Alterations in the Chemical Composition of Spinach (Spinacia oleracea L.) as Provoked by Season and Moderately Limited Water Supply in Open Field Cultivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material and Field Experimental Design
2.3. Cultivation and Water Supply
2.4. Harvest
2.5. Climatic Data
2.6. Sample Preparation
2.7. Chemical Analyses
2.7.1. Sugars and Polyols
2.7.2. Organic Acids
2.7.3. Inorganic Anions
2.7.4. Total Carbon and Nitrogen
2.7.5. Minerals and Trace Elements
2.7.6. Total Phenols
2.7.7. Flavonoids
2.7.8. Carotenoids
2.8. Statistical Analyses
3. Results and Discussion
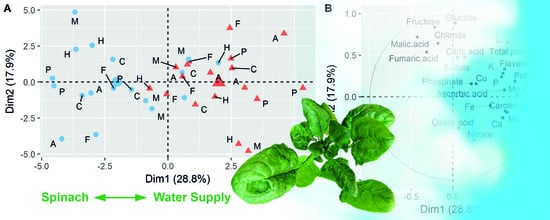
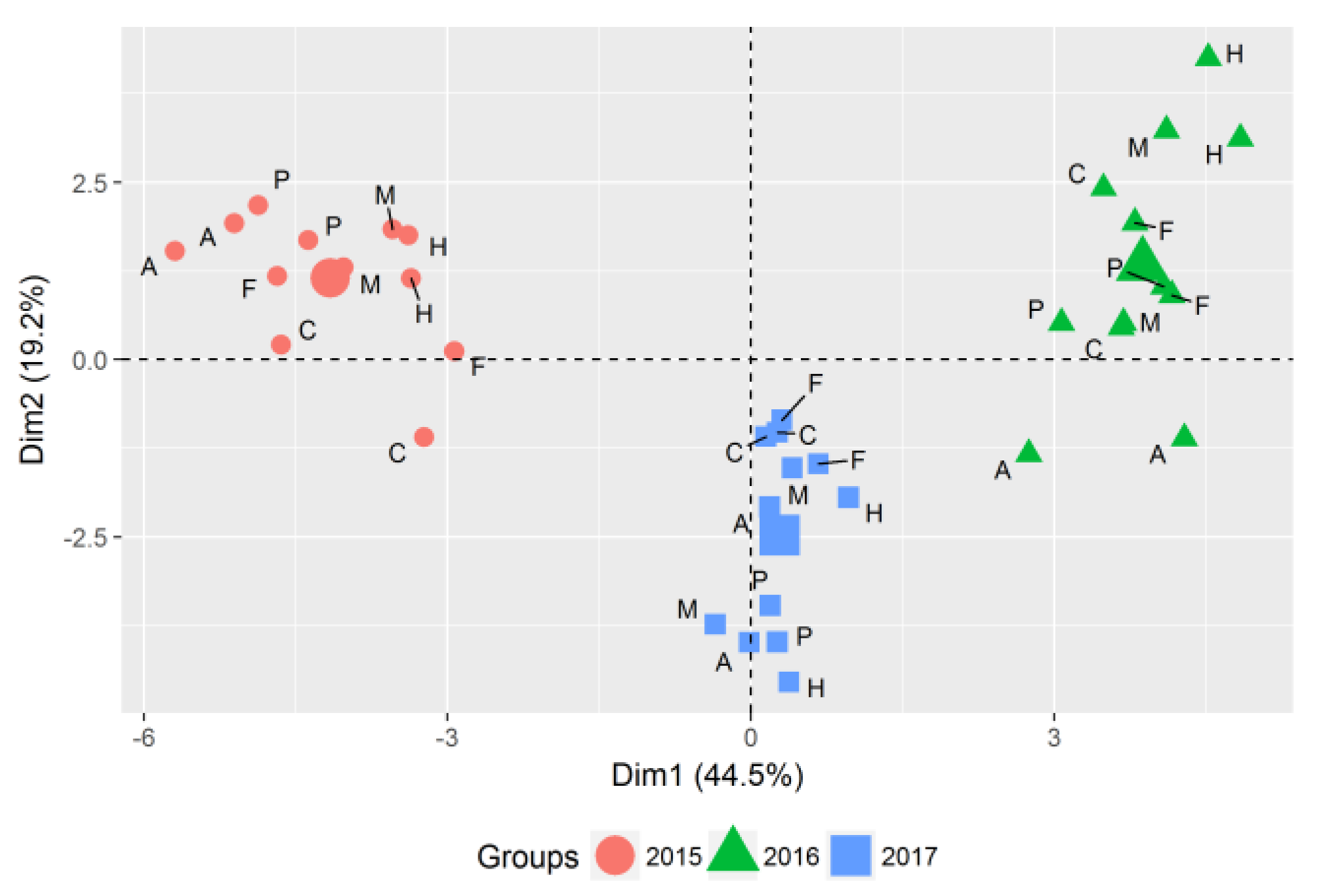
3.1. Evaluation of Years and Season
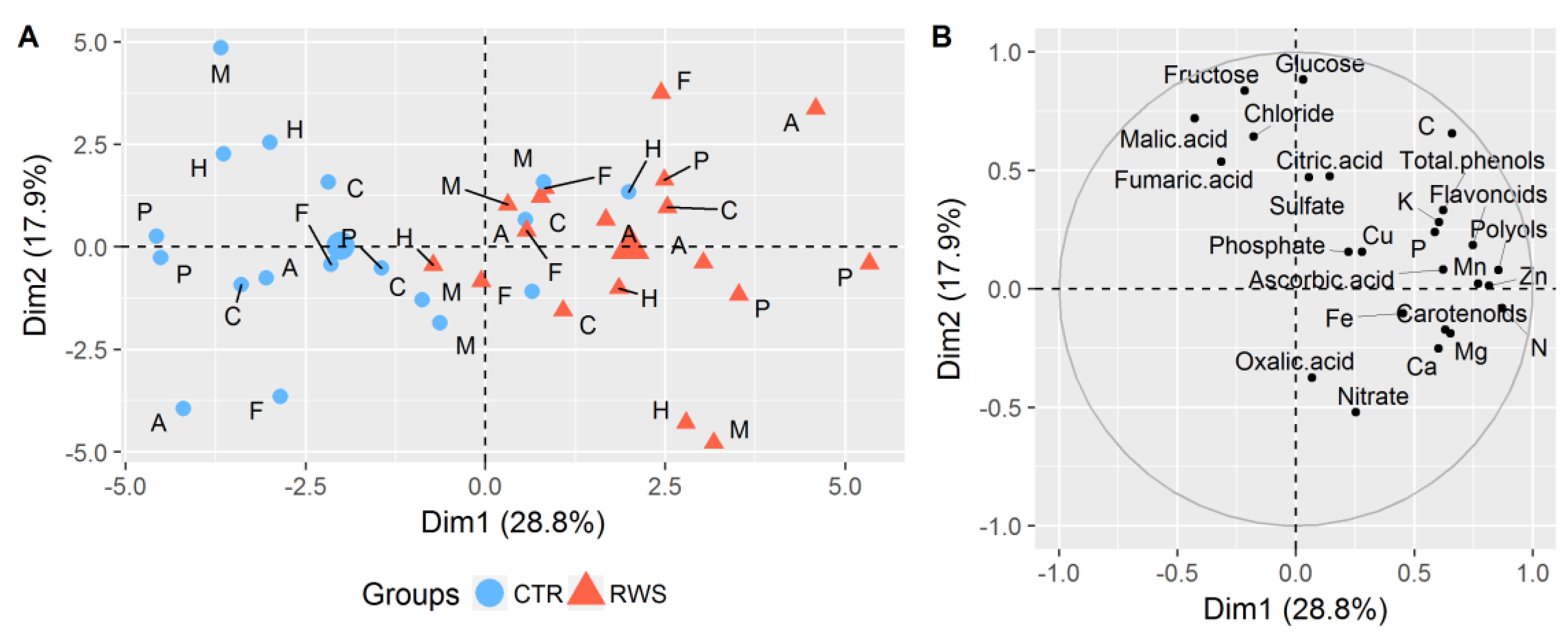
3.2. Effects of Reduced Water Supply on the Chemical Composition of Spinach Biomass
3.3. Sugars and Polyols
3.4. Organic Acids
3.5. Inorganic Anions
3.6. Carbon
3.7. Nitrogen
3.8. Potassium
3.9. Phosphorous
3.10. Calcium
3.11. Magnesium
3.12. Micronutrients (Fe, Zn, Mn, Cu)
3.13. Phenolic Compounds
3.14. Carotenoids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kundzewicz, Z.; Radziejewski, M.; Pińskwar, I. Precipitation Extremes in the Changing Climate of Europe. Clim. Res. 2006, 31, 51–58. [Google Scholar] [CrossRef]
- Kang, Y.; Khan, S.; Ma, X. Climate change impacts on crop yield, crop water productivity and food security—A review. Prog. Nat. Sci. 2009, 19, 1665–1674. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239. [Google Scholar] [CrossRef]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef] [Green Version]
- Parida, A.K.; Dagaonkar, V.S.; Phalak, M.S.; Umalkar, G.V.; Aurangabadkar, L.P. Alterations in photosynthetic pigments, protein and osmotic components in cotton genotypes subjected to short-term drought stress followed by recovery. Plant Biotech. Rep. 2007, 1, 37–48. [Google Scholar] [CrossRef]
