Construction and Phenotypic Characterization of a Recombination Inbred Line (RIL) Population from a Melo-agrestis Melon Hybrid
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Cultivation Conditions
2.2. Evaluation for Vegetative Growth of RILs
2.3. Assay for Premature Senescence, Sex Expression and Powdery Mildew Resistance of RILs
2.4. Evaluation for Fruit-Related Traits of RILs
2.5. Data Processing
3. Results
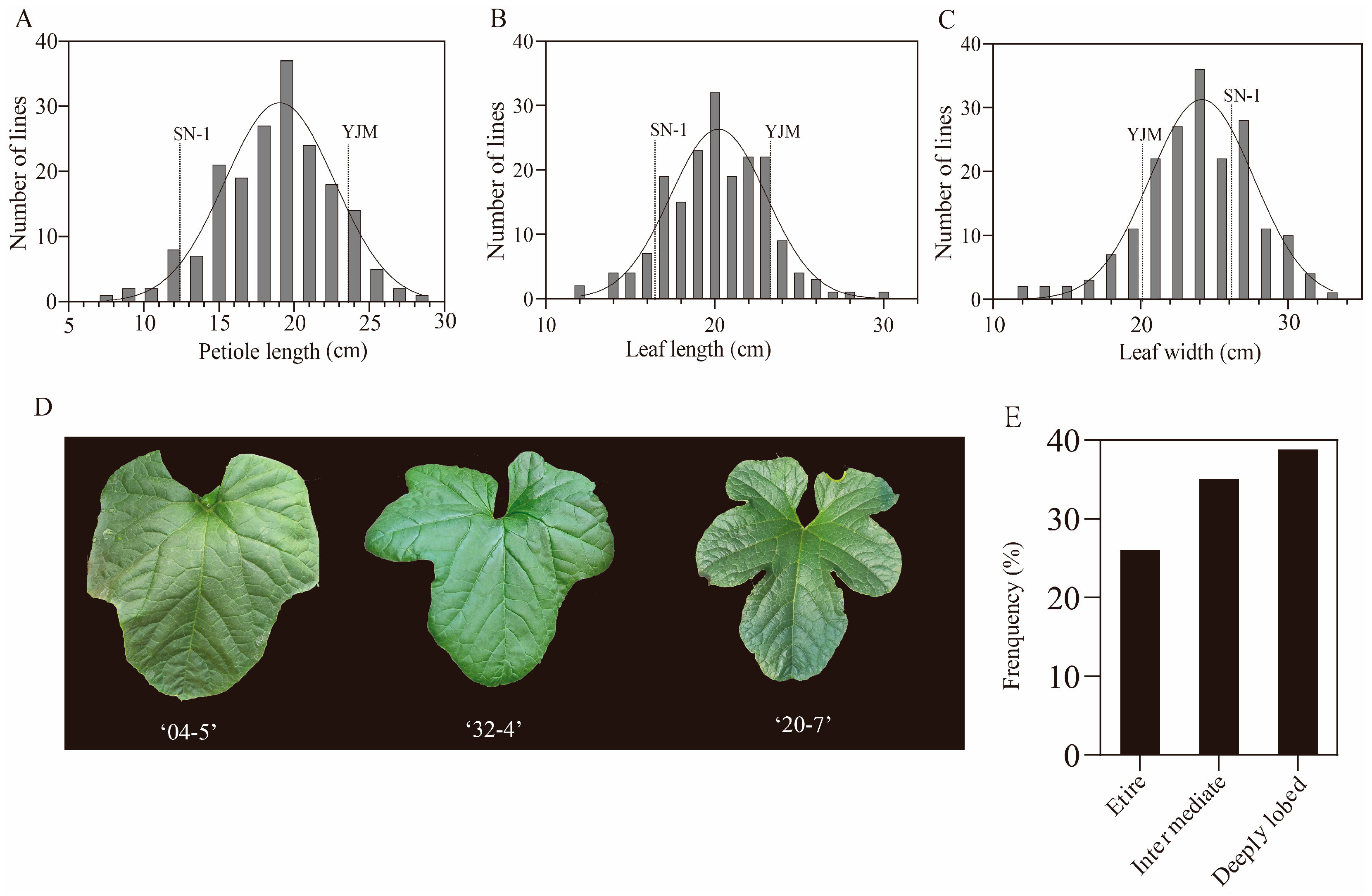
3.1. Variations of Leaf-Related Traits in the RIL Population
3.2. Variations of Stem Diameter and Internode Length in the RIL Population
3.3. Sex Expression and Premature Senescence in the RIL Population
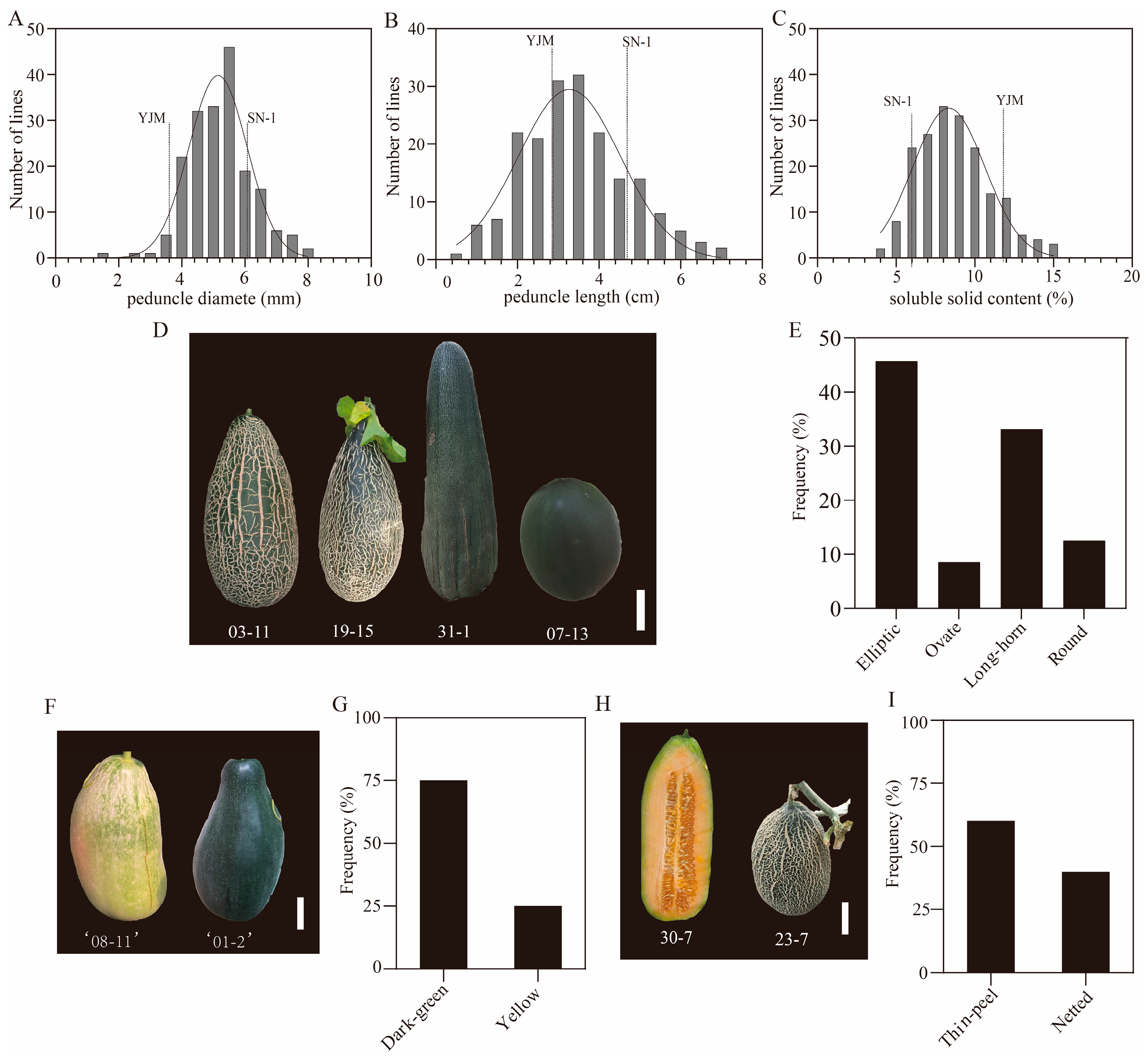
3.4. Variations of Fruit-Related Traits in the RIL Population
3.5. Resistance of Different RILs to Powdery Mildew Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Meng, Y.; Ding, F.; Yang, K.; Wang, C.; Zhang, H.; Jin, H. Comparative analysis of TPR gene family in Cucurbitaceae and expression profiling under abiotic stress in Cucumis melo L. Horticulturae 2024, 10, 83. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; Lv, F.; Chu, P.; Jin, H. Genome-wide identification of the OMT gene family in Cucumis melo L. and expression analysis under abiotic and biotic stress. PeerJ 2023, 11, e16483. [Google Scholar] [CrossRef]
- Kerje, T.; Grum, M. The origin of melon, Cucumis melo: A review of the literature. Acta Hortic. 2000, 510, 37–44. [Google Scholar] [CrossRef]
- Sebastian, P.; Schaefer, H.; Telford, I.R.; Renner, S.S. Cucumber (Cucumis sativus) and melon (C. melo) have numerous wild relatives in Asia and Australia, and the sister species of melon is from Australia. Proc. Natl. Acad. Sci. USA 2010, 107, 14269–14273. [Google Scholar] [CrossRef] [PubMed]
- Endl, J.; Achigan-Dako, E.G.; Pandey, A.K.; Monforte, A.J.; Pico, B.; Schaefer, H. Repeated domestication of melon (Cucumis melo) in Africa and Asia and a new close relative from India. Am. J. Bot. 2018, 105, 1662–1671. [Google Scholar] [CrossRef]
- Yılmaz, N.; Kaya, H.P.; Pınar, H.; Hancı, F.; Uzun, A. Detailed morphological and molecular characterizations of melon (Cucumis melo L.) accessions collected from Northern Cyprus and Turkey. Korean J. Hortic. Sci. Technol. 2021, 39, 471–481. [Google Scholar] [CrossRef]
- Cui, L.; Siskos, L.; Wang, C.; Schouten, H.J.; Visser, R.G.F.; Bai, Y. Breeding melon (Cucumis melo) with resistance to powdery mildew and downy mildew. Hortic. Plant J. 2022, 8, 545–561. [Google Scholar] [CrossRef]
