Light and Microbial Lifestyle: The Impact of Light Quality on Plant–Microbe Interactions in Horticultural Production Systems—A Review
Abstract
1. Introduction
- (i)
- Which light-dependent plant processes and mechanisms are decisive for phyllosphere colonizers?
- (ii)
- Which morphological plant characteristics are modified by light quality and consequently influence the structure and/or function of the phyllosphere microbiome?
- (iii)
- Which light-quality-dependent microbial processes and mechanisms affect plant traits?
- (iv)
- Which ecological principles and theories apply to microbiome effects in the phyllosphere with regard to artificial illumination?
2. Materials and Methods
3. Abiotic Effects of Light on the Leaf Microbiota
3.1. Impact of Lighting Technology on Leaf Temperature, Leaf Moisture, and Humidity
3.2. Effects of Ultraviolet (UV) Light
3.3. Effects of Far Red (FR) Light
4. Plant-Mediated Effects of Light on the Leaf Microbiota
4.1. Plant–Light Interactions
4.1.1. Plant Architecture and Leaf Morphology
4.1.2. Photosynthesis
4.1.3. Primary and Secondary Metabolism
4.1.4. Plant Defense Mechanisms
4.2. Direct Plant–Microbe Interactions Induced by Light
4.2.1. Leaf Leachate
4.2.2. Light-Triggered Pathways
4.2.3. Changes in Leaf Physiological Characteristics
5. Light-Quality-Mediated Effects on the Leaf Microbiota
5.1. Leaf Pathogens
5.2. Microbial Biocontrol Agents
5.3. Molecular Interactions
6. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Aleklett, K.; Hart, M.; Shade, A. The microbial ecology of flowers: An emerging frontier in phyllosphereresearch. Botany 2014, 92. [Google Scholar] [CrossRef]
- Andreote, F.D.; Gumiere, T.; Durrer, A. Exploring interactions of plant microbiomes. Sci. Agric. 2014, 71, 528–539. [Google Scholar] [CrossRef]
- Beilsmith, K.; Thoen, M.P.M.; Brachi, B.; Gloss, A.D.; Khan, M.H.; Bergelson, J. Genome-wide association studies on the phyllosphere microbiome: Embracing complexity in host-microbe interactions. Plant J. 2018, 97, 164–181. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Grube, M.; Schloter, M.; Smalla, K. Unraveling the plant microbiome: Looking back and future perspectives. Front. Microbiol. 2014, 5, 148. [Google Scholar] [CrossRef]
- Brader, G.; Compant, S.; Vescio, K.; Mitter, B.; Trognitz, F.; Ma, L.J.; Sessitsch, A. Ecology and genomic insights into plant-pathogenic and plant-nonpathogenic endophytes. Ann. Rev. Phytopathol. 2017, 55, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Bringel, F.; Couee, I. Pivotal roles of phyllosphere microorganisms at the interface between plant functioning and atmospheric trace gas dynamics. Front. Microbiol. 2015, 6, 486. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; van Themaat, E.V.L.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Ann. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.D.; Castillo, J.A. Influence of light on plant-phyllosphere interaction. Front. Plant Sci. 2018, 9, 1482. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, D.; Kumar, R.; Sihag, K.; Rashmi; Kumari, A. Phyllospheric microflora and its impact on plant growth: A review. Agric. Rev. 2017, 38, 51–59. [Google Scholar] [CrossRef]
- Farre-Armengol, G.; Filella, I.; Llusia, J.; Penuelas, J. Bidirectional interaction between phyllospheric Microbiotas and plant volatile emissions. Trends Plant Sci. 2016, 21, 854–860. [Google Scholar] [CrossRef]
- Finkel, O.M.; Castrillo, G.; Paredes, S.H.; Gonzalez, I.S.; Dangl, J.L. Understanding and exploiting plant beneficial microbes. Curr. Opin. Plant Biol. 2017, 38, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, X.; Dong, Z.; Liu, M.; Dai, L. Advance of phyllosphere microorganisms and their interaction with the outside environment. Plant Sci. J. 2016, 34, 654–661. [Google Scholar]
- Iguchi, H.; Yurimoto, H.; Sakai, Y. Interactions of methylotrophs with plants and other heterotrophic bacteria. Microorganisms 2015, 3, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.R.; Stone, B.W.G.; Tyler, H.L. Emerging perspectives on the natural microbiome of fresh produce vegetables. Agriculture 2015, 5, 170–187. [Google Scholar] [CrossRef]
- Kowalchuk, G.A.; Yergeau, E.; Leveau, J.H.J.; Sessitsch, A.; Bailey, M. Plant-Associated Microbial Communities. In Environmental Molecular Microbiology; Lui, W.-T., Jansson, J., Eds.; Caister Academic Press: Norfolk, UK, 2010; pp. 131–148. [Google Scholar]
- Lemanceau, P.; Barret, M.; Mazurier, S.; Mondy, S.; Pivato, B.; Fort, T.; Vacher, C. Plant communication with associated microbiota in the spermosphere, rhizosphere and phyllosphere. Adv. Bot. Res. 2017, 82, 101–133. [Google Scholar] [CrossRef]
- Leveau, J.H.J. Microbiology Life on leaves. Nature 2009, 461, 741. [Google Scholar] [CrossRef] [PubMed]
- Markland, S.M.; Kniel, K.E. Human pathogen-plant interactions: Concerns for food safety. Adv. Bot. Res. 2015, 75, 115–135. [Google Scholar] [CrossRef]
- Meyer, K.M.; Leveau, J.H.J. Microbiology of the phyllosphere: A playground for testing ecological concepts. Oecologia 2012, 168, 621–629. [Google Scholar] [CrossRef]
- Mueller, D.B.; Schubert, O.T.; Roest, H.; Aebersold, R.; Vorholt, J.A. Systems-level Proteomics of Two Ubiquitous Leaf Commensals Reveals Complementary Adaptive Traits for Phyllosphere Colonization. Mol. Cell. Proteom. 2016, 15, 3256–3269. [Google Scholar] [CrossRef]
- Mueller, T.; Ruppel, S. Progress in cultivation-independent phyllosphere microbiology. Fems Microbiol. Ecol. 2014, 87, 2–17. [Google Scholar] [CrossRef]
- Muller, D.B.; Vogel, C.; Bai, Y.; Vorholt, J.A. The plant microbiota: Systems-level insights and perspectives. Ann. Rev. Genet. 2016, 50, 211–234. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, G.; Sbodio, A.; Tech, J.J.; Suslow, T.V.; Coaker, G.L.; Leveau, J.H.J. Leaf microbiota in an agroecosystem: Spatiotemporal variation in bacterial community composition on field-grown lettuce. ISME J. 2012, 6, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Remus-Emsermann, M.N.P.; Tecon, R.; Kowalchuk, G.A.; Leveau, J.H.J. Variation in local carrying capacity and the individual fate of bacterial colonizers in the phyllosphere. ISME J. 2012, 6, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Saikkonen, K.; Mikola, J.; Helander, M. Endophytic phyllosphere fungi and nutrient cycling in terrestrial ecosystems. Curr. Sci. 2015, 109, 121–126. [Google Scholar]
- Schlaeppi, K.; Bulgarelli, D. The plant microbiome at work. Mol. Plant Microbe Interact. 2015, 28, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Prasanna, R. Prospecting the characteristics and significance of the phyllosphere microbiome. Ann. Microbiol. 2018, 68, 229–245. [Google Scholar] [CrossRef]
- Vacher, C.; Hampe, A.; Porte, A.J.; Sauer, U.; Compant, S.; Morris, C.E. The phyllosphere: Microbial jungle at the plant-climate interface. Ann. Rev. Ecol. Evol. Syst. 2016, 47, 1–24. [Google Scholar] [CrossRef]
- Vogel, C.; Bodenhausen, N.; Gruissem, W.; Vorholt, J.A. The Arabidopsis leaf transcriptome reveals distinct but also overlapping responses to colonization by phyllosphere commensals and pathogen infection with impact on plant health. New Phytol. 2016, 212, 192–207. [Google Scholar] [CrossRef] [PubMed]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef]
- Vorholt, J.A. The phyllosphere microbiome: Responses to and impacts on plants. Phytopathology 2014, 104, 155. [Google Scholar]
- Whipps, J.M.; Hand, P.; Pink, D.; Bending, G.D. Phyllosphere microbiology with special reference to diversity and plant genotype. J. Appl. Microbiol. 2008, 105, 1744–1755. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Chen, Y.; Wang, X.X.; Dai, C.C. Plant symbionts: Keys to the phytosphere. Symbiosis 2013, 59, 1–14. [Google Scholar] [CrossRef]
- Beattie, G.A.; Lindow, S.E. The secret life of bacterial pathogens on plants. Ann. Rev. Phytopathol. 1998, 33, 145–172. [Google Scholar] [CrossRef] [PubMed]
- Beattie, G.A.; Lindow, S.E. Bacterial colonization of leaves: A spectrum of strategies. Phytopathology 1999, 89, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Leveau, J.H.J. Microbial communities in the phyllosphere. In Biology of the Plant Cuticle; Riederer, M., Müller, C., Eds.; Blackwell Publishing: Oxford, UK, 2006; pp. 334–367. [Google Scholar]
- Lindow, S.E.; Brandl, M.T. Microbiology of the phyllosphere. Appl. Environ. Microbiol. 2003, 69, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Diaz, B.M.; Biurrun, R.; Moreno, A.; Nebreda, M.; Fereres, A. Impact of ultraviolet-blicking plastic films on insect vectors of virus diseases infesting crisp lettuce. HortScience 2006, 41, 711–716. [Google Scholar] [CrossRef]
- Hemming, S. Use of natural and artificial light in horticulture—Interaction of plant and technology. Acta Hortic. 2011, 907, 25–36. [Google Scholar] [CrossRef]
- Stamps, R.H. Use of colored shade netting in horticulture. HortScience 2009, 44, 239–241. [Google Scholar] [CrossRef]
- Philips. Master Agro 400W E40 1SL/12. 2018. Available online: http://www.lighting.philips.se/prof/konventionella-lampor-och-lysroer/urladdningslampor/hid-horticulture/horti/928144609201_EU/product (accessed on 7 January 2019).
