Serianthes nelsonii Seed Germination and Seedling Behavior are Minimally Influenced by Chemical and Light Treatment
Abstract
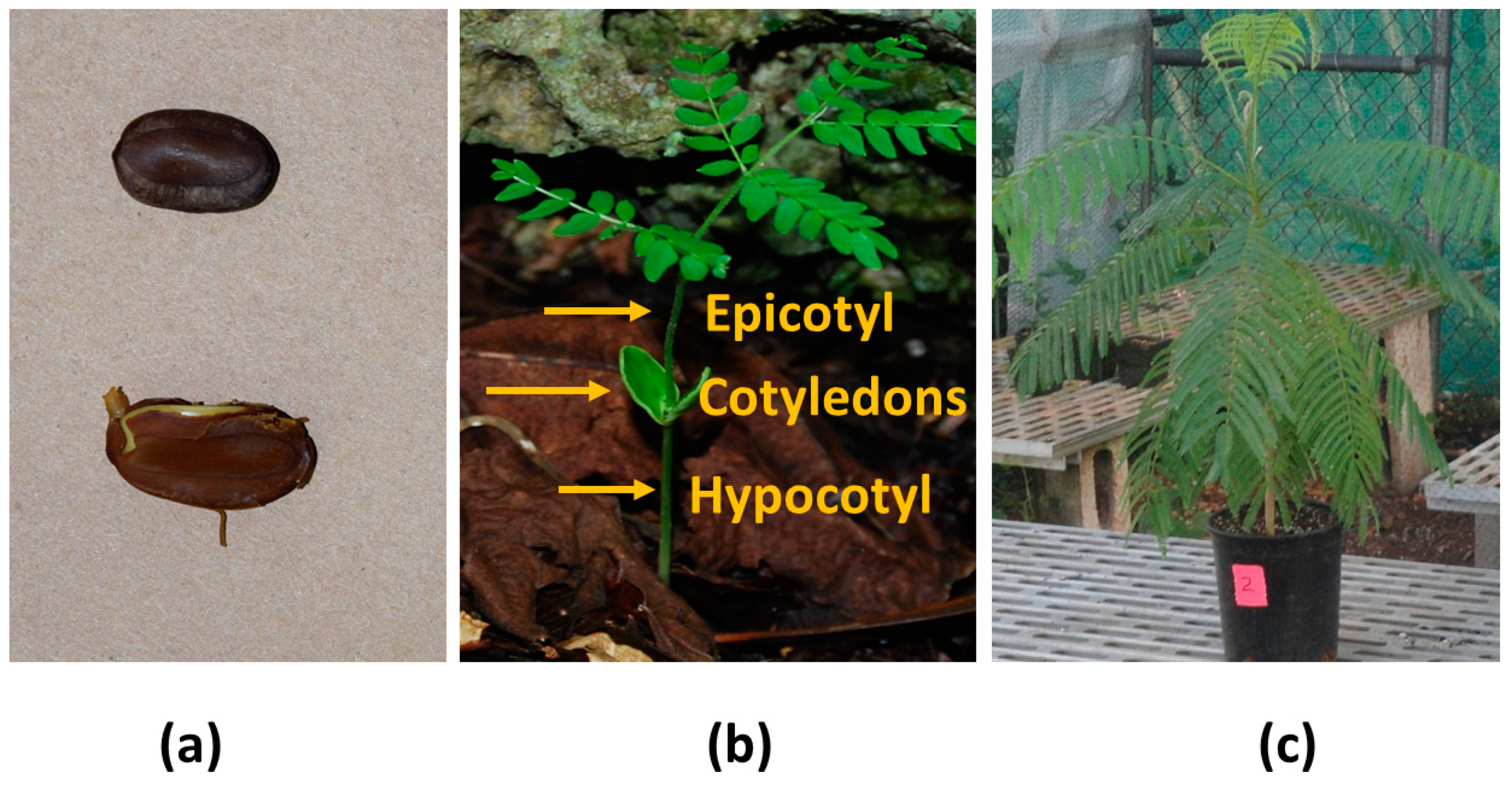
:1. Introduction
2. Materials and Methods
2.1. Light Methods
2.2. Chemical Pre-Soak Methods
2.3. Statistics
3. Results and Discussion
3.1. Light Treatments
3.2. Nitrogen Treatments
3.3. Gibberellic Acid Treatments
3.4. Interpretations
3.5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Harper, J.L. Population Biology of Plants; Academic Press: London, UK, 1977; ISBN 0-12-325850-2. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 1998; ISBN 0-12-080260. [Google Scholar]
- Baskin, J.M.; Baskin, C.C.; Li, X. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Spec. Biol. 2000, 15, 139–152. [Google Scholar] [CrossRef]
- Wiles, G.; Williams, E. Serianthes Nelsonii. The IUCN Red List of Threatened Species. 2017. Available online: https://www.iucnredlist.org/pdflink.98715973 (accessed on 10 March 2019).
