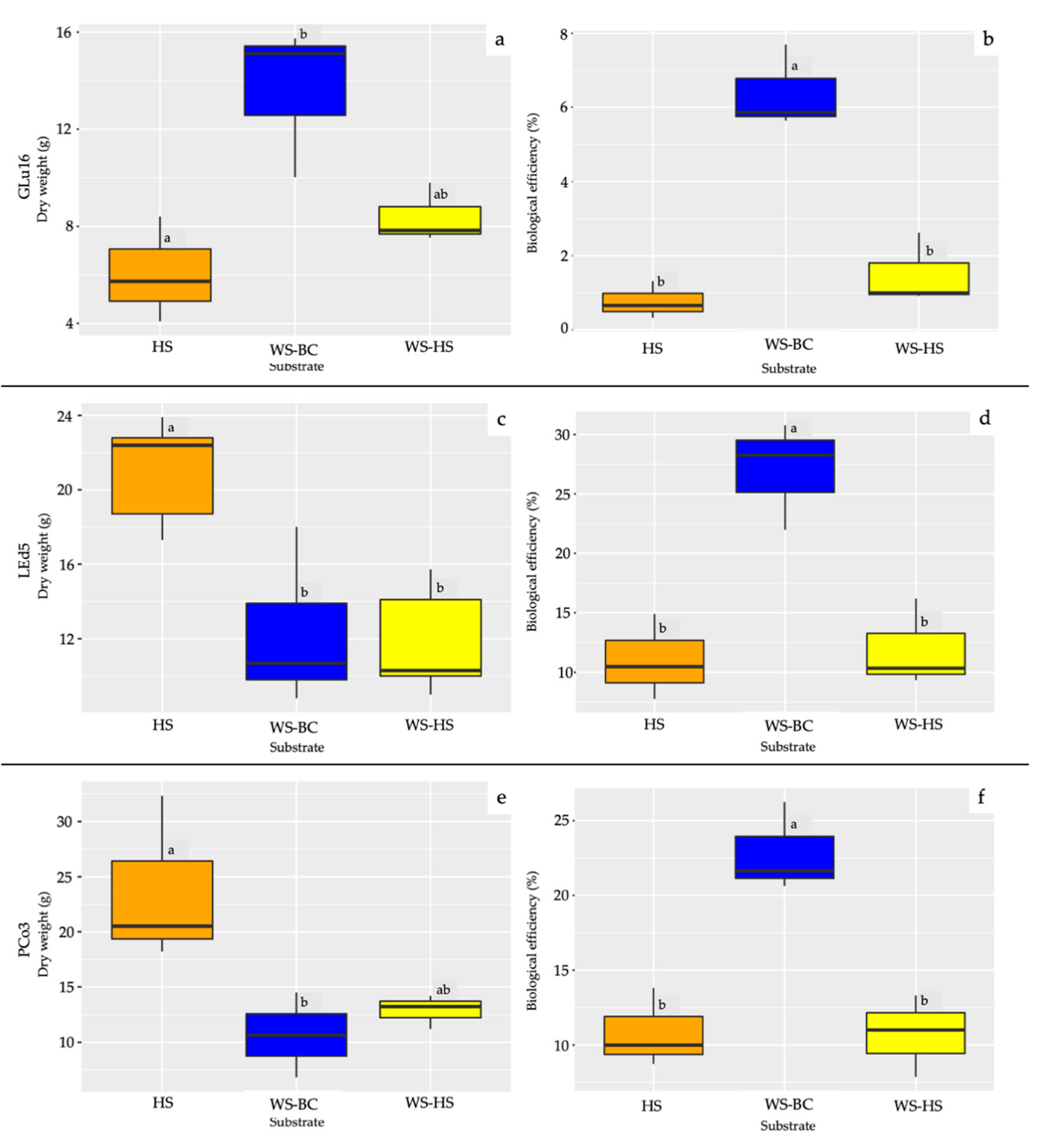
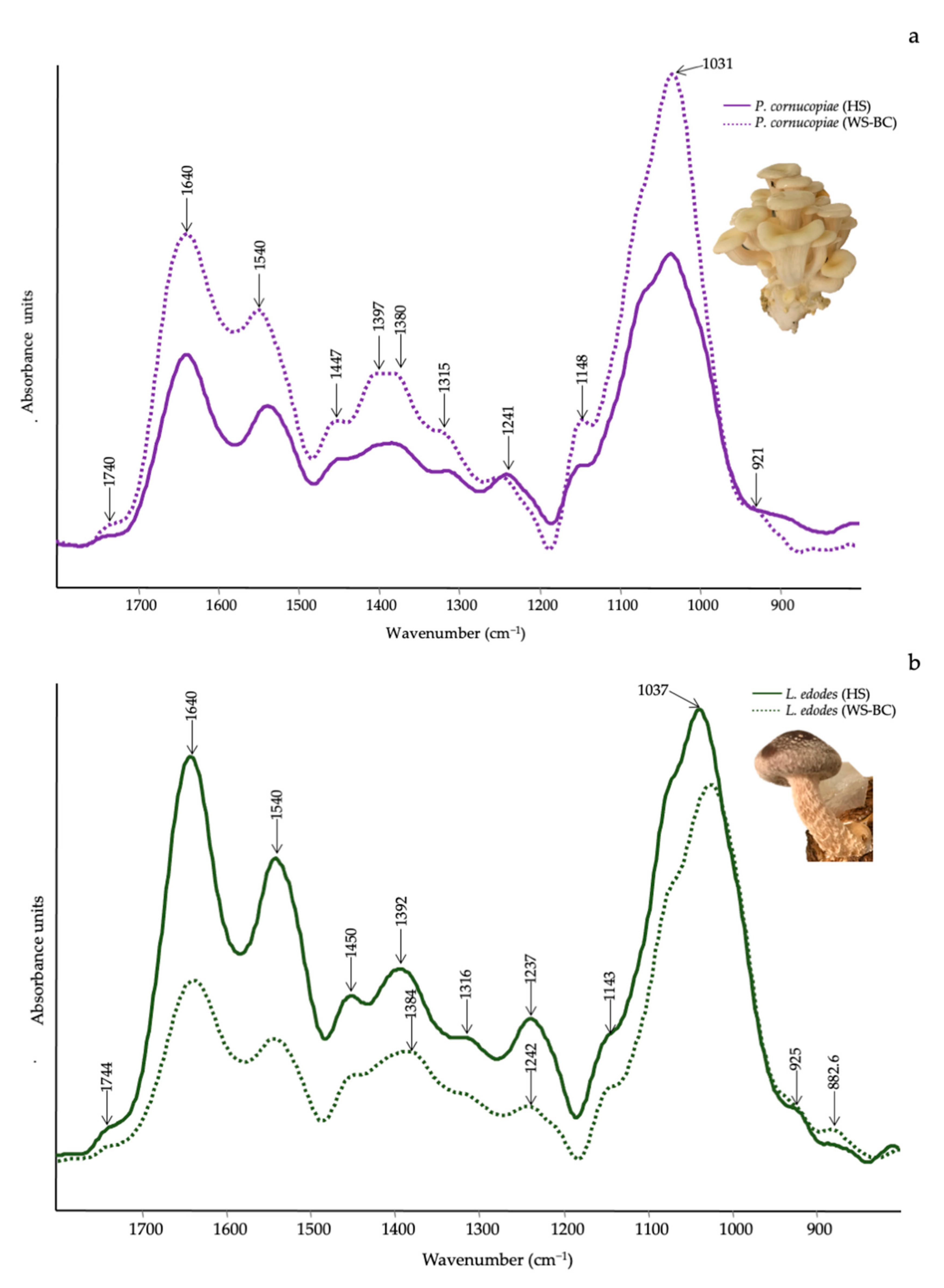
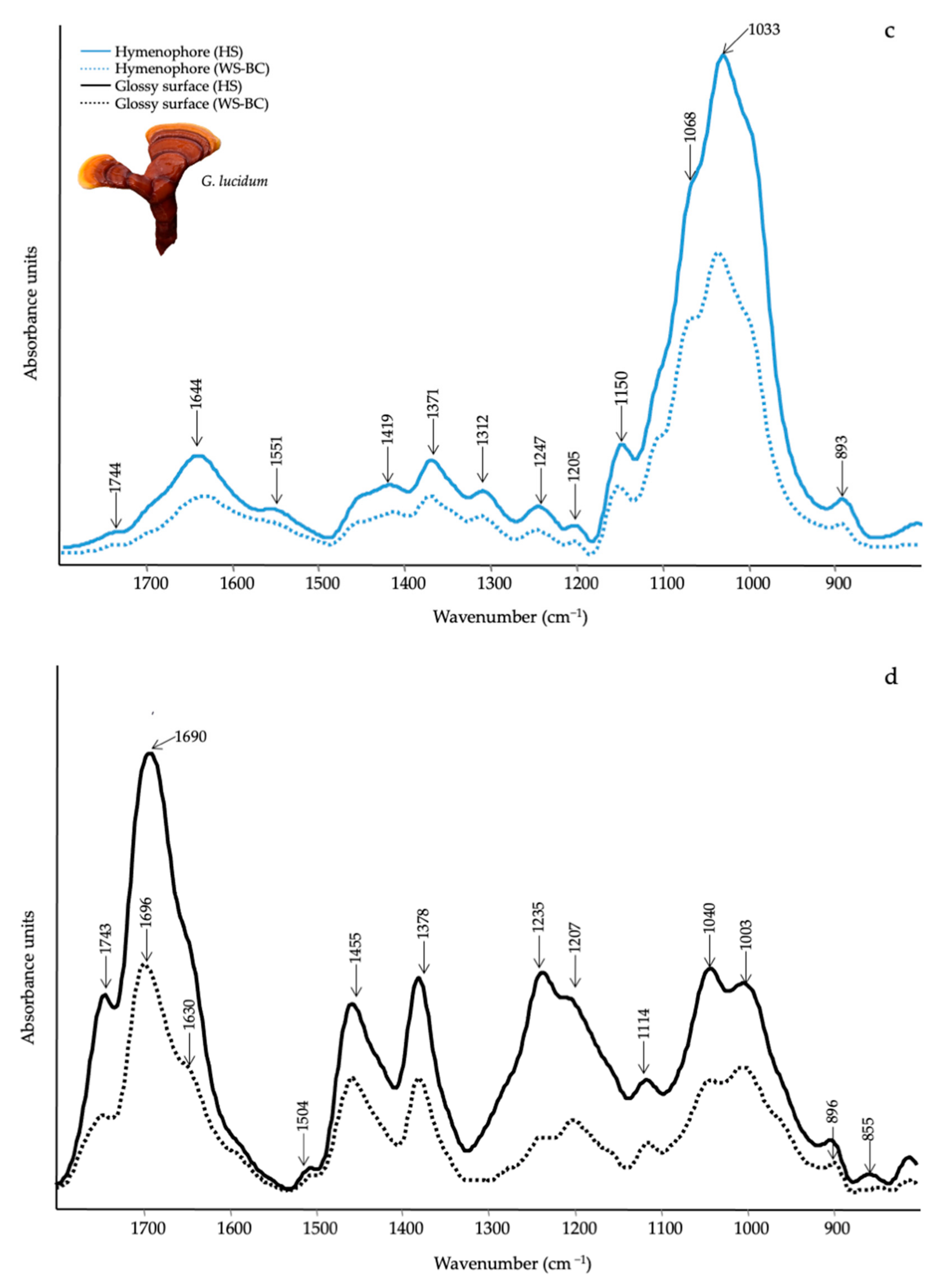
| Substrates and Mixing Ratio | Code | Average Dry Weight (g) |
|---|---|---|
| Wheat Straw 50%-Beech Chips 50% (control) | WS-BC | 400 |
| Wheat Straw 50%-Hazelnut Shells 50% | WS-HS | 900 |
| Hazelnut Shells 100% | HS | 1500 |
| Species | |||
|---|---|---|---|
| Ganoderma lucidum | Lentinula edodes | Pleurotus cornucopiae | |
| Temperature (°C) | 22–30 | 10–20 | 18–25 |
| Light intensity (Lux) | 500–1000 | 500–1000 | 500–1000 |
| Relative Humidity (%) | 85–95 | 60–95 | 80–95 |
| Wavenumber (cm−1) | Functional Groups Attributions | Reference |
|---|---|---|
| 1745–1738 | C=O stretching of lipids | [61] |
| 1696–1690 | CHO stretching in aromatic | [62] |