- Philips. Master HPI-T Plus 400 W/643. Philips Lighting Holding B.V. 2018. Available online: http://www.lighting.philips.com/main/prof/conventional-lamps-and-tubes/high-intensity-discharge-lamps/quartz-metal-halide/master-hpi-t-plus/928483500191_EU/product (accessed on 7 January 2019).
- Heliospectra. Heliospectra EOS. Heliospectra AB: 2018. Available online: https://www.heliospectra.com/led-grow-lights/eos/ (accessed on 7 January 2019).
- Senmatic. Senmatic FL300. Senmatic A/S: 2018. Available online: https://www.senmatic.com/horticulture/products/led-fixtures/fl300-grow-white (accessed on 7 January 2019).
- Valoya. Valoya Product Brochure. Valoya Oy: 2018. Available online: http://www.valoya.com/brochures/ (accessed on 7 January 2019).
- Ahn, S.Y.; Kim, S.A.; Yun, H.K. Inhibition of Botrytis cinerea and accumulation of stilbene compounds by light-emitting diodes of grapevine leaves and differential expression of defense-related genes. Eur. J. Plant Pathol. 2015, 143, 753–765. [Google Scholar] [CrossRef]
- Reuveni, R.; Raviv, M. Control of downy mildew in greenhouse-grown cucumbers using blue photoselective polyethylene sheets. Plant Dis. 1997, 81, 999–1004. [Google Scholar] [CrossRef]
- Suthaparan, A.; Stensvand, A.; Solhaug, K.A.; Torre, S.; Telfer, K.H.; Ruud, A.K.; Mortensen, L.M.; Gadoury, D.M.; Seem, R.C.; Gislerød, H.R. Suppression of cucumber powdery mildew by supplemental UV-B radiation in greenhouses can be augmented or reduced by background radiation quality. Plant Dis. 2014, 98, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Suthaparan, A.; Stensvand, A.; Torre, S.; Herrero, M.L.; Pettersen, R.; Gadoury, D.M.; Gislerød, H.R. Continuous lighting reduces conidial production and germinability in the rose powdery mildew pathosystem. Plant Dis. 2010, 94, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; McGrane, R.S.; Beattie, G.A. Light regulation of swarming motility in Pseudomonas syringae integrates signaling pathways mediated by a bacteriophytochrome and a LOV protein. mBio 2013, 4, e00334-13. [Google Scholar] [CrossRef] [PubMed]
- Alsanius, B.W.; Bergstrand, K.J.; Hartmann, R.; Gharaie, S.; Wohanka, W.; Dorais, M.; Rosberg, A.K. Ornamental flowers in new light: Artificial lighting shpares the microbial phyllosphere community structure of greenhouse grown sunflowers (Helianthus annuus L.). Sci. Hortic. 2017, 216, 234–247. [Google Scholar] [CrossRef]
- Alsanius, B.W.; Vaas, L.A.I.; Gharaie, S.; Karlsson, M.E.; Rosberg, A.K.; Grudén, M.; Wohanka, W.; Khalil, S.; Windstam, S. Dining in blue light impairs the appetite of some leaf epiphytes. Manuscript 2019. [Google Scholar]
- Gharaie, S.; Vaas, L.A.I.; Rosberg, A.K.; Windstam, S.T.; Karlsson, M.E.; Bergstrand, K.J.; Khalil, S.; Wohanka, W.; Alsanius, B.W. Light spectrum modifies the utilization pattern of energy sources in Pseudomonas sp. PLoS ONE 2017, 12, e0189862. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group, Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Nelson, J.A.; Bugbee, B. Analysis of environmental effects on leaf temperature under sunlight, high pressure sodium and light emitting diodes. PLoS ONE 2015, 10, e0138930. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2014, 121, 66–74. [Google Scholar] [CrossRef]
- Bergstrand, K.J.; Schüssler, H.K. Growth, development and photosynthesis of some horticultural plants as affected by different supplementary lighting technologies. Eur. J. Hortic. Sci. 2013, 78, 119–125. [Google Scholar]
- Demotes-Mainard, S.; Péron, T.; Corot, A.; Bertheloot, J.; Le Gourriere, J.; Pelleschi-Travier, S.; Crespel, L.; Morel, P.; Huché-Thélier, L.; Boumaz, R.; et al. Plant responses to red and far-red lights, applications in horticulture. Environ. Exp. Bot. 2016, 121, 4–21. [Google Scholar] [CrossRef]
- Mortensen, L.M. The effect of photon flux density and lighting period on growth, flowering, powdery mildew and water relations of miniature roses. Am. J. Plant Sci. 2014, 5, 1813–1818. [Google Scholar] [CrossRef][Green Version]
- O’Neill, T.M.; Shtienberg, D.; Elad, Y. Effect of some host and microclimate factors on infection of tomato stems by Botrytis cinerea. Plant Dis. 1997, 81, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.; Niu, G.; Takagaki, M. (Eds.) Physical environmental factors and their properties. In Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Elsevier Academic Press: Amsterdam, The Netherlands, 2016; pp. 129–140. [Google Scholar]
- Rünger, W. Licht und Temperatur im Zierpflanzenbau; Paul Parey: Berlin, Germany, 1964. [Google Scholar]
- Raviv, M.; Antignus, Y. UV radiation effects on pathogens and insect pests of greenhouse-grown crops. Photochem. Photobiol. 2004, 79, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Von Zabeltitz, C. Cladding material. In Integrated Greenhouse Systems for Mild Climates; Springer: Heidelberg, Germany, 2011; pp. 145–167. [Google Scholar]
- Waaijenberg, D. Design, construction and maintenance of greenhouse structures. Acta Hortic. 2004, 710, 31–42. [Google Scholar] [CrossRef]
- Ballaré, C.L. Light regulation of plant defense. Ann. Rev. Plant Biol. 2014, 65, 335–363. [Google Scholar] [CrossRef] [PubMed]
- Clifford, S.C.; Runkle, E.S.; Langton, F.A.; Mead, A.; Foster, S.A.; Pearson, S.; Heins, R.D. Height control of poinsettia using photoselective filters. HortScience 2004, 39, 383–387. [Google Scholar] [CrossRef]
