Abstract
Breadfruit (Artocarpus altilis) is a tropical fruit tree primarily grown as a staple crop for food security in Oceania. Significant wind damage has driven an interest in developing its dwarf phenotype. The presence of any dwarf breadfruit variety remains unknown. Little is known regarding the growth of the species on rootstocks. Here, we examined the phenotype of breadfruit plants growing on marang (Artocarpus odoratissimus) rootstocks within 18 months after grafting; we identified a rootstock-induced dwarf trait in the species. This dwarf phenotype was characterized by shorter stems, reduced stem thickness and fewer branches, with 73% shorter internode length, 51% fewer and 40% smaller leaves compared to standard size breadfruit plants. The height of breadfruit plants on marang rootstocks was reduced by 49% in 9 months, and 59% in 18 months after grafting. The results suggest marang rootstocks can be applied to breadfruit breeding program for tree vigor control. Further biochemical characterization showed plants on marang rootstocks displayed leaves without change of total chlorophyll content, but with lower total soluble sugars, and stems with reduced activity of plasma membrane H+-ATPase, a well-known primary proton pump essential for nutrient transport. The significance of the two parameters in rootstock dwarfing is discussed.
1. Introduction
Breadfruit [Artocarpus altilis (Parkinson) Fosberg)] is a tropical fruit tree present throughout Oceania. The species comprises fertile diploids and sterile triploids, and is primarily grown as a staple crop for food security in the tropics [1]. However, as an evergreen tree 15–30 m tall, breadfruit is prone to wind damage. Significant tree loss, due to intense tropical windstorm has driven an interest in the development of dwarf phenotype in breadfruit [2,3]. Small tree size is also increasingly sought for the transition toward high-density planting to maximize fruit production, and reducing the cost of management, such as pruning and harvesting [4]. Breadfruit has hundreds of cultivars with great diversity in morphological and agronomic characteristics [1,5,6], but the dwarf variety of the species has not been reported. Dwarfism in a wide range of other fruit tree species has been achieved by the use of dwarfing rootstocks. These include the dwarfing rootstock of ‘Malling’ series, M9 and M27 for apple trees [7], the Nanking cherry rootstocks for peach trees [8], the trifoliata ‘Flying Dragon’ orange rootstocks for citrus [9]. Breadfruit dwarfing through rootstocks has not been investigated. Vegetative propagation is required for the seedless varieties of breadfruit, but also preferred for the seeded varieties, due to their recalcitrant seeds [1]. Clonal propagation is predominantly through root suckers, root cuttings, or air layering [5]. Breadfruit intraspecific grafting has not been the main method for propagation [5]. In addition, vegetatively propagated rootstocks may not be ideal for wind resistance, due to their lack of taproot system [10,11,12]. Dwarfing rootstocks for breadfruit may come from other seeded species within the Artocarpus genus. Artocarpus comprises approximately 60 species of trees and shrubs, including A. camansi (breadnut), A. mariannensis (dugdug), A. heterophyllus) (jackfruit) and A. odoratissimus (marang) [13]. Breadfruit interspecific grafting has been focused on A. camansi and A. mariannensis rootstocks [14,15,16], but recently also achieved on rootstocks of marang (A. odoratissimus) and pedelai (A. sericicarpus) [3]. However, there is limited information regarding the growth pattern of breadfruit species on these rootstocks. Clearly, evaluation of the rootstock effect of these and other Artocarpus species on breadfruit growth habit is crucial for the identification of potential dwarfing rootstocks and development of a breeding program for breadfruit dwarfing.
In this study, we examined the morphological properties of breadfruit plants growing on marang rootstocks within the period of 18 months after grafting. These led to the discovery of a dwarf phenotype that was rootstock-dependent. Further investigation into the biochemical properties of the leaves and stems in the composite plants at several time points revealed marang rootstocks not only affected the total soluble sugar content, but also impact the activity of plasma membrane (PM) H+-ATPase, a well-known primary proton pump essential for nutrient transport, phloem loading and cell expansion [17]. The significance of the two parameters in breadfruit dwarfing through marang rootstocks is discussed.
