Performance and Hydroponic Tomato Crop Quality Characteristics in a Novel Greenhouse Using Dye-Sensitized Solar Cell Technology for Covering Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydroponic Tomato Cultivation Set Up in the CONV and DSSC Greenhouses
2.2. Evaluation of Cultivation in the Two Greenhouses
2.2.1. Fruit Yield and Morphological Characteristics
2.2.2. Plant Physiological Parameters
2.2.3. Fruit Physicochemical Parameters
2.2.4. Fruit Antioxidant Compounds
2.2.5. Antioxidant Capacity
2.3. Statistical Analysis
3. Results and Discussion
3.1. Microclimatic Conditions in the Examined Greenhouses
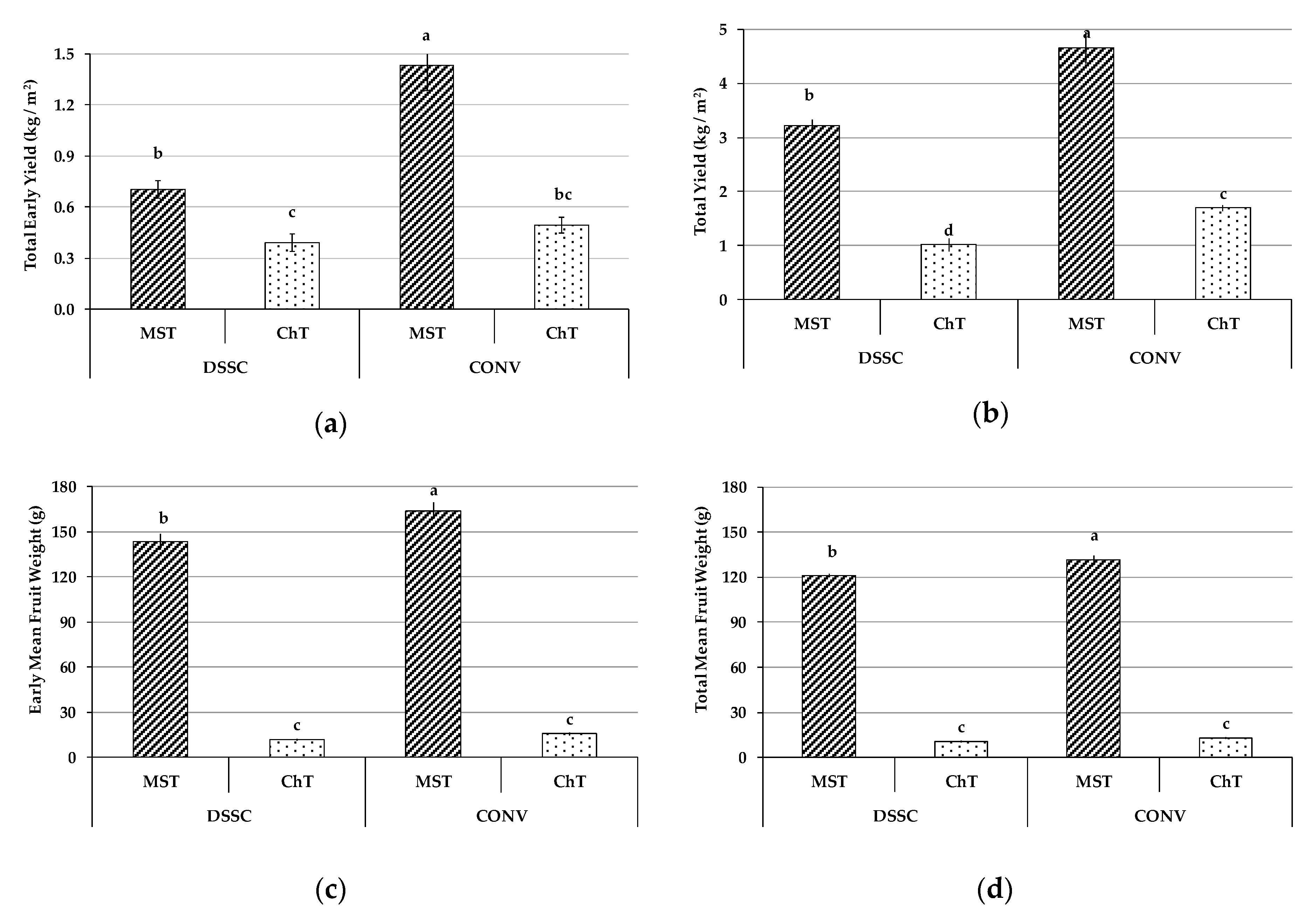
3.2. Yield and Mean Weight of Early and Total Fruit Production
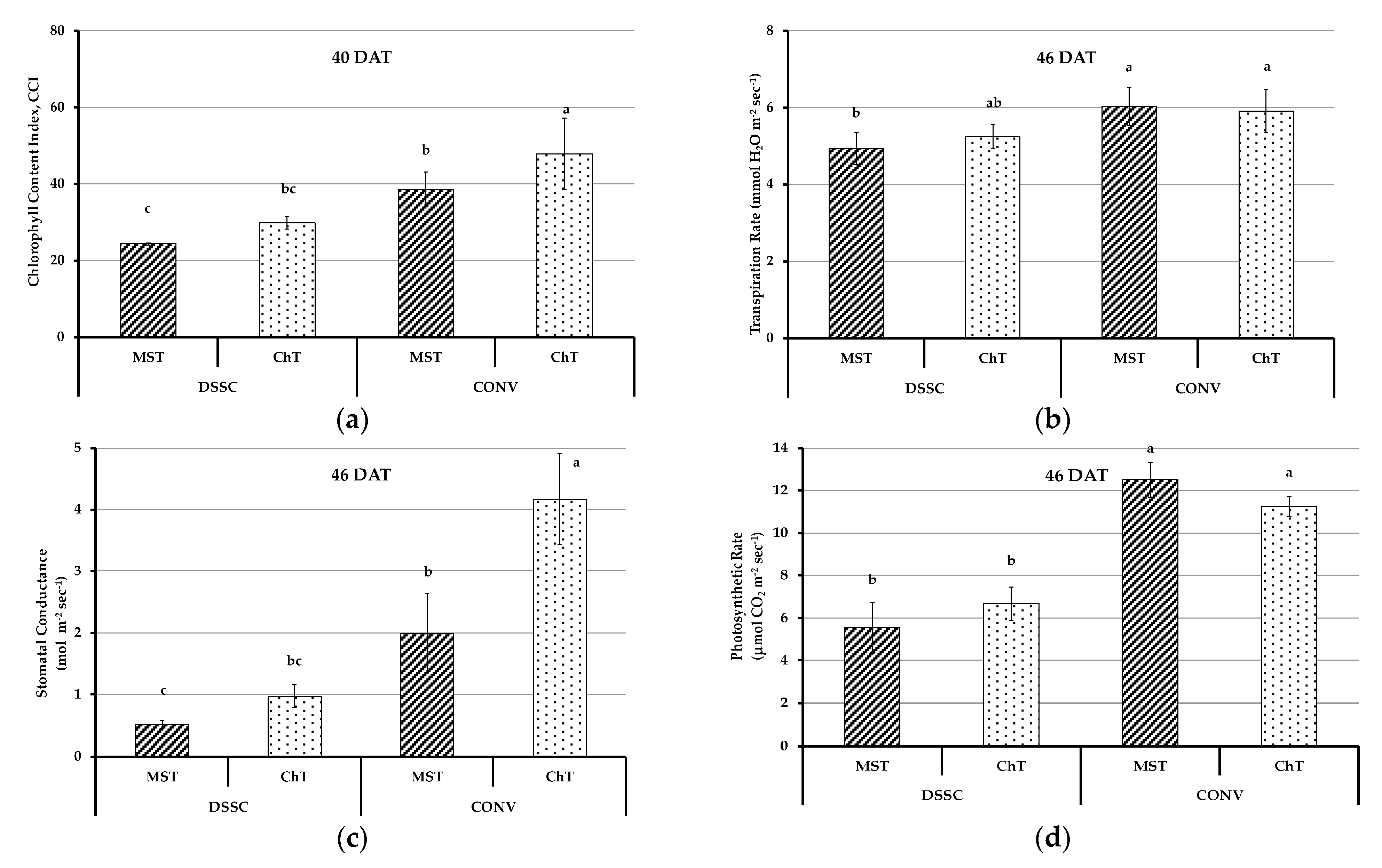
3.3. Physiological Parameters of Plants
3.4. Fruit Quality Parameters and Bioactive Compounds
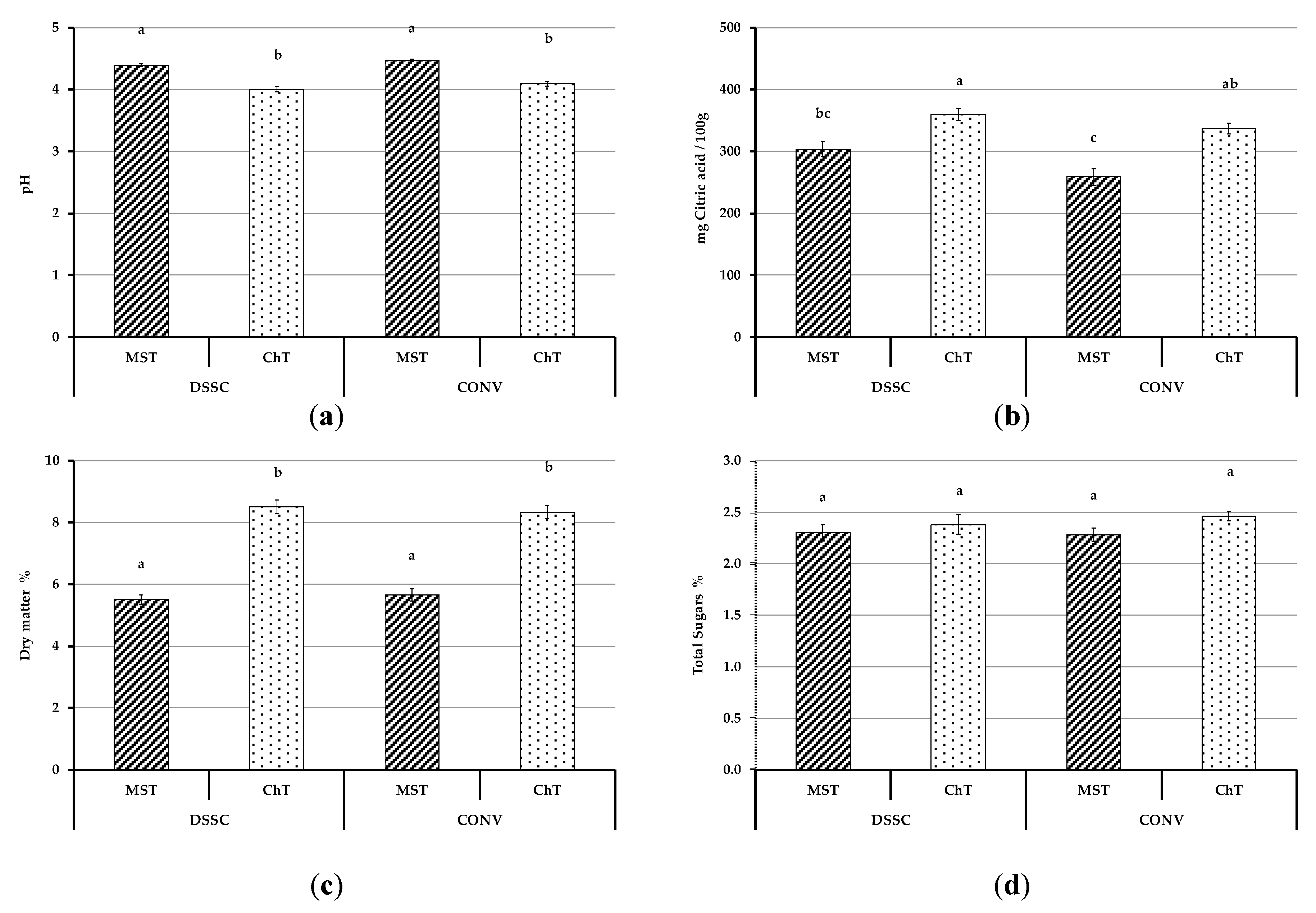
3.4.1. Physicochemical Parameters of Tomato Fruits
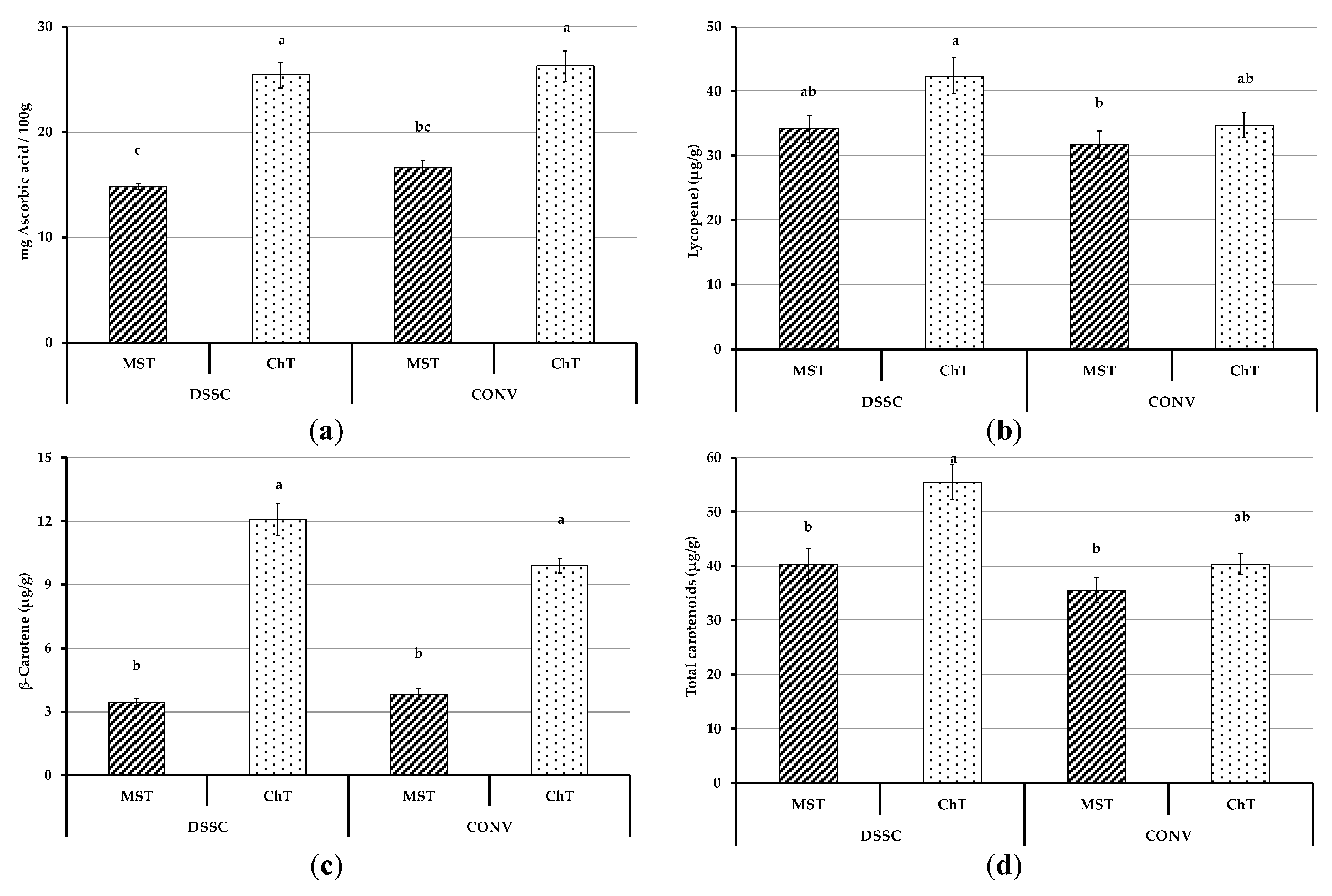
3.4.2. Bioactive Compounds of Tomato Fruits
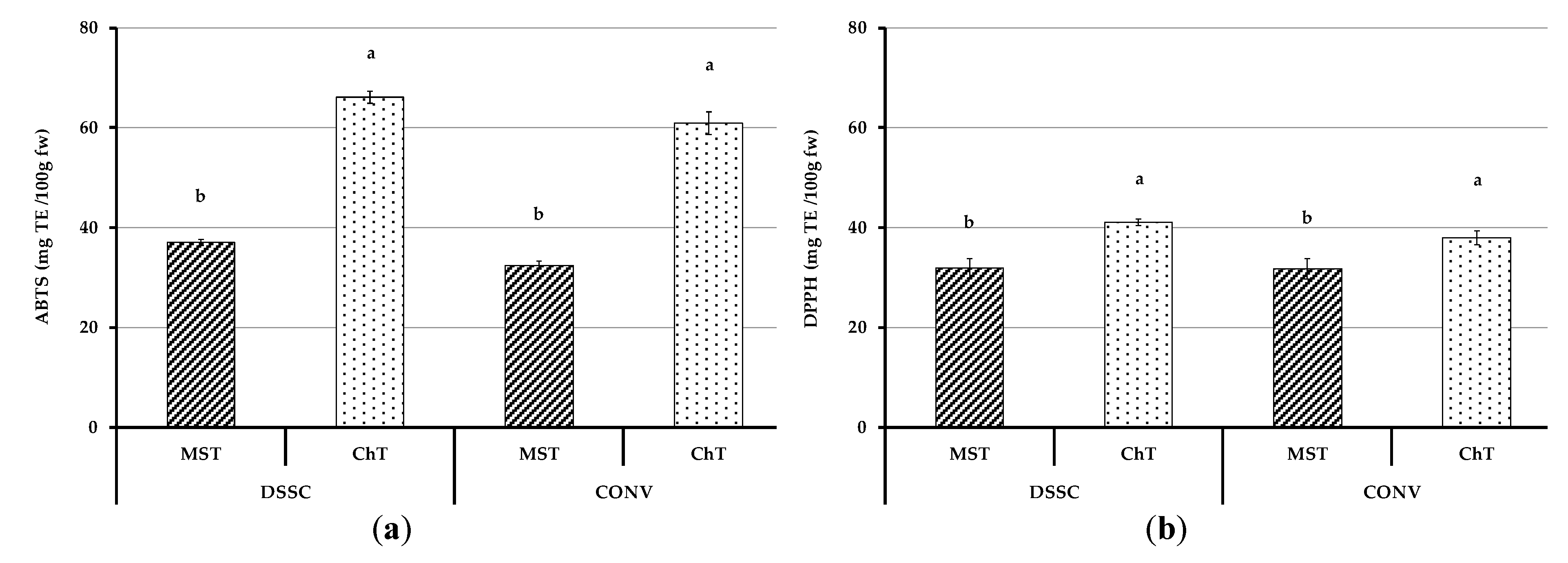
3.4.3. Antioxidant Capacity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ntinas, G.K.; Morichovitis, Z.; Nikita-Martzopoulou, C. The influence of a hybrid solar energy saving system on the growth and the yield of tomato crop in greenhouses. Acta Hortic. 2012, 952, 723–730. [Google Scholar] [CrossRef]