- Mata, D.A.; Botto, J.F. Manipulation of light environment to produce high-quality poinsettia plants. HortScience 2009, 44, 702–706. [Google Scholar] [CrossRef]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1972, 9, 191–216. [Google Scholar] [CrossRef]
- Massa, G.; Graham, T.; Haire, T.; Flemming, C.; Newsham, G.; Wheeler, R. Light-emitting diode light transmission through leaf tissue of seven different crops. HortScience 2015, 50, 501–506. [Google Scholar] [CrossRef]
- Bergstrand, K.J.; Mortensen, L.M.; Suthaparan, A.; Gislerød, H.R. Acclimatisation of greenhouse crops to differing light quality. Sci. Hortic. 2016, 204, 1–7. [Google Scholar] [CrossRef]
- Lin, K.H.; Huang, M.Y.; Huang, W.D.; Hsu, M.H.; Yang, Z.W.; Yang, C.M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Fankhauser, C.; Ulm, R. A photoreceptor’s on-off switch. Science 2016, 354, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Folta, K.M.; Carvalho, S.D. Photoreceptors and control of horticultural plant traits. HortScience 2015, 50, 1274–1280. [Google Scholar] [CrossRef]
- Huché-Thélier, L.; Crespel, L.; Le Gourrierec, J.; Morel, P.; Sakr, S.; Leduc, N. Light signaling and plant responses to blue light and UV radiation—Perspectives for applications in horticulture. Environ. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Lercari, B.; Bretzel, F.; Piazza, S. Effects of UV Treatments on Stem Growth of Some Greenhouse Crops. Act Hortic. 1992, 327, 99–104. [Google Scholar] [CrossRef]
- Abidi, F.; Girault, T.; Douillet, O.; Guillemain, G.; Sintes, G.; Laffaire, M.; Ahmed, H.B.; Smiti, S.; Huché-Thélier, L.; Leduc, N. Blue light effects on rose photosynthesis and photomorphogenesis. Plant Biol. 2013, 15, 67–74. [Google Scholar] [CrossRef]
- Glowacka, B. The effect of blue light on the height and habit of the tomato (Lycopersicon esculentum Mill.) transplant. Folia Hortic. 2004, 16, 3–10. [Google Scholar]
- Piszczek, P.; Głowacka, B. Effect of the colour of light on cucumber (Cucumis sativus L.) seedlings. Veg. Crop. Res. Bull. 2008, 68, 71–80. [Google Scholar] [CrossRef]
- Terfa, M.T.; Roro, A.G.; Olsen, J.E.; Torre, S. Effects of UV radiation on growth and postharvest characteristics of three pot rose cultivars grown at different altitudes. Sci. Hortic. 2014, 178, 184–191. [Google Scholar] [CrossRef]
- Torre, S.; Roro, A.G.; Bengtsson, S.; Mortensen, L.; Solhaug, K.A.; Gislerød, H.R.; Olsen, J.E. Control of plant morphology by UV-B and UV-B-temperature interactions. Acta Hortic. 2012, 956, 207–214. [Google Scholar] [CrossRef]
- Islam, M.A.; Kuwar, G.; Clarke, J.L.; Blystad, D.R.; Gislerød, H.R.; Olsen, J.E.; Torre, S. Artificial light from light emitting diodes (LEDs) with a high portion of blue light results in shorter poinsettias compared to high pressure sodium (HPS) lamps. Sci. Hortic. 2012, 147, 136–143. [Google Scholar] [CrossRef]
- Brown, C.S.; Schuerger, A.C.; Sager, J.C. Growth and photomorphogenesis of pepper plants under red light-emitting diodes with supplemental blue or far-red lighting. J. Am. Soc. Hortic. Sci. 1995, 120, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Ohashi-Kaneko, K.; Takase, M.; Kon, N.; Fujiwara, K.; Kurata, K. Effect of light quality on growth and vegetable quality in leaf lettuce, spinach and komatsuna. Environ. Control Biol. 2007, 45, 189–198. [Google Scholar] [CrossRef]
- Wargent, J.J.; Taylor, A.; Paul, N.D. UV supplementation for growth and disease control. Acta Hortic. 2006, 711, 333–338. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- O’Carrigan, A.; Babla, M.; Wang, F.; Liu, X.; Mak, M.; Thomas, R.; Bellotti, B.; Chen, Z.H. Analysis of gas exchange, stomatal behaviour and micronutrients uncovers dynamic response and adaptation of tomato plants to monochromatic light treatments. Plant Physiol. Biochem. 2014, 82, 105–115. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, C.A.; Kim, Y.H.; Yun, S.J. Shorter wavelength blue light promotes growth of green perilla (Perilla frutescens). Int. J. Agric. Biol. 2014, 16, 1177–1182. [Google Scholar]
- Nanya, K.; Ishigami, Y.; Hikosaka, S.; Goto, E. Effects of blue and red light on stem elongation and flowering of tomato seedlings. Acta Hortic. 2012, 956, 261–266. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Meinen, E.; van Ieperen, W. Finding the optimal growth-light spectrum for greenhouse crops. Acta Hortic. 2012, 956, 357–363. [Google Scholar] [CrossRef]
- Young, H.M.; George, S.; Narváez, D.F.; Srivastava, P.; Schuerger, A.C.; Wright, D.L.; Marois, J.J. Effect of solar radiation on severity of soybean rust. Phytopathology 2012, 102, 794–803. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamasaki, S.; Noguchi, N.; Mimaki, K. Continuous UV-B irradiation induces morphological changes and the accumulation of polyphenolic compounds on the surface of cucumber cotyledons. J. Radiat. Res. 2007, 48, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Casal, J.J.; Smith, H. The function, action and adaptive significance of phytochrome in light-grown plants. Plant Cell Environ. 1989, 12, 855–862. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Craig, D.; Runkle, E.S. Using leds to quantify the effect of the red to far-red ratio of night-interruption lighting on flowering of photoperiodic crops. Acta Hortic. 2012, 956, 179–185. [Google Scholar] [CrossRef]
