Effects of 1-MCP on Quality and Storability of Cherry Tomato (Solanum lycopersicum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Flesh Firmness and Cell Wall Thickness
2.3. Extraction of Ethanol Insoluble Solid (EIS) and Pectin Analysis
2.4. Weight Loss, Peel Color, and Lycopene Content
2.5. Ethylene, Acetaldehyde, Ethanol, and Respiration Rate Measurements
2.6. Statistical Analysis
3. Results
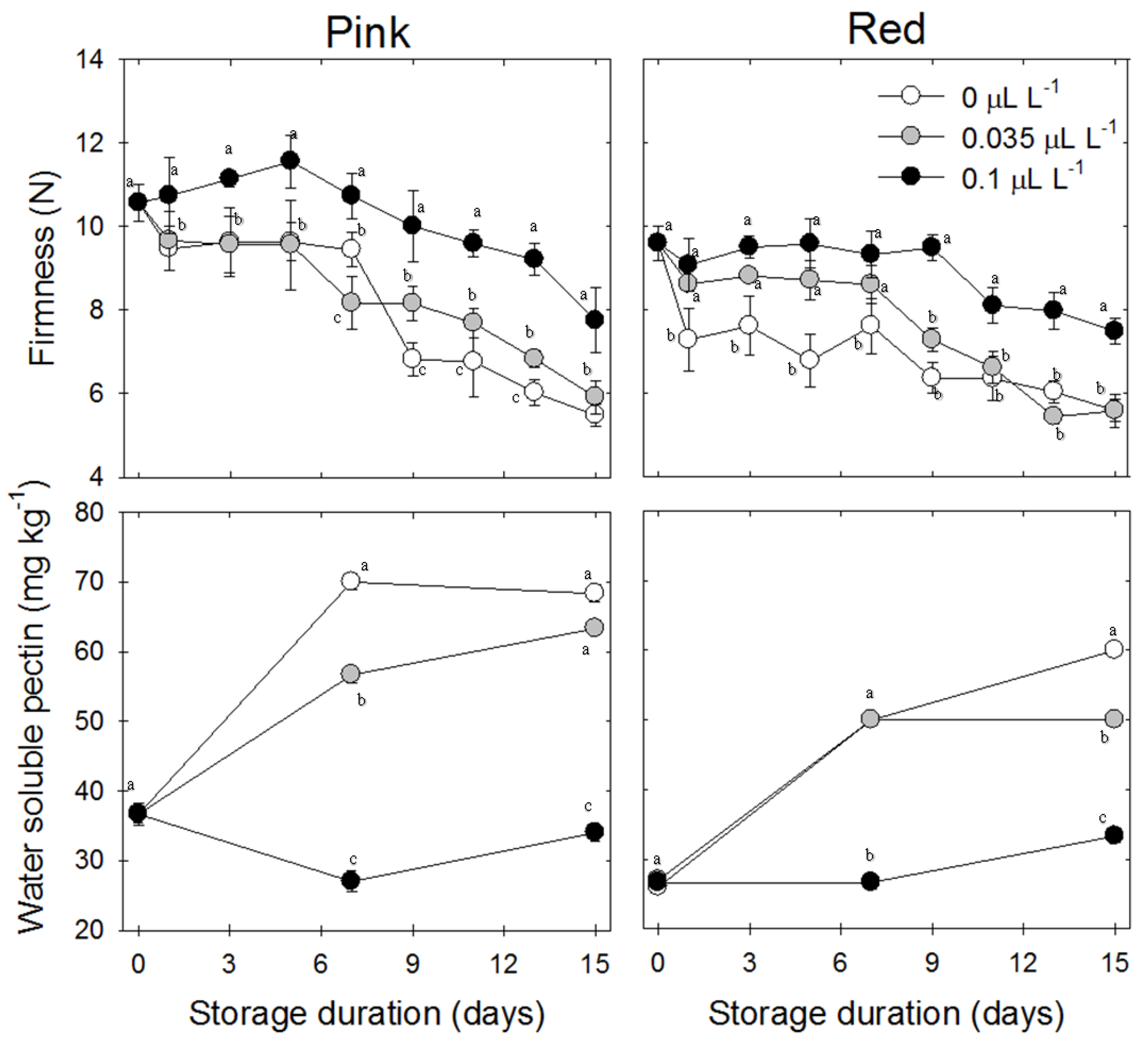
3.1. Firmness
3.2. Water-Soluble Pectin
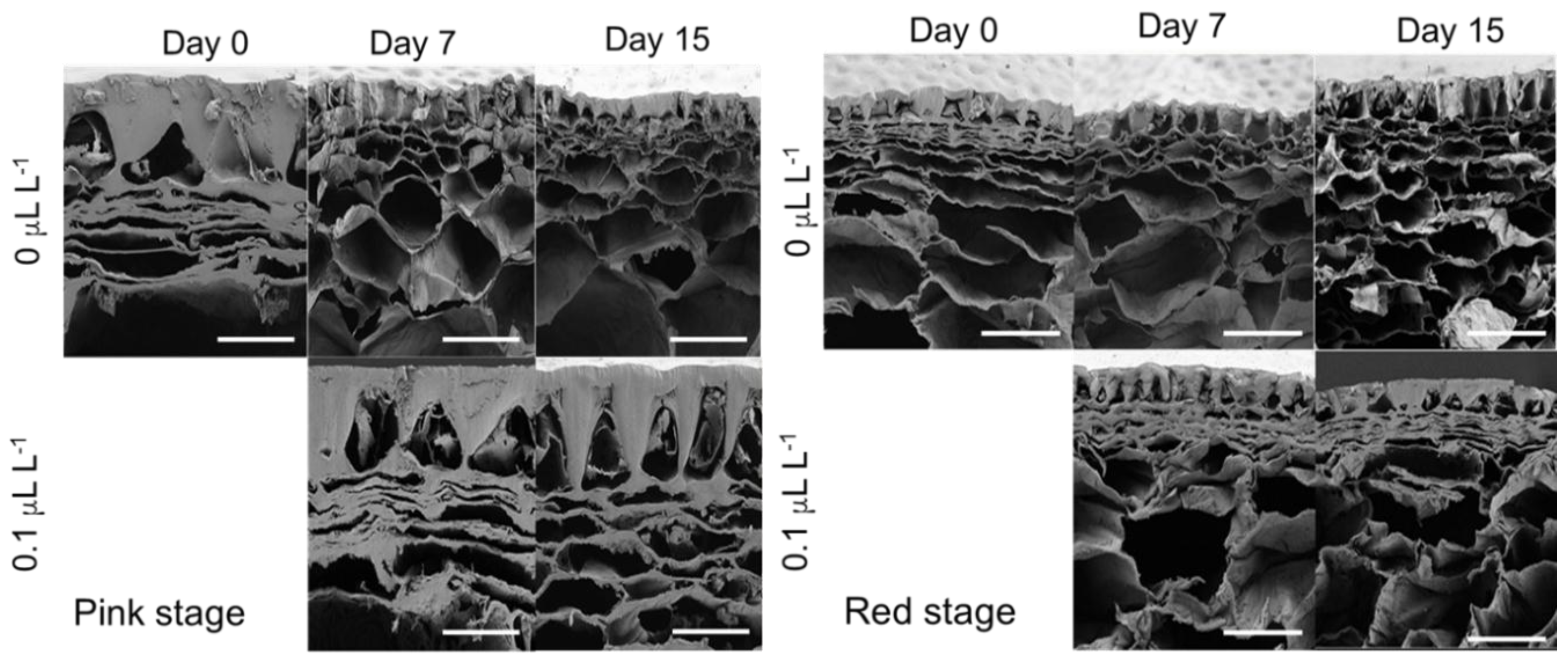
3.3. Cell Wall Thickness
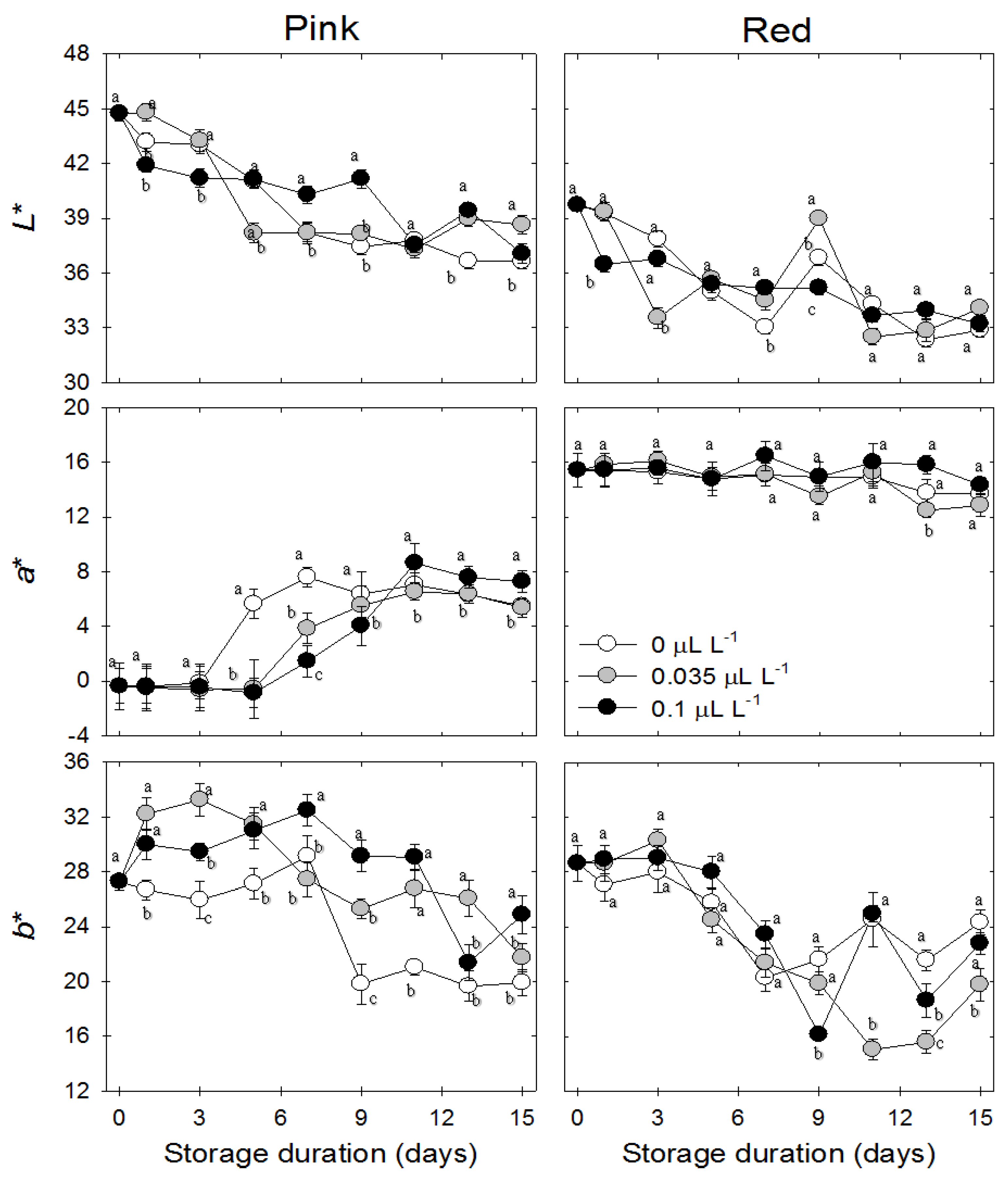
3.4. Peel Color
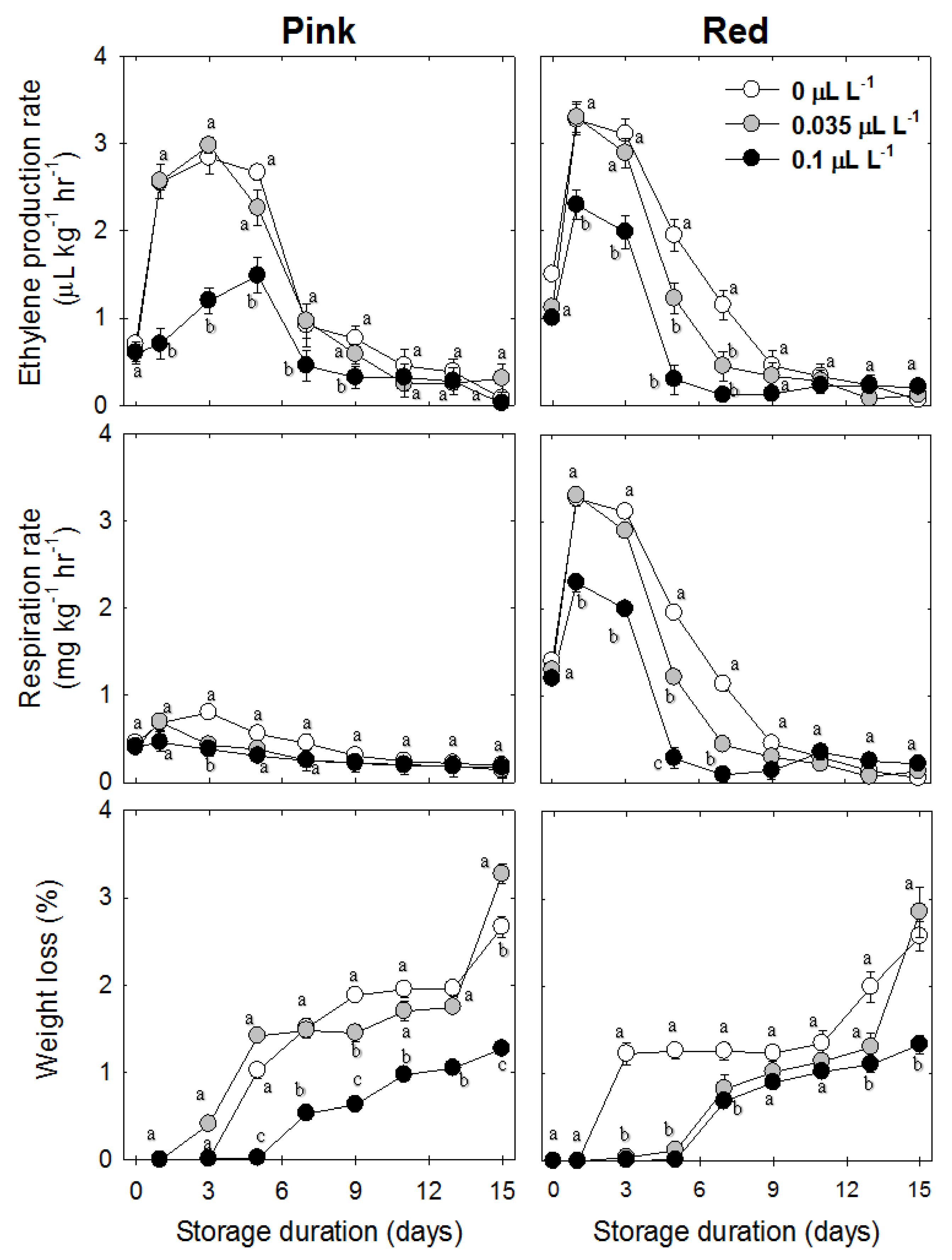
3.5. Weight Loss (%)
3.6. Ethylene, Acetaldehyde, Ethanol, and Respiration Rate
3.7. Lycopene Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lelievre, J.M.; Latche, A.; Jones, B.; Bouzayen, M.; Pech, J.C. Ethylene and fruit ripening. Physiol. Plant. 1997, 101, 727–739. [Google Scholar] [CrossRef]
- Gajewski, M.; Mazur, K.; Radzanowska, J.; Kowalczyk, K.; Marcinkowska, M.; Ryl, K.; Kalota, K. Sensory quality of cherry tomatoes in relation to 1-MCP treatment and storage duration. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 2014, 42, 30–35. [Google Scholar] [CrossRef][Green Version]
- Fan, X.T.; Mattheis, J.P. Yellowing of broccoli in storage is reduced by 1-methylcyclopropene. HortScience 2000, 35, 885–887. [Google Scholar] [CrossRef]