- Fernández, J.A.; Orsini, F.; Baeza, E.; Oztekin, G.B.; Muñoz, P.; Contreras, J.; Montero, J.I. Current trends in protected cultivation in Mediterranean climates. Eur. J. Hortic. Sci. 2018, 83, 294–305. [Google Scholar] [CrossRef]
- Ntinas, G.K.; Neumair, M.; Tsadilas, C.D.; Meyer, J. Carbon footprint and cumulative energy demand of greenhouse and open-field tomato cultivation systems under Southern and Central European climatic conditions. J. Clean. Prod. 2017, 142, 3617–3626. [Google Scholar] [CrossRef]
- Gruda, N.; Bisbis, M.; Tanny, J. Impacts of protected vegetable cultivation on climate change and adaptation strategies for cleaner production—A review. J. Clean. Prod. 2019, 225, 324–339. [Google Scholar] [CrossRef]
- Gruda, N.; Bisbis, M.; Tanny, J. Influence of climate change on protected cultivation: Impacts and sustainable adaptation strategies-A review. J. Clean. Prod. 2019, 225, 481–495. [Google Scholar] [CrossRef]
- Hassanien, R.H.E.; Li, M.; Dong Lin, W. Advanced applications of solar energy in agricultural greenhouses. Renew. Sustain. Energy Rev. 2016, 54, 989–1001. [Google Scholar] [CrossRef]
- Yano, A.; Onoe, M.; Nakata, J. Prototype semi-transparent photovoltaic modules for greenhouse roof applications. Biosyst. Eng. 2014, 122, 62–73. [Google Scholar] [CrossRef]
