Comparative Analysis of Phenolic Acid Metabolites and Differential Genes Between Browning-Resistant and Browning-Sensitive luffa During the Commercial Fruit Stage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Treatment
2.2. Determination of Antioxidant Enzyme (SOD, CAT), PPO, and PAL Activities
÷ VEL) ÷ T = 8.9 × [(Adetermination − Acontrol) − 0.0013] ÷ W
2.3. Determination of (1,1-Diphenyl-2-Picrylhydrazyl Radical 2,2-Diphenyl-1-(2,4,6-Trinitrophenyl)Hydrazyl)DPPH and (2,2′-Azino-Bis (3-Ethylbenzothiazoline-6-Sulfonic Acid))ABTS Scavenging Activity
2.4. Determination of Malondialdehyde (MDA), H2O2, and Total Phenolic Content
2.5. Real-Time Quantitative PCR Analysis
2.6. Transcriptome Sequencing
2.7. Gene Function Annotation, Differential Gene Expression, and Enrichment Analysis
2.8. Metabolite Determination
2.9. Data Processing
3. Results
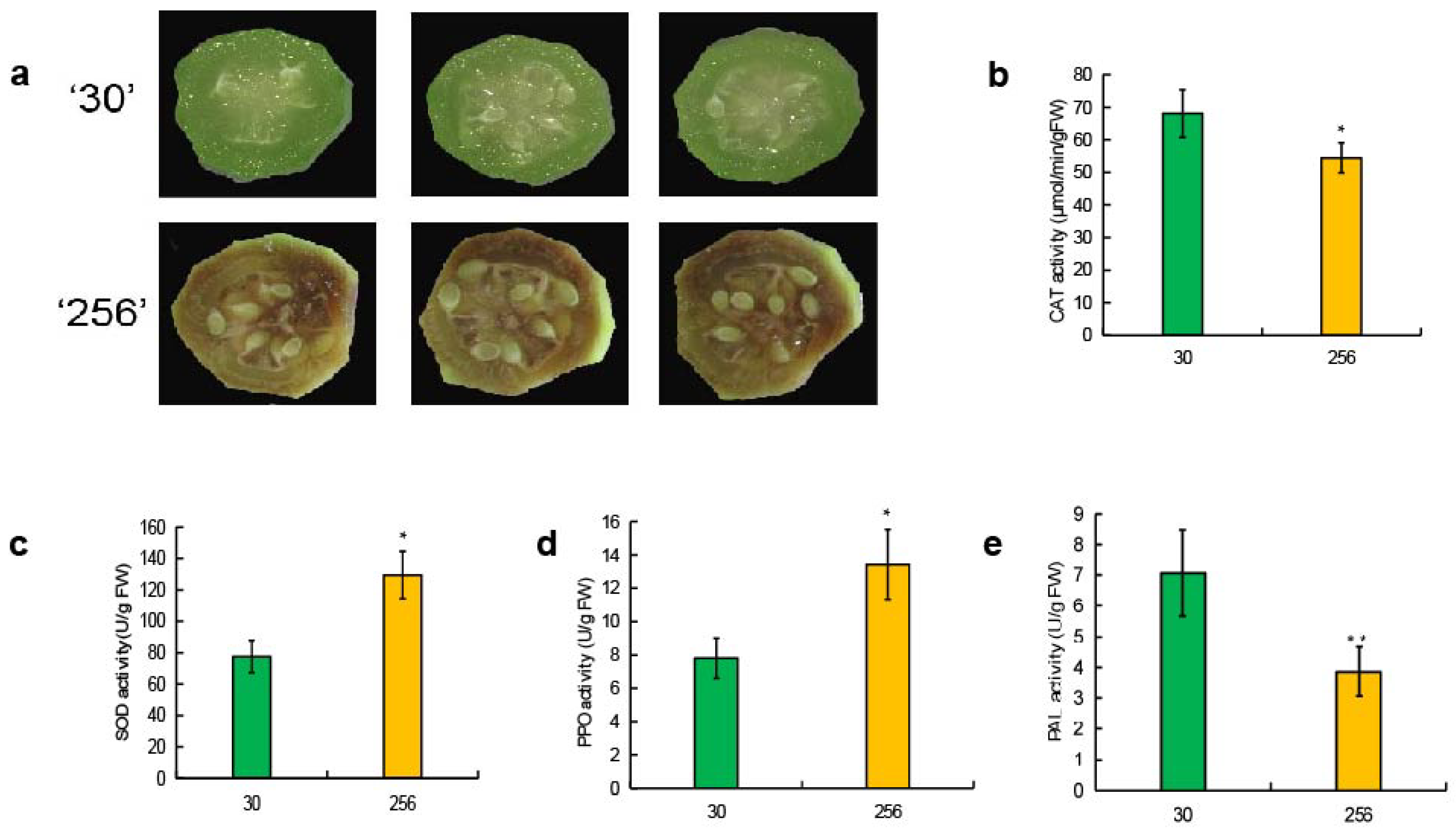
3.1. Analysis of Antioxidant Enzyme (SOD, CAT, PPO, and PAL) Activities
3.2. Analysis of Scavenging Activity of ABTS and DPPH Radicals
3.3. Analysis of H2O2, Total Phenols, and Malondialdehyde (MDA)
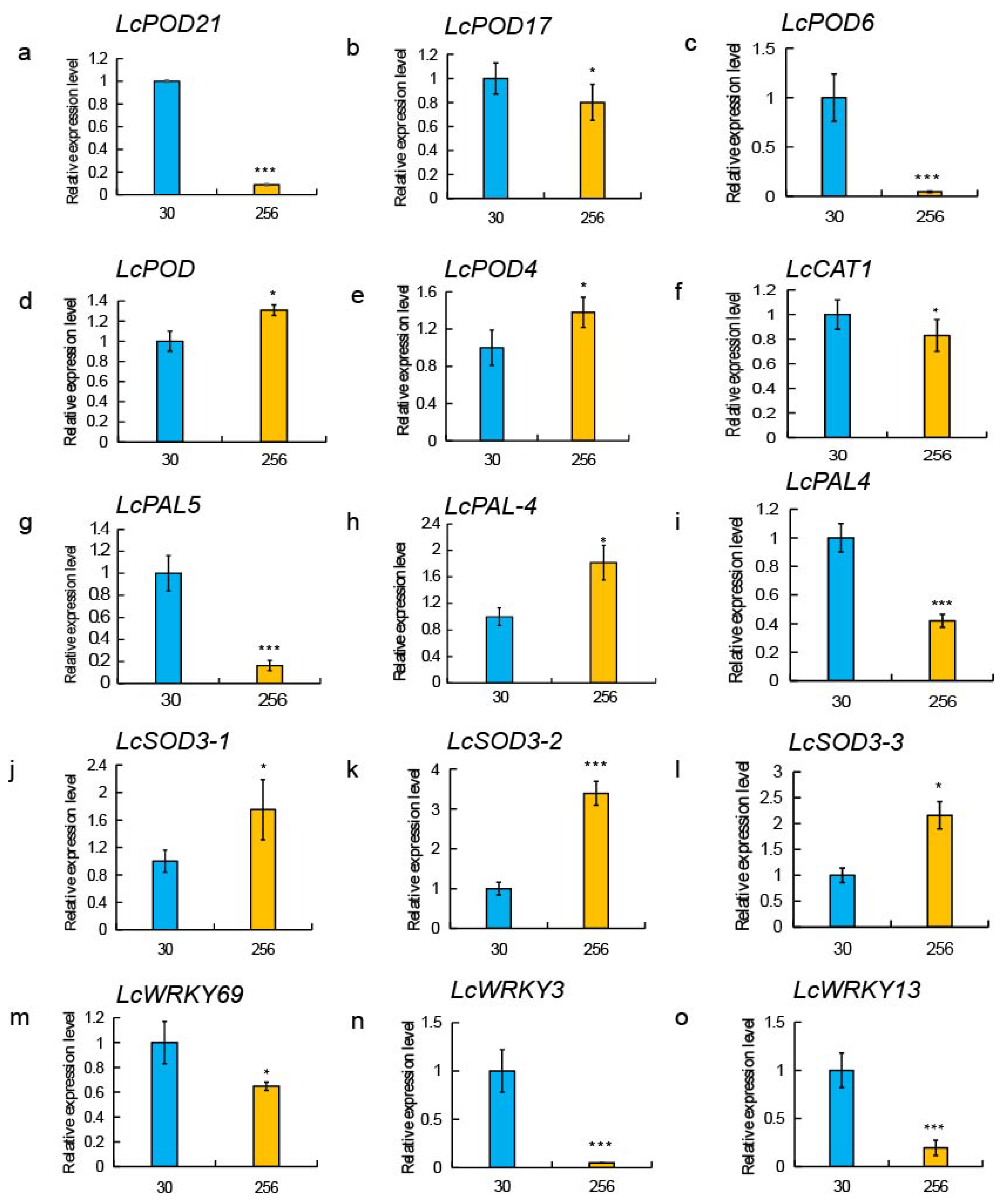
3.4. Verification of Expression Levels of luffa Browning-Related Genes
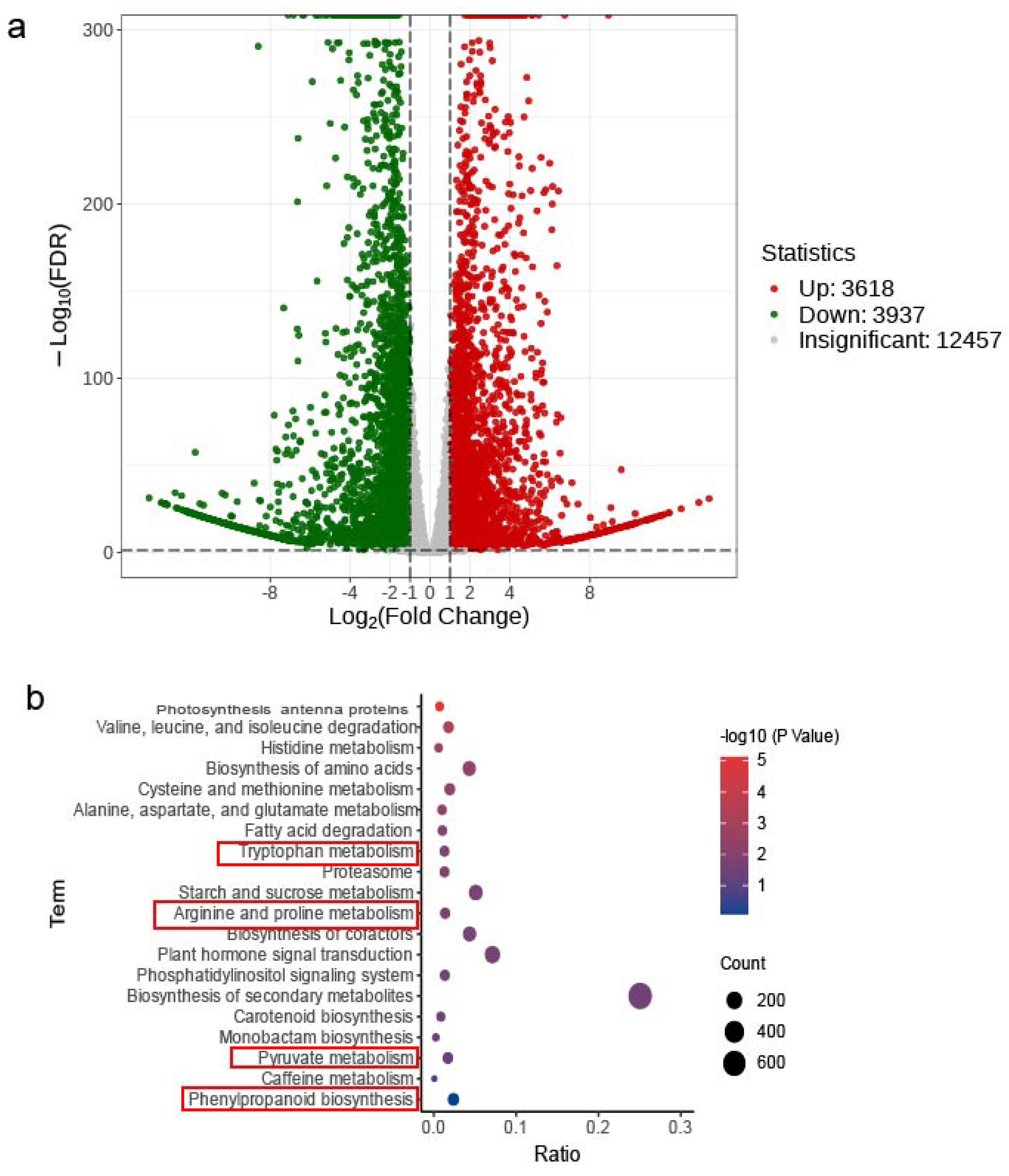
3.5. Transcriptome Analysis in Varieties ‘30’ vs. ‘256’
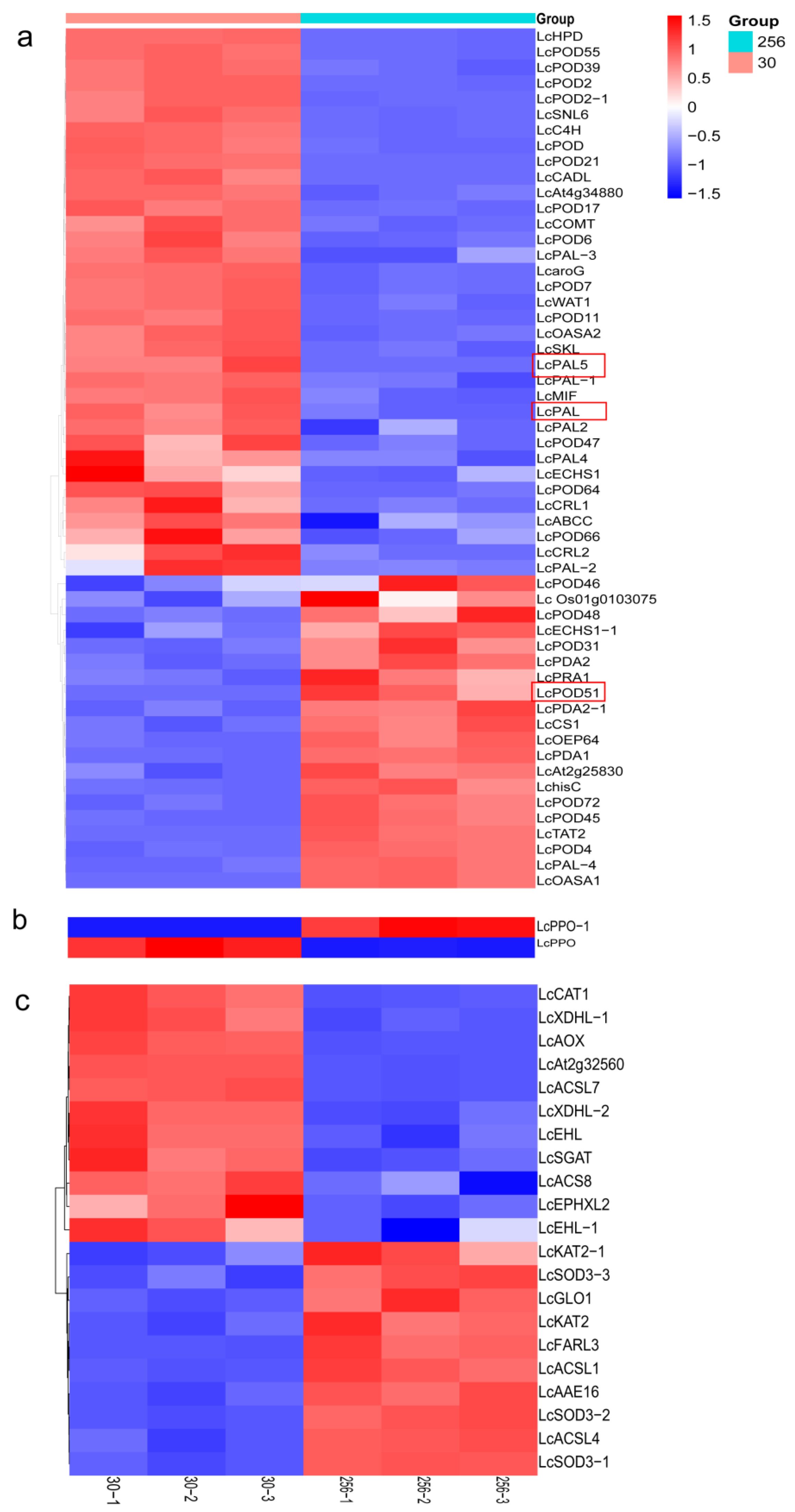
3.6. Identification of Potential Genes Related to Enzymatic Browning
3.7. Metabolomic Analysis of Varieties ‘30’ and ‘256’
3.8. DEGs and DEMs Involved in the Varieties ‘30’ and ‘256’
