Abstract
Crop production is directly associated with the improvement of germplasm, which is mainly reliant on genetic diversity. Diversity among various genotypes has been investigated employing a variety of statistical approaches. The most widely utilized of these methods for determining the genetic overlap of genotypes is multivariate. In the present investigation, a total of 27 onion genotypes/advanced lines/varieties comprising high and low total soluble solids (TSS) white onion lines along with red varieties were evaluated at the ICAR-Directorate of Onion and Garlic Research station. Data were recorded on seven biochemical parameters. In multivariate analysis, genotypes were clustered into three major groups: the first group comprised thirteen genotypes with high TSS; however, the second group (eight) comprised low TSS white onion genotypes, and in the third group (six), mostly red varieties were clubbed together. The analysis primarily focused on the trait TSS; it was significantly associated with the antioxidant assay 2, 2-diphenyl-1-picrylhydrazyl(DPPH) AE, while total sugar content was positively associated with the antioxidant assay 2, 2-azinobis (3-ethylbenzothiazoline- 6-sulfonic acid)ABTS. In principal component analysis (PCA), the first three principal components (PCs) with >1 Eigen value contributed 71.36% of the variability among genotypes. Characters with a maximum value at PC1 were total soluble solids (0.48), antioxidant capacity AE DPPH (0.46), and total sugar content (0.49). PC2 comprises total phenolic content (0.62) and total flavonoid content (0.62); for PC3, the major contributors were thiosulfinate content (0.82) and antioxidant capacity ABTS (0.32). From the findings of the present study, the best-performing high TSS lines can be used for advancement through strongly correlated traits using breeding strategies. These sorted high TSS lines (W-103, W-107, and W-123) (>22 °Brix.), high-sugar-content genotypes (W-108, W-111, and W-308), and W-361, which recorded high thiosulfinate content, can be advanced or used as parental material for the development of processing-suitable onion varieties.
1. Introduction
Within the genus Allium and family (Alliaceae), the onion (Allium cepa L.) is the most commonly grown species. India is the world’s second-largest producer of onions, behind China, with Maharashtra, Karnataka, and Madhya Pradesh being the main onion-producing states [1]. Whether in the form of a bulb, leaf, or pseudo stem, onions are used all year long as a salad ingredient, as a condiment, or in cooking alongside other vegetables [2,3]). Onions have been shown to have numerous anti-inflammatory and antioxidant properties. These characteristics lower blood sugar, strengthen bones, and lower the risk of developing a number of malignancies. Additionally, they prevent obesity, hypercholesterolemia, and coronary heart disease [4,5]. Onions are a good source of vitamin C, potassium, dietary fiber, minerals, and folic acid [6]. As a result, onion consumption has dramatically increased recently.
Onions are well known for their strong flavor and taste. Pungency level and total soluble solids are important quality attributes of onion bulbs. Compounds like sugars and organic acids contribute to the organoleptic test and contribute to the distinctive flavor and aroma [7]. The contents of soluble carbohydrates contribute to onion sweetness. The study of these parameters is a prerequisite for the export and processing of onion. Cultivation practices, location, and cultivar affect the total accumulation of taste components [8] and, ultimately, the quality of onions. In addition to this, according to Zhoa et al. [9], the three major constituents—flavonoids, oligosaccharides, and alkyl cysteine sulphoxides (ACSOs)—are thought to have a number of health benefits. There is a direct correlation between the generation of pyruvate and the pungency of onions. Because different cultivars have varying capacities to regulate sulfur uptake and assimilation in the biosynthesis pathway that produces the flavor, genetic background also appears as the most significant factor. Growing conditions, clones, and storage all affect pyruvic acid [10]. Therefore, it is vital to breed the short-day onion varieties based on their sweetness, pungency level, and storage and export qualities [4,8].
In short-day onions, previous research worked on biochemical aspects [11,12,13,14] on exotic and domesticated onion varieties and landraces. The present study includes onion accessions, which are being maintained by massing and selfing, with a special emphasis on the soluble solid content of white onions, which are popular red and white onion varieties possessing varying storage capacities. During storage, the bulb’s metabolic activity enhances the flavoring ingredient of onion until sprouting and the rise in sulfur compounds reaches its peak [5,15]. Generally, cultivars with a low storage quality have fewer flavors. Onion quality is determined by the amount of soluble solid content and thiosulfinate content, which directly correlate with pyruvic acid, sulfur content, or the pungency of onion. As low-pungency onions have grown in popularity, it is now important to estimate the pungency of bulbs [3].
Measuring the amount of genetic variation present is crucial for effectively using and assessing the germplasm [16]. A key tactic for classifying available accessions and analyzing the genetic relationships between breeding materials is the application of different statistical methods. Grouping by TSS levels allows breeders to identify and utilize parental lines with desirable biochemical profiles for specific breeding objectives, such as developing sweeter onions for fresh consumption or more pungent, high-TSS lines for processing, and extended storage. Advanced techniques like marker-assisted selection (MAS) can be employed to target genes associated with TSS accumulation and sulfur metabolism, improving breeding efficiency.