2. Materials and Methods
2.1. Plant Materials
Breadfruit (Artocarpus altilis cv. Noli) plants, propagated from root cuttings, and marang (Artocarpus odoratissimus) as seedlings of two to three months old, were obtained from a commercial nursery at Northern Queensland. The breadfruit cultivar, Noli is one of the main commercial breadfruit varieties in Australia [18]. The cultivar is seedless, up to 20 m tall with smooth, symmetrically round to oval fruits 1–2 kg in size [18,19]. In the current experiment, all breadfruit materials, including scions, breadfruit rootstocks (for homograft) and non-grafted breadfruit plants were from root-cuttings. Plants were grown in a glasshouse at 25~28 °C with natural daylight and daily water supply [3]. Before grafting, both scion and rootstock plants were grown as pot plants, with one plant per pot, single-stemmed, no adventitious shoots. These plants were grown in pots containing vermiculite and soil mixture (1:3), with one application of 10 g of 60-day-release fertiliser pellets (Osmocote; Scotts Australia Ltd, NSW, Australia) added to each pot every month. For grafting, scions selected from breadfruit plants 30~50 cm tall were grafted onto marang seedlings of a similar size through approach grafting, with graft union formed at a distance of 10~15 cm from the terminal bud of scions [3]. As a control, the breadfruit scions were also grafted onto the same breadfruit cultivar as rootstocks (homograft). Each established grafted plant was transferred to an 85-Litre pot six months after grafting, and continued to grow under the same glasshouse condition. The non-grafted breadfruits were selected with a similar height to homograft at three months after grafting and were grown under the same condition.
2.2. Morphology Characterisation of Grafted Breadfruit Plants
Stem elongation was measured monthly on scions from the graft junction to the terminal apex. For non-grafted plants, the measurement was started from a mark made on the stems at a similar height to the graft union on homograft at three months. Length of internodes was measured from the second internodes after the extension growth ceased. The number of nodes and branches (with at least one node) was counted at 18 months on the primary scion shoots. Stem thickness was based on the stem diameter calculated from the averaged stem circumference of the top five internodes. Leaf size was measured on the length and width of the widest points of each leaf bade from three fully matured leaves per plant, with other leaf characters, including leaf surface and vein, vein color, lobe number, the shape of leaf base, leaf apex and margin observed at the same time.
2.3. Determination of Total Chlorophyll Content in Leaves
Chlorophyll content was measured using a hand-held chlorophyll meters (atLeaf + Chl meter, FT GREEN LLC, Delaware, USA). The chlorophyll meter measured leaf absorbance of both 660 nm (red) and 940 nm (near-infrared) wavelengths. Three mature leaves were measured for each biological replicate, with five separate measurements on each leaf in the same leaf orientation. The optical values were converted to the total chlorophyll content following a calibration equation (y = 0.0067e0.0318x). The conversion equation was developed empirically by measuring the actual chlorophyll content of breadfruit leaves as described by Richardson, Duigan and Berlyn [20]. Briefly, twenty-five healthy leaves of different maturity were collected from glasshouse-grown breadfruit plants. After measurement by chlorophyll meter, three circular disks were punched from each leaf and extracted in dimethyl sulfoxide at 65 °C for 30 min. The chlorophyll concentration was calculated from the absorbance at 645 nm and 663 nm and expressed as mg Chl/cm2 leaf area [20].
2.4. Determination of Total Soluble Sugar in Leaves
Leaf discs were collected and stored at −80 °C until analysis. Leaf samples were homogenized with 80% ethanol, then incubated at 80 °C for 45 min followed by centrifugation at 15,000 g for 10 min. Pellets were re-extracted, and the supernatants were combined. Total soluble sugars were determined by the anthrone reagent according to the method by Yemm and Willis [21].
2.5. Measurement of Plasma Membrane H+-ATPase Activity in Stems
Stem tissues from the second internodes were prepared for microsomal fraction followed by measurement of the ATP hydrolytic activity at 38 °C for 30 min as previously described [3]. The activity of vanadate-sensitive ATPase was calculated from the difference between activity in the absence and presence of 1 mM Na3VO4. The activity was presented as µmol Pi/min/mg Protein.
2.6. Statistical Analyses
Significant differences were tested using analysis of variance (ANOVA) followed by Tukey’s multiple comparison test at p < 0.05 in the SPSS software package (IBM SPSS Statistics version 24, New York, USA).