- Lebeda, A.; Křístková, E.; Sedláková, B.; McCreight, J.D.; Coffey, M.D. Cucurbit powdery mildews: Methodology for objective determination and denomination of races. Eur. J. Plant Pathol. 2016, 144, 399–410. [Google Scholar] [CrossRef]
- Zhang, C.; Ren, Y.; Guo, S.; Zhang, H.; Gong, G.; Du, Y.; Xu, Y. Application of comparative genomics in developing markers tightly linked to the Pm-2F gene for powdery mildew resistance in melon (Cucumis melo L.). Euphytica 2013, 190, 157–168. [Google Scholar] [CrossRef]
- Ba, D.; Ding, Z.; Wang, S.; Shi, Y.; Li, Y.; Cui, H. Allantoin and jasmonic acid synergistically induce resistance response to powdery mildew in melon as revealed by combined hormone and transcriptome analysis. Sci. Hortic. 2024, 327, 112797. [Google Scholar]
- Rhouma, A.; Mehaoua, M.S.; Mougou, I.; Rhouma, H.; Shah, K.K.; Bedjaoui, H. Combining melon varieties with chemical fungicides for integrated powdery mildew control in Tunisia. Eur. J. Plant Pathol. 2023, 165, 189–201. [Google Scholar] [CrossRef]
- Warkentin, T.; Rashid, K.; Xue, A. Fungicidal control of powdery mildew in field pea. Can. J. Plant Sci. 1996, 76, 933–935. [Google Scholar] [CrossRef]
- Vijaykumar, K.N.; Kulkarni, S.; Hiremath, S.M. Management of powdery mildew in cluster bean using fungicides, botanicals and bioagents. Legume Res. 2025, 48, 1063–1067. [Google Scholar] [CrossRef]
- Hayashi, M.; Endo, Y.; Komura, T.; Kimura, S.; Oka, H. Synthesis and biological activity of a novel fungicide, flutianil. J. Pestic. Sci. 2020, 45, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, X.; Hu, J.; Wang, Q.; Zheng, J.; Yang, X.; Shi, Q. Positive involvement of HCO3− in modulation of melon resistance to powdery mildew. Veg. Res. 2023, 3, 1–11. [Google Scholar]
- Vielba-Fernández, A.; Polonio, Á.; Ruiz-Jiménez, L.; de Vicente, A.; Pérez-García, A.; Fernández-Ortuño, D. Fungicide resistance in powdery mildew fungi. Microorganisms 2020, 8, 1431. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Jorkesh, A.; Olfati, J. Chemical and biological products for control of powdery mildew on cucumber. Int. J. Veg. Sci. 2022, 28, 233–238. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Z.; Xu, H.; Liu, Q.; Jiang, Z.; Yin, C.; Han, G.; Zhang, W.; Zhang, Y. Biological control of wheat powdery mildew disease by the termite-associated fungus Aspergillus chevalieri BYST01 and potential role of secondary metabolites. Pest. Manag. Sci. 2024, 80, 2011–2020. [Google Scholar] [CrossRef] [PubMed]
- Kesh, H.; Kaushik, P. Advances in melon (Cucumis melo L.) breeding: An update. Sci. Hortic. 2021, 282, 110045. [Google Scholar] [CrossRef]
