Abstract
In the context of increasing demand for sustainable floriculture, this study evaluated the effects of salicylic acid (SA) on phenotypic traits of poinsettia (Euphorbia pulcherrima Willd.). A factorial experiment was conducted in a commercial glasshouse using rooted poinsettia cuttings treated with three SA concentrations (10−3, 10−4, 10−5 M) applied via foliar or root application. Morphological parameters, colorimetric traits (CIELAB), canopy development, and biomass accumulation were assessed throughout the cultivation cycle. SA had no significant influence on the plant height, leaf number, or biomass of stems, leaves, and roots. However, notable phenotypic changes were observed. Foliar applications, particularly at 10−5 M, induced visible changes in leaf and bract color, including reduced brightness, saturation, and red pigmentation, especially in newly developed tissues. Conversely, root applications had milder effects and were generally associated with a more stable bract color. The 10−4 M root treatment promoted greater bract surface and color saturation. Canopy expansion and dry matter accumulation were also influenced by SA in a dose- and method-dependent manner, with high-dose foliar treatments (10−3 M) exerting suppressive effects. These findings suggest that the application mode and concentration of SA are critical in modulating ornamental quality traits, with low-to-moderate doses—particularly via root application—offering promising strategies to enhance plant performance in sustainable poinsettia cultivation.
1. Introduction
Floriculture is a specialized agricultural sector that produces high-value ornamental products for a market that demands exceptional aesthetic quality and perfection. To support the transition toward sustainability, it is crucial to identify scientifically based cultivation techniques that maintain product quality while reducing environmental impact. Ornamental plant growers are often compelled to use chemical inputs to produce high-value products that enable adequate incomes [1]. However, there is an urgent need for an ecological transition that involves every productive sector. Sustainable and environmentally friendly production systems are widely studied in agriculture. Within this sector, floriculture is the production system probably least engaged in these studies and most challenging to convert into sustainable cultivation systems [2]. Studies have shown that consumers are more likely to purchase plants labeled as non-invasive, native, or packaged in biodegradable or low-carbon materials [3]. Environmentally friendly products have an intangible added value related to their quality and respect for the environment. Sustainable production is a real opportunity for flower growers who aim to diversify the quality level and expand product types. Demand for organic and sustainable flower products is increasing and as early as 2005, the ‘organic’ flower market was the fastest-growing sector in the organic non-food market in the United States [4]. Sustainable flower production aims to reduce environmental degradation [5], maintain productivity, promote economic viability, conserve resources and energy, and maintain stable communities and quality of life [1]. Sustainable practices include recycling irrigation water and plastics, implementing biological control, using alternative energy sources [6], and reducing chemical inputs. Barriers that limit the spread of sustainable floriculture include the cost of the technology, the lack of economic incentives, and also the age, education level, risk perception, and lack of knowledge of floriculturists [7,8,9,10,11], which depends on inadequate basic research. In this crucial phase of environmental transition, elicitors and plant growth regulators can be helpful [12]. Elicitors are compounds of natural or synthetic origin capable of activating plant defenses, representing an innovative strategy to reduce the use of chemical inputs in floriculture [13]. Their application in floriculture can improve resistance to biotic and abiotic stresses, enhance flower and bract color, extend flowering duration, and increase compactness—all characteristics that contribute to commercial value [14,15]. Plant growth regulators (PGRs) also play a significant role in the propagation of cuttings and seeds, in vitro propagation, and in vitro rooting of ornamental and foliage plants [16]. PGRs are also involved in prolonging flower life, the vase life of cut flowers, promoting plant growth, and regulating flowering [15], breaking dormancy in seeds, bulbs, corms, and tubers of flowering plants, enhancing apical dominance, lateral branching, controlling plant height, and delaying flowering [16,17]. Salicylic acid (SA) is among the most studied elicitors [18]. SA is a phenolic compound, synthesized from t-cinnamic acid, widely present in plants. SA is a multifunctional molecule that plays roles as both an elicitor and a growth promoter in plants. As an elicitor, it is involved in the activation of natural defenses in the plants [19]. When a plant is exposed to biotic stresses, such as pathogen attacks, SA accumulated in the plant tissues induces the production of pathogenesis-related proteins and other defensive compounds, strengthening plant resistance [20]. Therefore, it is also used to improve stress resistance of ornamental plants such as Impatiens walleriana and chrysanthemums [21,22]. African marigold plants treated with up to 10−3 M salicylic acid can elicit the production of natural defenses, improving resistance to fungal diseases even without an actual infection or direct stress, thus helping to enhance production quality [23]. As a growth regulator, it controls physiological processes influencing plant growth and development [24] by accelerating cell divisions; plays an essential role in photosynthesis, transpiration, ion uptake and transport; and also modifies the anatomy of leaves and chloroplasts [25]. Moreover, SA stimulates the enlargement of calyx and corolla whorls in flowers, and influences flowering, seed germination, post-harvest duration of cut flowers, leaf senescence, and the quality of cut flower stems and bedding plants [26,27].