- Koyama, R.; Itoh, H.; Kimura, S.; Morioka, A.; Uno, Y. Augmentation of Antioxidant Constituents by Drought Stress to Roots in Leafy Vegetables. HortTechnology 2012, 22, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Esteban, R.; Fleta-Soriano, E.; Buezo, J.; Míguez, F.; Becerril, J.M.; García-Plazaola, J.I. Enhancement of zeaxanthin in two-steps by environmental stress induction in rocket and spinach. Food Res. Int. 2014, 65, 207–214. [Google Scholar] [CrossRef]
- Costa, J.M.; Ortuño, M.F.; Chaves, M.M. Deficit Irrigation as a Strategy to Save Water: Physiology and Potential Application to Horticulture. J. Integr. Plant Biol. 2007, 49, 1421–1434. [Google Scholar] [CrossRef]
- Pérez-Bueno, M.L.; Pineda, M.; Barón, M. Phenotyping Plant Responses to Biotic Stress by Chlorophyll Fluorescence Imaging. Front. Plant Sci. 2019, 10, 1135. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Ainauer Associates Inc.: Sunderland, MA, USA, 2010; ISBN 978-0-87893-866-7. [Google Scholar]
- Preece, J.E.; Read, P.E. The biology of horticulture. In An Introductory Textbook, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; ISBN 0471465798. [Google Scholar]
- Bergquist, S.A.M.; Gertsson, U.E.; Olsson, M.E. Influence of growth stage and postharvest storage on ascorbic acid and carotenoid content and visual quality of baby spinach (Spinacia oleracea L.). J. Sci. Food Agric. 2006, 86, 346–355. [Google Scholar] [CrossRef]
- Toivonen, P.M.A.; Hodges, D.M. 10 Leafy Vegetables and Salads. In Health-Promoting Properties of Fruit and Vegetables, [Enhanced Credo Edition]; Terry, L.A., Ed.; CABI: Wallingford, UK; Cambridge, MA, USA; Boston, MA, USA, 2013; pp. 171–195. ISBN 978-1-78064-422-6. [Google Scholar]
- Herrmann, K. Inhaltsstoffe von Obst und Gemüse. 50 Tabellen und Übersichten, 97 Formeln; Ulmer: Stuttgart, Germany, 2001; ISBN 3-8001-3139-0. [Google Scholar]
- Gil, M.I.; Ferreres, F.; Tomás-Barberán, F.A. Effect of Modified Atmosphere Packaging on the Flavonoids and Vitamin C Content of Minimally Processed Swiss Chard (Beta vulgaris Subspecies cycla). J. Agric. Food Chem. 1998, 46, 2007–2012. [Google Scholar] [CrossRef]
- Gil, M.I.; Castañer, M.; Ferreres, F.; Artés, F.; Tomás-Barberán, F.A. Modified-atmosphere packaging of minimally processed “Lollo Rosso” (Lactuca sativa): Phenolic metabolites and quality changes. Z Lebensm Unters Forsch A 1998, 206, 350–354. [Google Scholar] [CrossRef]