- Villanueva, G.; Plazas, M.; Gramazio, P.; Moya, R.D.; Prohens, J.; Vilanova, S. Evaluation of three sets of advanced backcrosses of eggplant with wild relatives from different gene pools under low N fertilization conditions. Hortic. Res. 2023, 10, uhad141. [Google Scholar] [CrossRef]
- Tijskens, L.M.M.; Dos-Santos, N.; Jowkar, M.M.; Obando-Ulloa, J.M.; Moreno, E.; Schouten, R.E.; Monforte, A.J.; Fernández-Trujillo, J.P. Postharvest firmness behaviour of near-isogenic lines of melon. Postharvest Biol. Technol. 2009, 51, 320–326. [Google Scholar] [CrossRef]
- Galpaz, N.; Gonda, I.; Shem-Tov, D.; Barad, O.; Tzuri, G.; Lev, S.; Fei, Z.; Xu, Y.; Mao, L.; Jiao, C.; et al. Deciphering genetic factors that determine melon fruit-quality traits using RNA-Seq-based high-resolution QTL and eQTL mapping. Plant J. 2018, 94, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Branham, S.E.; Levi, A.; Katawczik, M.; Fei, Z.; Wechter, W.P. Construction of a genome-anchored, high-density genetic map for melon (Cucumis melo L.) and identification of Fusarium oxysporum f. sp. melonis race 1 resistance QTL. Theor. Appl. Genet. 2018, 131, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Toporek, S.M.; Branham, S.E.; Keinath, A.P.; Wechter, W.P. QTL mapping of resistance to Pseudoperonospora cubensis clade 2, mating type A1, in Cucumis melo and dual-clade marker development. Theor. Appl. Genet. 2023, 136, 91. [Google Scholar] [CrossRef]
- Luan, F.; Bai, J.; Zhu, Z.; Gao, P.; Liu, S.; Wu, C.; Fan, C.; Lyu, S. Establishment of Agrobacterium-mediated genetic transformation system in thin-skinned melon. J. Northeast. Agric. Univ. 2019, 50, 11–18. [Google Scholar]
- Bo, K.; Duan, Y.; Qiu, X.; Zhang, M.; Shu, Q.; Sun, Y.; He, Y.; Shi, Y.; Weng, Y.; Wang, C. Promoter variation in a homeobox gene, CpDll, is associated with deeply lobed leaf in Cucurbita pepo L. Theor. Appl. Genet. 2022, 135, 1223–1234. [Google Scholar] [CrossRef]
- Muchero, W.; Ehlers, J.D.; Close, T.J.; Roberts, P.A. Mapping QTL for drought stress-induced premature senescence and maturity in cowpea [Vigna unguiculata (L.) Walp.]. Theor. Appl. Genet. 2009, 118, 849–863. [Google Scholar] [CrossRef]
- Wang, Z.; Yadav, V.; Yan, X.; Cheng, D.; Wei, C.; Zhang, X. Systematic genome-wide analysis of the ethylene-responsive ACS gene family: Contributions to sex form differentiation and development in melon and watermelon. Gene 2021, 805, 145910. [Google Scholar] [CrossRef]