Poinsettia (Euphorbia pulcherrima Willd.) stands as one of the most economically significant ornamental crops worldwide, attracting exceptional market attention during the winter holiday season. In the United States of America, approximately 70 million plants are sold annually within a six-week period, generating nearly $200–250 million in retail value [28]. Its vivid bract colors—predominantly red, but also encompassing pink, white, and bicolored varieties—establish it as an iconic symbol of Christmas, significantly driving demand among both consumers and retailers [29]. With over 100 cultivars readily available and a consistent presence across major wholesale and retail channels, poinsettia remains a cornerstone of floriculture, particularly in December, when consumer preference and holiday aesthetics converge.
To successfully integrate salicylic acid into commercial floriculture, it is essential to determine the optimal concentrations and application methods that enhance visual quality without compromising plant growth. Consequently, this study aimed to evaluate how different concentrations and application methods (foliar vs. root) of salicylic acid influence key phenotypic and ornamental traits in poinsettia (Euphorbia pulcherrima). These traits included bract color, canopy development, and biomass accumulation. The goal was to identify treatment combinations that both improve visual quality and support more sustainable, low-input cultivation practices.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
The research was conducted in 2023 in a glass greenhouse of a commercial nursery (‘Conca d’Oro’ nursery, Palermo, Italy) (1000 m2—position 38°10′05″ N 13°40′03″ E; altitude 50 m above sea level). Rooted cuttings of poinsettia (Euphorbia pulcherrima cv Futura Brillant Red) were transplanted in the first week of October into 12 cm diameter plastic pots (volume 1046.15 cm3) filled with a commercial substrate (Utilis, GreenView srl, Crocetta del Montello, Italy) composed of a mixture of decomposed peat (pH 6.0—electrical conductivity EC 0.20 dS m−1; containing 850 g m−3 of a mineral fertilizer NPK 12-11-18). A factorial experimental design was adopted, where the two main factors were salicylic acid concentration (SA, four doses: 0 control, 10−3 M, 10−4 M, 10−5 M) and application method (application: F foliar and R root). These concentrations were selected based on previous studies on ornamental and vegetable species where SA showed physiological or morphological effect [30,31]. The experimental design included eight treatment combinations with four replicates of 10 pots for each treatment. Foliar and root control treatments were carried out with distilled water. The plants received two applications of salicylic acid solution for both application methods: the first on October 8th, immediately after transplanting into pots, and the second 10 days later. Irrigation and fertilization were applied uniformly using a micro-flow system. Each pot was equipped with one drip emitter (2 L h−1). Plants were fertilized once a week with the following nutrient solution: EC 1.6 dS m−1, pH 6, NH4 2.25 mmol L−1, K 3.5 mmol L−1, Ca 3.75 mmol L−1, Mg 1 mmol L−1, NO3 12.25 mmol L−1, SO4 1 mmol L−1, H2PO4 1 mmol L−1 [32]. During the treatment periods, all plants were temporarily disconnected from the daily irrigation system and irrigated via sub-irrigation. For the foliar treatment groups, sub-irrigation was performed using water only, while the root treatment groups were sub-irrigated with the corresponding salicylic acid solutions. Foliar applications of salicylic acid were carried out by spraying the leaves, and, to ensure uniform handling, the root-treated plants were sprayed with distilled water. The cultivation period, from transplanting to flowering, lasted 58 days. During the trial, air temperature and relative humidity in the greenhouse were monitored using a data logger (mod. 608-H1, Testo s.p.a., Settimo M.se, Italy). The average temperature was around 22 °C, with a maximum temperature of 28.0 °C and a minimum of 16.8 °C, while the relative humidity was, on average, 79 ± 1.5% and varied between 61.3% and 92.3%.