3. Results
3.1. Growth Analysis of Breadfruit Plants Growing on Different Rootstocks
Growth analysis was started from three months after grafting where the grafting procedure was completed. Both morphological and growth curve analysis in the period from 3 months to 18 months (Figure 1 and Figure 2) showed plants on marang rootstocks were significantly smaller than those on homograft and non-graft. Their significantly shorter stems started to appear from 9 months where a reduction of 52% and 49% respectively, was observed in comparison to those of non-graft and homograft. There was no significant difference in stem elongation between the homograft and non-graft during the tested period (Figure 2). These results were also reflected by the decreased average growth rate displayed in plants growing on marang rootstocks, where both homograft and non-graft elongated at a similar rate (Table 1). As a result, plants on marang rootstocks were significantly shorter at the end of 18 months, with a reduction of 61% and 59% in height respectively, compared those on homograft and non-graft, leading to a dwarf phenotype (Figure 2, Table 1). Plants on marang rootstocks also showed a reduction of 33% in stem thickness compared to those of homograft or non-graft (Table 1). There was no significant difference in the stem node number between rootstocks, but stems on marang rootstocks displayed strikingly shorter internodes, with a reduction of 43% in average internode length and a reduction of 73% in the final second internode length compared to those on homograft, while there was no difference between those of homograft and non-graft (Figure 1 and Figure 2, Table 1). By the end of 18 months, no branch was observed from plants on marang rootstocks while most of the homograft and non-graft had at least one branch (Table 1). Both leaf number and leaf size were decreased in plants on marang rootstocks, with leaf number being reduced by 51% and leaf size (both length and width) being reduced by about 40% from those of homograft (Table 1). There was no difference in other leaf characters, including leaf shape, leaf surface and vein, lobe number, leaf apex and margin between plants on different rootstocks (Figure 1).

Figure 1.
Representatives of breadfruit plants growing on different rootstocks. (Top) Displaying internode length of scion stems. Left to right: On marang rootstocks, on the same cultivar of breadfruit rootstocks (homograft), and on own roots (non-graft). (Bottom) Left to right: Breadfruit plants growing on marang rootstocks, homograft, and non-graft.
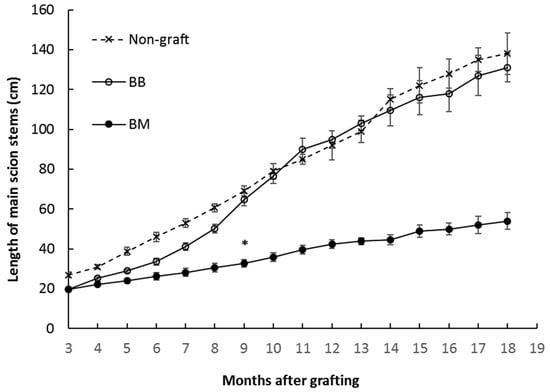
Figure 2.
Growth curves of breadfruit plants on different rootstocks in the period from 3 months to 18 months after grafting. non-graft, own-rooted breadfruit plants; BB, breadfruit plants on breadfruit rootstocks (homograft); BM, breadfruit plants on marang rootstocks. All values represent mean ± SE from five biological replicates. * Significant different (p < 0.05) from this time point onward.

Table 1.
Morphological assessment of breadfruit plants growing on different rootstocks.
3.2. Effect of Rootstocks on the Biochemical Properties of Scion Leaves
3.2.1. Total Soluble Sugar Content
Mature leaves (source leaves) were collected at several time points for total soluble sugar assay. Across the tested four times points from 9 to 18 months after grafting, leaves from scions on marang rootstocks showed significantly lower total soluble sugar content at three times points compared to those from homograft and non-graft, with a reduction of 27% at 9 months, 41% at 15 months and 24% at 18 months compared to those of homograft at the same time points (Figure 3). There was no significant difference in the total soluble sugar content between the leaves of homograft and non-grafted breadfruits.
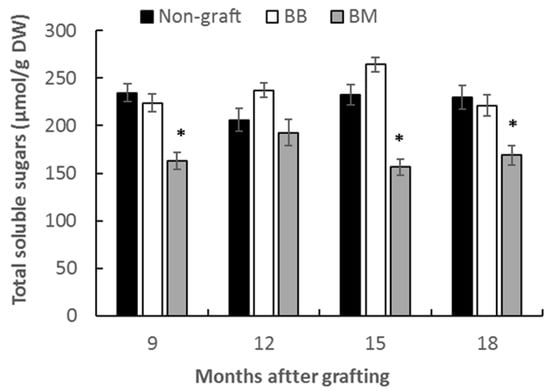
Figure 3.