- Wang, L.; Yang, X.; Ren, Z.; Wang, X. The co-involvement of light and air temperature in regulation of sex expression in monoecious cucumber (Cucumis sativus L.). Agric. Sci. 2014, 5, 858–863. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Wang, S.; Guo, Y.; Hu, Z.; Yin, M.; Shi, Q.; Yang, X. Efficient detection of melon-powdery mildew interactions by a medium-free inoculation. Veg. Res. 2024, 4, e024. [Google Scholar] [CrossRef]
- Park, E.; Luo, Y.; Marine, S.; Everts, K.; Micallef, S.A.; Bolten, S.; Stommel, J. Consumer preference and physicochemical evaluation of organically grown melons. Postharvest Biol. Technol. 2018, 141, 77–85. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, J. Delaying or promoting? Manipulation of leaf senescence to improve crop yield and quality. Planta 2023, 258, 48. [Google Scholar] [CrossRef]
- Yamamoto, K.; Guo, W.; Ninomiya, S. Node detection and internode length estimation of tomato seedlings based on image analysis and machine learning. Sensors 2016, 16, 1044. [Google Scholar] [CrossRef]
- Peng, L.; Liu, H.; Wu, Y.; Bing, J.; Zhang, G. New advances in the regulation of stem growth in vascular plants. Plant Growth Regul. 2024, 103, 65–80. [Google Scholar] [CrossRef]
- Ye, H.; Wang, L.; Lv, L.; Hu, Y.; Wang, B. Ethylene control of flowering and sex differentiation in three sex types of inbred melon lines. Hortic. Sci. Technol. 2020, 38, 512–521. [Google Scholar] [CrossRef]
- Rao, Y.; Jiao, R.; Ye, H.; Hu, J.; Lu, T.; Wu, X.; Fang, Y.; Li, S.; Lin, H.; Wang, S.; et al. Fine mapping and candidate gene analysis of leaf tip premature senescence and dwarf mutant dls-1 in rice. Plant Growth Regul. 2021, 94, 275–285. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, P.; Bai, W.; Chen, Z.; Cheng, Z.; Su, L.; Chen, X.; Bi, Y.; Feng, R.; Liu, Z. Fine mapping and functional validation of the candidate gene BhGA2ox3 for fruit pedicel length in wax gourd (Benincasa hispida). Theor. Appl. Genet. 2024, 137, 272. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; He, Y.; Tang, L.; Xu, Y.; Zhao, G. Genetics, genomics, and breeding in melon. Agronomy 2022, 12, 2891. [Google Scholar] [CrossRef]
- Grumet, R.; McCreight, J.D.; McGregor, C.; Weng, Y.; Mazourek, M.; Reitsma, K.; Labate, J.; Davis, A.; Fei, Z. Genetic resources and vulnerabilities of major Cucurbit crops. Genes 2021, 12, 1222. [Google Scholar] [CrossRef]
- Bazakos, C.; Hanemian, M.; Trontin, C.; Jiménez-Gómez, J.M.; Loudet, O. New strategies and tools in quantitative genetics: How to go from the phenotype to the genotype. Annu. Rev. Plant Biol. 2017, 68, 435–455. [Google Scholar] [CrossRef]