- Trypanagnostopoulos, G.; Kavga, A.; Souliotis, M.; Tripanagnostopoulos, Y. Greenhouse performance results for roof installed photovoltaics. Renew. Energy 2017, 111, 724–731. [Google Scholar] [CrossRef]
- Marcelis, L.F.M.; Broekhuijsen, A.G.M.; Meinen, E.; Nijs, E.M.F.M.; Raaphorst, M.G.M. Quantification of the growth response to light quantity of greenhouse grown crops. Acta Hortic. 2006, 711, 97–114. [Google Scholar] [CrossRef]
- Yamori, W.; Noguchi, K.; Hikosaka, K.; Terashima, I. Phenotypic Plasticity in Photosynthetic Temperature Acclimation among Crop Species with Different Cold Tolerances. Plant Physiol. 2010, 152, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Tewolde, F.T.; Lu, N.; Shiina, K.; Maruo, T.; Takagaki, M.; Kozai, T.; Yamori, W. Nighttime Supplemental LED Inter-lighting Improves Growth and Yield of Single-Truss Tomatoes by Enhancing Photosynthesis in Both Winter and Summer. Front. Plant Sci. 2016, 7, 448. [Google Scholar] [CrossRef]
- Roslan, N.; Radzi, M.A.M.; Chen, G.; Jamaludin, D.; Hashimoto, Y.; Ya’acob, M.E. Dye Sensitized Solar Cell (DSSC) greenhouse shading: New insights for solar radiation manipulation. Renew. Sustain. Energy Rev. 2018, 92, 171–186. [Google Scholar] [CrossRef]