Total soluble sugar content in leaves of breadfruit plants growing on different rootstocks. Non-graft, own-rooted breadfruit plants; BB, breadfruit plants on breadfruit rootstocks (homograft); BM, breadfruit plants on marang rootstocks. All values represent mean ± SE from five biological replicates (* p < 0.05).
3.2.2. Total Chlorophyll Content
Given that the optical values from the chlorophyll meter correlated well to the total chlorophyll content analytically measured from breadfruit leaves (R2 = 0.841, p < 0.0001, Supplemental Figure S1), the non-destructive method was used to examine the total chlorophyll content in mature leaves monthly in the period from 3 months to 18 months. These results showed there was a fluctuation in the total chlorophyll level on all plants over time, but there was no significant difference between plants grown on different rootstocks at any time point (Figure 4).
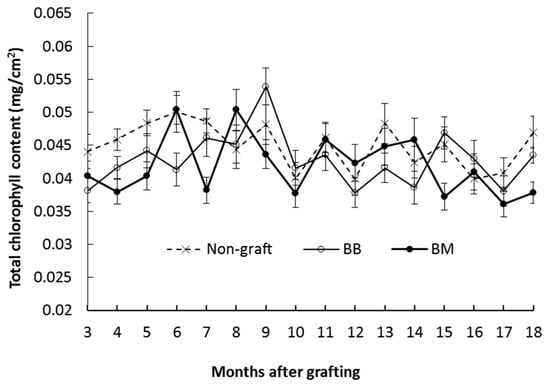
Figure 4.
Total chlorophyll content in leaves of breadfruit plants growing on different rootstocks in the period from 3 months to 18 months after grafting. The chlorophyll content was conversed from the chlorophyll meter reading following a calibration equation (y = 0.0067e0.0318x, see Supplemental Figure S1). All values represent mean ± SE from five biological replicates (* p < 0.05). Non-graft, own-rooted breadfruit plants; BB, breadfruit plants on breadfruit rootstocks (homograft); BM, breadfruit plants on marang rootstocks.
3.3. Effect of Rootstocks on the Plasma Membrane ATPase Activity of Stems
The reduced sugar level in scion leaves growing on marang rootstock prompted us to examine the activity of PM H+-ATPase, a primary proton pump that plays key roles in nutrient uptake and phloem loading. The enzyme generates the proton-motive force to drive the uptake of nutrients, such as sugars across the plasma membrane of growing cells [17]. Scion stems from the second internodes were assayed for PM-ATPase activity at 12 months and 15 months after grafting. The results found there were significantly lower levels of the enzyme activity in the scion stems grafted onto marang rootstocks, with a reduction of 33% and 35% respectively compared to those of the homograft at 15 months and 18 months (Figure 5). By contrast, the enzyme activities from the stems of homograft were not significantly different from those of the non-grafted plants (Figure 5).
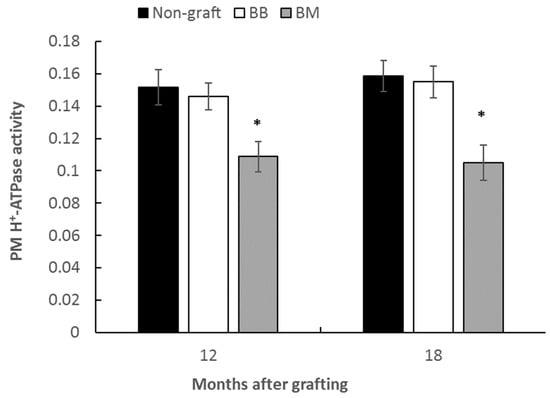
Figure 5.
Plasma membrane H+-ATPase activities in stems of breadfruit plants on different rootstocks. ATP hydrolytic activity was assayed in the microsomal fractions prepared from stems of the second internodes at 12 months and 18 months after grafting. Plasma membrane H+-ATPase activity is presented as µmol Pi/min/mg Protein. All values represent the mean ± SE of three biological replicates (* p < 0.05). PM, Plasma membrane; Non-graft, own-rooted breadfruit plants; BB, breadfruit plants on breadfruit rootstocks (homograft); BM, breadfruit plants on marang rootstocks.