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maamoun, A.A.; El-Akkad, R.H.; Farag, M.A. Mapping metabolome changes in luffa aegyptiaca Mill fruits at different maturation stages via MS-based metabolomics and chemometrics. J. Adv. Res. 2019, 29, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhao, G.; Gong, H.; Li, J.; Luo, C.; He, X.; Luo, S.; Zheng, X.; Liu, X.; Guo, J.; et al. A high-quality sponge gourd (luffa cylindrica) genome. Hortic. Res. 2020, 7, 128. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, M.; Luo, C.; Li, J.; Gong, H.; Zheng, X.; Liu, X.; Luo, J.; Wu, H. Metabolome and Transcriptome Analyses of Cucurbitacin Biosynthesis in luffa (luffa acutangula). Front. Plant Sci. 2022, 13, 886870. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Z.; Lou, L.; Su, X. Identification of browning-related microRNAs and their targets reveals complex miRNA-mediated browning regulatory networks in luffa cylindrica. Sci. Rep. 2018, 8, 16242. [Google Scholar] [CrossRef]
- Chen, X.; Tan, T.; Xu, C.; Huang, S.; Tan, J.; Zhang, M.; Wang, C.; Xie, C. Genome-wide transcriptome profiling reveals novel insights into luffa cylindrica browning. Biochem. Biophys. Res. Commun. 2015, 463, 1243–1249. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, J.; Wen, Q.; Chen, M.; Wang, B.; Zhang, Q.; Xue, Z. De novo sequencing and analysis of the transcriptome during the browning of fresh-cut luffa cylindrica ‘Fusi-3’ fruits. PLoS ONE 2017, 12, e0187117. [Google Scholar] [CrossRef]
- Zhang, S.; Tian, L.; Zhang, Y.; Zhao, H.; Zhu, G. De novo transcriptome assembly of the fresh-cut white husk of juglans cathayensis dode: Insights for enzymatic browning mechanism of fresh-cut husk of walnut. Sci. Hortic. 2019, 257, 108654. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, K.; Zhang, A.; Zhu, W.; Zhang, H.; Tan, F.; Huang, Q.; Wu, X.; Zha, D. Metabolomic Analysis, Combined with Enzymatic and Transcriptome Assays, to Reveal the Browning Resistance Mechanism of Fresh-Cut Eggplant. Foods 2022, 11, 1174. [Google Scholar] [CrossRef]