- Solymosi, K.; Schoefs, B. Etioplast and etio-chloroplast formation under natural conditions: The dark side of chlorophyll biosynthesis in angiosperms. Photosynth. Res. 2010, 105, 143–166. [Google Scholar] [CrossRef]
- Niyogi, K.K. Photoprotection revisited: Genetic and molecular approaches. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 33. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xu, X.M.; Cui, J. The importance of blue light for leaf area expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. Photosynthetica 2015, 53, 1–10. [Google Scholar] [CrossRef]
- Lefsrud, M.G.; Kopsell, D.A.; Sams, C.E. Irradiance from distinct wave length light-emitting diodes affect secondary metabolites in kale. HortScience 2008, 43, 2243–2244. [Google Scholar] [CrossRef]
- Hoffmann, A.M.; Noga, G.; Hunsche, M. High blue light improves acclimation and photosynthetic recovery of pepper plants exposed to UV stress. Environ. Exp. Bot. 2015, 109, 254–263. [Google Scholar] [CrossRef]
- Li, J.; Hikosaka, S.; Goto, E. Effects of light quality and photosynthetic photon flux on growth and carotenoid pigments in spinach (Spinacia oleracea L.). Acta Hortic. 2009, 907, 105–110. [Google Scholar] [CrossRef]
- Lidon, F.J.C.; Reboredo, F.H.; Leitão, A.E.; Silva, M.M.A.; Duarte, M.P.; Ramalho, J.C. Impact of UV-B radiation on photosynthesis—An overview. Emir. J. Food Agric. 2012, 24, 546–556. [Google Scholar] [CrossRef]
- Naznin, M.T.; Lefsrud, M.; Gagne, J.D.; Schwalb, M.; Bissonnette, B.H. Different wavelengths of LED light affect on plant photosynthesis. HortScience 2012, 47, S191. [Google Scholar]
- Opdam, J.G.; Schoonderbeek, G.G.; Heller, E.B.; Gelder, A. Closed green-house: A starting point for sustainable entrepreneurship in horticulture. Acta Hortic. 2005, 691, 517–524. [Google Scholar] [CrossRef]
- Trouwborst, G.; Hogewoning, S.W.; van Kooten, O.; Harbinson, J.; van Ieperen, W. Plasticity of photosynthesis after the ‘red light syndrome’ in cucumber. Environ. Exp. Bot. 2016, 121, 75–82. [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef]
- Samuolienè, G.; Viršilè, A.; Brazaitytè, A.; Jankauskienè, J.; Duchovskis, P.; Novickovas, A. Effect of supplementary pre-harvest LED lighting on the antioxidant and nutritional properties of green vegetables. Acta Hortic. 2010, 939, 85–91. [Google Scholar] [CrossRef]
- Britz, S.J.; Sager, J.C. Photomorphogenesis and photoassimilation in soybean and sorghum grown under broad spectrum or blue-deficient light sources. Plant Physiol. 1990, 94, 448–454. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Kim, K.; Kook, H.S.; Jang, Y.J.; Lee, W.H.; Kamala-Kannan, S.; Chae, J.C.; Lee, K.J. The effect of blue-light emitting diodes on antioxidant properties and resistance to Botrytis cinerea in tomato. J. Plant Pathol. Microbiol. 2013, 4, 203. [Google Scholar] [CrossRef]
- Li, H.M.; Tang, C.M.; Xu, Z.G.; Liu, X.Y.; Han, X.L. Effects of different light sources on the growth of non-heading Chinese cabbage (Brassica campestris L.). J. Agric. Sci. 2012, 4, 262–273. [Google Scholar] [CrossRef]
- Li, H.M.; Xu, Z.G.; Tang, C.M. Effect of light-emitting diodes on growth and morphogenesis of upland cotton (Gossypium hirsutum L.) plantlets in vitro. Plant Cell Tissue Organ Cult. 2010, 103, 155–163. [Google Scholar] [CrossRef]
- Soebo, A.; Krekling, T.; Applegren, M. Light quality affects photosynthesis and leaf anatomy of birch plantlets in vitro. Plant Cell Tissue Organ Cult. 1995, 41, 177–185. [Google Scholar] [CrossRef]
- Knief, C.; Delmotte, N.; Chaffron, S.; Stark, M.; Innerebner, G.; Wassmann, R.; von Mering, C.; Vorholt, J.A. Metaproteogenomic analysis of microbial communities in the phyllosphere and rhizosphere of rice. ISME J. 2012, 6, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Bantis, F.; Ouzounis, T.; Radoglou, K. Artificial LED lighting enhances growth characteristics and total phenolic content of Ocimum basilicum, but variably affects transplant success. Sci. Hortic. 2016, 198, 277–283. [Google Scholar] [CrossRef]
- Samuolienè, G.; Sirtautas, R.; Brazaitytè, A.; Duchovskis, P. LED lighting and seasonality effects antioxidant properties of baby leaf lettuce. Food Chem. 2012, 134, 1494–1499. [Google Scholar] [CrossRef]
- Chen, W.H.; Xu, Z.G.; Liu, X.Y.; Yang, Y.; Wang, Z.H.M.; Song, F.F. Effect of LED light source on the growth and quality of different lettuce varieties. Acta Bot. Boreali Occident. Sin. 2011, 31, 1434–1440. [Google Scholar]
- Liu, W.K.; Yang, Q.C. Effects of supplemental UV-A and UV-C irradiation on growth, photosynthetic pigments and nutritional quality of pea seedlings. Acta Hortic. 2012, 956, 657–663. [Google Scholar] [CrossRef]
- Mizuno, T.; Amaki, W.; Watanabe, H. Effects of monochromatic light irradiation by LED on the growth and anthocyanin contents in leaves of cabbage seedlings. Acta Hortic. 2009, 907, 179–184. [Google Scholar] [CrossRef]
- Zhang, T.; Folta, K.M. Green light signaling and adaptive response. Plant Signal Behav. 2012, 7, 75–78. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E. Increases in shoot tissue pigments, glucosinolates, and mineral elements in sprouting broccoli after exposure to short-duration blue light from light-emitting diodes. J. Am. Soc. Hortic. Sci. 2013, 138, 31–37. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E.; Barickman, T.C.; Morrow, R.C. Sprouting broccoli accumulate higher concentrations of nutritionally important metabolites under narrow-band light-emitting diode lighting. J. Am. Soc. Hortic. Sci. 2014, 139, 469–477. [Google Scholar] [CrossRef]
