Morphological and Molecular Insights into Genetic Variability and Heritability in Four Strawberry (Fragaria × ananassa) Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Site
2.2. Experimental Design and Morphological Evaluation Method
- 1
- Vegetative Traits:
- Plant height (cm), measured from the crown to the top of the highest leaf, using a measuring tape.
- Plant width (cm), measured horizontally at the widest point of the plant canopy.
- Number of leaves per plant, counted as fully developed trifoliate leaves.
- 2
- Reproductive Traits:
- Number of flowers per plant, counted at the peak flowering stage.
- Number of fruits per plant, cumulatively counted throughout the fruiting period.
- 3
- Fruit Traits:
- Fruit length and diameter (cm), measured using a digital caliper; average values were calculated from 10 randomly selected fruits per plant.
- Average fruit weight (g), determined by weighing 10 fruits per plant on a precision digital scale and calculating the mean.
- Skin color, visually evaluated based on a standardized color scale.
- Fruit shape index, calculated as the ratio of fruit length to diameter.
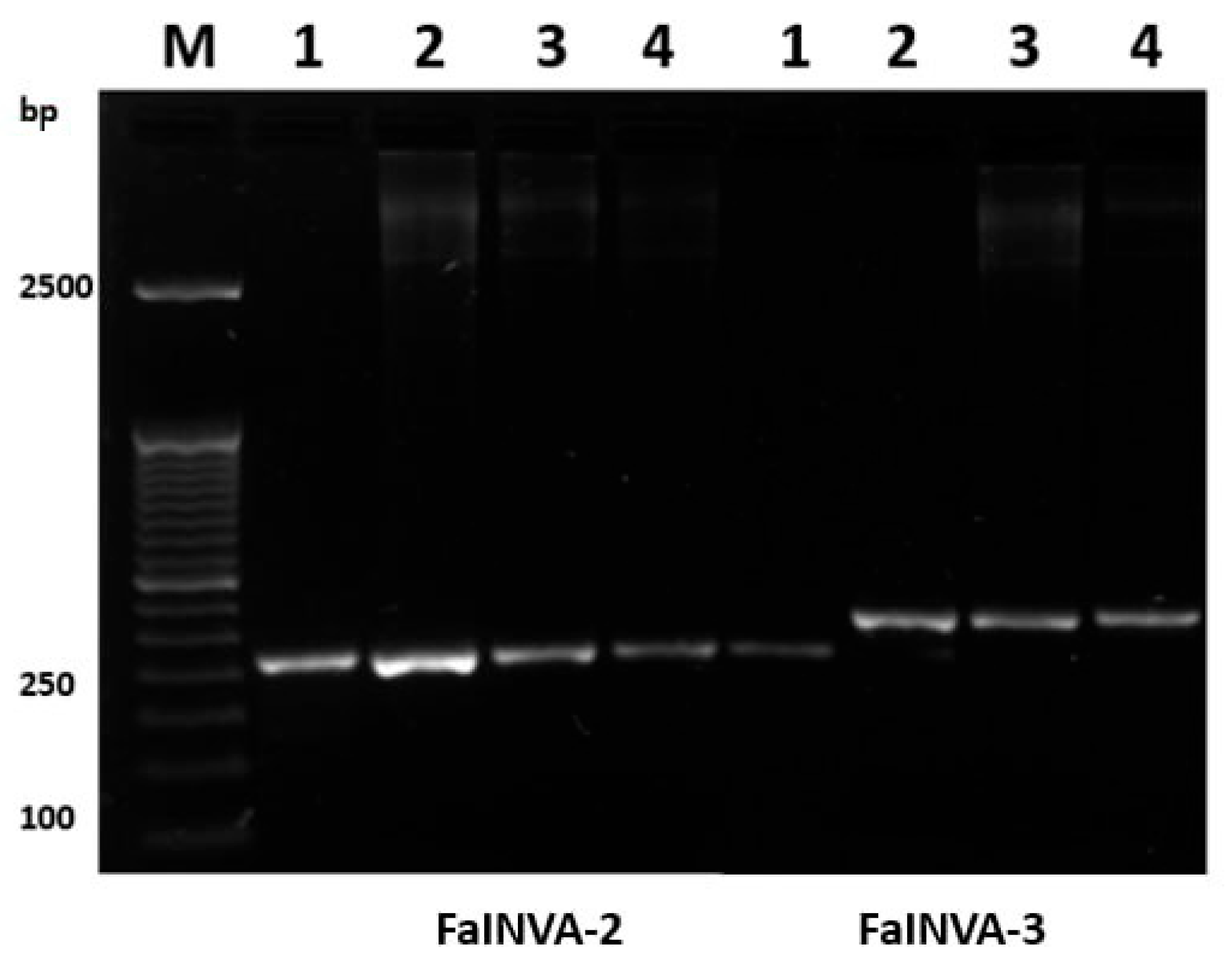
2.3. Genomic DNA Extraction and PCR Amplification
2.4. Marker Informativeness and Polymorphism Assessment
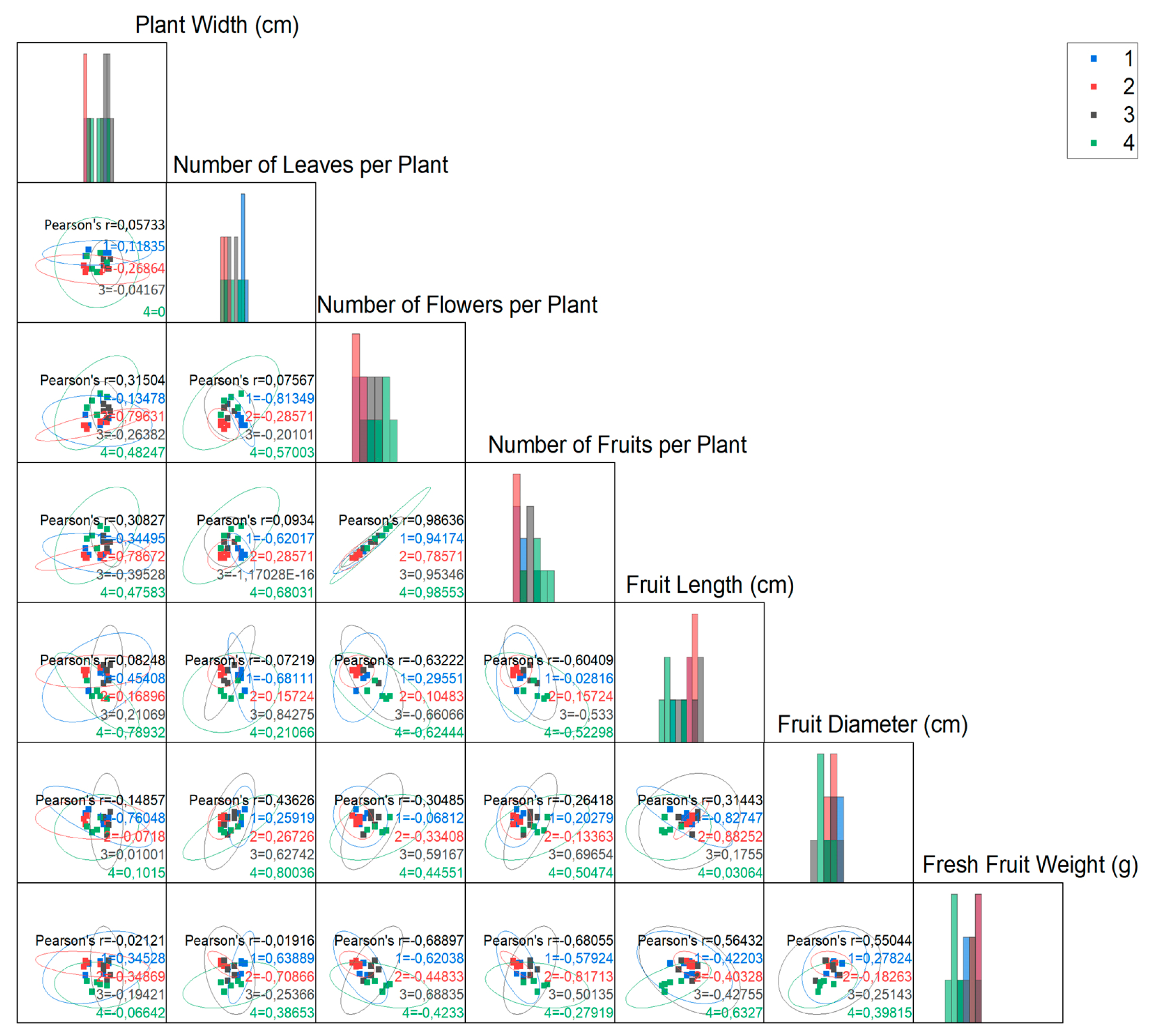
2.5. Statistical Analysis
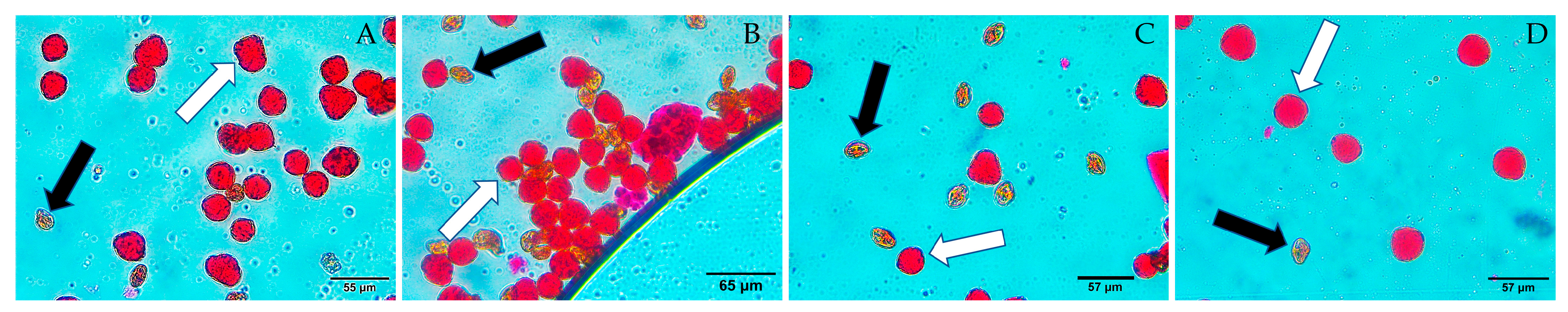
2.6. Pollen Fertility Analysis
2.7. Intercrossing of Strawberry Cultivars and Pollen Fertility
3. Results
3.1. Morphological Description
3.2. Statistical Evaluation of Results
3.3. Molecular Analysis Results
3.4. AMOVA Analysis
3.5. Pollen Viability Assessment and Reproductive Potential
3.6. Intercrossing of Strawberry Cultivars
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, P.; Li, H.-Q.; Li, X.-Y.; Zhou, X.-H.; Zhang, X.-X.; Zhang, A.-S.; Liu, Q.-Z. Transcriptomic analysis provides insight into defensive strategies in response to continuous cropping in strawberry (Fragaria × ananassa duch.) plants. BMC Plant Biol. 2022, 22, 476. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.R.; Araújo, F.H.V.; Ferreira, S.R.; dos Santos, J.C.B.; de Abreu, C.M.; Siqueira da Silva, R.; Regina da Costa, M. Strawberries in a warming world: Examining the ecological niche of Fragaria × ananassa Duch across different climate scenarios. J. Berry Res. 2024, 14, 193–208. [Google Scholar] [CrossRef]