- Qiao, L.; Gao, M.; Wang, Y.; Tian, X.; Lu, L.; Liu, X. Integrated transcriptomic and metabolomic analysis of cultivar differences provides insights into the browning mechanism of fresh-cut potato tubers. Postharvest. Biol. Technol. 2022, 188, 111905. [Google Scholar] [CrossRef]
- Hu, Z.; Fang, Y.; Yi, Z.; Tian, X.; Li, J.; Jin, Y.; He, K.; Liu, P.; Du, A.; Huang, Y.; et al. Determining the nutritional value and antioxidant capacity of duckweed (Wolffia arrhiza) under artificial conditions. LWT 2022, 153, 112477. [Google Scholar] [CrossRef]
- Kitima, S.; Souksanh, N.; Yupaporn, S. Paper-based DPPH Assay for Antioxidant Activity Analysis. Anal. Sci. 2018, 34, 795–800. [Google Scholar] [CrossRef]
- Lee, H.S.; Wicker, L. Anthocyanin Pigments in the Skin of Lychee Fruit. J. Food Sci. 2010, 56, 466–468. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Du, R. Nitric oxide delays chlorophyll degradation and enhances antioxidant activity in banana fruits after cold storage. Acta Physiol. Plant. 2015, 37, 74. [Google Scholar] [CrossRef]
- Zhu, L.; Zhou, J.; Zhu, S.; Guo, L. Inhibition of browning on the surface of peach slices by short-term exposure to nitric oxide and ascorbic acid. Food Chem. 2009, 114, 174–179. [Google Scholar] [CrossRef]
- Feng, Y.; Feng, C.; Wang, Y.; Gao, S.; Sun, P.; Yan, Z.; Su, X.; Sun, Y.; Zhu, Q. Effect of CaCl2 Treatment on Enzymatic Browning of Fresh-Cut luffa (luffa cylindrica). Horticulturae 2022, 8, 473. [Google Scholar] [CrossRef]