Therefore, studies on soluble solids, thiosulfinates, phenolic compounds, and sugar content of different white onion accessions in comparison with red- and white-colored domesticated popular onion varieties at the short-day condition in tropical climates is essential to derive the ideas for breeding processing oriented, good storage capacity, and nutritionally efficient onion varieties [17]. The primary goal of the current research was to document the biochemical characteristics that could be further altered in generation advancement through breeding and to use multivariate analysis to explain the degree of genetic diversity and correlations among onion genotypes based on quantitative variables. Overall, these activities will strengthen the short-day onion breeding program.
2. Materials and Methods
2.1. Plant Material
A total of 27 onion lines/varieties were used in this experiment. The breeding lines are equal to self-pollinated and massed (at least 20 selected bulbs) pure lines. The seeds of these genotypes were sourced from the germplasm collections of the Indian Council of Agriculture Research (ICAR)-Directorate of Onion and Garlic Research (DOGR), Pune and were both genetically and physically pure. The onion lines under study are Indian short-day lines/varieties, exhibiting a distinct capability to form bulbs and initiate flowering under short photoperiods of 10–12 h and a minimum temperature range of 10–12 °C. This precise photothermal response highlights their adaptive potential for cultivation in subtropical and tropical agro-climatic zones, where such environmental conditions prevail during critical phenological stages (Table 1).

Table 1.
Name and characteristics of onion genotypes used in study.
2.2. Field Evaluation
The place of the experiment is located at 18.32 N (latitude) and 73.51 E (longitude) at 553.8 m above MSL, with a temperature range of 5.5–42 °C, and having an annual rainfall of 669 mm. Each genotype’s seedlings were transplanted in a 3 × 1 m unit bed using standard agronomic practices. In the rabi/winter (Oct–Nov months), the seasons of 2021–2022 and 2022–2023, 49-day-old seedlings were transplanted into the main field using a Randomized Block Design (RBD) with three replications at ICAR-DOGR. Ten rows of two meters each, spaced 15cm by 10cm apart, were used to grow each genotype/line/variety. Harvest-produced bulbs weighing 80–100 g were selected for studying the biochemical properties of onion lines/varieties.
2.3. Data Recording
Data were recorded on eight parameters viz. total soluble solids (°Brix), thiosulfinate content (µmol/g fresh weight (FW)), total phenolic content GE (mg/g FW), total flavonoid content QE (mg/g FW), antioxidant capacity ABTS TEAC (µmol/g FW), antioxidant capacity DPPH AE (µmol/g FW), total sugar content (g/100 g FW), and reducing sugar (g/100 g FW) in three replicates each. In the sample preparation, 5 g of the sample’s paste was added to 50 mL of (80%) methanol and kept overnight at room temperature (25 ± 2 °C) on a shaker. After 24 h of incubation, the sample’s supernatantt was separated through centrifugation. This supernatant was used as an extract for further biochemical analysis/assay. The samples were biologically repeated three times for each parameter separately. All chemicals used for biochemical procedures were sourced from Thermo Fisher Scientific India Pvt. Ltd. Mumbai, India while analytical standards were obtained from Sigma –Aldrich (Merk Group), Mumbai, India. The parameter-wise detail procedure is as follows.
2.4. Total Soluble Solids Content
From the sample paste (2 bulbs of 80–100 g), extract a few drops of juice. Add the juice by hand to the refractometer plate after cleaning with distilled water (DW), drying with tissue paper, and then record the hand readings in degree Brix (°Brix) [12].
2.5. Thiosulfinate Content
In one gram sample paste, 15 mL of the HEPES buffer was added, and centrifuge the extract. In 80 µL of supernatant extract, 120 µL of L-cystein was mixed, and the mixture was incubated for 15–20 min at 37 °C. Then, 300 µL of HEPES and 100 µL of DTNB were added to the mixture. Then, absorbance was recorded at 412 nm using the Tecan Infinite 200 Pro M Nano+ spectrophotometer (Mannedorf, Switzerland); 200 µL of the sample was placed in the plate [18].
2.6. Total Phenolic Content
The total phenolic content (TPC) was estimated by using the Folin–Ciocalteu method [19]. First, 100 µL of the folin ciocalteus reagent was added to the 200 µL extract of the sample. Then, the sample was vortexedfor 1 min and incubated for 5 min. Then, 800 µL (700 mM) of sodium carbonate (Na2CO3) was mixed in the sample and incubated for 120 min in the dark. The sample (200 µL) was loaded in a microtitration plate to measure the absorbance at 765 nm using a Tecan Infinite 200 Pro M Nano+ spectrophotometer (Männedorf, Switzerland). The results were calculated utilizing a standard curve for gallic acid (y = 3.8765x + 0.0127; serial dilutions of gallic acid, 20 to 100 µg/mL; coefficient of determination, R2 = 0.9998). The TPC was expressed as mg GAE/g FW.