- Zukalova, H.; Vasak, J.; Nerad, D.; Stranc, P. The role of glucosinolates of Brassica genus in the crop system. Rostlinna Výroba 2002, 48, 181–189. [Google Scholar] [CrossRef]
- Colquhoun, T.A.; Schwieterman, M.L.; Gilbert, J.L.; Jaworski, E.A.; Langer, K.M.; Jones, C.R.; Rushing, G.V.; Hunter, T.M.; Olmstead, J.; Clark, D.G.; et al. Light modulation of volatile organic compounds from petunia flowers and select fruits. Postharvest Biol. Technol. 2013, 86, 37–44. [Google Scholar] [CrossRef]
- Vänninen, I.; Pinto, D.M.; Nissinen, A.I.; Johansen, N.S.; Shipp, L. In the light of new greenhouse technologies: 1. Plant-mediated effects of artificial lighting on arthropods and tritrophic interactions. Ann. Appl. Biol. 2010, 157, 393–414. [Google Scholar] [CrossRef]
- Wu, L.; Yang, H.Q. Cryptochrome 1 is implicated in promoting R protein- mediated plant resistance to Pseudomonas syringae in Arabidopsis. Mol. Plant 2010, 3, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, Y.P.; Yu, H.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Light quality affects incidence of powdery mildew, expression of defence-related genes and associated metabolism in cucumber plants. Eur. J. Plant Pathol. 2010, 127, 125–135. [Google Scholar] [CrossRef]
- Shibuya, T.; Itagaki, K.; Tojo, M.; Endo, R.; Kitaya, Y. Fluorescent illumination with high red-to-far-red ratio improves resistance of cucumber seedlings to powdery mildew. HortScience 2011, 46, 429–431. [Google Scholar] [CrossRef]
- Cargnel, M.D.; Demkura, P.V.; Ballare, C.L. Linking phytochrome to plant immunity: Low red: Far-red ratios increase Arabidopsis susceptibility to Botrytis cinerea by reducing the biosynthesis of indolic glucosinolates and camalexin. New Phytol. 2014, 204, 342–354. [Google Scholar] [CrossRef] [PubMed]
- De Wit, M.; Spoel, S.H.; Sanchez-Perez, G.F.; Gommers, C.M.M.; Pieterse, C.M.J.; Voesenek, L.; Pierik, R. Perception of low red:far-red ratio compromises both salicylic acid- and jasmonic acid-dependent pathogen defences in Arabidopsis. Plant J. 2013, 75, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.E.; Tao, Y.; Chory, J.; Ballare, C.L. Ecological modulation of plant defense via phytochrome control of jasmonate sensitivity. Proc. Natl. Acad. Sci. USA 2009, 106, 4935–4940. [Google Scholar] [CrossRef] [PubMed]
- Kurepin, L.V.; Emery, R.J.N.; Pharis, R.P.; Reid, D.M. Uncoupling light quality from light irradiance effects in Helianthus annuus shoots: Putative roles for plant hormones in leaf and internode growth. J. Exp. Bot. 2007, 58, 2145–2157. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Arasu, M.V.; Park, S.; Byeon, D.H.; Chung, S.O.; Park, S.U.; Lim, Y.P.; Kim, S.J. LED lights enhance metabolites and antioxidants in chinese cabbage and kale. Braz. Arch. Biol. Technol. 2016, 59, e16150546. [Google Scholar] [CrossRef]
- Muneer, S.; Kim, E.J.; Park, J.S.; Lee, J.H. Influence of green, red and blue light emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves (Lactuca sativa L.). Int. J. Mol. Sci. 2014, 15, 4657–4670. [Google Scholar] [CrossRef] [PubMed]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Hao, X. Different ratios of red and blue LEDs light affect on coriander productivity and antioxidant properties. Acta Hortic. 2016, 1134, 223–229. [Google Scholar] [CrossRef]
- Wu, M.C.; Hou, C.Y.; Jiang, C.M.; Wang, Y.T.; Wang, C.Y.; Chen, H.H.; Chang, H.M. A novel approach of LED light radiation improves the antioxidant activity of pea seedlings. Food Chem. 2007, 101, 1753–1758. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, F.; Zhang, M.; Hong-Hui, L.; Xi, D.H. Effects of light quality on the interaction between cucumber mosaic virus and Nicotiana tabacum. J. Phytopathol. 2015, 163, 1002–1013. [Google Scholar] [CrossRef]
- Xu, H.; Fu, Y.; Li, T.; Wang, R. Effects of different LED light wavelengths on the resistance of tomato against Botrytis cinerea and the corresponding physiological mechanisms. J. Integr. Agric. 2017, 16, 106–114. [Google Scholar] [CrossRef]
- Bavaresco, L.; Fregoni, C.; van Zeller de Macedo Basto Gonçalves, M.I.; Vezzulli, S. Physiology and molecular biology of grapevine stilbenes—An update. In Grapevine Molecular Physiology & Biotechnology, 2nd ed.; Roubelakis-Angelakis, K.A., Ed.; Springer Science and Business Media B.V.: Amsterdam, The Netherlands; New York, NY, USA, 2009; pp. 341–364. [Google Scholar]
- Ahn, S.Y.; Kim, S.A.; Choi, S.J.; Yun, H.K. Comparison of accumulation of stilbene compounds and stilbene related gene expression in two grape berries irradiated with different light sources. Hortic. Environ. Biotechnol. 2015, 56, 36–43. [Google Scholar] [CrossRef]
- Jeandet, P.; Douillt-Breuil, A.C.; Bessis, R.; Debord, S.; Sbaghi, M.; Adrian, M. Phytoalexins from the vitaceae: Biosynthesis, phytoalexin gene expression in transgenic plants, antifungal activity, and metabolism. J. Agric. Food Chem. 2002, 50, 2731–2741. [Google Scholar] [CrossRef]
- Parada, R.Y.; Mon-nai, W.; Ueno, M.; Kihara, J.; Arase, S. Red-light-induced resistance to brown spot disease caused by Bipolaris oryzae in rice. J. Phytopathol. 2014, 163, 116–123. [Google Scholar] [CrossRef]