4. Discussion
In this study, a dwarf phenotype was for the first time identified in breadfruit plants when they were grown on marang rootstocks. This dwarf phenotype was rootstock-dependent, as indicated by the distinct phenotypes between the same scion genotype growing on two different rootstock species, breadfruit (homograft) and marang. Breadfruit plants growing on marang rootstocks were characterised by shorter stems with reduced stem thickness and shorter internode length, fewer branches, fewer and smaller leaves compared to the homograft at the same age. These are consistent with the rootstock-induced dwarf traits of many other species [22,23]. The appearance of the dwarf traits on marang rootstocks during the early stage of growth is also consistent with previous findings that most rootstock effect on scion architecture occurs in the initial first or the second year of growth [7,24]. Particularly, previous quantitative modelling for the effects of dwarfing rootstocks on apple tree architecture has shown parameters, such as stem cross-sectional area, number of axillary shoots and internode length in the first one or two seasons after grafting correlate well with the long-term dwarfing potential of the rootstocks [7,25]. In this context, reduction in stem thickness, branch numbers and internode length found in our current study may predict a potentially lower tree form in breadfruit trees on marang rootstocks. However, continuous assessment for the long-term effect of marang rootstocks on breadfruit growth is required in the future. There was negligible difference in growth pattern between breadfruits on the same genotype of rootstocks (homograft) and the self-rooted breadfruits (non-graft), suggesting the effect of graft union on homograft was not significant during the period tested. Previously breadfruits were also grafted on pedalai (A. sericicarpus) rootstock, another distantly related Artocarpus species [3], the growth analysis on this rootstock was excluded in this study as preliminary results did not show a dwarf phenotype. Our results that the height of breadfruit was only reduced on marang rootstocks, with a reduction of 49% in 9 months and 59% in 18 months compared to the standard size, suggest marang rootstock may be applied to breeding program for breadfruit tree vigour control in order to develop wind-resistant tree crops or high density planting.
It is worth noting that the self-rooted marang species is known to grow into a standard tree of 10~25 m high, no dwarf phenotype has been identified in the species [13]. Preliminary results suggested marang seedlings were fast growing under our glasshouse condition, reaching over 1 m after 18 months, although monthly examination was not performed. There is little information regarding the properties of marang as rootstocks to other species. The intriguing effect that marang greatly reduced breadfruit tree size when used as rootstocks, agrees with previous findings in some citrus dwarfing rootstocks where their seedling genotypes showed little growth difference from standard rootstocks, but when used as rootstocks, greatly reduced tree size of the grafted scions [23]. Perhaps, rather than imparting the intrinsic dwarfing characteristics to scions as those naturally dwarf rootstocks [9], marang rootstocks may influence dwarfing depending on the interaction between rootstocks and scions [26]. Further investigation into the properties of marang rootstock on other related species may provide benefit to vigor control of other Artocarpus fruit crops.
It has been proposed that trees on dwarfing rootstocks display earlier termination of leaf production and earlier leaf senescence and abscission [7,22]. Both reduced leaf size and reduced leaf number observed in breadfruits growing on marring rootstock (Table 1) support this hypothesis. These suggest marang rootstocks may affect the total photosynthesis area and thus reduce the total capacity of carbohydrate production as observed in other rootstock-related dwarfing [23,24]. In the current experiment, leaf size was based on dimensional measurement, a further examination on the changes of leaf area would provide more predictable data on the changes of leaf size affected by rootstocks. While there was no significant difference in total chlorophyll content of leaves (Figure 4), and leaves on marang rootstocks were similar to those of the non-graft based on morphological characters, anatomical characteristics of leaves, such as cell number and structure associated with rootstock dwarfing appears worthy of further examination. Our results that significantly lower soluble sugar content was found in mature leaves on marang rootstocks (Figure 3) are comparable with previous findings on rootstock dwarfing of other species. For example, peach trees growing on dwarfing rootstocks had a lower level of glucose and fructose in leaves [27]. Levels of glucose and fructose are much lower in apple scions on dwarfing rootstocks, although sucrose and galactose concentrations remain unchanged [28]. For temperate species, the lower soluble sugar levels in the above-ground portion of trees growing on dwarfing rootstocks during the late autumn and dormancy periods have been associated with the low availability of carbohydrate reserve that reduces vegetative growth in the following spring season [26,29]. These pieces of evidence, together with the reduction of leaf number and leaf size in plants on marang rootstocks, further support the hypothesis that marang rootstocks may reduce the photosynthesis capacity of grafted breadfruits, leading to lower sugar accumulation, and thus reduction in vegetative growth. In addition to be carbon and energy sources, sugars also play vital roles as signaling molecules in plants. Sugars directly or indirectly control a wide range of processes, including photosynthesis, sugar transport itself, nitrogen uptake, defense reactions, secondary metabolism and hormonal balance [30]. For examples, intracellular sugars communicate metabolic demand to sucrose uptake that accounts for 90% of growth variation in pea cotyledons [31]. Sucrose applied to the aerial part of plants promote the morphogenesis at shoot apical meristem [32]. Sugar is also known to promote brassinosteroid (BR)-signaling, essential for shoot elongation driven by communication with other growth-promoting hormones [33]. Therefore, further investigation into the profile of soluble sugars with particular reference to metabolism and hormone balance may provide insight into the mechanism underlying the dwarfing effect of marang rootstock.