- Marler, T.E. Asexual reproduction to propel recovery efforts of the critically endangered Håyun Lågu tree (Serianthes nelsonii Merr.). Trop. Conserv. Sci. 2017, 10. [Google Scholar] [CrossRef]
- United States Fish and Wildlife Services. Recovery Plan for Serianthes nelsonii; United States Fish and Wildlife Services: Portland, OR, USA, 1994.
- Marler, T.E.; Cascasan, A.N.; Lawrence, J.H. Threatened native trees in Guam: Short-term seed storage and shade conditions influence emergence and growth of seedlings. HortScience 2015, 50, 1049–1054. [Google Scholar] [CrossRef]
- Marler, T.; Musser, C. Chemical and air pruning of roots influence posttransplant root traits of the critically endangered Serianthes nelsonii. Plant Root 2016, 10, 21–25. [Google Scholar] [CrossRef]
- Basra, A.S. Seed Quality Basic Mechanism and Agricultural Implications; Food Products Press: New York, NY, USA, 1995; ISBN 1-56022-850-4. [Google Scholar]
- Copeland, L.O.; McDonald, M.B. Principles of Seed Science and Technology, 4th ed.; Kluwer Academic Publishers: Norwell, MA, USA, 2001; ISBN 978-0792373223. [Google Scholar]
- Parera, C.A.; Cantliffe, D.J. Presowing seed priming. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 109–141. [Google Scholar] [CrossRef]
- Yadav, R.K.; Saini, P.K.; Pratap, M.; Tripathi, S.K. Techniques of seed priming in field crops. Int. J. Chem. Stud. 2018, 6, 1588–1594. [Google Scholar]
- Heydecker, W. Accelerated germination by osmotic seed treatment. Nature 1973, 246, 42–44. [Google Scholar] [CrossRef]
- Nimir, N.E.A.; Lu, S.; Zhou, G.; Guo, W.; Ma, B.; Wang, Y. Comparative effects of gibberellic acid, kinetin and salicylic acid on emergence, seedling growth and the antioxidant defence system of sweet sorghum (Sorghum bicolor) under salinity and temperature stresses. Crop Pasture Sci. 2015, 66, 145–157. [Google Scholar] [CrossRef]
- Brian, P.W.; Hemming, H.G. Promotion of cucumber hypocotyl growth by two new gibberellins. Nature 1961, 189, 74. [Google Scholar] [CrossRef]
- Crozier, A.; Kuo, C.C.; Durley, R.C.; Pharis, R.P. The biological activities of 26 gibberellins in nine plant bioassays. Can. J. Bot. 1970, 48, 867–877. [Google Scholar] [CrossRef]
- Derkx, M.P.M.; Vermeer, E.; Karssen, C.M. Gibberellins in seeds of Arabidopsis thaliana: Biological activities, identification and effects of light and chilling on endogenous levels. Plant Growth Regul. 1994, 15, 223–234. [Google Scholar] [CrossRef]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Shinomura, T. Phytochrome regulation of seed germination. J. Plant Res. 1997, 110, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Pons, T.L. Seed responses to light. In Seed: The Ecology of Regeneration in Plant Communities; Fenner, M., Ed.; CABI Publishing: New York, NY, USA, 2000; pp. 237–260. [Google Scholar]