- Wills, R.B.H.; Ku, V.V.V.; Warton, M.A. Use of 1-methylcyclopropene to extend the postharvest life of lettuce. J. Sci. Food Agric. 2002, 82, 1253–1255. [Google Scholar] [CrossRef]
- Watkins, C.B. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables research review paper. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef] [PubMed]
- Blankenship, S.M.; Dole, J.M. 1-Methylcyclopropene: A review. Postharvest Biol. Technol. 2003, 28, 1–25. [Google Scholar] [CrossRef]
- Sisler, E.C. The discovery and development of compounds counteracting ethylene at the receptor level. Biotechnol. Adv. 2006, 24, 357–367. [Google Scholar] [CrossRef]
- Madhavi, D.L.; Salunkhe, D.K. Tomato in Handbook of Vegetable Science and Technology Production, Storage and Processing; Salunkhe, D.K., Kadam, S.S., Eds.; Marcel Dekker: New York, NY, USA, 1998; pp. 171–201. [Google Scholar]
- Klee, H.J.; Giovannoni, J.J. Genetics and control of tomato fruit ripening and quality attributes. Annu. Rev. Genet. 2011, 45, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Opiyo, A.M.; Ying, T.J. The effects of 1-methylcyclopropene treatment on the shelf life and quality of cherry tomato (Lycopersicon esculentum var. cerasiforme) fruit. Int. J. Food Sci. Technol. 2005, 40, 665–673. [Google Scholar] [CrossRef]
- Colelli, G.; Sánchez, M.T.; Torralbo, F.J. Effects of treatment with 1-methylcyclopropene (1-MCP) on tomato. Alimentaria 2003, 342, 67–70. [Google Scholar]