| 1640–1638 | Amide I, water | [61] |
| 1540–1548 | Amide II | [61] |
| 1507 | Aromatic ring | [62] |
| 1455 | CH2 bending in polysaccharides | [39] |
| 1400–1378 | C–H in-plane bending vibration | [63] |
| 1245–1235 | Amide III and C–O stretching | [40] |
| 1150–1157 | C–O–C asymmetric stretching of glycosidic linkage | [43] |
| 1040–1025 | stretching vibration of C–O–C group | [43] |
| 930–882 | Glucan band β anomer; C–H deformation | [64] |
| 807–796 | α (1–6) linked backbone with α (1–3); CH out-of plane bending | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puliga, F.; Leonardi, P.; Minutella, F.; Zambonelli, A.; Francioso, O. Valorization of Hazelnut Shells as Growing Substrate for Edible and Medicinal Mushrooms. Horticulturae 2022, 8, 214. https://doi.org/10.3390/horticulturae8030214
Puliga F, Leonardi P, Minutella F, Zambonelli A, Francioso O. Valorization of Hazelnut Shells as Growing Substrate for Edible and Medicinal Mushrooms. Horticulturae. 2022; 8(3):214. https://doi.org/10.3390/horticulturae8030214
Chicago/Turabian StylePuliga, Federico, Pamela Leonardi, Francesco Minutella, Alessandra Zambonelli, and Ornella Francioso. 2022. "Valorization of Hazelnut Shells as Growing Substrate for Edible and Medicinal Mushrooms" Horticulturae 8, no. 3: 214. https://doi.org/10.3390/horticulturae8030214
APA StylePuliga, F., Leonardi, P., Minutella, F., Zambonelli, A., & Francioso, O. (2022). Valorization of Hazelnut Shells as Growing Substrate for Edible and Medicinal Mushrooms. Horticulturae, 8(3), 214. https://doi.org/10.3390/horticulturae8030214