2.2. Phenotypic Traits Determination
During the test, leaf and bract color were determined with a colorimeter (Chroma Meter CR-400C, Minolta Corporation, Ltd., Osaka, Japan) on the upper surface of four randomly selected leaves and bracts for each plant. Colorimetric measurements were performed at four time points: before the treatment (8 October), 10 days after the first salicylic acid treatment (18 October), 10 days after the second treatment (28 October), and finally, during the pre-sale phase (early December) corresponding to the post-veraison bract stage. Measurements at the final time point included the most basal (oldest) and most apical (youngest) leaves and bracts on the main stem. The instrument was first standardized with a white ceramic tile (L* = 89.06, a* = −00.13, b* = 06.28). The CIELab parameters L*, a*, and b* were recorded. L* values indicate darkness and lightness (black: L* = 0; white: L* = 100), a* values range from green (−) to red (+), and b* values range from blue (−) to yellow (+). Chroma and hue angle (Hue°) were then calculated as Chroma = (a*2 + b*2)1/2, Hue° = 180° + arctan(b*/a*) for quadrant II (−a*, +b* for all leaves), and Hue° = arctan(b*/a*) for quadrant I (+a*, +b* for bract color) [33,34]. Chroma is a measure of saturation and describes the vividness, purity, or intensity of a color. Hue° refers to the type or shade of color expressed in degrees on a circular scale from 0° to 360° (0° red; 90° yellow; 180° green; 270° blue). To provide a more detailed analysis of the colorimetric variations induced by the treatments respect to the control, the individual component differences were also calculated as ΔL* = L* − L*0, Δa* = a* − a*0, Δb* = b* − b*0, ΔChroma = Chroma − Chroma0, ΔHue° = [ (Δa*)2 + (Δb*)2 − (ΔChroma)2]1/2, ΔE = [(L* − L*0)2 + (a* − a*0)2 + (b* − b*0)2]1/2, where L*0, a*0, b*0, and Chroma0 are the values of the control plants not treated with salicylic acid [35,36].
Growth was monitored from transplanting until the plants reached marketable size for the Christmas holiday season (early December), as determined by measuring plant height. Moreover, at the end of the trial, canopy width, the number of green leaves and red bracts per plant, the number of shoots, and stem diameter (1 cm below the first node) were also recorded. The total area covered by the canopy (plant canopy area) was measured for each plant by taking digital images 1 m above the plant using a digital camera (Canon EOS 300D, Canon Inc., Tokyo, Japan) that were then processed with the Easy Leaf Area software (software version 2.0) to calculate the area of canopy projected to the ground [37]. At the end of the trial, plants were subjected to destructive samplings: leaves, bracts, and stems were separated, and roots were gently washed to remove substrate. Each part was weighed, then oven-dried at 85 °C until constant weight and weighed again to calculate dry biomass. Before drying, the leaf and bract were scanned at 300 dpi (Epson Perfection 4180 Photo, Seiko Epson Corp., Suwa, Japan) to obtain digital images that were processed with the Easy Leaf Area software [37]. Specific leaf area (SLA cm2 g−1 DW) was calculated as leaf area/leaf dry weight; specific bract area was calculated similarly.
2.3. Statistical Analyses
The effects of salicylic acid levels and mode of application on morpho-physiological and qualitative parameters were determined by performing a two-way ANOVA. Significant differences between means and significant interactions between factors were identified by comparing mean values using the least significant difference (LSD) test (p ≤ 0.05).
Percentages were subjected to angular transformation before statistical analysis (Φ = arcsin (p/100)1/2).
3. Results
3.1. Colorimetric Parameters of Poinsettia Plants
The colorimetric parameters of the plants were monitored throughout the trial; before the first treatment (8 October); 10 days after the first treatment (18 October); 10 days after the second treatment (28 October); and at the end of the trial, after the veraison of the bracts (5 December). The colorimetric parameters of poinsettia green leaves in the pre-veraison phase are reported in Table 1. No significant difference was found until the second treatment, and only L* and b* showed changes due to the treatments after the second application. Foliar application of SA at 10−4 M and 10−3 M significantly increased leaf color brightness (L*), whereas a significantly darker color was found in the leaves of plants treated with the same doses but via root and supplementing a 10−5 M foliar treatment. (Figure 1). At the end of the pre-veraison stage, the foliar treatment determined a more yellowish leaf color, as indicated by the higher b* values recorded.