Accumulating evidence has indicated dwarfing rootstocks reduce nutrient transport from source to sink tissues, such as roots, stems and young leaves [27,28]. Our results that scion stems on marang rootstocks had lower PM H+-ATPase activity (Figure 5) for the first time provided evidence for a potential link of rootstock dwarfing to a primary transporter with fundamental roles in phloem loading and nutrient uptake [17]. PM H+-ATPase generates the proton-motive force that drives the uptake of nutrients, such as sugars and ions across the plasma membrane of growing cells [17]. As the uptake of most nutrients into sink tissues is an energy-dependent process under the control of PM H+-ATPase [17], a lower activity of PM H+-ATPase in scion stems growing on marang rootstocks (Figure 5) may suggest there is not enough proton-motive force to sustain the uptake of nutrients essential for cell growth in stem tissues. Furthermore, PM H+-ATPase is a key enzyme for H+ efflux resulting in cell wall loosening and cell extension in differentiating tissues [17]. A lower level of PM H+-ATPase activity was found to be associated with low cell extensibility [34]. In this context, a reduced PM ATPase activity in stems on marang rootstocks may reflect a reduction in cell expansion leading to reduced stem internode length as displayed in the dwarf phenotype. Finally, PM H+-ATPase also integrates with diverse signals, including sugars and growth-promoting hormones, auxin and BR [17,35,36]. Some of these signals have long been proposed to play a role in rootstock dwarfing [7,37]. In this context, the nature of PM H+-ATPase change and its function in rootstock-induced dwarfing deserve further investigation.
In conclusion, we reported the first dwarf phenotype found in breadfruit plants growing on marang rootstocks. The dwarf traits included a reduction in stem height, stem thickness and internode length, branch number, leaf number and leaf size. The dwarf breadfruit plants had lower total soluble sugars in leaves, and lower PM H+-ATPase activity in stems at tested time points. Our results can be applied to breadfruit breeding program for conferring vigor control and developing dwarf phenotype.
Supplementary Materials
The following are available online at https://www.mdpi.com/2311-7524/5/2/40/s1. Figure S1: Relationship between chlorophyll meter values and the content of total content chlorophyll in breadfruit leaves. Chl meter value—value from chlorophyll meters.
Author Contributions
Conceptualization, Y.Z. and S.J.R.U.; methodology, Y.Z.; investigation, Y.Z.; writing—original draft preparation, Y.Z.; writing—review and editing, Y.Z. and S.J.R.U.
Funding
This work was funded by the Australia Centre for International Agriculture Research (ACIAR) through project HORT 2014/077.