- Krammes, J.G.; Megguer, C.A.; Argenta, L.C.; Amarante, C.V.T.; Grossi, D. Use of 1-methycyclopropene to delay fruit ripening of tomato. Hortic. Bras. 2003, 21, 611–614. [Google Scholar] [CrossRef]
- Hoeberichts, F.A.; Van Der Plas, L.H.W.; Woltering, E.J. Ethylene perception is required for the expression of tomato ripening-related genes and associated physiological changes even at advanced stages of ripening. Postharvest Biol. Technol. 2002, 26, 125–133. [Google Scholar] [CrossRef]
- Mir, N.; Canoles, M.; Beaudry, R. Inhibiting tomato ripening with 1-methylcyclopropene. J. Am. Soc. Hort. Sci. 2004, 129, 112–120. [Google Scholar] [CrossRef]
- Mostofi, Y.; Toivonen, P.M.A.; Lessani, H.; Babalar, M.; Lu, C.W. Effect of 1-methylcyclopropene on ripening of greenhouse tomatoes at three storage temperatures. Postharvest Biol. Technol. 2003, 27, 285–292. [Google Scholar] [CrossRef]
- Sisler, E.C.; Blankenship, S.M. Methods of Counteracting an Ethylene Response in Plants. U.S. Patent 5,518,988, 21 May 1996. [Google Scholar]
- Moretti, C.L.; Araujo, A.L.A.; Marouelli, W.A.; Silva, W.L.C. 1-Methylcyclopropene delays tomato fruit ripening. Hortic. Bras. 2002, 20, 659–663. [Google Scholar] [CrossRef][Green Version]
- Sayed, A.; Nakano, K.; Maezawa, S. Combined effect of heat treatment and modified atmosphere packaging on the color development of cherry tomato. Postharvest Biol. Technol. 2004, 34, 113–116. [Google Scholar]