- Balint-Kurti, P.; Simmons, S.J.; Blum, J.E.; Ballaré, C.L.; Stapleton, A.E. Maize leaf epiphytic bacteria diversity patterns are genetically correlated with resistance to fungal pathogen infection. Mol. Plant Microbe Interact. 2010, 23, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Aruscavage, D.; Phelan, P.L.; Lee, K.; LeJeune, J.T. Impact of changes in sugar exudate created by biological damage to tomato plants on the persistence of Escherichia coli O157:H7. J. Food Sci. 2010, 75, M187–M192. [Google Scholar] [CrossRef] [PubMed]
- Leveau, J.H.J.; Lindow, S.E. Appetite of an epiphyte: Quantitative monitoring of bacterial sugar consumption in the phyllosphere. Proc. Natl. Acad. Sci. USA 2001, 98, 3446–3453. [Google Scholar] [CrossRef] [PubMed]
- Aschenbrenner, A.K.; Amrehn, E.; Bechtel, L.; Spring, O. Trichome differentiation on leaf primordia of Helianthus annuus (Asteraceae): Morphology, gene expression and metabolite profile. Planta 2015, 41, 837–846. [Google Scholar] [CrossRef]
- Aschenbrenner, A.K.; Horakh, S.; Spring, O. Linear glandular trichomes of Helianthus (Asteraceae): Morphology, localization, metabolite activity and occurrence. AoB Plants 2013, 5. [Google Scholar] [CrossRef]
- Huang, S.S.; Kirchoff, B.K.; Liao, J.P. The capitate and peltate glandular trichomes of Lavandula pinnata L. (Lamiaceae): Histochemistry, ultrastructure, and secretion. J. Torrey Bot. Soc. 2008, 135, 155–167. [Google Scholar] [CrossRef]
- Rico, A.; Preston, G.M. Pseudomonas syringae pv. tomato DC3000 uses constitutive and apoplast-induced nutrient assimilation pathways to catabolize nutrients that are abundant in the tomato apoplast. Mol. Plant Microbe Interact. 2008, 21, 269–282. [Google Scholar] [CrossRef]
- Kniskern, J.M.; Traw, M.B.; Bergelson, J. Salicylic acid and jasmonic acid signaling defense pathways reduce natural bacterial diversity on Arabidopsis thaliana. Mol. Plant Microbe Interact. 2007, 20, 1512–1522. [Google Scholar] [CrossRef]
- Nagendran, R.; Lee, Y.H. Green and red light reduces the disease severity by Pseudomonas cichorii JBC1 in tomato plants via upregulation of defense-related gene expression. Phytopathology 2015, 105, 412–418. [Google Scholar] [CrossRef]
- Islam, S.Z.; Babadoost, M.; Bekal, S.; Lambert, K. Red light-induced systemic disease resistance against root-knot nematode Meloidogyne javanica and Pseudomonas syringae pv. tomato DC 3000. J. Phytopathol. 2008, 156, 708–714. [Google Scholar] [CrossRef]
- Yadav, R.K.P.; Karamanoli, K.; Vokou, D. Bacterial colonization of the phyllosphere of Mediterranean perennial species as influenced by leaf structural and chemical features. Microb. Ecol. 2005, 50, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Gratani, L.; Covone, F.; Larcher, W. Leaf plasticity in response to light of three evergreen species of the Mediterranean maquis. Trees 2006, 20, 549–558. [Google Scholar] [CrossRef]
- Steinmüller, D.; Tevini, M. Action of ultraviolet radiation (UV-B) upon cuticular waxes in some crop plants. Planta 1985, 164, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Gomelsky, M.; Hoff, W.D. Light helps bacteria make important lifestyle decisions. Trends Microbiol. 2011, 19, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Losi, A.; Gärtner, W. Bacterial bilin- and flavin-binding photoreceptors. Photochem. Photobiol. Sci. 2008, 7, 1168–1178. [Google Scholar] [CrossRef]
- Pertot, I.; Fiamingo, F.; Amsalem, L.; Maymon, M.; Freeman, S.; Gobbin, D.; Elad, Y. Sensitivity of two Podosphaera aphanis populations to disease control agents. J. Plant Pathol. 2007, 89, 85–96. [Google Scholar]
- Xiao, C.; Chandler, C.; Price, J.; Duval, J.; Mertely, J.; Legard, D. Comparison of epidemics of Botrytis fruit rot and powdery mildew of strawberry in large plastic tunnel and field production systems. Plant Dis. 2001, 85, 901–909. [Google Scholar] [CrossRef]
- Kraiselburd, I.; Alet, A.I.; Tondo, M.L.; Petrocelli, S.; Daurelio, L.D.; Monzón, J.; Ruiz, O.A.; Losi, A.; Orellano, E.G. A LOV protein modulates the physiological attributes of Xanthomonas axonopodis pv. citri relevant for host plant colonization. PLoS ONE 2012, 7, e38226. [Google Scholar] [CrossRef]
- Mao, D.; Tao, J.; Li, C.; Luo, C.; Zheng, L.; He, C. Light signalling mediated by Per-ARNT-Sim domain-containing proteins in Xanthomonas campestris pv. campestris. FEMS Microbiol. Lett. 2012, 326, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.L.; Tuttobene, M.; Altilio, M.; Martínez Amezaga, M.; Nguyen, M.; Cribb, P.; Cybulski, L.E.; Ramírez, M.S.; Altabe, S.; Mussi, M.A. Light modulates metabolic pathways and other novel physiological traits in the human pathogen Acinetobacter baumannii. J. Bacteriol. 2017, 199, e00011-17. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.M.; Lee, Y.H. Effect of light quality on Bacillus amyloliquefaciens JBC36 and its biocontrol efficacy. Biol. Control 2013, 64, 203–210. [Google Scholar] [CrossRef]
- Imada, K.; Tanaka, S.; Ibaraki, Y.; Yoshimura, K.; Ito, S. Antifungal effect of 405-nm light on Botrytis cinerea. Lett. Appl. Microbiol. 2014, 59, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Wilde, A.; Mullineaux, C.W. Light-controlled motility in prokaryotes and the problem of directional light perception. FEMS Microbiol. Rev. 2017, 41, 900–922. [Google Scholar] [CrossRef] [PubMed]