- Sabahat, S.; Abbasi, J.; Ahmad, M.; Mumtaz, S.; Khan, T.N.; Tariq, S.; Imran, M. Role of micronutrients in improving fruit quality and yield of strawberry cv. Chandler under microclimatic conditions. Pak. J. Agric. Res. 2022, 34, 897–904. [Google Scholar] [CrossRef]
- Edger, P.P.; Poorten, T.J.; VanBuren, R.; Hardigan, M.A.; Colle, M.; McKain, M.R.; Smith, R.D.; Teresi, S.J.; Nelson, A.D.L.; Wai, C.M.; et al. Origin and evolution of the octoploid strawberry genome. Nat. Genet. 2019, 51, 541–547. [Google Scholar] [CrossRef]
- Staudt, G. Taxonomic studies in the genus Fragaria. Typification of Fragaria species known at the time of Linnaeus. Canad. J. Bot. 1962, 40, 869–886. [Google Scholar] [CrossRef]
- Liston, A.; Cronn, R.; Ashman, T.L. Fragaria: A genus with deep historical roots and ripe for evolutionary and ecological insights. Am. J. Bot. 2014, 101, 1686–1699. [Google Scholar] [CrossRef]
- Staudt, G. The species of Fragaria, their taxonomy and geographical distribution. Acta Hortic. 1989, 265, 23–33. [Google Scholar] [CrossRef]
- Staudt, G. Systematics and Geographic Distribution of the American Strawberry Species: Taxonomic Studies in the Genus Fragaria; University of California Press: Oakland, CA, USA, 1999; Volume 81. [Google Scholar]
- Naruhashi, N.; Iwata, T. Taxonomic Reevaluation of Fragaria nipponica Makino and Allied Species. J. Phytogeogr. Taxon. 1988, 36, 59–64. [Google Scholar] [CrossRef]
- Song, Y.; Peng, Y.; Liu, L.; Li, G.; Zhao, X.; Wang, X.; Cao, S.; Muyle, A.; Zhou, Y.; Zhou, H. Phased gap-free genome assembly of octoploid cultivated strawberry illustrates the genetic and epigenetic divergence among subgenomes. Hortic. Res. 2024, 11, uhad252. [Google Scholar] [CrossRef]
- Safiullina, A.K.; Ernazarova, D.K.; Turaev, O.S.; Rafieva, F.U.; Ernazarova, Z.A.; Arslanova, S.K.; Toshpulatov, A.K.; Oripova, B.B.; Kudratova, M.K.; Khalikov, K.K.; et al. Genetic Diversity and Subspecific Races of Upland Cotton (Gossypium hirsutum L.). Genes 2024, 15, 1533. [Google Scholar] [CrossRef] [PubMed]
- Khidirov, M.T.; Ernazarova, D.K.; Rafieva, F.U.; Ernazarova, Z.A.; Toshpulatov, A.K.; Umarov, R.F.; Kholova, M.D.; Oripova, B.B.; Kudratova, M.K.; Gapparov, B.M.; et al. Genomic and Cytogenetic Analysis of Synthetic Polyploids between Diploid and Tetraploid Cotton (Gossypium) Species. Plants 2023, 12, 4184. [Google Scholar] [CrossRef] [PubMed]
- Arslanova, S.K.; Ernazarova, Z.A.; Ernazarova, D.K.; Turaev, O.S.; Safiullina, A.K.; Toshpulatov, A.K.; Kholova, M.D.; Azimova, L.A.; Rafiyeva, F.U.; Gapparov, B.M.; et al. Development and Characterization of Synthetic Allotetraploids Between Diploid Species Gossypium herbaceum and Gossypium nelsonii for Cotton Genetic Improvement. Plants 2025, 14, 1620. [Google Scholar] [CrossRef]