- Kim, J.-J.; Kang, M.; Kwak, O.K.; Yoon, Y.-J.; Min, K.S.; Chu, M.-J. Fabrication and Characterization of Dye-Sensitized Solar Cells for Greenhouse Application. Int. J. Photoenergy 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Jones, B.J. Tomato Plant Culture: In the Field, Greenhouse, and Home Garden, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9781420007398. [Google Scholar]
- Beckles, D.M. Factors affecting the postharvest soluble solids and sugar content of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2012, 63, 129–140. [Google Scholar] [CrossRef]
- Friedman, M. Anticarcinogenic, cardioprotective, and other health benefits of tomato compounds lycopene, α-tomatine, and tomatidine in pure form and in fresh and processed tomatoes. J. Agric. Food Chem. 2013, 61, 9534–9550. [Google Scholar] [CrossRef]
- Dumas, Y.; Dadomo, M.; Di Lucca, G.; Grolier, P. Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. J. Sci. Food Agric. 2003, 382, 369–382. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Ruiz, J.M.; Ferreres, F.; Moreno, D.A. Phenolic profiles of cherry tomatoes as influenced by hydric stress and rootstock technique. Food Chem. 2012, 134, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Lenucci, M.S.; Cadinu, D.; Taurino, M.; Piro, G.; Dalessandro, G. Antioxidant Composition in Cherry and High-Pigment Tomato Cultivars. J. Agric. Food Chem. 2006, 54, 2606–2613. [Google Scholar] [CrossRef]