Table 1.
Effect of different levels of salicylic acid (0, 10−5, 10−4, and 10−3 M) and different application methods (root or foliar) on the colorimetric parameters of poinsettia leaves in the pre-veraison phase.
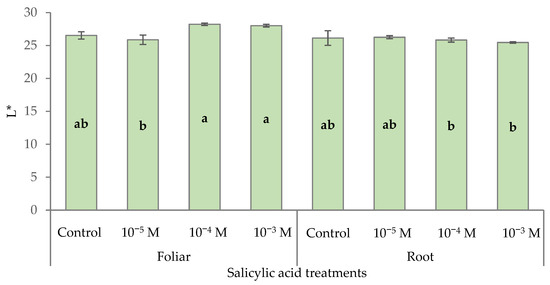
Figure 1.
Effect of different levels of salicylic acid (0, 10−5, 10−4, and 10−3 M) and different application methods (root or foliar) on the L* parameter of poinsettia green leaves 10 days after the second treatment (black bars represent standard errors of the means; bars with different letters are significantly different at p ≤ 0.05 according to the LSD test).
Table 2 reports the color differences using the CIELAB color space parameters between treated and control plants for both the most basal (oldest) and most apical (youngest) leaves on the main stem.

Table 2.
Differences in colorimetric parameters of green leaves at trial end between control plants (0 m SA; reference color) and salicylic acid-treated plants (10−5, 10−4, and 10−3 M SA) via root or foliar application.
In the CIELAB color space, positive ΔL* values indicate increased lightness compared to the reference color, while negative values indicate decreased lightness (darker color). Foliar application of SA consistently resulted in negative ΔL* values, indicating a darkening of leaves compared to the control, especially when using the highest SA dose (10−3 M) (Figure 2). Conversely, root application of SA at 10−4 M and 10−3 M led to the highest positive ΔL* values, signifying an increase in leaf lightness compared to the control. These findings emphasize that both the method and concentration of SA application significantly affect the lightness of the youngest leaves (ΔL*), a key factor for the visual quality of ornamental plants.
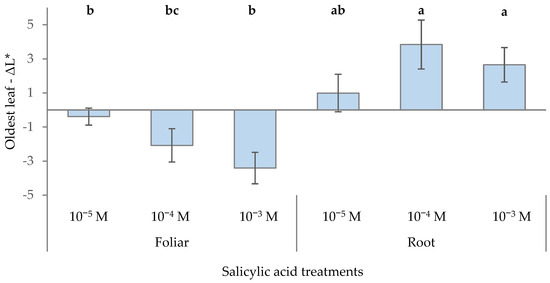
Figure 2.
Effect of different levels of salicylic acid (0, 10−5, 10−4, and 10−3 M) and different application methods (root or foliar) on ΔL* of the oldest green leaves of poinsettia plants at the end of the trial (black bars represent standard errors of the means; bars with different letters are significantly different at p ≤ 0.05 according to the LSD test).
A significant effect of the SA dose was observed in the red–green axis (Δa*) and yellow–blue axis (Δb*). The highest reduction in Δa∗ (averaging −2.5 for both foliar and root applications) and increase in Δb∗ (averaging +2.6) was recorded when applying 10−4 M SA, which corresponded to an increase in the green tone and yellowness of the oldest leaves. Conversely, applying 10−5 M SA resulted in an inverse trend, showing a slight but significant increase in redness (Δa∗ +0.7) and reduced yellowness (Δb∗ −0.4) (Table 3). The application of 10−4 M SA significantly increased color saturation of the basal leaves (+3.6 on average), while a modest reduction was observed when using 10−5 M SA (−0.7).

Table 3.
Differences in colorimetric parameters of red bracts at trial end between control plants (0 m SA; reference color) and salicylic acid-treated plants (10−5, 10−4, and 10−3 M SA) via root or foliar application.
The ΔL* values of the youngest leaves were mainly affected by the application method. Foliar application of SA resulted in the most significant color variation, leading to a notable reduction in lightness (−1.7), whereas root application caused a slight increase (+0.6). The ΔChroma decreased significantly with decreasing SA concentration, with the greatest reduction (–4.0) observed at 10−5 M compared to the control. The foliar application method significantly reduced ΔChroma (–4.0) more than the root application (–1.7).