- Ainsworth, E.; Gillespie, K. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Ceccanti, C.; Landi, M.; Incrocci, L.; Pardossi, A.; Guidi, L. Suitability of hydroponically-grown Rumex acetosa L. as fresh-cut produce. Horticulturae 2020, 6, 4. [Google Scholar] [CrossRef]
- Trapnell, C.; Pachter, L.; Salzberg, S. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.; Schlesinger, F.; Drenkow, J.; Gingeras, T. Star: Ultrafast universal rna-seq aligner. Bioinformatics 2012, 29, 15–21. [Google Scholar] [CrossRef]
- Zhang, T.; Ren, X.; Zhang, Z.; Ming, Y.; Yang, Z.; Hu, J.; Li, S.; Wang, Y.; Sun, S.; Sun, K.; et al. Long-read sequencing and de novo assembly of the luffa cylindrica (L.) Roem. genome. Mol. Ecol. Resour. 2020, 20, 511–519. [Google Scholar] [CrossRef]
- Jo, Y.; Choi, H.; Lee, B.; Hong, J.; Kim, S.; Cho, W. Exploring tomato fruit viromes through transcriptome data analysis. Viruses 2023, 15, 2139. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ji, R.; Huang, Z.; Ye, X.; Huang, L.; Deng, H.; Lu, G.; Wei, S.; Liu, C.; Li, Z.; et al. Transcriptomic and Metabolomic Characterization of Volatile Flavor Compound Dynamics in Dragon Fruit (Selenicereus spp.) Development. Horticulturae 2025, 11, 599. [Google Scholar] [CrossRef]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Clowers, B.; Moore, R.; Zink, E. Signature-discovery approach for sample matching of a nerve-agent precursor using liquid chromatography-mass spectrometry, XCMS, and chemometrics. Anal Chem. 2010, 82, 4165–4173. [Google Scholar] [CrossRef] [PubMed]