- Borguini, R.G.; Ferraz, E.A.; Silva, D.; Ferraz, E.A.; Silva, D.A. Tomatoes and Tomato Products as Dietary Sources of Antioxidants Tomatoes and Tomato Products as Dietary Sources of Antioxidants. Food Rev. Int. 2009, 25, 313–325. [Google Scholar] [CrossRef]
- Gómez-Romero, M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Analytical determination of antioxidants in tomato: Typical components of the Mediterranean diet. J. Sep. Sci. 2007, 30, 452–461. [Google Scholar] [CrossRef]
- Ilahy, R.; Hdider, C.; Lenucci, M.S.; Tlili, I.; Dalessandro, G. Antioxidant activity and bioactive compound changes during fruit ripening of high-lycopene tomato cultivars. J. Food Compos. Anal. 2011, 24, 588–595. [Google Scholar] [CrossRef]
- Helyes, L.D.; Lugasi, A. Formation of certain compounds having technological and nutritional importance in tomato fruits during maturation. Acta Aliment. 2006, 35, 183–193. [Google Scholar] [CrossRef]
- Pék, Z.; Helyes, L.; Lugasi, A. Color Changes and Antioxidant Content of Vine and Postharvest- ripened Tomato Fruits. HortScience 2010, 45, 466–468. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; AOAC: Rockville, MD, USA, 2000; ISBN 0935584544. [Google Scholar]