- Vayda, K.; Donohue, K.; Auge, G.A. Within- and trans-generational plasticity: Seed germination responses to light quantity and quality. AoB Plants 2018, 10, ply023. [Google Scholar] [CrossRef] [PubMed]
- Ballaré, C.L.; Scopel, A.L.; Sanchez, R.A. Far-red radiation reflected from adjacent leaves: An early signal of competition in plant canopies. Science 1990, 247, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E.; Cascasan, A.N. Number of emerged seedlings and seedling longevity of the non-recruiting, Critically Endangered Hayan Lagu tree Serianthes nelsonii Merr. (Fabales: Leguminosae) are influenced by month of emergence. J. Threat. Taxa 2015, 7, 8221–8225. [Google Scholar] [CrossRef]
| Response Variable | Darkness | Incandescent | Fluorescent | Mean | F | p |
|---|---|---|---|---|---|---|
| Germination (%) | 70.0 ± 4.5 | 66.7 ± 4.2 | 70.0 ± 4.5 | 68.9 | 0.1923 | 0.8271 |
| Initial germination (h) | 42.0 ± 2.7 | 44.0 ± 4.0 | 38.0 ± 2.0 | 41.3 | 1.0294 | 0.3811 |
| Final germination (h) | 58.0 ± 2.0 | 58.0 ± 2.0 | 54.0 ± 2.7 | 56.7 | 1.0526 | 0.3734 |
| Germination duration (h) | 16.0 ± 4.0 | 16.0 ± 4.0 | 14.0 ± 4.8 | 15.3 | 0.0725 | 0.9304 |
| Hypocotyl length (mm) | 22.8 ± 1.7 | 22.2 ± 2.1 | 19.8 ± 1.6 | 21.6 | 1.0038 | 0.3898 |
| Epicotyl length (mm) | 15.7 ± 1.2 | 16.3 ± 1.7 | 16.2 ± 1.3 | 16.1 | 0.0590 | 0.9429 |
| Cotyledon death (d) | 8.3 ± 0.7 | 8.7 ± 0.6 | 8.8 ± 0.6 | 8.6 | 0.1977 | 0.8227 |
| Growth resumption (d) | 8.7 ± 0.6 | 8.3 ± 0.8 | 8.2 ± 0.6 | 8.4 | 0.1643 | 0.8499 |
| Time to 30-cm height (w) | 17.3 ± 0.6 | 16.8 ± 0.9 | 17.8 ± 0.9 | 17.3 | 0.2848 | 0.7561 |
| Response Variable | Darkness | Leaf Shade | Cloth Shade | Mean | F | p |
|---|---|---|---|---|---|---|
| Germination (%) | 73.3 ± 4.2 | 70.0 ± 4.5 | 73.3 ± 4.2 | 72.2 | 1.5513 | 0.2441 |
| Initial germination (h) | 38.0 ± 2.0 | 38.0 ± 2.0 | 38.0 ± 2.0 | 38.0 | 0 | 1.0000 |
| Final germination (h) | 52.0 ± 4.0 | 52.0 ± 4.0 | 56.0 ± 2.5 | 53.3 | 0.4617 | 0.6666 |
| Germination duration (h) | 14.0 ± 4.8 | 14.0 ± 4.8 | 18.0 ± 2.7 | 15.3 | 0.2985 | 0.7462 |
| Hypocotyl length (mm) | 22.7 ± 1.8 | 22.2 ± 2.0 | 20.8 ± 1.9 | 21.8 | 0.2503 | 0.7818 |
| Epicotyl length (mm) | 16.3 ± 1.8 | 16.0 ± 1.1 | 16.2 ± 1.9 | 16.2 | 0.0145 | 0.9856 |
| Cotyledon death (d) | 8.7 ± 0.8 | 8.3 ± 0.6 | 8.5 ± 0.5 | 8.5 | 0.0664 | 0.9361 |
| Growth resumption (d) | 8.2 ± 0.7 | 7.7 ± 0.7 | 8.3 ± 0.8 | 8.1 | 0.2490 | 0.7827 |
| Time to 30-cm height (w) | 17.5 ± 1.5 | 17.0 ± 1.3 | 17.3 ± 0.9 | 17.3 | 0.0426 | 0.9584 |
| Response Variable | Water | NH4NO3 | KNO3 | F | p |