- Mie, A.; Laursen, K.; Åberg, K.; Forshed, J.; Lindahl, A.; Thorup-Kristensen, K.; Olsson, M.; Knuthsen, P.; Larsen, E.; Husted, S. Discrimination of conventional and organic white cabbage from a long-term field tr ial study using untargeted LC-MS-based metabolomics. Anal Bioanal Chem. 2014, 406, 2885–2897. [Google Scholar] [CrossRef]
- Leng, T.; Hu, X.; Chen, Y.; Gan, B.; Xie, J.; Yu, Q. Rapid identification and quantitation of pork and duck meat of binary and ternary adulteration in minced beef by 1H NMR combined with multivariate data fusion. Food Control 2023, 154, 110018. [Google Scholar] [CrossRef]
- Sui, X.; Meng, Z.; Dong, T.; Fan, X.; Wang, Q. Enzymatic browning and polyphenol oxidase control strategies. Curr. Opin. Biotechnol. 2023, 81, 102921. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B.; del Río, A.L. Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells. Trends Plant Sci. 2001, 6, 145–150. [Google Scholar] [CrossRef]
- Averill-Bates, D. Reactive oxygen species and cell signaling. Review. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119573. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Li, F.; Yuan, D. Physiological, metabolome, and transcriptome analysis revealed the effect of ethyl 3-amino-3-thioxopropanoate on the browning of fresh-cut banana during storage. Postharvest Biol. Technol. 2024, 218, 113176. [Google Scholar] [CrossRef]
- Saghaie, L.; Pourfarzam, M.; Fassihi, A.; Sartippour, B. Synthesis and tyrosinase inhibitory properties of some novel derivatives of kojic acid. Res. Pharm. Sci. 2013, 8, 233–242. [Google Scholar] [PubMed]
- Dong, T.; Shi, J.; Jiang, C.Z.; Feng, Y.; Wang, Q. A short-term carbon dioxide treatment inhibits the browning of fresh-cut burdock. Postharvest Biol. Technol. 2015, 110, 96–102. [Google Scholar] [CrossRef]