- Irakli, M.; Chatzopoulou, P.; Kadoglidou, K.; Tsivelika, N. Optimization and development of a high-performance liquid chromatography method for the simultaneous determination of vitamin E and carotenoids in tomato fruits. J. Sep. Sci. 2016, 39, 3348–3356. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggenete, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Yen, G.; Chen, H. Antioxidant Activity of Various Tea Extracts in Relation to Their Antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]



| Stock Solution (Tank A) | Stock Solution (Tank B) | Micronutrients (Tank C) | |||
|---|---|---|---|---|---|
| Macronutrients | (g/100 L) | Macronutrients | (g/100 L) | Micronutrients | (g/20 L) |
| Ca(NO3)2 | 6800 | K2SO4 | 2780 | MnSO4 | 585 |
| KNO3 | 1000 | KNO3 | 1320 | Na2B4O7 | 530 |
| EDTA-Fe (13%) | 200 | MgSO4 | 1300 | CuSO4 | 40 |
| KH2PO4 | 2340 | ZnSO4 | 270 | ||
| (NH4)6Mo7O24 | 25 | ||||
| Source | Significance of F Ratio | ||||
|---|---|---|---|---|---|
| df z | Early Yield | Total Yield | |||
| Early Yield | Fruit Weight | Yield | Fruit Weight | ||
| Replications | 4 | NS y | NS | NS | NS |
| Greenhouse (G) | 1 | ** | NS | ** | ** |
| Error (a) | 4 | ||||
| Hybrid (H) | 1 | ** | ** | ** | ** |
| G × H | 1 | ** | * | NS | NS |
| Error (b) | 8 | ||||
| CV % | 23.87 | 9.30 | 17.59 | 5.64 | |
| Source | Significance of F ratio | ||||