Acknowledgments
In this section you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ragone, D. Breadfruit, Artocarpus Altilis (Parkinson) Fosberg-Promoting the Conservation and Use of Underutilized and Neglected Crops. 10; Leibniz Institute of Plant Genetics and Crop Plant Research (IPK): Gatersleben, Germany, 1997; pp. 1–77. [Google Scholar]
- Daley, O.; Gloster, M.; Roberts-Nkrumah, L.B. Assessment and characterization of damage by hurricane Tomas to major tree crops with special emphasis on breadfruit (Artocarpus altilis) and breadnut (Artocarpus camansi) in St. Lucia and St. Vincent and the Grenadines. Proc. Caribb. Food Crop Soc. 2012, 48, 124–131. [Google Scholar]
- Zhou, Y.; Underhill, S.J.R. Plasma membrane H-ATPase activity and graft success of breadfruit (Artocarpus altilis) onto interspecific rootstocks of marang (A. odoratissimus) and pedalai (A. sericicarpus). Plant Biol. 2018, 20, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Taylor, M.B.; Underhill, S.J.R. Dwarfing of breadfruit (Artocarpus altilis) trees: Opportunities and challenges. Am. J. Exp. Agric. 2014, 4, 1743–1763. [Google Scholar] [CrossRef]
- Roberts-Nkrumah, L.B. Breadnut and Breadfruit Propagation, a Manual for Commercial Propagation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2012; pp. 1–18. [Google Scholar]
- Jones, A.M.P.; Ragone, D.; Tavana, N.G.; Bernotas, D.W.; Murch, S.J. Beyond the bounty: Breadfruit (Artocarpus altilis) for food security and novel foods in the 21st century. Ethnobot. Res. Appl. 2011, 9, 129–149. [Google Scholar] [CrossRef]
- Marini, R.P.; Fazio, G. Apple rootstocks: History, physiology, management, and breeding. Hortic. Rev. 2017, 45, 197–312. [Google Scholar]
- Nimbolkar, P.K.; Awachare, C.; Reddy, Y.T.N.; Chander, S.; Hussain, F. Role of Rootstocks in Fruit Production—A Review. J. Agric. Eng. Food Technol. 2016, 3, 183–188. [Google Scholar]
- Cheng, F.S.; Roose, M.L. Origin and inheritance of dwarfing by the citrus rootstock poncirus trifoliata ‘Flying Dragon’. J. Am. Soc. Hortic. Sci. 1995, 120, 286–291. [Google Scholar] [CrossRef]
- Haq, M.Z.; Robbani, M.; Ali, M.; Hasan, M.M.; Hasan, M.M.; Uddin, M.J.; Begum, M.; da Silva, J.A.T.; Pan, X.-Y.; Karim, M.R. Damage and management of cyclone Sidr-affected homestead tree plantations: A case study from Patuakhali, Bangladesh. Nat. Hazards 2012, 64, 1305–1322. [Google Scholar] [CrossRef]
- Parthiban, K.T.; Krishnakumar, N.; Karthick, N. (Eds.) Introduction to Forestry & Agroforestry; Scientific Publishers: Jodhpur, India, 2018; p. 367. [Google Scholar]
- Calvert, G. An Assessment of Tree Susceptibility and Resistance to Cyclones—with Particular Reference to Severe Tropical Cyclone Yasi; Greening Australia, Townsville City Council and Ergon Energy: Queensland, Australia, 2011; Available online: https://www.greeningaustralia.org.au/wp-content/uploads/2017/11/RESEARCH_Yasi_TreeReport_NewFormat.pdf (accessed on 15 January 2019).
- Tropicos. Available online: http://www.tropicos.org/NameSearch.aspx (accessed on 15 January 2019).
- Nandwani, D.; Kuniyuki, A.H. Grafting and improvement of breadfruit production in micronesia. In Proceedings of the International Symposium on Harnessing the Potential of Horticulture in the Asian-Pacific Region, Coolum, Australia, 1–3 September 2005; Volume 694, pp. 307–310. [Google Scholar]
- Medagoda, I.; Chandrarathna, W.M. Grafting of breadfruit (Artocarpus altilis) using breadnut (Artocarpus camansi) as root stock. Acta Hortic. 2007, 757, 149–152. [Google Scholar] [CrossRef]
- Solomon, F.J.; Roberts-Nkrumah, L.B. An evaluation of factors influencing successful grafting of breadfruit on chataigne rootstock. Proc. Caribb. Food Crop. Soc. 2008, 44, 304–312. [Google Scholar]
- Palmgren, M.G. Plant plasma membrane H+-ATPase: Powerhouses for nutrient uptake. Annu. Rev. Plant Biol. 2001, 52, 817–845. [Google Scholar] [CrossRef] [PubMed]
- Goebel, R. Breadfruit-the Australian scene. In Proceedings of the International Symposium on Breadfruit Research and Development, Nadi, Fiji, 16–19 April 2007; Volume 757, pp. 141–148. [Google Scholar]
- Goebel, R.; Breadfruit. Peninsula Gardening Notes, Department of Primary Industries and Fisheries, Queensland. 2004. Available online: http://plant.daleysfruit.com.au/l/breadfruit-tress-677.pdf (accessed on 15 January 2019).