The color difference (ΔE) value quantifies the overall difference in color between treated and control plants (the reference color). High ΔE values (whether positive or negative) indicate a greater deviation in color from the untreated control, while lower values suggest a more similar appearance. The ΔE of the youngest leaves was affected by both SA concentration and application method. ΔE increased with decreasing SA concentration, with the highest value observed at 10−5 M (5.2), showing a marked color change. As regards the application method, the color difference was higher in foliar treatments (4.8) than in the root (2.9). A ΔE value greater than 2.0–3.0 is generally noticeable to the human eye, so these changes were visibly detectable, particularly for the lowest SA concentration and foliar-based supply.
Table 3 presents the effects of the different SA treatments on the colorimetric parameters of the oldest and youngest red bract. The application of SA had limited effects in modifying the color of the oldest bracts, except for ΔL*, Δa*, and ΔChroma.
The application method played a role in modifying the red–green component and chroma. Root application led to significantly greater changes in the red–green axis (Δa* = −4.1) compared to the foliar method (Δa* = 0.8). Likewise, root application induced a more pronounced reduction in chroma (ΔChroma = −4.64) than foliar application (−0.3). ΔL* was influenced by the SA concentration as a function of the application method with an increase in lightness using 10−4 M SA via foliar application (+2.7) and 10−5 M SA via root application (+2.3), whereas the color of the oldest bracts became darker (−2.7) using 10−5 M SA via foliar spray (Figure 3). These findings highlighted that both SA concentration and application method significantly influenced the oldest bract lightness, and the same concentrations could either lighten or darken bracts depending on how they were applied.
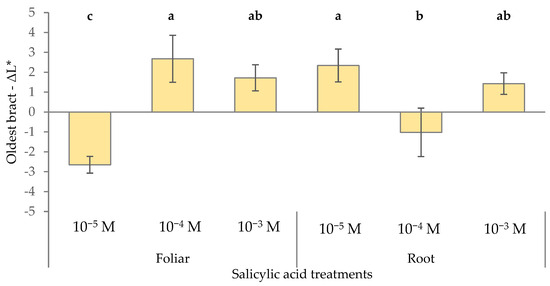
Figure 3.
Effect of different levels of salicylic acid (0, 10−5, 10−4, and 10−3 M) and different application methods (root or foliar) on ΔL* of the oldest red bracts of poinsettia plants at the end of the trial (black bars represent standard errors of the means; bars with different letters are significantly different at p ≤ 0.05 according to the LSD test).
The youngest bracts showed to be more affected by the SA treatments. The changes in lightness compared to the control were mainly due to the application method, with a darker bract color using root application (−5.7) and a lighter color using foliar application (+1.7).
The Δa* values were all negative, indicating a loss of redness compared to the control (Figure 4). The 10−5 M foliar treatment and the 10−3 M root treatment caused the most pronounced reduction in a* (–11.1 and −5.3, respectively). These substantial negative values suggested that these treatments significantly reduced red pigmentation. The 10−5 and 10−4 M root treatments and 10−3 SA foliar treatment showed only a mild loss of red pigmentation compared to the control (−1.9 on average). The results highlighted that the method and concentration of salicylic acid application significantly influenced the red color intensity of poinsettia bracts, with low-dose foliar and high-dose root applications having the most detrimental effect on red pigment retention.
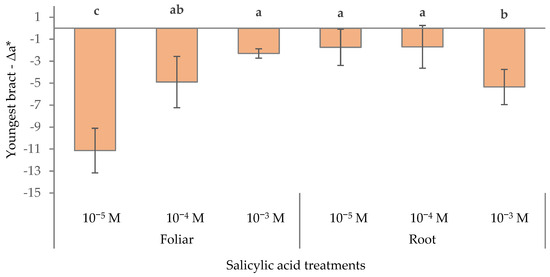
Figure 4.
Effect of different levels of salicylic acid (0, 10−5, 10−4, and 10−3 M) and different application methods (root or foliar) on Δa* of the youngest red bracts of poinsettia plants at the end of the trial (black bars represent standard errors of the means; bars with different letters are significantly different at p ≤ 0.05 according to the LSD test).