- Han, H.; Barbey, C.R.; Fan, Z.; Verma, S.; Whitaker, V.M.; Lee, S. Telomere-to-telomere and haplotype-phased genome assemblies of the heterozygous octoploid “Florida Brilliance” strawberry (Fragaria × ananassa). GigaScience 2025, 14, giaf005. [Google Scholar] [CrossRef] [PubMed]
- Hardigan, M.A.; Lorant, A.; Pincot, D.D.; Feldmann, M.J.; Famula, R.A.; Acharya, C.B.; Lee, S.; Verma, S.; Whitaker, V.M.; Bassil, N.; et al. Unraveling the complex hybrid ancestry and domestication history of cultivated strawberry. Mol. Biol. Evol. 2021, 38, 2285–2305. [Google Scholar] [CrossRef]
- Oosumi, T.; Gruszewski, H.A.; Blischak, L.A.; Baxter, A.J.; Wadl, P.A.; Shuman, J.L.; Veilleux, R.E.; Shulaev, V. High-efficiency transformation of the diploid strawberry (Fragaria vesca) for functional genomics. Planta 2006, 223, 1219–1230. [Google Scholar] [CrossRef]
- Shulaev, V.; Sargent, D.; Crowhurst, R. The genome of woodland strawberry (Fragaria vesca). Nat. Genet. 2011, 43, 109–116. [Google Scholar] [CrossRef]
- Costa, R.C.d.; Calvete, E.O.; Spengler, N.C.L.; Chiomento, J.L.T.; Trentin, N.S.; de Paula, J.E.C. Morpho-phenological and agronomic performance of strawberry cultivars with different photoperiodic flowering responses. Acta Sci. Agron. 2020, 43, e45189. [Google Scholar] [CrossRef]
- Urrutia, M.; Meco, V.; Rambla, J.L.; Martín-Pizarro, C.; Pillet, J.; Andrés, J.; Posé, D. Diversity of the volatilome and the fruit size and shape in European woodland strawberry (Fragaria vesca). Plant J. 2023, 116, 1201–1217. [Google Scholar] [CrossRef]
- Sánchez-Sevilla, J.F.; Horvath, A.; Botella, M.A.; Gaston, A.; Folta, K.M.; Kilian, A.; Amaya, I. Diversity arrays technology (DArT) marker platforms for diversity analysis and linkage mapping in a complex crop, the octoploid cultivated strawberry (Fragaria × ananassa). PLoS ONE 2015, 10, e0144960. [Google Scholar] [CrossRef] [PubMed]
- Bradford, E.; Hancock, J.F.; Warner, R.M. Interactions of temperature and photoperiod determine expression of repeat flowering in strawberry. J. Am. Soc. Hortic. Sci. 2010, 135, 102–107. [Google Scholar] [CrossRef]
- Knee, M. Fruit Quality and Its Biological Basis (Sheffield Biological Siences); Blackwell: Sheffield, UK, 2001. [Google Scholar]