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]
- Yemm, E.; Willis, A. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1956, 57, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.D. Rootstock and interstock effects on deciduous fruit tree vigour, precocity, and yield productivity. N. Z. J. Crop Hortic. Sci. 1995, 23, 373–382. [Google Scholar] [CrossRef]
- Forner-Giner, M.A.; Rodriguez-Gamir, J.; Martinez-Alcantara, B.; Quinones, A.; Iglesias, D.J.; Primo-Millo, E.; Forner, J. Performance of Navel orange trees grafted onto two new dwarfing rootstocks (Forner-Alcaide 517 and Forner-Alcaide 418). Sci. Hortic. 2014, 179, 376–387. [Google Scholar] [CrossRef]
- Clearwater, M.; Seleznyova, A.; Thorp, T.; Blattmann, P.; Barnett, A.; Lowe, R.; Austin, P. Vigor-controlling rootstocks affect early shoot growth and leaf area development of kiwifruit. Tree Physiol. 2006, 26, 505–515. [Google Scholar] [CrossRef]
- Seleznyova, A.N.; Thorp, T.G.; White, M.; Tustin, S.; Costes, E. Application of architectural analysis and AMAPmod methodology to study dwarfing phenomenon: The branch structure of ‘Royal Gala’ apple grafted on dwarfing and non-dwarfing rootstock/interstock combinations. Ann. Bot. 2003, 91, 665–672. [Google Scholar] [CrossRef]
- Lochard, R.G.; Schneider, G.W. Stock and scion growth relationships and the dwarfing mechanism in apple. Hortic. Rev. 1981, 3, 315–375. [Google Scholar]
- Salvatierra, M.A.; Gemma, H.; Iwahori, S. Partitioning of Carbohydrates and Development of Tissues in the Graft Union of Peaches Grafted on Prunus tomentosa Thunb. Rootstock. J. Jpn. Soc. Hortic. Sci. 1998, 67, 175–182. [Google Scholar] [CrossRef]
- Foster, T.M.; McAtee, P.A.; Waite, C.N.; Boldingh, H.L.; McGhie, T.K. Apple dwarfing rootstocks exhibit an imbalance in carbohydrate allocation and reduced cell growth and metabolism. Hortic. Res. 2017, 4, 17009. [Google Scholar] [CrossRef]
- Brown, C.S.; Young, E.; Pharr, D.M. Rootstock and scion effects on the seasonal distribution of dry weight and carbohydrates in young apple trees. J. Am. Soc. Hortic. Sci. 1985, 110, 696–701. [Google Scholar]
- Smeekens, S.; Ma, J.; Hanson, J.; Rolland, F. Sugar signals and molecular networks controlling plant growth. Curr. Opin. Plant Biol. 2010, 13, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chan, K.; Wang, T.; Hedley, C.; Offler, C.; Patrick, J. Intracellular sucrose communicates metabolic demand to sucrose transporters in developing pea cotyledons. J. Exp. Bot. 2009, 60, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Roldán, M.; Gómez-Mena, C.; Ruiz-García, L.; Salinas, J.; Martínez-Zapater, J.M. Sucrose availability on the aerial part of the plant promotes morphogenesis and flowering of Arabidopsis in the dark. Plant J. 2002, 20, 581–590. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, J.-Y.; Roh, J.; Marchive, C.; Kim, S.-K.; Meyer, C.; Sun, Y.; Wang, W.; Wang, Z.-Y. TOR signaling promotes accumulation of BZR1 to balance growth with carbon availability in Arabidopsis. Curr. Biol. 2016, 26, 1854–1860. [Google Scholar] [CrossRef] [PubMed]
- Sveinsdottira, H.; Yan, F.; Zhu, Y.Y.; Peiter-Volk, T.; Schubert, S. Seed ageing-induced inhibition of germination and post-germination root growth is related to lower activity of plasma membrane H+-ATPase in maize roots. J. Plant Physiol. 2009, 166, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Okumura, M.; Inoue, S.; Kuwata, K.; Kinoshita, T. Photosynthesis Activates Plasma Membrane H+-ATPase via Sugar Accumulation. Plant Physiol. 2016, 171, 580–589. [Google Scholar] [CrossRef]
- Caesar, K.; Elgass, K.; Chen, Z.H.; Huppenberger, P.; Witthoft, J.; Schleifenbaum, F.; Blatt, M.R.; Oecking, C.; Harter, K. A fast brassinolide-regulated response pathway in the plasma membrane of Arabidopsis thaliana. Plant J. 2011, 66, 528–540. [Google Scholar] [CrossRef]
- Noda, K.; Okuda, H.; Iwagaki, I. Indole acetic acid and abscisic acid levels in new shoots and fibrous roots of citrus scion-rootstock combinations. Sci. Hortic. 2000, 84, 245–254. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).