|---|---|---|---|---|---|
| df z | Chlorophyll Content Index | Transpiration Rate | Stomatal Conductance | Photosynthetic Rate | |
| 40 DAT y | 46 DAT | 46 DAT | 46 DAT | ||
| Replications | 5 | NS x | NS | NS | NS |
| Greenhouse (G) | 1 | * | * | ** | ** |
| Error (a) | 5 | ||||
| Hybrid (H) | 1 | ** | NS | ** | NS |
| G × H | 1 | NS | NS | NS | * |
| Error (b) | 10 | ||||
| CV % | 19.38 | 13.31 | 12.71 | 12.74 | |
| Source | Significance of F ratio | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| df z | pH | CA y | SUG | ASC | LUC | β-CAR | CARs | DPPH | ABTS | |
| Replications | 2 | NS x | NS | NS | NS | NS | NS | NS | ** | ** |
| Greenhouse (G) | 1 | ** | NS | NS | * | ** | * | * | ** | ** |
| Error (a) | 2 | |||||||||
| Hybrid (H) | 1 | ** | NS | NS | ** | ** | ** | ** | ** | ** |
| G × H | 1 | NS | ** | NS | NS | NS | ** | NS | ** | ** |
| Error (b) | 4 | |||||||||
| Inflorescence (I) | 4 | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| G x I | 4 | NS | ** | ** | NS | ΝS | ** | NS | ** | ** |
| H × I | 4 | ** | ** | ** | ** | NS | ** | * | ** | ** |
| G × H × I | 4 | NS | NS | ** | NS | * | * | * | * | ** |
| Error (c) | 32 | |||||||||