- Prohaska, A.; Rey-Serra, P.; Petit, J.; Petit, A.; Perrotte, J.; Rothan, C.; Denoyes-Rothan, B. Exploration of a European-centered strawberry diversity panel provides markers and candidate genes for the control of fruit quality traits. Hortic. Res. 2024, 11, uhae137. [Google Scholar] [CrossRef] [PubMed]
- Paterson, A.H.; Brubaker, C.; Wendel, J.F. A rapid method for extraction of cotton (Gossypium spp.) genomic DNA suitable for RFLP or PCR analysis. Plant Mol. Biol. Rep. 1993, 11, 122–127. [Google Scholar] [CrossRef]
- Liu, K.; Muse, S.V. PowerMarker: An integrated analysis environment for genetic marker analysis. Bioinformatics 2005, 21, 2128–2129. [Google Scholar] [CrossRef] [PubMed]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar] [PubMed Central]
- Nei, M. Analysis of gene diversity in subdivided populations. Proc. Natl. Acad. Sci. USA 1973, 70, 3321–3323. [Google Scholar] [CrossRef]
- Maxwell, S.E.; Delaney, H.D. Designing Experiments and Analyzing Data: A Model Comparison Perspective, 3rd ed.; Routledge: New York, NY, USA, 2003. [Google Scholar]
- Stevenson, K.J. Review of OriginPro 8.5. J. Am. Chem. Soc. 2011, 133, 5621. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Gao, Q.; Luo, H.; Li, Y.; Liu, Z.; Kang, C. Genetic modulation of RAP alters fruit coloration in both wild and cultivated strawberry. Plant Biotechnol. J. 2020, 18, 1550–1561. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.-B.; Noh, Y.-H.; Min, S.R.; Lee, H.-S.; Jung, J.; Park, K.-H.; Kim, D.-S.; Nam, M.H.; Kim, T.I.; et al. Sugar content and expression of sugar metabolism-related genes in strawberry fruits from various cultivars. J. Plant Biotechnol. 2018, 45, 90–101. [Google Scholar] [CrossRef]
- Giovanetti, G.; Marcellini, M.; Pergolotti, V.; Mecozzi, F.; Mezzetti, B.; Capocasa, F.; Sabbadini, S. Standardization of an effective scarification and germination protocol for strawberry seeds that is useful for gamic propagation. Horticulturae 2024, 10, 1345. [Google Scholar] [CrossRef]
- Lata, S.; Sharma, G.; Garg, S.; Mishra, G. Pollen viability, germination and stigma receptivity studies in different strawberry cultivars. Agric. Res. J. 2018, 55, 627–632. [Google Scholar] [CrossRef]
- Zingaretti, L.; Monfort, A.; Pérez-Enciso, M. Automatic fruit morphology phenome and genetic analysis: An application in the octoploid strawberry. Plant Phenomics 2021, 2021, 9812910. [Google Scholar] [CrossRef]
- Resende, J.V.; Gabriel, A.; Moreira, A.F.; Gonçalves, P.; Resende, A.; de Goes, C.D.M.; Zanin, D.S. Application of mixed models in the study of the adaptability and stability of short-day and neutral-day strawberry cultivars. Res. Soc. Dev. 2020, 9, e110953104. [Google Scholar] [CrossRef]