- Marak, K.A.; Mir, H.; Siddiqui, M.W.; Singh, P.; Homa, F.; Alamri, S. Exogenous melatonin delays oxidative browning in litchi during cold storage by regulating biochemical attributes and gene expression. Front. Plant Sci. 2024, 15, 1402607. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant. 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, C.; Wang, X.; Liu, X.; Zhu, X.; Zhang, J. Integration of Metabolome and Transcriptome Profiling Reveals the Effect of Modified Atmosphere Packaging (MAP) on the Browning of Fresh-Cut Lanzhou Lily (Lilium davidii var. unicolor) Bulbs during Storage. Foods 2023, 12, 1335. [Google Scholar] [CrossRef]
- Supapvanich, S.; Pimsaga, J.; Srisujan, P. Physicochemical changes in fresh-cut wax apple (Syzygium samarangenese [Blume] Merrill & L.M. Perry) during storage. Food Chem. 2011, 127, 912–917. [Google Scholar]
- Huang, Q.; Liu, J.; Peng, C.; Han, X.; Tan, Z. Hesperidin ameliorates H2O2-induced bovine mammary epithelial cell oxidative stress via the Nrf2 signaling pathway. Anim. Sci. Biotechnol. 2024, 15, 57. [Google Scholar] [CrossRef]
- Wang, W.; Bi, J.; Hu, J.; Li, X. Metabolomics comparison of four varieties apple with different browning characters in response to pretreatment during pulp processing. Food Res. Int. 2024, 190, 114600. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Zhang, R.; Yan, Z.; Xiong, A.; Su, X. Sequencing, assembly, annotation, and gene expression: Novel insights into browning-resistant luffa cylindrica. PeerJ 2020, 8, e9661. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, A.; Zhao, J.; Shang, J.; Zhu, Z.; Wu, X.; Zha, D. Transcriptome profiling reveals potential genes involved in browning of fresh-cut eggplant (Solanum melongena L.). Sci. Rep. 2021, 11, 16081. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Chen, Y.; Hu, W.; Zhao, S. Integrated metabolomic and transcriptomic analysis provides insights into the browning of walnut endocarps. Front, Plant Sci. 2025, 16, 1582209. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Lin, Z.; Wang, M.; Liang, H.; Zhao, Y.; Li, Y.; Yan, B.; He, Y.; Wu, X.; Wang, Q.; et al. Mechanisms underlying the effect of high-temperature curing treatments on the browning response of fresh-cut yams. Food Chem. 2025, 476. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; El-Gizawy, A.; El-Bassiouny, R.; Saleh, M. Browning inhibition mechanisms by cysteine, ascorbic acid and citric acid, and identifying PPO-catechol-cysteine reaction products. Food Sci. Technol. 2015, 52, 3651–3659. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aquino-Bolaños, E.; Mercado-Silva, E. Effects of polyphenol oxidase and peroxidase activity, phenolics and lignin content on the browning of cut jicama. Postharvest Biol. Technol. 2004, 33, 275–283. [Google Scholar] [CrossRef]
- Dharmawardhana, D.; Ellis, B.; Carlson, J. A beta-glucosidase from lodgepole pine xylem specific for the lignin precursor coniferin. Plant Physiol. 1995, 107, 331–339. [Google Scholar] [CrossRef]
- Min, T.; Bao, Y.; Zhou, B.; Yi, Y.; Wang, L.; Hou, W.; Ai, Y.; Wang, H. Transcription profiles reveal the regulatory synthesis of phenols during the development of lotus rhizome (Nelumbo nucifera gaertn). Int. J. Mol. Sci. 2019, 20, 2735. [Google Scholar] [CrossRef]