- Rajalingam, N.; Lee, J.H. Effects of green light on the gene expression and virulence of the plant pathogen Pseudomonas cichorii JBC1. Eur. J. Plant Pathol. 2018, 150, 223–236. [Google Scholar] [CrossRef]
- Someya, N.; Nakajima, M.; Hamamoto, H.; Yamaguchi, I.; Akutsu, K. Effects of light conditions on prodigiosin stability in the biocontrol bacterium Serratia marcescens strain B2. J. Gen. Plant Pathol. 2004, 70, 367–370. [Google Scholar] [CrossRef]
- Guffy, J.S.; Wilborn, J. In vitro bactericidal effects of 405-nm and 470-nm blue light. Photobiomodulation Photomed. Laser Surg. 2006, 24, 684–688. [Google Scholar] [CrossRef]
- Berrocal-Tito, G.; Sametz-Baron, L.; Eichenberg, K.; Horwitz, B.A.; Herrera-Estrella, A. Rapid blue light regulation of a Trichoderma harzianum photolyase gene. J. Biol. Chem. 1999, 274, 14288–14294. [Google Scholar] [CrossRef]
- Papavizas, G.C. Trichoderma and Gliocladium: Biology, ecology and potential for biocontrol. Ann. Rev. Phytopathol. 1985, 23, 23–54. [Google Scholar] [CrossRef]
- Van der Horst, M.A.; Hellingwerf, K.J. Photoreceptor proteins, “star actors of modern times”: A review of the functional dynamics in the structure of representative members of six different photoreceptor families. Acc. Chem. Res. 2004, 37, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Van der Horst, M.A.; Key, J.; Hellingwerf, K.J. Photosensing in chemotrophic, non-phototrophic bacteria: Let there be light sensing too. Trends Microbiol. 2007, 15, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Jenal, U.; Malone, J. Mechanisms of Cyclic-di-GMP signaling in bacteria. Ann. Rev. Genet. 2006, 40, 385–407. [Google Scholar] [CrossRef] [PubMed]
- Hengge, R.; Galperin, M.Y.; Ghigo, J.M.; Gomelsky, M.; Green, J.; Hughes, K.T.; Jenal, U.; Landini, P. Systematic Nomenclature for GGDEF and EAL Domain-Containing Cyclic Di-GMP Turnover Proteins of Escherichia coli. J. Bacteriol. 2016, 198, 7–11. [Google Scholar] [CrossRef]
- Bumah, V.V.; Masson-Meyers, D.S.; Cashin, S.; Enwemeka, C.S. Optimization of the antimicrobial effect of blue light on methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers Surg. Med. 2015, 47, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Halstead, F.D.; Thwaite, J.E.; Burt, R.; Laws, T.R.; Raguse, M.; Moeller, R.; Webber, M.A.; Oppenheim, B.A. Antibacterial activity of blue light against nosocomial wound pathogens growing planktonically and as mature biofilms. Appl. Environ. Microbiol. 2016, 82, 4006–4016. [Google Scholar] [CrossRef] [PubMed]
- Glantz, S.T.; Carpenter, E.J.; Melkonian, M.; Gardner, K.H.; Boyden, E.S.; Wong, G.K.S.; Chow, B.Y. Functional and topological diversity of LOV domain photoreceptors. Proc. Natl. Acad. Sci. USA 2016, 113, E1442–E1451. [Google Scholar] [CrossRef]
- Zayner, J.P.; Antoniou, C.; French, A.R.; Hause, R.J., Jr.; Sosnick, T.R. Investigating models of protein function and allostery with a widespread mutational analysis of a light-activated protein. Biophys. J. 2013, 105, 1027–1036. [Google Scholar] [CrossRef]
- Kraiselburd, I.; Moyano, L.; Carrau, A.; Tano, J.; Orellano, E.G. Bacterial photosensory proteins and their role in plant–pathogen interactions. Photochem. Photobiol. 2017, 93, 666–674. [Google Scholar] [CrossRef]
- Masuda, S. Light detection and signal transduction in the BLUF photoreceptors. Plant Cell Physiol. 2013, 54, 171–179. [Google Scholar] [CrossRef]
- Tschowri, N.; Busse, S.; Hengge, R. The BLUF-EAL protein YcgF acts as a direct anti-repressor in a blue-light response of Escherichia coli. Genes Dev. 2009, 23, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.E.; Yakali, E.; Cusanovich, M.A.; Tollin, F. Properties of a water soluble yellow protein isolated from a halophilic phototrophic bacterium that has photochemical activity analogous to sensory rhodopsin. Biochemistry 1987, 26, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.E.; Kyndt, J.A.; Memmi, S.; Moser, T.; Colon-Acevedo, B.; Devreese, B.; Van Beeumen, J. The growing family of photoactive yellow proteins and their presumed functional roles. Photochem. Photobiol. Sci. 2012, 11, 1495–1514. [Google Scholar] [CrossRef] [PubMed]
- Imamoto, Y.; Kataoka, M. Structure and Photoreaction of Photoactive Yellow Protein, a Structural Prototype of the PAS Domain Superfamily†. Photochem. Photobiol. 2007, 83, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, I.B.; Boyé-Péronne, S.; El Ghazaly, M.O.A.; Kristensen, M.B.; Brøndsted Nielsen, S.; Andersen, L.H. Absorption spectra of photoactive yellow protein chromophores in vacuum. Biophys. J. 2005, 89, 2597–2604. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.V.; Connor, E.W. Translating phytobiomes from theory to practice: Ecological and evolutionary considerations. Phytobiomes 2017, 1, 57–69. [Google Scholar] [CrossRef]
- Werner, G.D.A.; Strassmann, J.E.; Ivens, A.B.F.; Engelmoor, D.J.P.; Verbryggen, E.; Queller, D.C.; Roë, R.; Collins Johnson, N.; Hammerstein, P.; Kiers, E.T. Evolution of microbial markets. Proc. Natl. Acad. Sci. USA 2014, 111, 1237–1244. [Google Scholar] [CrossRef]
- Mercier, J.; Lindow, S.E. Role of leaf surface sugars in colonization of plants by bacterial epiphytes. Appl. Environ. Microbiol. 2000, 66, 369–374. [Google Scholar] [CrossRef]