- Toor, R.K.; Savage, G.P. Changes in major antioxidant components of tomatoes during post-harvest storage. Food Chem. 2006, 99, 724–727. [Google Scholar] [CrossRef]
- Darwin, H.P.; Irving, D. Application of 1-MCP to intact tomatoes differing in maturity delays quality changes in the stored slices. J. Agron. Indones. 2011, 39, 92–96. [Google Scholar]
- Somayeh, S.M.; Sani, A.M. Effects of 1-methyl cyclopropene (1-MCP) on ethylen production and physicochemical properties of tomato type Rapsona. Int. J. Biol. Sci. 2015, 6, 55–62. [Google Scholar]
- Tilahun, S.; Seo, M.H.; Park, D.S.; Jeong, C.S. Effect of cultivar and growing medium on the fruit quality attributes and antioxidant properties of tomato (Solanum lycopersicum L.). Hortic. Environ. Biotechnol. 2018, 59, 215–223. [Google Scholar] [CrossRef]
- USDA. United Stages Standards for Grade for Fresh Tomatoes; United States Department of Agriculture, Agricultural Marketing Service: Washington, DC, USA, 1991; p. 13.
- Islam, M.Z.; Mele, M.K.; Baek, J.P.; Kang, H.M. Cherry tomato qualities affected by foliar spraying with boron and calcium. Hortic. Environ. Biotechnol. 2016, 57, 46–52. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Kim, M.S.; Min, J.H.; Jong, P.C.; Jin, G.K.; Eun, M.L.; Ji, Y.L. Effect of 1-MCP and high percentage CO2 treatment on the firmness and pectin changes in peach (Prunus persica) fruit during Shelf-life. J. Agric. Sci. 2010, 37, 209–216. [Google Scholar]