- Prasad, K.; Sharma, R.; Srivastav, M. Postharvest treatment of antioxidant reduces lenticel browning and improves cosmetic appeal of mango (Mangifera indica L.) fruits without impairing quality. Food Sci. Technol. 2016, 53, 2995–3001. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Gao, S.; Wang, R.; Liu, Y.; Yan, Z.; Yong, M.; Feng, C.; Ni, W.; Fang, Y.; Zhu, S.; et al. Comparative Analysis of Phenolic Acid Metabolites and Differential Genes Between Browning-Resistant and Browning-Sensitive luffa During the Commercial Fruit Stage. Horticulturae 2025, 11, 903. https://doi.org/10.3390/horticulturae11080903
Feng Y, Gao S, Wang R, Liu Y, Yan Z, Yong M, Feng C, Ni W, Fang Y, Zhu S, et al. Comparative Analysis of Phenolic Acid Metabolites and Differential Genes Between Browning-Resistant and Browning-Sensitive luffa During the Commercial Fruit Stage. Horticulturae. 2025; 11(8):903. https://doi.org/10.3390/horticulturae11080903
Chicago/Turabian StyleFeng, Yingna, Shuai Gao, Rui Wang, Yeqiong Liu, Zhiming Yan, Mingli Yong, Cui Feng, Weichen Ni, Yichen Fang, Simin Zhu, and et al. 2025. "Comparative Analysis of Phenolic Acid Metabolites and Differential Genes Between Browning-Resistant and Browning-Sensitive luffa During the Commercial Fruit Stage" Horticulturae 11, no. 8: 903. https://doi.org/10.3390/horticulturae11080903
APA StyleFeng, Y., Gao, S., Wang, R., Liu, Y., Yan, Z., Yong, M., Feng, C., Ni, W., Fang, Y., Zhu, S., Liu, L., & Wang, Y. (2025). Comparative Analysis of Phenolic Acid Metabolites and Differential Genes Between Browning-Resistant and Browning-Sensitive luffa During the Commercial Fruit Stage. Horticulturae, 11(8), 903. https://doi.org/10.3390/horticulturae11080903