- Nascimento, D.A.; Gomes, G.C.; de Oliveira, L.V.B.; de Paula Gomes, G.F.; Ivamoto-Suzuki, S.T.; Ziest, A.R.; Mariguele, K.H.; Roberto, S.R.; de Resende, J.T.V. Adaptability and Stability Analyses of Improved Strawberry Genotypes for Tropical Climate. Horticulturae 2023, 9, 643. [Google Scholar] [CrossRef]
- Żebrowska, J. The viability and storage of strawberry pollen. Plant Breed. 1995, 114, 469–470. [Google Scholar] [CrossRef]
- Mishra, P.K.; Ram, R.B.; Kumar, N. Genetic variability, heritability, and genetic advance in strawberry (Fragaria × ananassa Duch.). Turk. J. Agric. For. 2012, 39, 451–458. [Google Scholar] [CrossRef]
- Galleta, G.J.; Maas, J.L. Strawberry genetics. Hort Sci. 1990, 25, 871–878. [Google Scholar] [CrossRef]
- Keniry, A.; Hopkins, C.L.; Jewell, E.; Morrison, B.; Spangenberg, G.C.; Edwards, D.; Batley, J. Identification and characterization of simple sequence repeat (SSR) markers from Fragaria × ananassa expressed sequence. Mol. Ecol. Notes 2005, 6, 319–322. [Google Scholar] [CrossRef]
- Bassil, N.V.; Gunn, M.; Folta, K.; Lewers, K. Microsatellite markers for Fragaria from ‘Strawberry Festival’ expressed sequence tags. Mol. Ecol. Notes 2006, 6, 473–476. [Google Scholar] [CrossRef]
- Ashley, M.V.; Wilk, J.A.; Styan, S.M.N.; Craft, K.J.; Jones, K.L.; Fedkheim, K.A.; Lewers, K.S.; Ashman, T.L. High variability and disomic segregation of microsatellites in octoploid Fragaria virginiana Mill. (Rosaceae). Theor. Appl. Genet. 2003, 107, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Njuguna, W.; Hummer, K.E.; Richards, C.M.; Davis, T.M.; Bassil, N.V. Genetic diversity of diploid Japanese strawberry species based on microsatellite markers. Genet. Resour. Crop Evol. 2011, 58, 1187–1198. [Google Scholar] [CrossRef]
- Chen, L.-X.; Xu, S.-T.; Ding, W.-H.; Li, J.-M.; Alpert, P.; Zhang, D.-Y. Genetic diversity and offspring fitness in the red and white fruit color morphs of the wild strawberry Fragaria pentaphylla. J. Plant Ecol. 2020, 13, 36–41. [Google Scholar] [CrossRef]
- Biswas, A.; Melmaiee, K.; Elavarthi, S.; Jones, J.; Reddy, U. Characterization of strawberry (Fragaria spp.) accessions by genotyping with SSR markers and phenotyping by leaf antioxidant and trichome analysis. Sci. Hortic. 2019, 256, 108561. [Google Scholar] [CrossRef]