- Lyu, X.; Xia, Y.; Wang, C.; Zhang, K.; Deng, G.; Shen, Q.; Gao, W.; Zhang, M.; Liao, N.; Ling, J.; et al. Pan-genome analysis sheds light on structural variation-based dissection of agronomic traits in melon crops. Plant Physiol. 2023, 193, 1330–1348. [Google Scholar] [CrossRef] [PubMed]
- Merkouropoulos, G.; Hilioti, Z.; Abraham, E.M.; Lazaridou, M. Evaluation of Lotus corniculatus L. accessions from different locations at different altitudes reveals phenotypic and genetic diversity. Grass Forage Sci. 2017, 72, 851–856. [Google Scholar] [CrossRef]
- Shet, R.M.; Gunnaiah, R.; Tirkannanavar, S.; Hongal, S.; Hiremata, V.; Venugopal, K.C. Phenotypic characterization of Mangalore melon (Cucumis melo subsp. agrestis var. acidulus) accessions from Southern India based on qualitative and quantitative traits. Genet. Resour. Crop Evol. 2025, 72, 1–12. [Google Scholar] [CrossRef]
- Rime, J.; Dinesh, M.R.; Sankaran, M.; Shivashankara, K.S.; Rekha, A.; Ravishankar, K.V. Evaluation and characterization of EMS derived mutant populations in mango. Sci. Hortic. 2019, 254, 55–60. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, J.; Liu, S.; Ding, Z.; Luan, F.; Gao, P. Bulked-segregant analysis identified a putative region related to short internode length in melon. HortScience 2019, 54, 1293–1298. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, K.; Zhu, H.; Zhang, X.; Yan, W.; Xu, N.; Liu, D.; Hu, J.; Wu, Y.; Weng, Y.; et al. Melon short internode (CmSi) encodes an ERECTA-like receptor kinase regulating stem elongation through auxin signaling. Hortic. Res. 2020, 7, 202. [Google Scholar] [CrossRef]
- Fallik, E.; Ilic, Z. Pre- and postharvest treatments affecting flavor quality of fruits and vegetables. In Preharvest Modulation of Postharvest Fruit and Vegetable Quality; Siddiqui, M.W., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 139–168. [Google Scholar]
- da Silva, J.M.C.; Viana, E.R.D.C.; de Morais, P.L.D.; Tomaz, F.L.D.S.; Martins, A.F.; Nunes, G.H.D.S. Inheritance of soluble solids content and sucrose in melon. Hortic. Bras. 2022, 40, 295–301. [Google Scholar] [CrossRef]
- Adedze, Y.M.N.; Fan, W.Y.; Lu, X.; Zhang, W.T.; Yang, X.; Deng, Z.J.; Teng, L.H.; Xu, G.L.; Wang, X.L.; Li, W.H. Towards the development of the PCR-based InDel markers associated with fruit skin quality traits in melon (Cucumis melo L.) using bulked segregant analysis. Can. J. Plant Sci. 2023, 103, 312–318. [Google Scholar] [CrossRef]
- Ma, J.; Yuan, G.; Xu, X.; Zhang, H.; Qiu, Y.; Li, C.; Zhang, H. Identification and molecular marker development for peel color gene in melon (Cucumis melo L.). J. Integr. Agric. 2025, 24, 2589–2600. [Google Scholar] [CrossRef]