2.7. Total Flavonoid Content
The total flavonoid content was determined via an aluminum chloride colorimetric assay developed by Sembiring et al. in 2018 [20], with some modifications. In short, 100 µL of the sample extract was added to 400 µL of distilled water, 300 µL (10%) of aluminum chlorite (AlCl3), 300 µL (5%) of sodium nitrite (NaNo2) and the mixture was incubated for 5 min. Then, 200 µL of 1N sodium hydroxide (NaOH) and 240 µL of DW were added to the extract, and absorbance at 510 nm was observed [21]. The results were determined via the standard curve of quercetin (y = 7.1021x − 0.005; serial dilutions of quercetin: 20, 40, 60, 80, and 100 mg/L; coefficient of determination; R2 = 0.9999). The total flavonoid content was expressed as mg QE/g FW.
2.8. Antioxidant Content ABTS
Dilute the extract with distilled water in a ratio of 1:5. Take 50 µL of the diluted sample, add 950 µL of ABTS, and record the absorbance at 734 nm [22].
2.9. Total Antioxidant Capacity
For the DPPH (1,1-diphenyl-1-picrylhydrazyl) assay [23]: 100 µL sample extract was mixed in 390 µL DPPH (0.1 mM) solution, then incubate in dark at 25 °C for 30 min and observe the absorbance at 515 nm with a Tecan Infinite 200 Pro M Nano+ spectrophotometer (Männedorf, Switzerland). The results were calculated using a Trolox standard curve (y = −21.0864x + 21.2202; series of Trolox dilutions: 20, 40, 60, 80, and 100 µM; coefficient of determination, R2 = 0.9999) and were expressed as µmol TE/g FW [22].
2.10. Total Sugar Content
Dilute the 50 µL of the extract with 450 µL of DW, and then add 500 µL of phenol and 500 µL of sulfuric acid. Incubate for 15min at room temperature, and record the absorbance at 480-490 nm (Lane and Eynon method, Ranganna) using a Tecan Infinite 200 Pro M Nano+ spectrophotometer (Männedorf, Switzerland), expressed as g/100 g FW [24].
2.11. Reducing Sugar Content
In 100 µL of the sample extract and 100 µL of the DNS reagent was added and boiled for 5 min. Then, absorbance was recorded at 540nm by a Tecan Infinite 200 Pro M Nano + spectrophotometer (Männedorf, Switzerland) and expressed as g/100 g FW [24].
2.12. Statistical Analysis
Statistical analysis was performed using SAS 9.3 Proc GLM software. The mean replicated data of all the parameters used for analysis. The significance of differences in the biochemical profile was assessed using a one-way ANOVA with Tukey’s post hoc test (p < 0.05) to evaluate the variation between the bulbs in relation to the genotype/variety. A further cluster analysis by Ward, the 1963 method, a dendrogram, principal component analysis (PCA), and Pearson’s correlation were generated by JMP 10.0 (SAS institute) software.
3. Results
In the results, the analysis of variance explained that the means of the recorded seven traits were significantly different for high and low TSS onion lines and varieties for the recorded seven biochemical traits except thiosulfinate content. The ANOVA results provide significant insights into the factors that affected the different biochemical parameters evaluated.
3.1. Total Soluble Solids Content
Concerning the total soluble solids content (TSSC), among the total 27 onion genotypes/breeding lines or varieties, a total of seven varieties reported TSS in the range of 11–15 °Brix and nine lines recorded low TSS (>18 °Brix); however, 11 lines noted TSS < 18 °Brix (Table 2, Figure 1). These lines/genotypes were previously advanced through the “massing and selection of single bulb progeny” breeding method for seven generations (data not reported). This TSS-based grouping of onion lines was further utilized for verifying the effects of other biochemical parameters on TSS variability.

Table 2.
Variation in the biochemical traits of the bulbs of the tested lines/varieties of onion. Data are reported as the mean ± standard error.
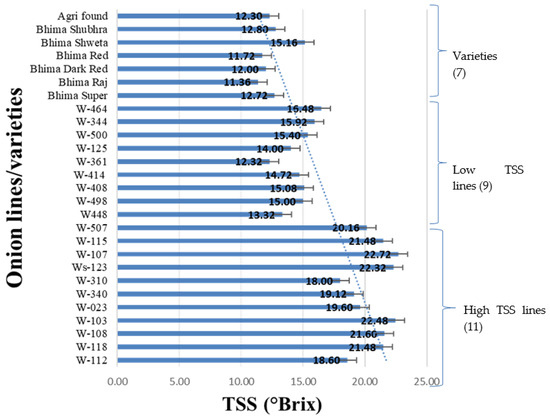
Figure 1.
Total soluble solids (TSS) variability in onion genotypes/varieties.
3.2. Thiosulfinate Content
This parameter represents the total amount of sulfur-containing compounds present in the onion line/genotype. However, there was no significant difference found among high, low TSS lines, and varieties. The average thiosulfinate content ranged between 20 and 21 µmol/g FW (Table 2, Figure 2).