- Gil, M.I.; Ferreres, F.; Tomás-Barberán, F.A. Effect of Postharvest Storage and Processing on the Antioxidant Constituents (Flavonoids and Vitamin C) of Fresh-Cut Spinach. J. Agric. Food Chem. 1999, 47, 2213–2217. [Google Scholar] [CrossRef]
- Santamaria, P.; Elia, A.; Serio, F.; Todaro, E. A survey of nitrate and oxalate content in fresh vegetables. J. Sci. Food Agric. 1999, 79, 1882–1888. [Google Scholar] [CrossRef]
- Schlering, C.B.; Dietrich, H.; Frisch, M.; Schreiner, M.; Schweiggert, R.; Will, F.; Zinkernagel, J. Chemical composition of field grown radish (Raphanus sativus L. var. sativus) as influenced by season and moderately reduced water supply. J. Appl. Bot. Food Qual. 2019, 343–354. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144. [Google Scholar]
- Koh, E.; Charoenprasert, S.; Mitchell, A.E. Effect of Organic and Conventional Cropping Systems on Ascorbic Acid, Vitamin C, Flavonoids, Nitrate, and Oxalate in 27 Varieties of Spinach (Spinacia oleracea L.). J. Agric. Food Chem. 2012, 60, 3144–3150. [Google Scholar] [CrossRef]
- Bergquist, S.A.M.; Gertsson, U.E.; Knuthsen, P.; Olsson, M.E. Flavonoids in baby spinach (Spinacia oleracea L.): Changes during plant growth and storage. J. Agric. Food Chem. 2005, 53, 9459–9464. [Google Scholar] [CrossRef]
- Britton, G. UV/visible spectroscopy. In Carotenoids: Volume 1B, Spectroscopy; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 1995; pp. 13–62. ISBN 3-7643-2909-2. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using {lme4}. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R.; RStudio, Inc.: Boston, MA, USA, 2016. [Google Scholar]
- Lenth, R.V. Least-Squares Means: The R Package lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82. [Google Scholar] [CrossRef] [Green Version]
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses, R package version 1.0.5; 2017; Available online: https://CRAN.R-project.org/package=factoextra (accessed on 27 March 2018).