- Tukey, H.B.J.; Morgan, J.V. Injury to foliage and its effect upon the leaching of nutrients from above-ground plant parts. Physiol. Plant. 1963, 16, 557–564. [Google Scholar] [CrossRef]
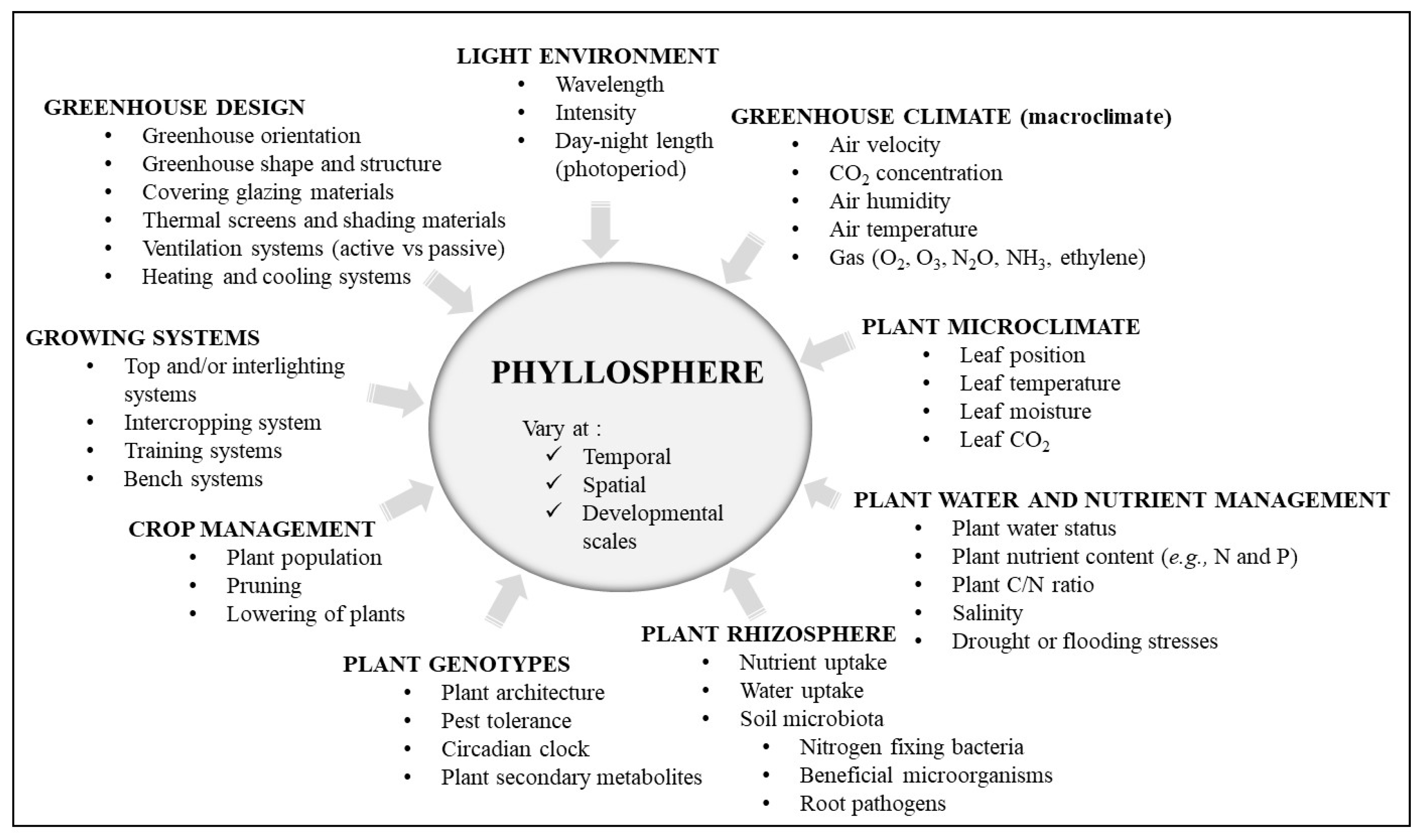
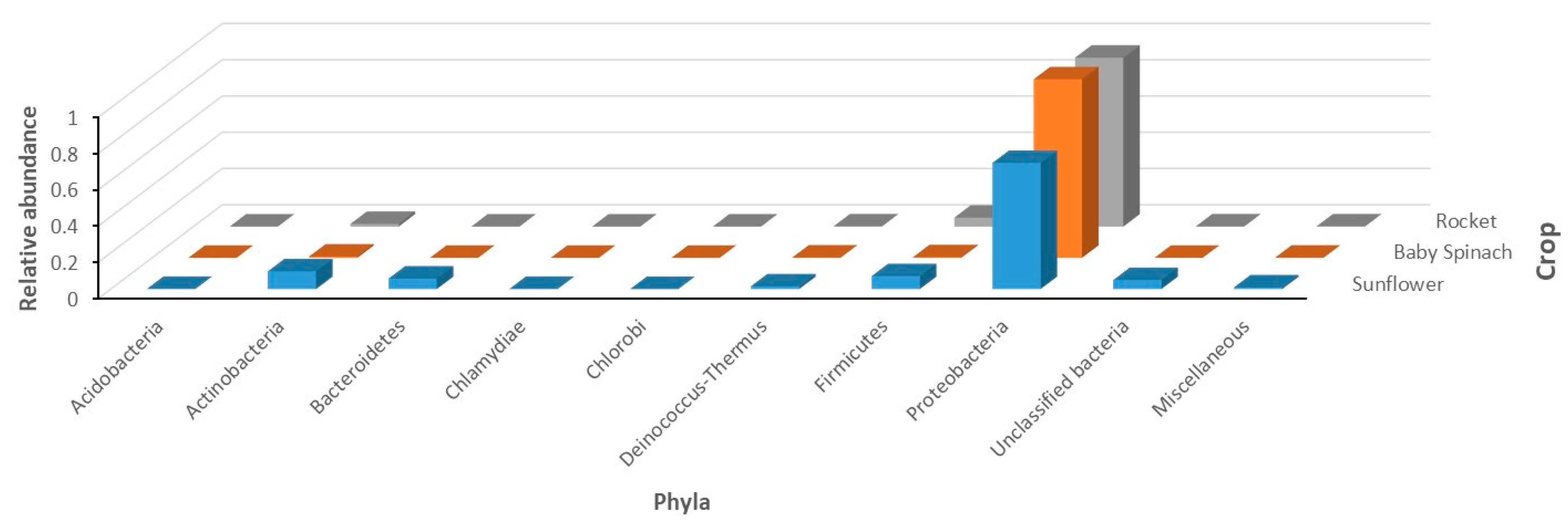
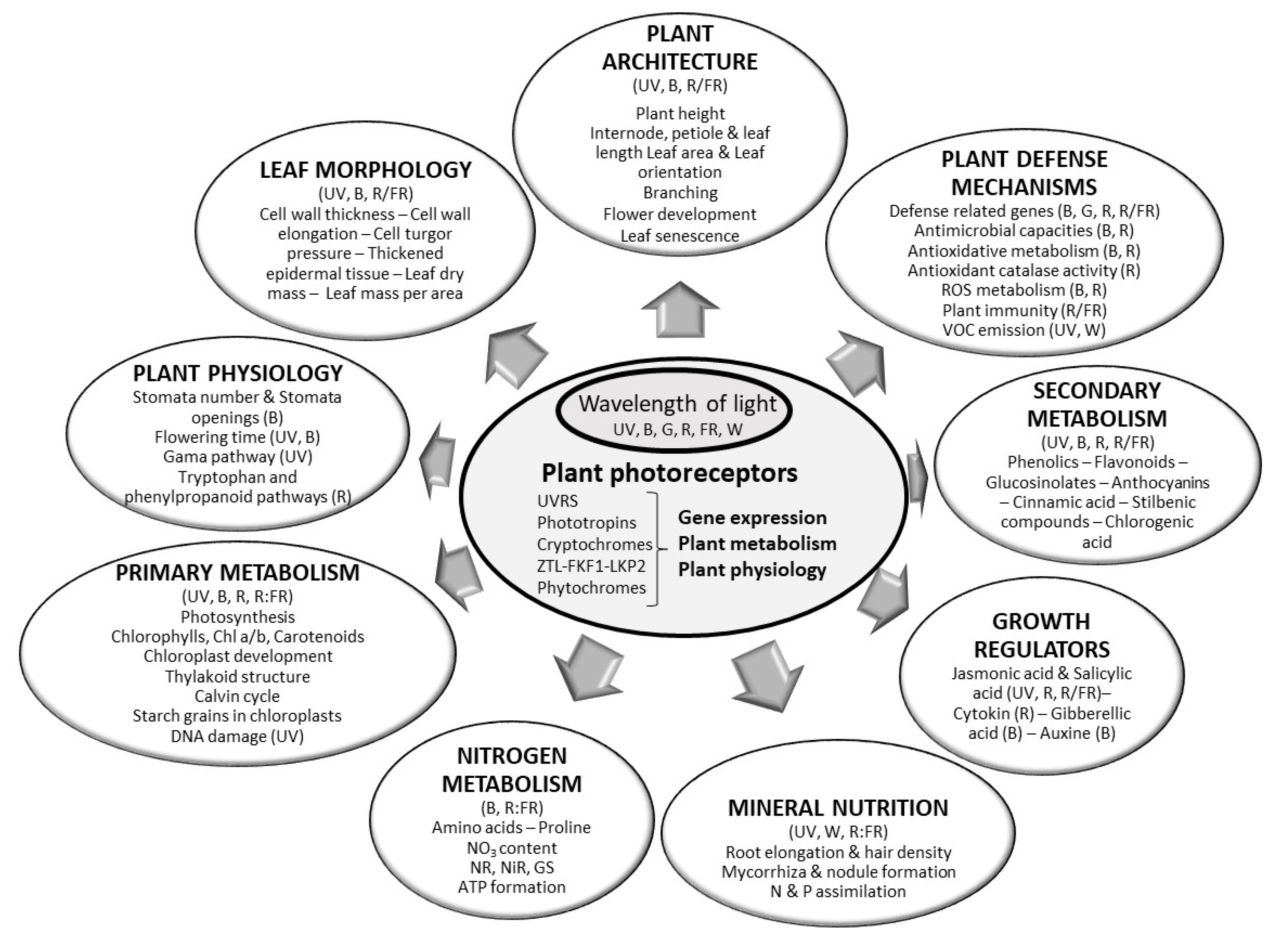
| Lamp Type | Effect (W) | Infra-Red | UV | PAR 1 | Direction of Heat Emissions | References |
|---|---|---|---|---|---|---|
| HPS 2 | 400 | High | Low | 1.6 | ↓ | [41] |
| Metal halide 3 | 400 | High | Low | n/a | ↓ | [42] |
| Light tube 4 | 58 | Medium | Low | n/a | ↓ | |
| LED 1 5 | 630 | None | None | 1.85 | ↑ | [43] |
| LED 2 6 | 550 | None | None | 2.5 | ↑ | [44] |
| LED 3 7 | 400 | None | None | 2.3 | ↑ | [45] |
| Organism | Light Quality | Wave Length (nm) | Photoreceptor | Photoreceptor Architecture | Effect | Ref. |
|---|---|---|---|---|---|---|
| Acinetobacter baumannii | Blue | 415 | BLUF, LOV | EAL-GAF-GGDEF-LOV-GGDEF | Biofilm formation, metabolism, virulence | [163] |
| Bacillus amylolique-faciens | Red Blue | 645 458 | LOV | LOV-STAS | Swarming motility, biofilm formation, antifungal activity | [164] |
| Botrytis cinerea | Blue | 405 | PHY, LOV | PAS-GAF-PHY-HK LOV-PAS, short LOV | Inhibited mycelial growth, virulence | [165] |
| Pseudomonas aeringiunosa | Blue | 405 | PHY, LOV | PAS-GAF-PHY-kinase Short LOV | Survival, virulence factors | [166] |
| P. cichorii | Green | NI | LOV | HATP-HisKA-LOV-RR | Siderophore and phytotoxic lipopeptide production | [167] |
| P. syringae | Red/Far-red Blue White | 680/750 470 | PHY, LOV | PAS-GAF-PHY-kinase HATP-HisKA-LOV-RR Short LOV | Decreased swarming motility | [50] |
| Podosphaera pannosa | Blue | 420–520 | Reduced germination and conidia formation | [49] | ||
| Serratia marcescens | Blue White | 470 | Antibiotic production | [168] | ||
| Sphaerotheca fuliginea | Red | NI 1 | Disease suppression | [127] | ||
| Staphylococcus aureus | Blue | 405, 470 | Growth | [169] | ||
| Trichoderma harzianum | Blue | NI | Induced gene expression of phr1 | [170] | ||
| Xanthomonas axonopodis | Light/ dark | PHY, LOV, BLUF | PAS-GAF-PHY-PAS LOV-HK | Motility, adhesion, biofilm formation | [161] | |
| Xanthomonas campestris | Red/ Far-red Blue White | NI | PHY, LOV | PAS-GAF-PHY-PAS HATP-HisKA-LOV-RR | Growth, motility | [162] |
| Theory/Principles | Modes of Action | Potential Research Questions | Light Spectra of Interest |
|---|---|---|---|
| Niche theory | |||
| Priority effects | Pre-emptying of space and resources by the first arriving species | Heterotrophic utilization of leaf lysates/organic compounds and their impact on secondary metabolites | B 1, G 2, Y 3, R 4, R:FR 5 |
| Competitive dominance | Dominance due to efficient resource use under prevailing stable conditions | ||
| Niche partitioning | Coexistence | Light-quality-associated impact on biofilm community structure Bacterial–fungi symbionts/Suitable microbe combination Plant–microbe and microbe–microbe compatibility | B, G, Y, R, R:FR B, R:FR |
| Storage effect | Coexistence of microbes within the same ecological community | Storage effects in non-phototrophic non-spore-forming bacterial leaf colonizers | B, G, Y, R, R:FR |
| Niche modification | Invasion of leaf interior | Light quality as a driver towards an endophytic lifestyle | B, G, Y, R, R:FR |
| Biofilm formation | Light quality as a driver for switch from planktonic to biofilm lifestyle | ||
| Complementarity | Diversification of resource requirements leading to less competition between interspecific than conspecific neighbors | Mechanisms of coexistence under various light qualities | B, G, Y, R, R:FR |
| Resource-based interactions | |||
| Resource competition | Heterotrophic utilization of leaf lysates/organic compounds and their impact on secondary metabolites in microbial aggregate communities | B, G, Y, R, R:FR | |
| Phenotypic plasticity | Formation of different phenotypes under various conditions | Complementary microbe pair for stimulating plant growth and pathogen control | B, R:FR |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsanius, B.W.; Karlsson, M.; Rosberg, A.K.; Dorais, M.; Naznin, M.T.; Khalil, S.; Bergstrand, K.-J. Light and Microbial Lifestyle: The Impact of Light Quality on Plant–Microbe Interactions in Horticultural Production Systems—A Review. Horticulturae 2019, 5, 41. https://doi.org/10.3390/horticulturae5020041
Alsanius BW, Karlsson M, Rosberg AK, Dorais M, Naznin MT, Khalil S, Bergstrand K-J. Light and Microbial Lifestyle: The Impact of Light Quality on Plant–Microbe Interactions in Horticultural Production Systems—A Review. Horticulturae. 2019; 5(2):41. https://doi.org/10.3390/horticulturae5020041
Chicago/Turabian StyleAlsanius, Beatrix W., Maria Karlsson, Anna Karin Rosberg, Martine Dorais, Most Tahera Naznin, Sammar Khalil, and Karl-Johan Bergstrand. 2019. "Light and Microbial Lifestyle: The Impact of Light Quality on Plant–Microbe Interactions in Horticultural Production Systems—A Review" Horticulturae 5, no. 2: 41. https://doi.org/10.3390/horticulturae5020041
APA StyleAlsanius, B. W., Karlsson, M., Rosberg, A. K., Dorais, M., Naznin, M. T., Khalil, S., & Bergstrand, K.-J. (2019). Light and Microbial Lifestyle: The Impact of Light Quality on Plant–Microbe Interactions in Horticultural Production Systems—A Review. Horticulturae, 5(2), 41. https://doi.org/10.3390/horticulturae5020041