As regards the modification of b* parameter, the foliar treatments determined a clear trend toward bluer hues in the youngest bracts when lowering SA concentrations (−10.1 with 10−5 M SA), resulting in a colder, more magenta-toned color (Figure 5). Root applications, on the other hand, produced small variations at every SA concentration. Specifically, the 10−4 M and 10−5 M root treatments determined positive ∆b* values, indicating a shift toward yellow in the youngest bract and a warmer, more orange color. Treatments that significantly alter b* can, therefore, be strategically employed to fine-tune color in red-bracted or cream-colored poinsettia varieties, making the visual appearance either warmer or cooler to suit commercial or aesthetic preferences.
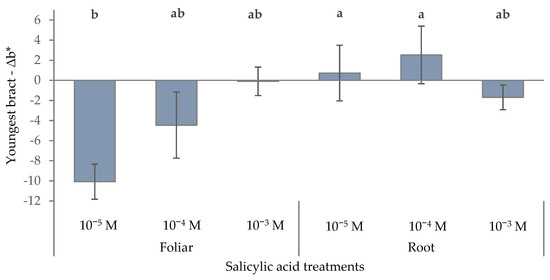
Figure 5.
Effect of different levels of salicylic acid (0, 10−5, 10−4, and 10−3 M) and different application methods (root or foliar) on Δb* of the youngest red bracts of poinsettia plants at the end of the trial (black bars represent standard errors of the means; bars with different letters are significantly different at p ≤ 0.05 according to the LSD test).
Negative ∆Chroma values indicate a reduction in color vividness, while positive values suggest an enhancement in color saturation compared to the red bracts of the control plants. As found for ∆a* and ∆b*, the foliar treatments determined a negative trend of ∆Chroma when lowering SA concentrations (−14.8 with 10−5 M SA), resulting in a more muted bract appearance and a strong desaturation or dulling of color in the youngest bracts (Figure 6). Conversely, the 10−4 M root application slightly increased the chroma of the youngest red bracts (+2.85), enhancing bract color saturation compared to the control.
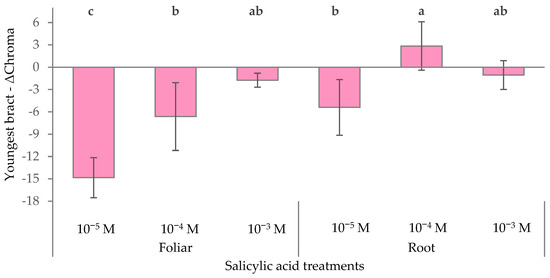
Figure 6.
Effect of different levels of salicylic acid (0, 10−5, 10−4, and 10−3 M) and different application methods (root or foliar) on ΔChroma of the youngest red bracts of poinsettia plants at the end of the trial (black bars represent standard errors of the means; bars with different letters are significantly different at p ≤ 0.05 according to the LSD test).
The foliar application of 10−5 M SA caused the highest ΔE (18.5) (Figure 7), reflecting the most substantial color alteration relative to the control bracts. In contrast, the foliar 10−3 M treatment showed the lowest ΔE (3.6), indicating a much smaller color change from the control, although not statistically different compared with the remaining treatments. These results indicate that low-dose foliar SA triggered the most evident color modification in the youngest red bracts, while high-dose foliar application (10−3 M) preserved a color appearance more similar to that of the control.
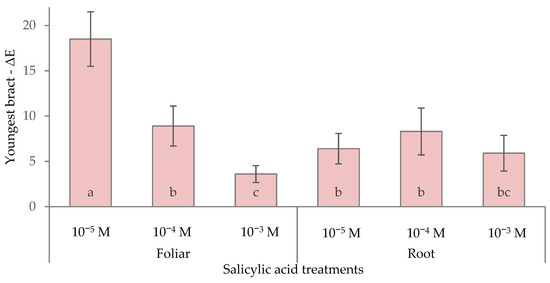
Figure 7.
Effect of different levels of salicylic acid (0, 10−5, 10−4, and 10−3 M) and different application methods (root or foliar) on ΔE of the youngest red bracts of poinsettia plants at the end of the trial (black bars represent standard errors of the means; bars with different letters are significantly different at p ≤ 0.05 according to the LSD test).