- Blumenkrantz, N.; Asoe-Hansen, H.M. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Tilahun, S.; Park, D.S.; Taye, A.M.; Jeong, C.S. Effects of storage duration on physicochemical and antioxidant properties of tomato (Lycopersicon esculentum Mill.). Korean J. Hortic. Sci. Technol. 2017, 35, 88–97. [Google Scholar]
- Fish, W.W.; Veazie, P.P.; Collins, J.K. A quantitative assay for lycopene that utilizes reduced volumes of organic solvents. J. Food Compos. Anal. 2002, 15, 309–317. [Google Scholar] [CrossRef]
- Park, K.W.; Kang, H.M.; Kim, C.H. Comparison of storability on film sources and storage temperature for fresh Japanese mint in MA storage. J. Bio-Environ. Control 2000, 9, 40–46. [Google Scholar]
- Miranda, M.R.A.; Silva, F.S.; Filgueiras, H.A.C.; Alves, R.E.; Aráujo, N.C.C. Storage of two types of sapodilla under ambient condition. Rev. Bras. Frutic. 2002, 24, 644–646. [Google Scholar] [CrossRef]
- Jeong, J.; Brecht, J.K.; Huber, D.J.; Sargent, S.A. 1-methylcyclopropene (1-MCP) for maintain texture quality of fresh-cut tomato. HortScience 2004, 39, 1359–1362. [Google Scholar] [CrossRef]
- Miranda, M.R.A.; Silva, F.S.; Filgueiras, H.A.C.; Alves, R.E.; Aráujo, N.C.C. Enzymes and pectin breakdown of sapodilla during modified atmosphere storage. Proc. Int. Soc. Trop. Hortic. 2002, 45, 18–21. [Google Scholar]