- Peng, M.; Zong, X.; Wang, C.; Meng, F. Genetic diversity of strawberry (Fragaria × ananassa Duch.) from the Motuo County of the Tibet Plateau determined by AFLP markers. Biotechnol. Equip. 2015, 29, 876–881. [Google Scholar] [CrossRef]
- Gil-Ariza, D.; Amaya, I.; Lopez Aranda, J.M.; Sánchez-Sevilla, J.F.; Botella, M.; Valpuesta, V. Impact of plant breeding on the genetic diversity of cultivated strawberry as revealed by expressed sequence tag-derived simple sequence repeat markers. J. Am. Soc. Hortic. Sci. 2009, 134, 337–347. [Google Scholar] [CrossRef]
- Carrasco, B.; Garces, M.; Rojas, P.; Saud, G.; Herrera, R.; Retamales, J.B.; Caligari, P.D.S. The Chilean strawberry [Fragaria chiloensis (L.) Duch.]: Genetic diversity and structure. J. Am. Soc. Hortic. Sci. 2007, 132, 501–506. [Google Scholar] [CrossRef]
- Milutinović, M.; Nikolić, D.; Fotirić, M.; Rakonjac, V. The relation between pollen functional ability and fruit set in grapevine (Vitis sp.). Genetika 2000, 32, 81–87. [Google Scholar]
- Gupta, A.K.; Singh, M.; Marboh, E.S.; Nath, V.; Verma, J.P. Pollen production, viability and in vitro pollen germination of different litchi (Litchi chinensis) genotypes. Indian J. Agric. Sci. 2018, 88, 884–888. [Google Scholar] [CrossRef]
- Martín-Pizarro, C.; Vallarino, J.G.; Osorio, S.; Meco, V.; Urrutia, M.; Pillet, J.; Casanal, A.; Merchante, C.; Amaya, I.; Willmitzer, L.; et al. The NAC transcription factor FaRIF controls fruit ripening in strawberry. Plant Cell 2021, 33, 1574–1593. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Liu, D.J.; Jiang, Y.M.; Zhao, M.L.; Shan, W.; Kuang, J.F.; Lu, W.J. Molecular Characterization of a Strawberry FaASR Gene in Relation to Fruit Ripening. PLoS ONE 2011, 6, e24649. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Cai, W.; Chen, X.; Pang, F.; Wang, J.; Zhao, M. Heterozygous frameshift mutation in FaMYB10 is responsible for the natural formation of red and white-fleshed strawberry (Fragaria x ananassa Duch). Front. Plant Sci. 2022, 13, 1027567. [Google Scholar] [CrossRef]


| № | Research Samples | Country of Origin | Biological Characteristics |
|---|---|---|---|
| 1 | Seolhyang | Republic of Korea | High yield |
| 2 | Cinderella | Republic of Korea | Aesthetically attractive, pineapple-flavored, white-colored, and sweet |
| 3 | Alba | Italy | Relatively large fruit |
| 4 | Vitaberry | Republic of Korea | Sour-tasting fruit |
| No. | Samples | Minimum | Maximum | SD | V | |
|---|---|---|---|---|---|---|
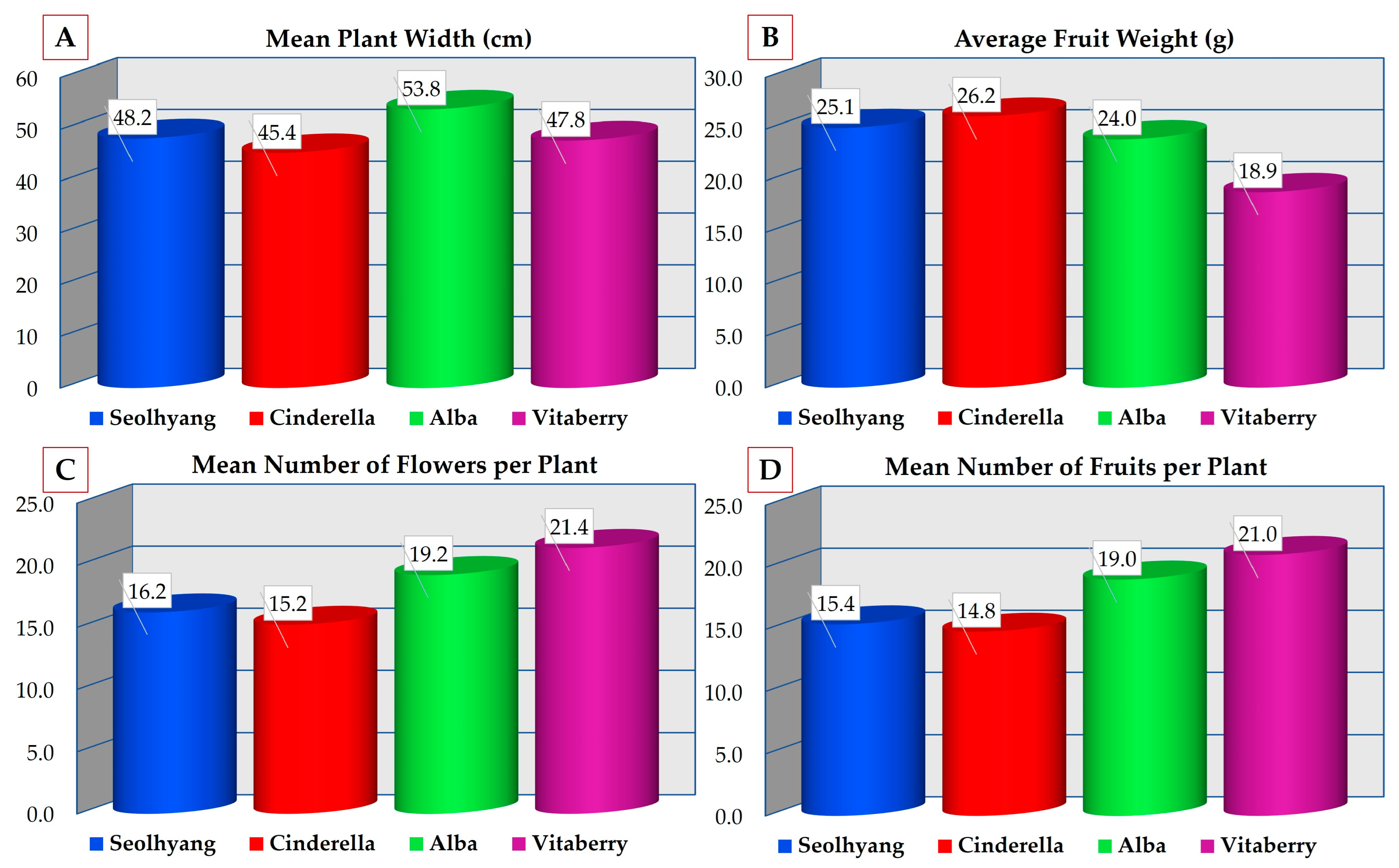
| Plant Width (cm) | ||||||
| 1. | Seolhyang | 48.2 ± 2.67 | 41 | 55 | 5.97 | 12.3% |