- Liang, X.; Wang, P.; Luo, C.; Li, X.; Mao, W.; Hou, J.; Fan, J.; Guo, Y.; Cheng, Z.; Li, Q.; et al. CmSN regulates fruit skin netting formation in melon. Horticulturae 2024, 10, 1115. [Google Scholar] [CrossRef]
- Fang, L.; Li, Y.; Gong, X.; Sang, X.; Ling, Y.; Wang, X.; Cong, Y.; He, G. Genetic analysis and gene mapping of a dominant presenescing leaf gene PSL3 in rice (Oryza sativa L.). Chin. Sci. Bull. 2010, 55, 2517–2521. [Google Scholar] [CrossRef]
- Qiu, L.; Fang, R.; Jia, Y.; Xiong, H.; Xie, Y.; Zhao, L.; Gu, J.; Zhao, S.; Ding, Y.; Li, C.; et al. The allelic mutation of NBS-LRR gene causes premature senescence in wheat. Plant Sci. 2025, 352, 112395. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, X.; Yin, W.; Yang, S.; Zhang, J.; Xu, N.; Bai, D.; Huang, Q.; Li, Y.; Qi, P.; et al. OsFK1 encodes C-14 sterol reductase, which is involved in sterol biosynthesis and affects premature aging of leaves in rice. Crop J. 2024, 12, 1010–1021. [Google Scholar] [CrossRef]
- Ingram, R.J.; Donaldson, J.T.; Levy, F. Impacts, prevalence, and spatiotemporal patterns of lily leaf spot disease on Lilium grayi (Liliaceae), Gray’s lily. J. Torrey Bot. Soc. 2018, 145, 296–310. [Google Scholar] [CrossRef]
- Jin, F.; Hua, M.; Song, L.; Cui, S.; Sun, H.; Kong, W.; Hao, Z. Transcriptome analysis of gene expression in the tomato leaf premature senescence mutant. Physiol. Mol. Biol. Plants 2022, 28, 1501–1513. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Wang, J.; Cao, S.; Guo, Y.; Shi, Q.; Yang, X. Construction and Phenotypic Characterization of a Recombination Inbred Line (RIL) Population from a Melo-agrestis Melon Hybrid. Horticulturae 2025, 11, 1087. https://doi.org/10.3390/horticulturae11091087
Liu H, Wang J, Cao S, Guo Y, Shi Q, Yang X. Construction and Phenotypic Characterization of a Recombination Inbred Line (RIL) Population from a Melo-agrestis Melon Hybrid. Horticulturae. 2025; 11(9):1087. https://doi.org/10.3390/horticulturae11091087
Chicago/Turabian StyleLiu, He, Jianquan Wang, Shoujun Cao, Yongjie Guo, Qinghua Shi, and Xiaoyu Yang. 2025. "Construction and Phenotypic Characterization of a Recombination Inbred Line (RIL) Population from a Melo-agrestis Melon Hybrid" Horticulturae 11, no. 9: 1087. https://doi.org/10.3390/horticulturae11091087
APA StyleLiu, H., Wang, J., Cao, S., Guo, Y., Shi, Q., & Yang, X. (2025). Construction and Phenotypic Characterization of a Recombination Inbred Line (RIL) Population from a Melo-agrestis Melon Hybrid. Horticulturae, 11(9), 1087. https://doi.org/10.3390/horticulturae11091087