- Patrícia, L.D.D.; Lima, L.C.D.; De Miranda, M.R.A.; Alves, J.D.; Alves, R.E.; Silva, J.D. Enzyme activities and pectin breakdown of sapodilla submittedto 1-methylcyclopropene. Pesqui. Agropecu. Bras. 2008, 43, 15–20. [Google Scholar]
- Pinheiro, A.C.M.; Vilas Boas, E.V.D.B.; De Paiva, A.; La Selva, M. Ripening of ‘apple’ banana Submitted to 1-methylcyclopropene (1-MCP). Rev. Bras. Frutic. 2007, 29, 1–4. [Google Scholar] [CrossRef]
- Liu, H.X.; Jiang, W.B.; Zhou, L.G.; Wang, B.G.; Luo, Y.B. The effects of 1-methylcyclopropene on peach fruit (Prunus persica, L. cv. Jiubao) ripening and disease resistance. Int. J. Food Sci. Technol. 2005, 40, 1–17. [Google Scholar] [CrossRef]
- Guillen, F.; Castillo, S.; Zapata, P.J.; Martinez-Romero, D.; Serrano, M.; Valero, D. Efficacy of 1-MCP treatment in tomato fruit 1. Duration and concentration of 1-MCP treatment to gain an effective delay of postharvest ripening. Postharvest Biol. Technol. 2007, 43, 23–27. [Google Scholar]
- Porat, R.; Weiss, B.; Cohen, L.; Daus, A.; Goren, R. Effects of ethylene and 1-methylcyclopropene on the postharvest qualities of ‘Shamouti’ oranges. Postharvest Biol. Technol. 1999, 15, 155–163. [Google Scholar] [CrossRef]
- Pék, Z.; Helyes, L.; Lugasi, A. Color changes and antioxidant content of vine and postharvest-ripened tomato fruits. HortScience 2010, 45, 466–468. [Google Scholar] [CrossRef]