| 2. | Cinderella | 45.4 ± 2.79 | 41 | 54 | 6.22 | 13.7% |
| 3. | Alba | 53.8 ± 0.80 | 52 | 56 | 1.78 | 3.32% |
| 4. | Vitaberry | 47.8 ± 2.06 | 42 | 54 | 4.60 | 9.63% |
| Number of Leaves per Plant | ||||||
| 1. | Seolhyang | 17.0 ± 0.31 | 16 | 18 | 0.70 | 4.15% |
| 2. | Cinderella | 11.8 ± 0.37 | 11 | 13 | 0.83 | 7.0% |
| 3. | Alba | 13.6 ± 0.60 | 12 | 15 | 1.34 | 9.86% |
| 4. | Vitaberry | 14 ± 1.14 | 11 | 17 | 2.54 | 18.2% |
| Number of Flowers per Plant | ||||||
| 1. | Seolhyang | 16.2 ± 0.58 | 15 | 18 | 1.30 | 8.04% |
| 2. | Cinderella | 15.2 ± 0.37 | 14 | 16 | 0.83 | 5.50% |
| 3. | Alba | 19.2 ± 0.66 | 17 | 21 | 1.48 | 7.72% |
| 4. | Vitaberry | 21.4 ± 1.07 | 18 | 24 | 2.40 | 11.2% |
| Number of Fruits per Plant | ||||||
| 1. | Seolhyang | 15.4 ± 0.51 | 14 | 17 | 1.14 | 7.40% |
| 2. | Cinderella | 14.8 ± 0.37 | 14 | 16 | 0.83 | 5.65% |
| 3. | Alba | 19 ± 0.63 | 17 | 21 | 1.41 | 7.44% |
| 4. | Vitaberry | 21 ± 1.22 | 17 | 24 | 2.73 | 13.0% |
| Fruit Length (cm) | ||||||
| 1. | Seolhyang | 4.32 ± 0.14 | 3.8 | 4.6 | 0.31 | 7.32% |
| 2. | Cinderella | 4.56 ± 0.07 | 4.5 | 4.7 | 0.15 | 3.46% |
| 3. | Alba | 4.5 ± 0.14 | 4.1 | 4.8 | 0.31 | 7.02% |
| 4. | Vitaberry | 3.74 ± 0.12 | 3.5 | 4.2 | 0.27 | 7.32% |
| Fruit Diameter (cm) | ||||||
| 1. | Seolhyang | 3.12 ± 0.07 | 2.87 | 3.35 | 0.15 | 5.06% |
| 2. | Cinderella | 3.02 ± 0.07 | 2.87 | 3.15 | 0.15 | 5.23% |
| 3. | Alba | 2.97 ± 0.12 | 2.55 | 3.27 | 0.27 | 9.22% |
| 4. | Vitaberry | 2.78 ± 0.07 | 2.62 | 3.1 | 0.15 | 5.68% |
| Fresh Fruit Weight (g) | ||||||
| 1. | Seolhyang | 25.1 ± 1.04 | 22.3 | 27.2 | 2.32 | 9.25% |
| 2. | Cinderella | 26.2 ± 0.36 | 25.3 | 27.3 | 0.82 | 3.13% |
| 3. | Alba | 24.0 ± 1.26 | 20.4 | 27.6 | 2.81 | 11.7% |
| 4. | Vitaberry | 18.9 ± 0.66 | 16.5 | 20.3 | 1.47 | 7.80% |
| No. | Source | Df | Sum of Squares | Mean Squares | Estimated Variation | Total Variation (%) |
|---|---|---|---|---|---|---|
| 1. | Among cultivars | 2 | 21.438 | 10.719 | 0.339 | 3% |
| 3. | Within cultivars | 8 | 75.500 | 9.438 | 9.438 | 97% |
| Total | 10 | 96.938 | 20.157 | 9.777 | 100% |
| No. | Research Samples | Number of Analyzed Pollens | Pollen Fertility, % | |||
|---|---|---|---|---|---|---|
| Range | S | V | ||||
| 1 | Seolhyang | 1301 | 78.55 ± 1.23 | 74.71–93.33 | 5.03 | 6.40 |
| 2 | Cinderella | 1501 | 86.88 ± 2.48 | 67.31–96.91 | 2.48 | 12.77 |
| 3 | Alba | 931 | 79.81 ± 1.18 | 76.77–83.16 | 5.13 | 6.43 |
| 4 | Vitaberry | 1497 | 77.09 ± 0.67 | 72.83–83.09 | 2.99 | 3.89 |