- Mercadante, A.Z.; Rodriguez-Amaya, D.B. Carotenoid composition of a leafy vegetable in relation to some agricultural variables. J. Agric. Food Chem. 1991, 39, 1094–1097. [Google Scholar] [CrossRef]
- Schreiner, M. Vegetable crop management strategies to increase the quantity of phytochemicals. Eur. J. Nutr. 2005, 44, 85–94. [Google Scholar] [CrossRef]
- Britton, G. Functions of Intact Carotenoids. In Carotenoids: Volume 4: Natural Functions; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Basel: Basel, Switzerland, 2008; pp. 189–212. ISBN 978-3-7643-7499-0. [Google Scholar]
- Schweiggert, R.; Ziegler, J.; Metwali, E.; Mohamed, F.; Almaghrabi, O.; Kadasa, N.; Carle, R. Carotenoids in mature green and ripe red fruits of tomato (Solanum lycopersicum L.) grown under different levels of irrigation. Arch. Biol. Sci. 2017, 69, 305–314. [Google Scholar] [CrossRef]
- Radovich, T.J.K.; Kleinhenz, M.D.; Streeter, J.G. Irrigation Timing Relative to Head Development Influences Yield Components, Sugar Levels, and Glucosinolate Concentrations in Cabbage. J. Am. Soc. Hortic. Sci. 2005, 130, 943–949. [Google Scholar] [CrossRef]
- Proietti, S.; Moscatello, S.; Giacomelli, G.A.; Battistelli, A. Influence of the interaction between light intensity and CO2 concentration on productivity and quality of spinach (Spinacia oleracea L.) grown in fully controlled environment. Adv. Space Res. 2013, 52, 1193–1200. [Google Scholar] [CrossRef]
- Quick, W.P.; Chaves, M.M.; Wendler, R.; David, M.; Rodriguez, M.L.; Passaharinho, J.A.; Pereira, J.S.; Adcock, M.D.; Leegood, R.C.; Stitt, M. The effect of water stress on photosynthetic carbon metabolism in four species grown under field conditions. Plant Cell Environ. 1992, 15, 25–35. [Google Scholar] [CrossRef]
- Cutler, J.M.; Rains, D.W. Effects of Water Stress and Hardening on the Internal Water Relations and Osmotic Constituents of Cotton Leaves. Physiol. Plant. 1978, 42, 261–268. [Google Scholar] [CrossRef]
- Raschke, K. Stomatal Action. Annu. Rev. Plant Physiol. 1975, 26, 309–340. [Google Scholar] [CrossRef]
- Lester, G.E.; Makus, D.J.; Hodges, D.M.; Jifon, J.L. Summer (subarctic) versus winter (subtropic) production affects spinach (Spinacia oleracea L.) leaf bionutrients: Vitamins (C, E, folate, K1, provitamin A), lutein, phenolics, and antioxidants. J. Agric. Food Chem. 2013, 61, 7019–7027. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.C.; Nogueira, R.J.M.C.; Silva, M.; Albuquerque, M. Drought Stress and Plant Nutrition. Plant Stress 2010, 5, 32–41. [Google Scholar]
- Abdel Rahman, A.A.; Shalaby, A.F.; El Monayeri, M.O. Effect of moisture stress on metabolic products and ions accumulation. Plant Soil 1971, 34, 65–90. [Google Scholar] [CrossRef]
- Sørensen, J.N.; Jørgensen, U.; Kühn, B.F. Drought Effects on the Marketable and Nutritional Quality of Carrots. J. Sci. Food Agric. 1997, 74, 379–391. [Google Scholar] [CrossRef]
- Dubey, R.S.; Pessarakli, M. Physiological mechanisms of nitrogen absorption and assimilation in plants under stressful conditions. In Handbook of Plant and Crop Physiology, 2nd ed.; Pessarakli, M., Ed.; Dekker: New York, NY, USA, 2002; pp. 637–655. ISBN 0-8247-0546-7. [Google Scholar]
- Morilla, C.A.; Boyer, J.S.; Hageman, R.H. Nitrate reductase activity and polyribosomal content of corn (Zea mays L.) having low leaf water potentials. Plant Physiol. 1973, 51, 817. [Google Scholar] [CrossRef] [Green Version]
- Qiu, W.; Wang, Z.; Huang, C.; Chen, B.; Yang, R. Nitrate accumulation in leafy vegetables and its relationship with water. J. Soil Sci. Plant Nutr. 2014, 14, 761–768. [Google Scholar] [CrossRef]
