Aeroponic Cloning of Capsicum spp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Propagation of Mother Plants
2.2. Cloning Experiments
2.3. Statistical Analysis
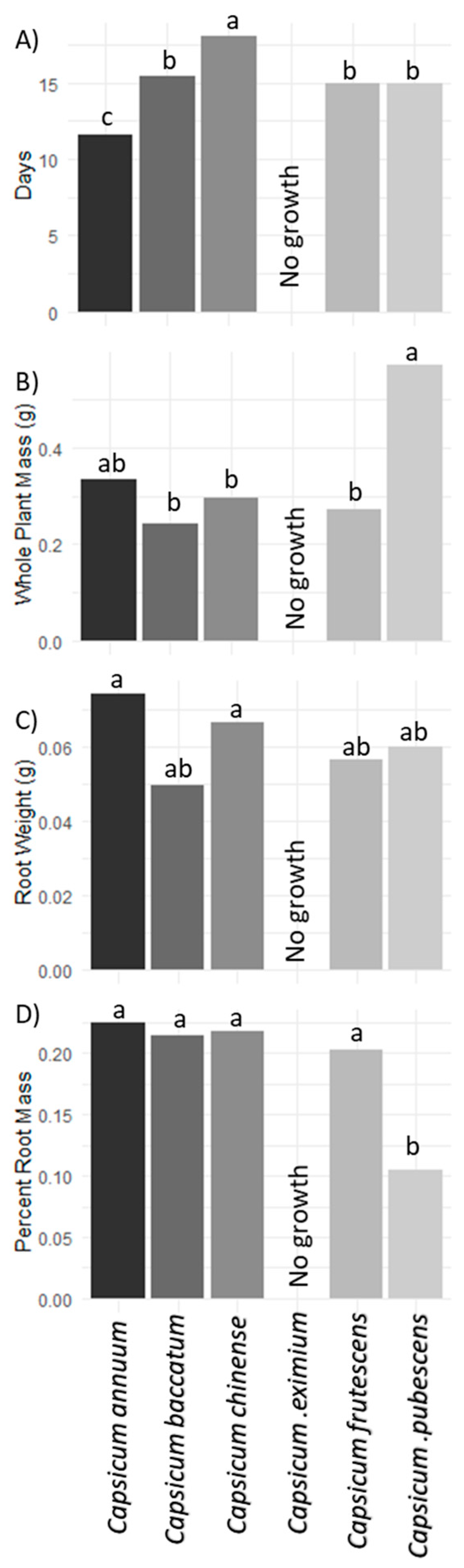
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bosland, P.W.; Votava, E.J. Peppers: Vegetable and Spice Capsicums; CAB Intl.: Wallingford, UK, 2012. [Google Scholar]
- Omolo, M.O.; Wong, Z.Z.; Mergen, A.K.; Hastings, J.C.; Le, N.C.; Reiland, H.A.; Case, K.A.; Baumler, D.J. Antimicrobial properties of chili peppers. J. Infect. Dis. Ther. 2014, 2, 145. [Google Scholar] [CrossRef]
- Sherman, P.W.; Billing, J. Darwinian Gastronomy: Why We Use Spices Spices taste good because they are good for us. BioScience 1999, 49, 453–463. [Google Scholar] [CrossRef]
- Smith, S.H. In the shadow of a pepper-centric historiography: Understanding the global diffusion of capsicums in the sixteenth and seventeenth centuries. J. Ethnopharmacol. 2015, 167, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Kraft, K.H.; Brown, C.H.; Nabhan, G.P.; Luedeling, E.; Ruiz, J.D.J.L.; d’Eeckenbrugge, G.C.; Hijmans, R.J.; Gepts, P. Multiple lines of evidence for the origin of domesticated chili pepper, Capsicum annuum, in Mexico. Proc. Natl. Acad. Sci. USA 2014, 111, 6165–6170. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.; Dickau, R.; Zarrillo, S.; Holst, I.; Pearsall, D.M.; Piperno, D.R.; Berman, M.J.; Cooke, R.G.; Rademaker, K.; Ranere, A.J.; et al. Starch fossils and the domestication and dispersal of chili peppers (Capsicum spp. L.) in the Americas. Science 2007, 315, 986–988. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Final Data 2014. 2014. Available online: http://www.fao.org/faostat/en/#home (accessed on 17 January 2017).
- USDA ERS. 2017. Available online: https://www.nass.usda.gov/Data_and_Statistics/index.php (accessed on 17 February 2017).