|---|---|---|---|---|---|
| Germination (%) | 73.3 ± 6.7 | 76.7 ± 6.1 | 80.0 ± 5.2 | 0.3061 | 0.7408 |
| Initial germination (h) | 46.0 ± 2.0 | 44.0 ± 2.5 | 46.0 ± 2.0 | 0.6919 | 0.5159 |
| Final germination (h) | 62.0 ± 2.0 | 62.0 ± 2.0 | 62.0 ± 2.0 | 0 | 1.0000 |
| Germination duration (h) | 16.0 ± 4.0 | 18.0 ± 4.1 | 16.0 ± 2.5 | 0.1020 | 0.9036 |
| Hypocotyl length (mm) | 21.5 ± 0.9a 1 | 26.5 ± 1.3b | 27.2 ± 1.2b | 6.8609 | 0.0077 |
| Epicotyl length (mm) | 16.2 ± 0.5a | 20.0 ± 0.3b | 19.8 ± 0.3b | 12.5495 | 0.0006 |
| Cotyledon death (d) | 8.3 ± 0.8 | 7.8 ± 0.7 | 9.1 ± 0.8 | 0.5490 | 0.5887 |
| Growth resumption (d) | 9.8 ± 0.7 | 10.3 ± 0.6 | 11.0 ± 0.6 | 0.7312 | 0.4977 |
| Time to 30-cm height (w) | 16.3 ± 0.4 | 16.5 ± 0.6 | 16.3 ± 0.4 | 0.0376 | 0.9632 |
| Response Variable | 0 | 50 | 100 | 200 | 300 | Significance1 |
|---|---|---|---|---|---|---|
| Germination (%) | 76.7 ± 6.1 | 73.3 ± 4.2 | 73.3 ± 6.7 | 76.7 ± 3.3 | 83.3 ± 6.1 | NS |
| Initial germination (h) | 46.0 ± 2.0 | 44.0 ± 2.5 | 46.0 ± 2.0 | 46.0 ± 2.0 | 46.0 ± 2.0 | NS |
| Final germination (h) | 62.0 ± 2.0 | 62.0 ± 2.0 | 62.0 ± 2.0 | 62.0 ± 2.0 | 62.0 ± 2.0 | NS |
| Germination duration (h) | 16.0 ± 4.0 | 16.0 ± 3.0 | 16.0 ± 2.5 | 16.0 ± 2.5 | 16.0 ± 2.5 | NS |
| Hypocotyl length (mm) | 23.3 ± 1.8 | 26.2 ± 1.9 | 28.7 ± 1.2 | 29.2 ± 1.4 | 33.8 ± 2.7 | L *** |
| Epicotyl length (mm) | 16.2 ± 1.8 | 16.3 ± 1.4 | 16.5 ± 1.8 | 16.3 ± 1.8 | 16.2 ± 1.9 | NS |
| Cotyledon death (d) | 8.2 ± 0.6 | 8.8 ± 0.5 | 9.8 ± 0.5 | 10.2 ± 0.5 | 10.7 ± 0.4 | L *** |
| Growth resumption (d) | 10.7 ± 0.3 | 8.5 ± 0.4 | 7.8 ± 0.6 | 7.3 ± 0.5 | 7.2 ± 0.4 | L *** Q ** |
| Time to 30-cm height (w) | 16.7 ± 1.0 | 17.0 ± 1.0 | 16.7 ± 0.8 | 16.0 ± 1.1 | 17.3 ± 0.8 | NS |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marler, T.E. Serianthes nelsonii Seed Germination and Seedling Behavior are Minimally Influenced by Chemical and Light Treatment. Horticulturae 2019, 5, 31. https://doi.org/10.3390/horticulturae5020031
Marler TE. Serianthes nelsonii Seed Germination and Seedling Behavior are Minimally Influenced by Chemical and Light Treatment. Horticulturae. 2019; 5(2):31. https://doi.org/10.3390/horticulturae5020031
Chicago/Turabian StyleMarler, Thomas E. 2019. "Serianthes nelsonii Seed Germination and Seedling Behavior are Minimally Influenced by Chemical and Light Treatment" Horticulturae 5, no. 2: 31. https://doi.org/10.3390/horticulturae5020031
APA StyleMarler, T. E. (2019). Serianthes nelsonii Seed Germination and Seedling Behavior are Minimally Influenced by Chemical and Light Treatment. Horticulturae, 5(2), 31. https://doi.org/10.3390/horticulturae5020031