| CV % | 1.5 | 6.2 | 3.7 | 11.1 | 18.8 | 11.3 | 16.7 | 5.9 | 3.2 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntinas, G.K.; Kadoglidou, K.; Tsivelika, N.; Krommydas, K.; Kalivas, A.; Ralli, P.; Irakli, M. Performance and Hydroponic Tomato Crop Quality Characteristics in a Novel Greenhouse Using Dye-Sensitized Solar Cell Technology for Covering Material. Horticulturae 2019, 5, 42. https://doi.org/10.3390/horticulturae5020042
Ntinas GK, Kadoglidou K, Tsivelika N, Krommydas K, Kalivas A, Ralli P, Irakli M. Performance and Hydroponic Tomato Crop Quality Characteristics in a Novel Greenhouse Using Dye-Sensitized Solar Cell Technology for Covering Material. Horticulturae. 2019; 5(2):42. https://doi.org/10.3390/horticulturae5020042
Chicago/Turabian StyleNtinas, Georgios K., Kalliopi Kadoglidou, Nektaria Tsivelika, Konstantinos Krommydas, Apostolos Kalivas, Parthenopi Ralli, and Maria Irakli. 2019. "Performance and Hydroponic Tomato Crop Quality Characteristics in a Novel Greenhouse Using Dye-Sensitized Solar Cell Technology for Covering Material" Horticulturae 5, no. 2: 42. https://doi.org/10.3390/horticulturae5020042
APA StyleNtinas, G. K., Kadoglidou, K., Tsivelika, N., Krommydas, K., Kalivas, A., Ralli, P., & Irakli, M. (2019). Performance and Hydroponic Tomato Crop Quality Characteristics in a Novel Greenhouse Using Dye-Sensitized Solar Cell Technology for Covering Material. Horticulturae, 5(2), 42. https://doi.org/10.3390/horticulturae5020042