- Bosland, P.W. Capsicums: Innovative uses of an ancient crop. In Progress in New Crops; Janick, J., Ed.; ASHS Press: Arlington, VA, USA, 1996; pp. 479–487. [Google Scholar]
- Zewdie, Y.; Bosland, P.W. Capsaicinoid profiles are not good chemotaxonomic indicators for Capsicum species. Biochem. Syst. Ecol. 2001, 29, 161–169. [Google Scholar] [CrossRef]
- Lillywhite, J.M.; Simonsen, J.E.; Uchanski, M.E. Spicy Pepper Consumption and Preferences in the United States. HortTechnology 2013, 23, 868–876. [Google Scholar] [CrossRef]
- Bosland, P.W.; Coon, D.; Reeves, G. ‘Trinidad Moruga Scorpion’ Pepper is the World’s Hottest Measured Chile Pepper at More Than Two Million Scoville Heat Units. HortTechnology 2012, 22, 534–538. [Google Scholar] [CrossRef]
- Kantar, M.B.; Anderson, J.E.; Lucht, S.A.; Mercer, K.; Bernau, V.; Case, K.A.; Le, N.C.; Frederiksen, M.K.; DeKeyser, H.C.; Wong, Z.Z.; et al. Vitamin Variation in Capsicum Spp. Provides Opportunities to Improve Nutritional Value of Human Diets. PLoS ONE 2016, 11, e0161464. [Google Scholar] [CrossRef] [PubMed]
- Tewksbury, J.J.; Reagan, K.M.; Machnicki, N.J.; Carlo, T.A.; Haak, D.C.; Peñaloza, A.L.C.; Levey, D.J. Evolutionary ecology of pungency in wild chilies. Proc. Natl. Acad. Sci. USA 2008, 105, 11808–11811. [Google Scholar] [CrossRef] [PubMed]
- Kraft, K.H.; de Luna-Ruíz, J.; Gepts, P. Different seed selection and conservation practices for fresh market and dried chile farmers in Aguascalientes, Mexico. Econ. Bot. 2010, 64, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Mehandru, P.; Shekhawat, N.S.; Rai, M.K.; Kataria, V.; Gehlot, H.S. Evaluation of aeroponics for clonal propagation of Caralluma edulis, Leptadenia reticulata and Tylophora indica—Three threatened medicinal Asclepiads. Physiol. Mol. Biol. Plants 2014, 20, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Harlan, J.R.; de Wet, J.M.J.; Price, E.G. Comparative evolution of cereals. Evolution 1973, 27, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, S.; Acharjee, S.; Devi, J. In vitro plantlet regeneration of Capsicum chinense Jacq. cv.’Bhut jalakia’: Hottest chili of northeastern India. In Vitro Cell. Dev. Biol. Plant 2014, 50, 235–241. [Google Scholar] [CrossRef]
- Ritter, E.; Angulo, B.; Riga, P.; Herran, C.; Relloso, J.; San Jose, M. Comparison of hydroponic and aeroponic cultivation systems for the production of potato minitubers. Potato Res. 2001, 44, 127–135. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Tye, A. The status of the endemic flora of Galapagos: The number of threatened species is increasing. Galápagos Rep. 2006, 2007, 96–103. [Google Scholar]
- Hernández-Verdugo, S.; Oyama, K.; Vázquez-Yanes, C. Differentiation in seed germination among populations of Capsicum annuum along a latitudinal gradient in Mexico. Plant Ecol. 2001, 155, 245–257. [Google Scholar] [CrossRef]
- Randle, W.M.; Honma, S. Dormancy in peppers. Sci. Hortic. 1981, 14, 19–25. [Google Scholar] [CrossRef]
- Dabauza, M.; Pena, L. High efficiency organogenesis in sweet pepper (Capsicum annuum L.) tissues from different seedling explants. Plant Growth Regul. 2001, 33, 221–229. [Google Scholar] [CrossRef]
- Kumar, V.; Gururaj, H.B.; Prasad, B.N.; Giridhar, P.; Ravishankar, G.A. Direct shoot organogenesis on shoot apex from seedling explants of Capsicum annuum L. Sci. Hortic. 2005, 106, 237–246. [Google Scholar] [CrossRef]


| Species | Cultivar | Seed Source |
|---|---|---|
| Capsicum annuum | Ruby King | Seed Savers |
| Capsicum annuum | Sweet Big Daddy Hybrid | Burpee |
| Capsicum baccatum | Dedo De Moca | PepperLover.com |
| Capsicum baccatum | Aji Limon | Chile Pepper Institute |
| Capsicum chinense | Tobago Yellow Scotch | PepperLover.com |
| Capsicum chinense | Trin. Scorpion Sweet | PepperLover.com |
| Capsicum chinense | Hot Zavory | Burpee |
| Capsicum eximium | CGN 19198 | PepperLover.com |
| Capsicum eximium | CGN 24332 | PepperLover.com |
| Capsicum frutescens | Bradley | PepperLover.com |
| Capsicum frutescens | Tobasco Heirloom | Burpee |
| Capsicum frutescens | Tobasco | Seed Savers |
| Capsicum pubescens | Red Rocoto | Seed Savers |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Valle-Echevarria, A.R.; Kantar, M.B.; Branca, J.; Moore, S.; Frederiksen, M.K.; Hagen, L.; Hussain, T.; Baumler, D.J. Aeroponic Cloning of Capsicum spp. Horticulturae 2019, 5, 30. https://doi.org/10.3390/horticulturae5020030
Del Valle-Echevarria AR, Kantar MB, Branca J, Moore S, Frederiksen MK, Hagen L, Hussain T, Baumler DJ. Aeroponic Cloning of Capsicum spp. Horticulturae. 2019; 5(2):30. https://doi.org/10.3390/horticulturae5020030
Chicago/Turabian StyleDel Valle-Echevarria, Angel R., Michael B. Kantar, Julianne Branca, Sarah Moore, Matthew K. Frederiksen, Landon Hagen, Tanveer Hussain, and David J. Baumler. 2019. "Aeroponic Cloning of Capsicum spp." Horticulturae 5, no. 2: 30. https://doi.org/10.3390/horticulturae5020030
APA StyleDel Valle-Echevarria, A. R., Kantar, M. B., Branca, J., Moore, S., Frederiksen, M. K., Hagen, L., Hussain, T., & Baumler, D. J. (2019). Aeroponic Cloning of Capsicum spp. Horticulturae, 5(2), 30. https://doi.org/10.3390/horticulturae5020030