- Ćustić, M.; Poljak, M.; Čoga, L.; Ćosić, T.; Toth, N.; Pecina, M. The influence of organic and mineral fertilization on nutrient status, nitrate accumulation, and yield of head chicory. Plant Soil Environ. 2003, 49, 218–222. [Google Scholar] [CrossRef]
- Anjana, S.U.; Iqbal, M. Nitrate accumulation in plants, factors affecting the process, and human health implications. A review. Agron. Sustain. Dev. 2007, 27, 45–57. [Google Scholar] [CrossRef]
- Santamaria, P.; Gonnella, M.; Elia, A.; Parente, A.; Serio, F. Ways if reducing rocket salad nitrate content. Acta Hortic. 2001, 529–536. [Google Scholar] [CrossRef]
- Proietti, S.; Moscatello, S.; Colla, G.; Battistelli, Y. The effect of growing spinach (Spinacia oleracea L.) at two light intensities on the amounts of oxalate, ascorbate and nitrate in their leaves. J. Hortic. Sci. Biotech. 2004, 79, 606–609. [Google Scholar] [CrossRef]
- He, M.; Dijkstra, F.A. Drought effect on plant nitrogen and phosphorus: A meta-analysis. New Phytol. 2014, 204, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Cardarelli, M.; Schwarz, D.; Franken, P.; Colla, G. Effects of Drought on Nutrient Uptake and Assimilation in Vegetable Crops. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 171–195. ISBN 978-3-642-32653-0. [Google Scholar]
- Junjittakarn, J.; Pimratch, S.; Jogloy, S.; Htoon, W.; Singkham, N.; Vorasoot, N.; Toomsan, B.; Holbrook, C.C.; Patanothai, A. Nutrient uptake of peanut genotypes under different water regimes. IJPP 2013, 7, 677–692. [Google Scholar] [CrossRef]
- Ashraf, M.; Shahbaz, M.; Qasim, A. Drought-induced modulation in growth and mineral nutrients of canola (Brassica napus L.). Pak. J. Bot. 2013, 45, 93–98. [Google Scholar]
- Utrillas, M.J.; Alegre, L.; Simon, E. Seasonal changes in production and nutrient content of Cynodon dactylon (L.) Pers. subjected to water deficits. Plant Soil 1995, 175, 153–157. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; del Mar Rubio-Wilhelmi, M.; Cervilla, L.M.; Blasco, B.; Rios, J.J.; Leyva, R.; Romero, L.; Ruiz, J.M. Study of the ionome and uptake fluxes in cherry tomato plants under moderate water stress conditions. Plant Soil 2010, 335, 339–347. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Maathuis, F.J.M. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef]
- McLaughlin, S.B.; Wimmer, R. Tansley Review No. 104 Calcium physiology and terrestrial ecosystem processes. New Phytol. 1999, 142, 373–417. [Google Scholar] [CrossRef]
- Pulupol, L.U.; Behboudian, M.H.; Fisher, K.J. Growth, Yield, and Postharvest Attributes of Glasshouse Tomatoes Produced under Deficit Irrigation. HortScience 1996, 31, 926–929. [Google Scholar] [CrossRef] [Green Version]
- Hertog, M.G.L.; Hollman, P.C.H.; Katan, M.B. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J. Agric. Food Chem. 1992, 40, 2379–2383. [Google Scholar] [CrossRef]
- Schmidt, S.; Zietz, M.; Schreiner, M.; Rohn, S.; Kroh, L.W.; Krumbein, A. Genotypic and climatic influences on the concentration and composition of flavonoids in kale (Brassica oleracea var. sabellica). Food Chem. 2010, 119, 1293–1299. [Google Scholar] [CrossRef]
- Aritomi, M.; Komori, T.; Kawasaki, T. Flavonol glycosides in leaves of Spinacia oleracea. Phytochemistry 1985, 25, 231–234. [Google Scholar] [CrossRef]

| Growing Season | 2015 | 2016 | 2017 |
|---|---|---|---|
| Sowing date (Year-month-day) | 2015-09-07 | 2016-04-19 | 2017-04-20 |
| Beginning of water supply differentiation (DAS) | 23 | 23 | 26 |
| Harvest date (DAS) | 51 | 44 | 39 |
| Temperature sum (°C) | 626.0 | 593.2 | 549.8 |
| Daily mean air temperature (°C) | 12.0 | 13.2 | 13.8 |
| Mean relative air humidity (%) | 78.8 | 68.7 | 67.8 |
| Global irradiation sum (MJ/m2) | 453.5 | 799.9 | 756.9 |
| Daily mean global irradiation (MJ/m2) | 8.7 | 17.8 | 18.9 |
| Wind speed sum (m/s) at height of 2 m | 46.0 | 69.3 | 50.8 |