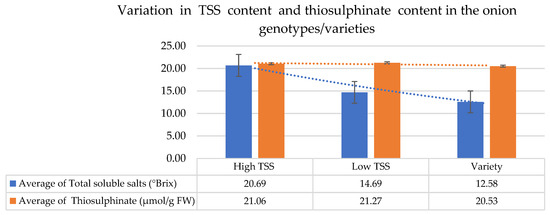
Figure 2.
Variation in TSS content and thiosulfinate content in the onion genotypes/varieties. Data are presented in mean ± standard error form.
3.3. Total Phenolic Content
A higher content of TPC was observed in the onion varieties, followed by high TSS onion lines. However, a significant difference was noted among the studied onion genotypes/varieties (Table 2, Figure 3).
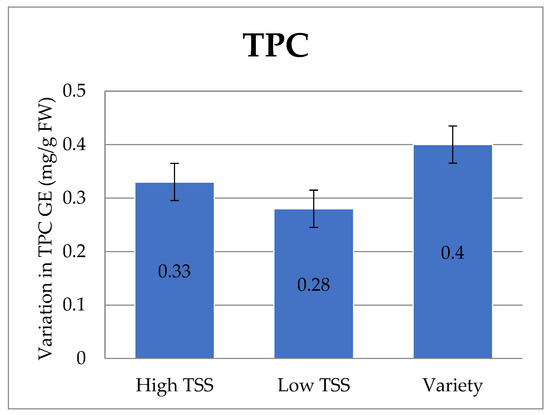
Figure 3.
Variation in TPC GAE (mg/g) in the onion genotypes/varieties. Data are presented in mean ± standard error form.
3.4. Total Flavonoid Content
In the case of TFC, onion varieties possess significantly higher TFC than other genotypes. However, within onion lines, genotypes possessing low TSS had the lowest TFC (Table 2, Figure 4)
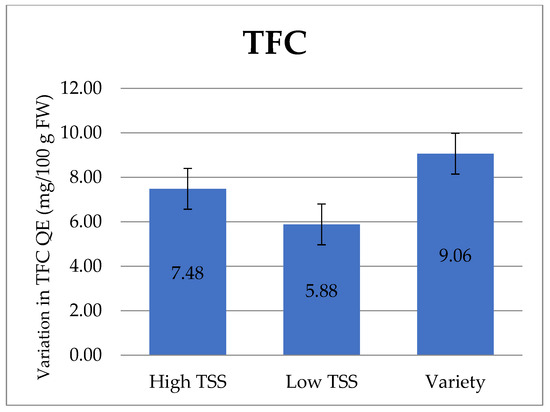
Figure 4.
Variation in total flavanoid content (TFC), QE (mg/100 g), and FW in the onion genotypes/varieties. The data are represented in mean ± standard error.
3.5. Total Sugar Content
The total sugar content present in onion represents the sweet taste of onion, and also correlates with its storage value. In the studied population, a higher total sugar content was recorded in high TSS genotypes, while domesticated onion varieties showed the lowest total sugar content (Table 2; Figure 5).
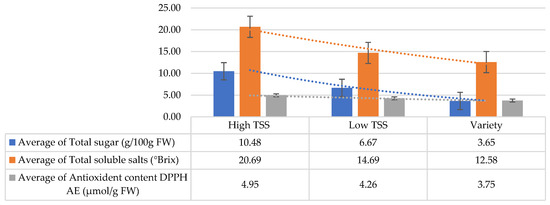
Figure 5.
Variation in total sugar content, TSS, and antioxidant content of DPPH AE (u mol/g FW) in the onion genotypes/varieties. The data are presented in mean ± standard error form.
There was a significant difference found between onion genotypes and the domesticated varieties for reducing sugar content (Table 2). However, domesticated onion varieties used in the study recorded a higher percent of reducing sugar content (Figure 6), as compared to the percent of total sugar content.
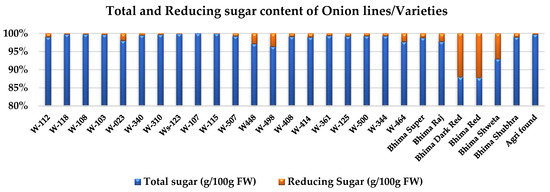
Figure 6.
Variation in total sugar content and reducing sugar content of onion genotypes/varieties. Data are presented in the percent form and derived from the relative mean data value of the respective genotype/variety.