- Tilahun, S.; Park, D.S.; Seo, M.H.; Hwang, I.G.; Kim, S.H.; Choi, H.R.; Jeong, C.S. Prediction of lycopene and β-carotene in tomatoes by portable chroma-meter and VIS/NIR spectra. Postharvest Biol. Technol. 2018, 136, 50–56. [Google Scholar] [CrossRef]
- Agarwal, S.; Rao, A.V. Tomato lycopene and low density lipoprotein oxidation: A human dietary intervention study. Lipids 1998, 33, 981–984. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taye, A.M.; Tilahun, S.; Seo, M.H.; Park, D.S.; Jeong, C.S. Effects of 1-MCP on Quality and Storability of Cherry Tomato (Solanum lycopersicum L.). Horticulturae 2019, 5, 29. https://doi.org/10.3390/horticulturae5020029
Taye AM, Tilahun S, Seo MH, Park DS, Jeong CS. Effects of 1-MCP on Quality and Storability of Cherry Tomato (Solanum lycopersicum L.). Horticulturae. 2019; 5(2):29. https://doi.org/10.3390/horticulturae5020029
Chicago/Turabian StyleTaye, Adanech Melaku, Shimeles Tilahun, Mu Hong Seo, Do Su Park, and Cheon Soon Jeong. 2019. "Effects of 1-MCP on Quality and Storability of Cherry Tomato (Solanum lycopersicum L.)" Horticulturae 5, no. 2: 29. https://doi.org/10.3390/horticulturae5020029
APA StyleTaye, A. M., Tilahun, S., Seo, M. H., Park, D. S., & Jeong, C. S. (2019). Effects of 1-MCP on Quality and Storability of Cherry Tomato (Solanum lycopersicum L.). Horticulturae, 5(2), 29. https://doi.org/10.3390/horticulturae5020029