| Evapotranspiration sum (mm) z | 68.7 | 126.9 | 126.6 |
| Precipitation sum (mm) | 65 | 101 | 68 |
| Number of differentiated irrigation events | 4 | 5 | 2 |
| Total irrigation (mm) y | 39 | 95 | 45 |
| Total water amount (mm) incl. precipitation | 104 | 184 | 113 |
| Total water amount of RWS (% of CTR) | 90% | 94% | 96% |
| Spinach 2015 | Spinach 2016 | Spinach 2017 | Spinach Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTR | RWS | p-Value | CTR | RWS | p-Value | CTR | RWS | p-Value | CTR | RWS | p-Value | |
| Leaf water potential Ψ (-MPa) | nd | nd | nd | 0.14 | 0.18 | 0.1136 | 0.07 | 0.093 | 0.0842 | nd | nd | nd |
| Dry biomass (%) | 8.54 | 8.60 | 0.6186 | 5.40 | 5.48 | 0.6736 | 6.13 | 6.83 | 0.0032 ** | 6.69 | 6.97 | 0.0216 * |
| Sugars and Polyols (mg/g) | ||||||||||||
| Glucose | 56.71 | 59.55 | 0.3342 | 72.92 | 62.70 | 0.1521 | 50.92 | 57.26 | 0.3902 | 60.18 | 59.84 | 0.9207 |
| Fructose | 43.35 | 43.59 | 0.9073 | 37.58 | 30.94 | 0.0721 | 27.70 | 29.41 | 0.6028 | 36.21 | 34.65 | 0.3965 |
| Polyols total | 5.91 | 5.91 | 0.9565 | 3.87 | 4.09 | 0.0302 * | 4.96 | 4.91 | 0.4331 | 4.91 | 4.97 | 0.2929 |
| Inositol | 1.66 | 1.68 | 0.3461 | 0.99 | 1.06 | 0.0830 | 1.48 | 1.54 | 0.1647 | 1.38 | 1.42 | 0.0119 * |
| Glycerol | 2.56 | 2.58 | 0.3724 | 1.67 | 1.70 | 0.2236 | 2.31 | 2.28 | 0.3984 | 2.18 | 2.19 | 0.7136 |
| Polyol unknown | 1.37 | 1.38 | 0.7343 | 0.71 | 0.79 | 0.0530 | 0.67 | 0.61 | 0.1224 | 0.91 | 0.93 | 0.5387 |
| Erythritol | 0.33 | 0.28 | 0.5110 | 0.51 | 0.55 | 0.2218 | 0.50 | 0.47 | 0.2676 | 0.44 | 0.43 | 0.6779 |
| Organic acids (mg/g) | ||||||||||||
| Oxalic acid | 49.89 | 44.51 | 0.0863 | 62.56 | 62.56 | 1.0000 | 53.70 | 52.46 | 0.8734 | 55.38 | 53.18 | 0.4438 |
| Total acidity | 16.73 | 18.73 | 0.1270 | 17.20 | 15.71 | 0.5452 | 16.87 | 18.26 | 0.2514 | 16.93 | 17.57 | 0.5028 |
| Malic acid | 12.81 | 12.63 | 0.7896 | 22.08 | 18.47 | 0.0103 * | 14.08 | 13.31 | 0.6206 | 16.33 | 14.80 | 0.0389 * |
| Ascorbic acid | 9.41 | 9.90 | 0.0493 * | 4.42 | 4.84 | 0.4642 | 6.85 | 6.70 | 0.7087 | 6.89 | 7.15 | 0.2866 |
| Citric acid | 6.19 | 6.14 | 0.8849 | 3.18 | 3.15 | 0.8647 | 2.94 | 3.40 | 0.1219 | 4.11 | 4.23 | 0.4872 |
| Fumaric acid | 0.61 | 0.57 | 0.0761 | 2.26 | 1.69 | 0.1530 | 1.30 | 1.11 | 0.5026 | 1.39 | 1.12 | 0.0803 |
| Anions (mg/g) | ||||||||||||
| Nitrate | 8.57 | 7.73 | 0.2291 | 28.16 | 28.60 | 0.5373 | 12.98 | 12.53 | 0.8169 | 16.57 | 16.29 | 0.6854 |
| Phosphate | 13.65 | 11.08 | 0.0377 * | 16.69 | 17.13 | 0.1913 | 13.12 | 12.44 | 0.2995 | 14.49 | 13.55 | 0.0594 |
| Sulfate | 3.44 | 2.64 | 0.0192 * | 7.73 | 6.56 | 0.0910 | 3.30 | 3.88 | 0.1040 | 4.82 | 4.36 | 0.1151 |
| Chloride | 5.38 | 4.95 | 0.1268 | 7.28 | 5.83 | 0.0692 | 8.61 | 7.98 | 0.1430 | 7.09 | 6.25 | 0.0053 ** |
| Elements (mg/g) | ||||||||||||
| Carbon | 381.17 | 380.38 | 0.7578 | 360.43 | 356.70 | 0.3442 | 359.68 | 362.83 | 0.3491 | 367.09 | 366.64 | 0.8316 |
| Macronutrients | ||||||||||||
| Potassium | 78.27 | 81.38 | 0.0297 * | 87.88 | 85.60 | 0.2540 | 90.68 | 89.89 | 0.7354 | 85.61 | 85.62 | 0.9932 |
| Nitrogen | 50.92 | 51.64 | 0.0682 | 38.98 | 38.07 | 0.7042 | 42.47 | 41.91 | 0.7088 | 44.12 | 43.87 | 0.7769 |
| Phosphorous | 5.59 | 5.93 | 0.0596 | 5.58 | 5.72 | 0.4096 | 5.22 | 5.00 | 0.1205 | 5.46 | 5.55 | 0.3650 |
| Calcium | 11.69 | 10.85 | 0.1553 | 14.78 | 14.94 | 0.8400 | 14.03 | 13.78 | 0.7268 | 13.50 | 13.19 | 0.4079 |
| Magnesium | 7.77 | 7.22 | 0.0196 * | 6.20 | 6.29 | 0.7254 | 7.57 | 7.45 | 0.7784 | 7.18 | 6.99 | 0.2684 |
| Micronutrients (µg/g) | ||||||||||||
| Iron | 323.89 | 273.68 | 0.2516 | 300.40 | 406.83 | 0.0586 | 348.97 | 336.95 | 0.6028 | 324.42 | 339.15 | 0.5711 |
| Zinc | 73.63 | 80.85 | 0.0234 * | 127.00 | 142.33 | 0.0245 * | 101.87 | 111.03 | 0.0458 * | 100.83 | 111.40 | 0.0001 *** |