3.6. Correlation Studies
The results divulged that total soluble solids (TSS) is positively associated with the antioxidant assay (ABTS), while a significantly positive association was found with the antioxidant assay (DPPH AE), and the total sugar; on the other hand, there was a negative association with the total phenolic content, the total flavonoid content, and reducing sugar. A correlation of thiosulfinate was positive with the antioxidant assay (ABTS and DPPH), while a significant positive association was recorded with the total sugar content (Table 3). Total phenolic content showed a significantly positive association with total flavonoid content while a positive association with antioxidant capacity (ABTS) and reducing sugar. However, a negative association was observed between antioxidant capacity, DPPH, and total sugar content. Antioxidant capacity (ABTS) recorded a positive association with the total sugar content, while a negative association was recorded with reducing sugar content. A strong positive association was noted between antioxidant capacity (DPPH) and total sugar content (Table 3).

Table 3.
Correlation studies for various biochemical attributes of onion genotypes/varieties, which were used in the study.
3.7. Clustering of Genotypes Based on Biochemical Traits
In multivariate analysis, clustering was carried out by using the Ward (1963) [25] method with the seven biochemical traits of 27 total onion genotypes/varieties through JMP.10 software. Based on genetic distance, genotypes were clustered into three distinct groups. Group one contains thirteen genotypes (W-112, W-108, W-507, W-118, W-123, W-107, W-103, W-115, W-340, W-344, W-310, W-500, and W-125); the second group comprises eight genotypes/varieties (W-448, W-408, W-498, Bhima Shubhra, Bhima Shweta, W-381, W-464, and Agri Found Red); while the third group includes six genotypes/varieties (W-023, W-441, Bhima Super, Bhima Raj, Bhima Red, and Bhima Dark Red) (Figure 7). In group I, the majority of the high TSS genotypes (19.48 °Brix) were clustered together (Table 3), with the highest antioxidant capacity for DPPH AE (4.97 µmol/g FW and total sugar content (10.45 g/100 g FW) (Table 3). In the second group, the mean values of all the traits were less than the first group except for thiosulfinate content (26–17 µmol/g FW), as this group comprises low TSS genotypes and few varieties (Table 4). However, in the third group, genotypes/varieties were high in the total phenolic content as well as reducing content. The means of other respective traits were the lowest when compared to other groups (Table 4). Therefore, this clustering assists breeders in selecting trait-wise onion genotypes as a parental breeding material for the advancement of a particular trait.
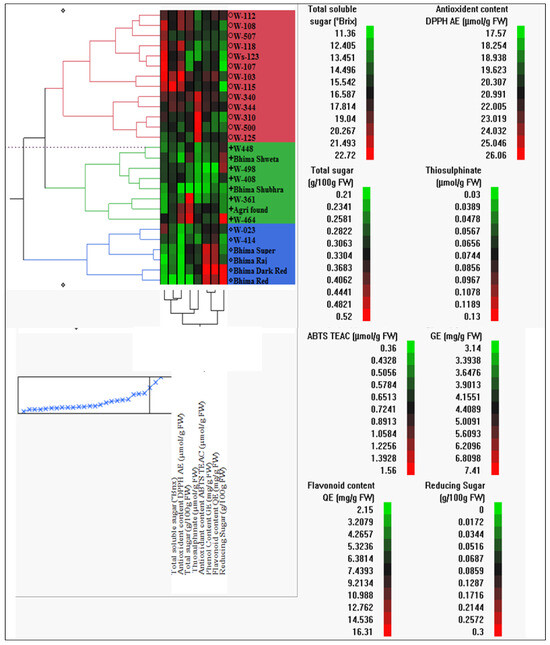
Figure 7.
Dendrogram generated through genetic distance (Ward, 1963 method) based clustering of 27 onion genotypes/varieties using JMP 10.0 software. Means of eight biochemical traits were used for sorting groups based on genetic distance. First group (red colour) contains thirteen genotypes, second (green colour) comprises eight genotypes while six genotypes/varieties in third group (Blue colour). Heatmap-red colour indicated highest value of particular trait with respect to genotype, fluorescent green colour- lowest value of particular trait with respect to genotype and mixed colour (overlapping of red and green) indicated middle range i.e., average value for particular trait with respect to genotype.

Table 4.
Cluster-wise means and other related parameters.
3.8. Principal Component Analysis
Principal component analysis (PCA), showed the distribution of studied onion accessions/varieties based on analysis of biochemical parameters (Table 5 and Table 6). The PCA had grouped the estimated onion genotypes/variables into three main components. The first three components with Eigen values>1 accounted for 71.36% of the total variation (Table 6). PC1 absorbs 34.89% of total variation, while PC2 represents 22.79% of the total variation. In PC1 main positive source of variation in data comes from total soluble solids content, antioxidant capacity DPPH and total sugar content while negative contribution were received from total phenolic content, total flavonoid content and reducing sugar content. However, total phenolic content and total flavonoid content significantly contributing for total variation in PC2 while no negative effect of any parameter noted for this component. Trait thiosulfinate content followed by antioxidant capacity ABTS showed vital effect for PC3 variation with negative effect of total soluble solids content and total flavonoid content (Figure 8).

Table 5.
Principal component analysis for biochemical traits in Onion genotypes/varieties.

Table 6.
Eigen value and relative percent towards variation created by cumulative effect of studied traits.