| No. | Research Samples | Number of Crosses | Number of Obtained Hybrid Fruits | Crossing Rate, % |
|---|---|---|---|---|
| 1. | Seolhyang × Cinderella | 23 | 22 | 95.6 |
| 2. | Cinderella × Seolhyang | 25 | 25 | 100.0 |
| 3. | Seolhyang × Vitaberry | 22 | 20 | 90.9 |
| 4. | Vitaberry × Seolhyang | 24 | 21 | 87.5 |
| 5. | Seolhyang × Alba | 24 | 21 | 87.5 |
| 6. | Alba × Seolhyang | 24 | 20 | 83.3 |
| 7. | Cinderella × Alba | 23 | 20 | 86.9 |
| 8. | Alba × Cinderella | 24 | 22 | 91.6 |
| 9. | Cinderella × Vitaberry | 27 | 24 | 88.8 |
| 10. | Vitaberry × Cinderella | 26 | 23 | 88.4 |
| 11. | Alba × Vitaberry | 23 | 20 | 86.9 |
| 12. | Vitaberry × Alba | 25 | 21 | 84.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ernazarova, D.K.; Safiullina, A.K.; Kholova, M.D.; Azimova, L.A.; Hasanova, S.A.; Nematullaeva, E.F.; Rafieva, F.U.; Akhmedova, N.S.; Khursandova, M.S.; Turaev, O.S.; et al. Morphological and Molecular Insights into Genetic Variability and Heritability in Four Strawberry (Fragaria × ananassa) Cultivars. Horticulturae 2025, 11, 1195. https://doi.org/10.3390/horticulturae11101195
Ernazarova DK, Safiullina AK, Kholova MD, Azimova LA, Hasanova SA, Nematullaeva EF, Rafieva FU, Akhmedova NS, Khursandova MS, Turaev OS, et al. Morphological and Molecular Insights into Genetic Variability and Heritability in Four Strawberry (Fragaria × ananassa) Cultivars. Horticulturae. 2025; 11(10):1195. https://doi.org/10.3390/horticulturae11101195
Chicago/Turabian StyleErnazarova, Dilrabo K., Asiya K. Safiullina, Madina D. Kholova, Laylo A. Azimova, Shalola A. Hasanova, Ezozakhon F. Nematullaeva, Feruza U. Rafieva, Navbakhor S. Akhmedova, Mokhichekhra Sh. Khursandova, Ozod S. Turaev, and et al. 2025. "Morphological and Molecular Insights into Genetic Variability and Heritability in Four Strawberry (Fragaria × ananassa) Cultivars" Horticulturae 11, no. 10: 1195. https://doi.org/10.3390/horticulturae11101195
APA StyleErnazarova, D. K., Safiullina, A. K., Kholova, M. D., Azimova, L. A., Hasanova, S. A., Nematullaeva, E. F., Rafieva, F. U., Akhmedova, N. S., Khursandova, M. S., Turaev, O. S., Oripova, B. B., Kudratova, M. K., Doshmuratova, A. A., Kubeisinova, P. A., Rakhimova, N. M., Erjigitov, D. S., Komilov, D. J., Ruziyev, F. A., Khamraev, N. U., ... Kushanov, F. N. (2025). Morphological and Molecular Insights into Genetic Variability and Heritability in Four Strawberry (Fragaria × ananassa) Cultivars. Horticulturae, 11(10), 1195. https://doi.org/10.3390/horticulturae11101195