| Manganese | 60.93 | 58.30 | 0.1549 | 85.46 | 100.41 | 0.0039 ** | 76.31 | 81.83 | 0.0711 | 74.23 | 80.18 | 0.0138 * |
| Copper | 15.21 | 15.75 | 0.8240 | 12.92 | 13.70 | 0.0112 * | 13.70 | 12.64 | 0.2121 | 13.94 | 14.03 | 0.9141 |
| Phenolic compounds (mg/g) | ||||||||||||
| Total phenols | 10.31 | 10.09 | 0.4565 | 11.26 | 11.80 | 0.3630 | 11.42 | 11.38 | 0.9294 | 11.00 | 11.08 | 0.7317 |
| Flavonoids | 9.91 | 10.08 | 0.9490 | 9.91 | 10.56 | 0.1919 | 9.82 | 10.16 | 0.4886 | 10.08 | 9.88 | 10.27 |
| Carotenoids (mg/g) | ||||||||||||
| Violaxanthin | 1.13 | 1.18 | 0.1067 | 0.82 | 0.84 | 0.5267 | 1.78 | 1.71 | 0.5954 | 1.24 | 1.24 | 0.9793 |
| Neoxanthin | 0.38 | 0.39 | 0.1106 | 0.27 | 0.26 | 0.3837 | 0.65 | 0.64 | 0.8515 | 0.43 | 0.43 | 0.8136 |
| Lutein | 1.35 | 1.39 | 0.1431 | 0.94 | 0.90 | 0.6399 | 2.53 | 2.54 | 0.9492 | 1.61 | 1.61 | 0.9091 |
| ß-Carotin | 1.00 | 1.04 | 0.0558 | 0.91 | 0.95 | 0.3429 | 2.05 | 2.09 | 0.7092 | 1.32 | 1.36 | 0.2836 |
| 9-cis-ß-Carotin | 0.11 | 0.12 | 0.0756 | 0.097 | 0.102 | 0.2031 | 0.248 | 0.250 | 0.9031 | 0.15 | 0.16 | 0.3772 |
| Total carotenoids | 3.98 | 4.11 | 0.0972 | 3.03 | 3.05 | 0.8677 | 7.25 | 7.22 | 0.9587 | 4.75 | 4.80 | 0.7542 |
| Spinach 2015 | Spinach 2016 | Spinach 2017 | Spinach Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTR | RWS | p-Value | CTR | RWS | p-Value | CTR | RWS | p-Value | CTR | RWS | p-Value | |
| Leaf water potential Ψ (-MPa) | nd | nd | nd | 0.14 | 0.18 | 0.1136 | 0.07 | 0.093 | 0.0842 | nd | nd | nd |
| Dry biomass (%) | 8.54 | 8.60 | 0.6186 | 5.40 | 5.48 | 0.6824 | 6.13 | 6.83 | 0.0032 ** | 6.69 | 6.97 | 0.0216 * |
| Sugars and Polyols (mg/100 g) | ||||||||||||
| Glucose | 536.71 | 563.58 | 0.3345 | 402.68 | 346.22 | 0.1522 | 318.31 | 357.98 | 0.3903 | 345.00 | 372.50 | 0.4335 |
| Fructose | 410.34 | 412.52 | 0.9085 | 207.51 | 170.87 | 0.0721 | 173.19 | 183.83 | 0.6033 | 223.89 | 240.56 | 0.3937 |
| Polyols total | 49.83 | 49.99 | 0.8777 | 20.66 | 23.29 | 0.0065 ** | 32.81 | 34.80 | 0.0408 * | 34.43 | 36.03 | 0.0048 ** |
| Inositol | 14.16 | 14.29 | 0.5232 | 5.26 | 6.02 | 0.0127 * | 9.09 | 10.53 | 0.0170 * | 9.50 | 10.28 | 0.0013 ** |
| Glycerol | 21.73 | 21.97 | 0.5287 | 8.91 | 9.67 | 0.0022 ** | 14.16 | 15.56 | 0.0115 * | 14.93 | 15.73 | 0.0013 ** |
| Polyol unknown | 11.63 | 11.76 | 0.7103 | 3.78 | 4.48 | 0.0088 ** | 4.09 | 4.22 | 0.5782 | 6.50 | 6.82 | 0.0476 * |
| Erythritol | 2.32 | 1.96 | 0.4704 | 2.72 | 3.12 | 0.0809 | 5.46 | 4.49 | 0.0264 * | 3.50 | 3.19 | 0.2038 |
| Organic acids (mg/100 g) | ||||||||||||
| Oxalic acid | 472.07 | 421.23 | 0.0863 | 345.47 | 345.47 | 1.0000 | 335.73 | 327.99 | 0.8732 | 384.42 | 364.90 | 0.3067 |
| Total acidity | 158.32 | 177.21 | 0.1271 | 94.97 | 86.74 | 0.5455 | 105.46 | 114.17 | 0.2515 | 119.58 | 126.04 | 0.3662 |
| Malic acid | 121.21 | 119.54 | 0.7926 | 121.93 | 101.96 | 0.0103 * | 88.05 | 83.21 | 0.6203 | 110.39 | 101.57 | 0.0528 |
| Ascorbic acid | 80.49 | 85.30 | 0.0568 | 23.62 | 27.50 | 0.2745 | 41.96 | 46.02 | 0.1822 | 48.69 | 52.94 | 0.0093 ** |
| Citric acid | 58.61 | 58.10 | 0.8880 | 17.57 | 17.42 | 0.8771 | 18.39 | 21.29 | 0.1219 | 31.52 | 32.27 | 0.5873 |
| Fumaric acid | 5.72 | 5.12 | 0.1202 | 11.91 | 9.48 | 0.2684 | 7.81 | 7.62 | 0.9169 | 8.48 | 7.41 | 0.2225 |
| Anions (mg/100 g) | ||||||||||||
| Nitrate | 72.08 | 65.80 | 0.2778 | 150.48 | 162.49 | 0.0238 * | 79.17 | 84.78 | 0.6029 | 100.58 | 104.35 | 0.4025 |
| Phosphate | 115.97 | 95.26 | 0.0421 * | 88.92 | 97.02 | 0.0027 ** | 80.25 | 84.77 | 0.3201 | 95.04 | 92.35 | 0.5248 |
| Sulfate | 29.12 | 22.64 | 0.0189 * | 41.28 | 37.07 | 0.2234 | 20.18 | 26.39 | 0.0371 * | 30.20 | 28.70 | 0.4426 |
| Chloride | 46.13 | 43.02 | 0.1800 | 39.00 | 32.69 | 0.1556 | 52.82 | 54.48 | 0.6515 | 45.98 | 43.40 | 0.1913 |