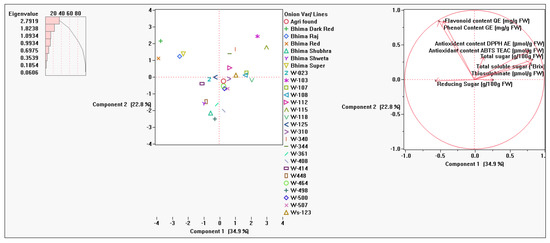
Figure 8.
Principal component analysis carried out by using the means of the data of 27 onion varieties on eight biochemical traits using JMP 10.0 software.
4. Discussion
Today, consumers increasingly seek vegetables that are rich in bioactive compounds like polyphenols, ascorbic acid, and vitamins. Onions, known for their versatility, are widely used in the human diet as condiments or incorporated into various dishes as fresh produce. The findings highlight that biodiversity in the nutritional composition of onions is substantial. The data indicate significant interactions between the genotype and the biochemical profile of the onion. Several scientific studies have highlighted the specific health benefits of onions, including their antioxidant, antimicrobial, anticholesterolemic, and anticancer properties [26]. The primary antioxidant compounds in onions are flavonoids, including quercetin and kaempferol, and their derivatives. The onion’s antioxidant activity is linked to its capacity to inhibit the formation of free radicals and reactive oxygen species (ROS) [27].
The PCA showed distinct effects on antioxidant properties and sugar components. The bulbs were characterized by total soluble solids, high antioxidant capacity (DPPH and ABTS), total flavonoid content (TFC), total phenolic content, and thiosulfinate content as well as sugars [10,28]. In our results, a significantly high antioxidant capacity and sugar content were found in bulbs with high TSS white onion lines. This genetic relationship between the traits and bulbs of high TSS accessions was also justified by correlation studies as well as principal component analysis [29].
The crucial function of phenolic compounds, which are recognized as antioxidants, is to defend cells and tissues from the damaging effects of oxygen radicals and other extremely reactive oxygen species. Based on the study by Wang et al. [5], which indicates variations in phenolic content and antioxidant capacity across different genotypes, our findings offer valuable insights into the profiles of both high and low TSS white skin onion accessions, as well as red onion varieties [12,15]. Additionally, Velioglu et al. [30] reported a positive and highly significant correlation between total phenolic content and antioxidant activity, suggesting that a molecule with higher phenol content had better antioxidant activity.
Notably, the organosulfur compounds were the most predominant and volatile compounds in the onion bulb. Therefore, thiosulfinate content was determined, and no significant difference was observed throughout the various studied genotypes, comprising white high and low TSS lines and red onion varieties [8,10,31]. The total flavonoid content was determined. In line with the previous study, red onion varieties exhibited a high total flavonoid content [9] compared to white, high and low TSS accessions [7]. The antioxidant capacity was assessed using various assays, including ABTS and DPPH. Our results confirm that high TSS onion genotypes showed the highest DPPH value and DPPH capacity when compared to low TSS, as well as white and red onion varieties [32,33]. Our results align with the literature data, as the antioxidant capacity varied in relation to the genotypes and the different methods utilized [33].
Consistent with previous studies, our results demonstrated that onions with higher TSS also exhibited a greater antioxidant capacity (DPPH), indicating that the genotypes studied possess enhanced nutritional properties [32]. The data also provide valuable insights into the diversity of onion biochemical traits, offering opportunities for exploring metabolite variation and guiding targeted breeding efforts to optimize onion varieties for specific applications, such as culinary or medicinal purposes [27]. Soluble sugar content plays a crucial role in onions, contributing to their sweetness and impacting both their flavor and nutritional profile [34]. The primary sugars—FOS, glucose, sucrose, and fructose—can vary significantly depending on factors such as cultivar, environmental conditions, plant parts, and growth stage [34]. Our results on total soluble solids and their strong correlation with total sugar, along with the presence of a higher percentage of reducing sugar in domesticated onion varieties, signified the need for a detailed study on FOS, glucose, sucrose, and fructose, especially in high TSS white onion lines in relation to genotype, plant organ, etc. [27]. Furthermore, the importance and preference of consumers for prebiotics are well recognized, with vegetables high in FOS content being particularly valued.
5. Conclusions
In this study, among the biochemical traits evaluated, total soluble solid contents were identified as a pivotal attribute with considerable commercial value for the processing industries, whereas other traits contributed substantially to the nutritional quality of onion. The results demonstrated that onion genotypes with a higher yield potential generally exhibited lower TSS levels (10–12 °Brix), while certain accessions and breeding lines recorded significantly higher TSS levels (18–20 °Brix). This presents a substantial opportunity for breeders to introgress high TSS traits into high-yielding cultivars or to develop novel genotypes with high TSS.