| Elements (mg/100 g) | ||||||||||||
| Carbon | 3.26 | 3.28 | 0.8151 | 1.94 | 1.95 | 0.9071 | 2.20 | 2.48 | 0.0105 * | 2.47 | 2.57 | 0.0687 |
| Macronutrients | ||||||||||||
| Potassium | 662.16 | 694.89 | 0.0185 * | 468.56 | 485.03 | 0.1523 | 555.12 | 613.09 | 0.0103 * | 561.95 | 597.67 | 0.0002 *** |
| Nitrogen | 407.22 | 410.14 | 0.7606 | 176.17 | 202.64 | 0.0065 ** | 238.58 | 262.42 | 0.0097 ** | 273.99 | 291.73 | 0.0013 ** |
| Phosphorous | 47.65 | 50.96 | 0.0984 | 29.77 | 32.39 | 0.0135 * | 31.94 | 34.09 | 0.0297 * | 36.45 | 39.15 | 0.0004 *** |
| Calcium | 99.76 | 93.89 | 0.1860 | 79.13 | 84.88 | 0.2441 | 86.12 | 94.28 | 0.1152 | 88.34 | 91.02 | 0.3357 |
| Magnesium | 66.71 | 62.63 | 0.0117 * | 33.16 | 35.67 | 0.1156 | 46.48 | 50.92 | 0.1760 | 48.78 | 49.74 | 0.4889 |
| Micronutrients (mg/100 g) | ||||||||||||
| Iron | 2.75 | 2.34 | 0.2768 | 1.60 | 2.31 | 0.0314 * | 2.14 | 2.30 | 0.2856 | 2.16 | 2.31 | 0.4017 |
| Zinc (µg/100 g) | 632.02 | 699.59 | 0.0021 ** | 678.36 | 807.78 | 0.0030 ** | 625.07 | 760.62 | 0.0064 ** | 645.15 | 756.00 | 0.0001 *** |
| Manganese (µg/100 g) | 519.79 | 501.32 | 0.2620 | 456.93 | 568.91 | 0.0009 *** | 467.35 | 558.30 | 0.0112 * | 481.35 | 542.84 | 0.0022 ** |
| Copper (µg/100 g) | 128.94 | 135.43 | 0.7665 | 68.96 | 77.63 | 0.0004 *** | 84.02 | 86.30 | 0.6883 | 93.97 | 99.79 | 0.4075 |
| Phenolic compounds (mg/100 g) | ||||||||||||
| Total phenols | 88.00 | 86.23 | 0.4172 | 60.78 | 64.93 | 0.4388 | 70.09 | 77.85 | 0.1354 | 72.95 | 76.34 | 0.1695 |
| Flavonoids | 86.52 | 86.93 | 0.8607 | 54.45 | 59.17 | 0.3192 | 62.81 | 73.40 | 0.0495 * | 67.93 | 73.17 | 0.0308 * |
| Carotenoids (mg/100 g) | ||||||||||||
| Violaxanthin | 9.66 | 10.05 | 0.1835 | 5.13 | 5.99 | 0.0462 * | 11.15 | 12.09 | 0.3774 | 8.65 | 9.38 | 0.0439 * |
| Neoxanthin | 3.18 | 3.28 | 0.1826 | 1.70 | 1.86 | 0.1231 | 4.07 | 4.55 | 0.1484 | 2.98 | 3.23 | 0.0289 * |
| Lutein | 11.52 | 11.87 | 0.2574 | 5.83 | 6.39 | 0.2920 | 15.91 | 18.00 | 0.1553 | 11.09 | 12.09 | 0.0474 * |
| ß-Carotene | 8.54 | 8.88 | 0.1166 | 5.68 | 6.73 | 0.0311 * | 12.85 | 14.81 | 0.1022 | 9.02 | 10.14 | 0.0074 ** |
| 9-cis-ß-Carotene | 0.98 | 1.00 | 0.4962 | 0.60 | 0.71 | 0.0123 * | 1.55 | 1.77 | 0.1128 | 1.04 | 1.16 | 0.0138 * |
| Total carotenoids | 33.88 | 35.07 | 0.1866 | 18.94 | 21.68 | 0.0680 | 45.53 | 51.21 | 0.1728 | 32.78 | 35.99 | 0.0233 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlering, C.; Zinkernagel, J.; Dietrich, H.; Frisch, M.; Schweiggert, R. Alterations in the Chemical Composition of Spinach (Spinacia oleracea L.) as Provoked by Season and Moderately Limited Water Supply in Open Field Cultivation. Horticulturae 2020, 6, 25. https://doi.org/10.3390/horticulturae6020025
Schlering C, Zinkernagel J, Dietrich H, Frisch M, Schweiggert R. Alterations in the Chemical Composition of Spinach (Spinacia oleracea L.) as Provoked by Season and Moderately Limited Water Supply in Open Field Cultivation. Horticulturae. 2020; 6(2):25. https://doi.org/10.3390/horticulturae6020025
Chicago/Turabian StyleSchlering, Christine, Jana Zinkernagel, Helmut Dietrich, Matthias Frisch, and Ralf Schweiggert. 2020. "Alterations in the Chemical Composition of Spinach (Spinacia oleracea L.) as Provoked by Season and Moderately Limited Water Supply in Open Field Cultivation" Horticulturae 6, no. 2: 25. https://doi.org/10.3390/horticulturae6020025
APA StyleSchlering, C., Zinkernagel, J., Dietrich, H., Frisch, M., & Schweiggert, R. (2020). Alterations in the Chemical Composition of Spinach (Spinacia oleracea L.) as Provoked by Season and Moderately Limited Water Supply in Open Field Cultivation. Horticulturae, 6(2), 25. https://doi.org/10.3390/horticulturae6020025