Furthermore, a robust positive correlation was observed between total soluble solids content, total sugar content, and antioxidant activity (DPPH), highlighting the importance of these interrelated traits in enhancing TSSC levels in onions. The application of multivariate statistical methods viz. PCA and clustering of genotypes provided practical insights for breeding programs, enabling the identification and selection of superior parental materials for the development of improved onion varieties suitable for processing industries.
6. Recommendation
Representative genotypes may be chosen from the particular groups for hybridization programs with other approved cultivars. This will aid in identification, selection, and combining genotypes to obtain important traits in one line with a broad genetic base.
Author Contributions
Conceptualization, A.P.B., D.N.M. and V.M.; methodology, A.P.B.; software, A.P.B.; validation, A.P.B. and D.N.M.; formal analysis, A.P.B.; investigation, V.M.; resources, A.P.B.; data curation, A.P.B.; writing—original draft preparation, A.P.B.; writing—review and editing, A.P.B.; visualization, D.N.M.; supervision, D.N.M.; project administration, V.M.; funding acquisition, V.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare and thank the ICAR-DOGR for funding the entire research work.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to thank ICAR-DOGR for supporting this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAO; STAT. Food and Agriculture Organization of the United Nations-FAO Statistics Division. 2019. Available online: http://www.fao.org (accessed on 1 June 2024).
- Havey, M.J. Onion breeding. Plant Breed. Rev. 2018, 42, 39–85. [Google Scholar]
- Singh, A.K.; Janakiram, T.; Singh, M.; Mahajan, V. Onion cultivation in India- a way forward. Indian Hortic. 2017, 62, 3–8. [Google Scholar]
- Benkeblia, N.; Varoquaux, P. Effects of gamma-irradiation, temperature and storage time on the status of the glucose, fructose and sucrose in onion bulbs (Allium cepa L.). Int. Agrophys. 2003, 17, 1–5. [Google Scholar]
- Wang, C.K. Health benefits of onion bioactives on hypercholesterolemia, cardiovascular diseases, and bone mineral density. Food Front. 2020, 1, 107–108. [Google Scholar] [CrossRef]
- Zhao, X.X.; Linc, F.J.; Li, H.; Li, H.B.; Wu, D.T.; Geng, F.; Ma, W.; Wang, Y.; Miao, B.H.; Gan, R.Y. Recent advances in bioactive compounds, health functions, and safety concerns of onion (Allium cepa L.). Front. Nutr. 2021, 8, 669805. [Google Scholar] [CrossRef]
- Sato, A.T.; Zhang, L.; Yonekura, H.; Tamura, A. Antiallergic activities of eleven onions (Allium cepa) were attributed to quercetin 4’-glucoside using QuEChERS method and Pearson’s correlation coefficient. J. Funct. Foods 2015, 14, 581–589. [Google Scholar] [CrossRef]
- Dhumal, K.; Datir, S.; Pandey, R. Assessment of bulb pungency level in different Indian cultivars of onion (Allium cepa L.). Food Chem. 2007, 100, 1328–1330. [Google Scholar] [CrossRef]
- Zhang, S.L.; Peng, D.E.N.G.; Xu, Y.C.; Lü, S.W.; Wang, J.J. Quantification and analysis of anthocyanin and flavonoids compositions, and antioxidant activities in onions with three different colors. J. Integr. Agric. 2016, 15, 2175–2181. [Google Scholar] [CrossRef]
- Lee, E.J.; Patil, B.S.; Yoo, K.S. Antioxidants of 15 onions with white, yellow, and red colors and their relationship with pungency, anthocyanin, and quercetin. LWT—Food Sci. Technol. 2015, 63, 108–114. [Google Scholar] [CrossRef]
- Dangi, R.; Khar, A.; Islam, S.; Kumar, A. Characterization and association of phenotypic and biochemical traits in onion under short day tropical conditions. Indian J. Hortic. 2018, 75, 226–236. [Google Scholar] [CrossRef]
- Gupta, A.J.; Benke, A.P.; Gorrepati, K.; Mahajan, V.; Singh, M. Trait association and variability study for biochemical and yield related traits in onion (Allium cepa L.). Veg. Sci. 2024, 51, 49–55. [Google Scholar] [CrossRef]
- Lyngkhoi, F.; Saini, N.; Gaikwad, A.B.; Thirunavukkarasu, N.; Verma, P.; Silvar, C.; Yadav, S.; Khar, A. Genetic diversity and population structure in onion (Allium cepa L.) accessions based on morphological and molecular approaches. Physiol. Mol. Biol. Plants 2021, 27, 2517–2525. [Google Scholar] [CrossRef]
- Raj, A.C.; Sharangi, A.B.; Das, A.; Pramanik, K.; Upadhyay, T.K.; Almutairi, M.; Khan, M.I.; Ahmad, I.; Kausar, M.A.; Saeed, M. Assessing the genetic divergence of onion (Allium cepa L.) through morpho-physiological and molecular markers. Sustainability 2022, 14, 1131. [Google Scholar] [CrossRef]
- Priyadarshini, G.; Amarananjundeswara, H.; Doddabasappa, B.; Vasudeva, K.R.; Anjanappa, M.; Prasad, P.S. Studies on identification of suitable onion genotypes for processing. J. Pharmacol. Phytochem. 2020, 9, 3091–3094. [Google Scholar]
- Singh, S.R.; Ahamed, N.; Srivastava, K.K.; Kumar, D.; Yousuf, S. Assessment of genetic divergence in long day onion (Allium cepa L.) through principal component and single linkage cluster analysis. J. Hortic. Sci. 2020, 15, 17–26. [Google Scholar]
- Abbasi, Z.; Darabi, A.; Shahmansouri, E. Evaluation of short day onion (Allium cepa L.) genotypes for quantity and quality traits. J. Hortic. Postharvest Res. 2022, 5, 379–390. [Google Scholar]
- Cantwell, M.I.; Kang, J.; Hong, G. Heat treatments control sprouting and rooting of garlic cloves. Posth. Bio. Tech. 2003, 30, 57–65. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Sembiring, E.N.; Elya, B.; Sauriasari, R. Phytochemical screening, total flavonoid and total phenolic content and antioxidant activity of different parts of Caesalpinia bonduc (L.) Roxb. Pharmacogn. J. 2018, 10, 123–127. [Google Scholar] [CrossRef]
- Kumaran, A.; Karunakaran, R.J. In vitro antioxidant activities of methanol extracts of five phyllanthus species from India. LWT-Food Sci. Technol. 2007, 40, 344–352. [Google Scholar] [CrossRef]
- Lee, J.Y.; Hwang, W.I.; Lim, S.T. Antioxidant and anticancer activities of organic extracts from Platycodon grandiflorum A. De Candolle roots. J. Ethnopharma 2004, 93, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Nuutila, A.M.; Puupponen-Pimiä, R.; Aarni, M.; Oksman-Caldentey, K.M. Comparison of antioxidant activities of onion and garlic extracts by inhibition of lipid peroxidation and radical scavenging activity. Food Chem. 2003, 81, 485–493. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E. Studies on the antioxidant activities of cinnamon (Cinnamom umverum) bark extracts, through various in vitro models. Food Chem. 2006, 94, 520–528. [Google Scholar] [CrossRef]
- Ward, J.H., Jr.; Hook, M.E. Application of an hierarchical grouping procedure to a problem of grouping profiles. Educ. Psychol. Meas. 1963, 23, 69–81. [Google Scholar] [CrossRef]
- Benkeblia, N. Free-radical scavenging capacity and antioxidant properties of some selected onions (Allium cepa L.) and garlic (Allium sativum L.) extracts. Braz. Arch. Biol. Technol. 2005, 48, 753–759. [Google Scholar] [CrossRef]
- Kahane, R.; Vaillle-Guerin, E.; Boukema, I.; Tzanoudakis, D.; Bellamy, C.; Chamaux, C.; Kik, C. Changes in non- structural carbohydrate composition during bulbing in sweet and high-solid onions in field experiments. Environ. Exp. Bot. 2001, 45, 72–83. [Google Scholar] [CrossRef]
- Benke, A.P.; Krishna, R.; Mahajan, V.; Ansari, W.A.; Gupta, A.J.; Khar, A.; Shelke, P.; Thangasamy, A.; Shabeer, T.A.; Singh, M.; et al. Genetic diversity of Indian garlic core germplasm using agro-biochemical traits and SRAP markers. Saudi J. Biol.Sci. 2021, 28, 4833–4844. [Google Scholar] [CrossRef]
- Sahoo, B.B.; Panda, D.; Panda, A.; Gupta, A.J.; Tripathy, P.; Senapati, N.; Bhupenchandra, I.; Singh, S.; Mohanty, S.; Dasgupta, M.; et al. Exploring the morphological and biochemical characteristics of Kharif onion (Allium cepa L.): Principal component and path coefficient analysis. Vegetos 2024, 37, 2041–2050. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113. [Google Scholar] [CrossRef]
- Benke, A.P.; Dukare, S.; Mahajan, V.; Singh, M. Genetic divergence studies for bulbing and related traits in garlic germplasm during kharif season. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2920–2927. [Google Scholar] [CrossRef]
- Islam, S.; Khar, A.; Singh, S.; Tomar, B.S. Variability, heritability and trait association studies for bulb and antioxidant traits in onion (Allium cepa L.) varieties. Ind. J. Agric. Sci. 2019, 89, 450–457. [Google Scholar] [CrossRef]
- Kaur, C.; Joshi, S.; Kapoor, H.C. Antioxidants in onion (Allium cepa L.) cultivars grown in India. J. Food Biochem. 2009, 33, 184–200. [Google Scholar] [CrossRef]
- Clark, C.J.; Shaw, M.L.; Wright, K.M.; McCallum, J.A. Quantification of free sugars, fructan, pungency and sweetness indices in onion populations by FT-MIR spectroscopy. J. Sci. Food. Agric. 2018, 98, 5525–5533. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).