3.2. Plant and Canopy Parameters of Poinsettia Plants
The salicylic acid, regardless of concentration and application mode, did not significantly impact the plant morphological parameters observed during the crop cycle (Table 4).

Table 4.
Morphological parameters of poinsettia plants treated with different levels of salicylic acid (0, 10−5, 10−4, and 10−3 M) and different application methods (root or foliar).
SA dose and its mode of application did not influence the horizontal spread of the plant canopy (Table 5). Root SA treatments did not affect the plant canopy area, which was consistent with the control plants. However, foliar SA treatment showed a concentration-dependent effect (Figure 8), with the 10−5 M SA foliar treatment significantly increasing canopy area by 20.5%. These results indicate that a low concentration (10−5 M) of foliar-applied SA enhanced canopy development, while the root treatments exhibited less variation and were generally less effective in stimulating canopy expansion.

Table 5.
Leaf and canopy characteristics of poinsettia plants treated with different levels of salicylic acid (0, 10−5, 10−4, and 10−3 M) and different application methods (root or foliar).
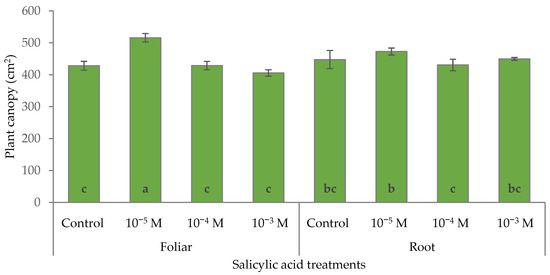
Figure 8.
Effect of different levels of salicylic acid (0, 10−5, 10−4, and 10−3 M) and different application methods (root or foliar) on ΔE of the canopy area of poinsettia plants at the end of the trial (black bars represent standard errors of the means; bars with different letters are significantly different at p ≤ 0.05 according to the LSD test).
The green leaf area and the SLA (specific leaf area) were not influenced by the SA treatments (Table 5), while a marked concentration- and application method-dependent effect was observed in the red bract area. The 10−4 M SA treatment led to the largest bract area (1808.0 cm2 plant−1, +8.5% compared to control), while the highest SA level (10−3 M) determined a significant reduction compared to the control plants (1421.7 cm2 plant−1, −14.7% compared to control). Moreover, root treatments were more effective in increasing red bract area than the foliar treatments (Table 5). Thus, SA root treatments at moderate doses positively influenced bract development, suggesting an enhancement in ornamental quality. Regardless of treatment, the bract SLA remained consistent.
The analysis of biomass partitioning of poinsettia plants confirmed the trends found for the red bract area. Leaves, roots, and total fresh and dry weight were not affected by the treatments, whereas the red bract biomass showed modifications related to the dose and mode of application (Table 6). Moderate doses of SA enhanced bract development and contributed to improving ornamental value. The 10−4 M dose led to the highest bract weight (45.4 g) while the highest dose showed a suppressive effect, resulting in a lower bract fresh weight (36.6 g). As regards bract dry weight, the effect of the treatments was less evident but still the highest SA concentration via foliar application produced the lowest bract dry weight (Figure 9).

Table 6.
Biomass partitioning of poinsettia plants treated with different levels of salicylic acid (0, 10−5, 10−4, and 10−3 M) and different application methods (root or foliar).
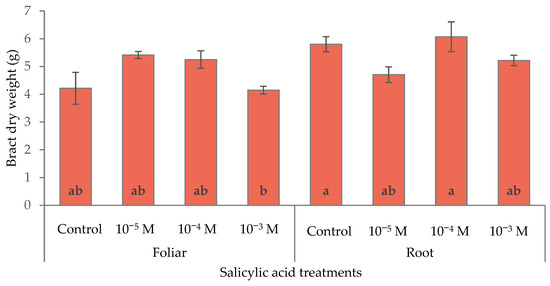
Figure 9.
Effect of different levels of salicylic acid (0, 10−5, 10−4, and 10−3 M) and different application methods (root or foliar) on the dry weight of the red bracts of poinsettia plants at the end of the trial (black bars represent standard errors of the means; bars with different letters are significantly different at p ≤ 0.05 according to the LSD test).
Epigeal dry matter ranged from 16.0% to 17.2%, while root dry matter ranged from 12.6% to 13.4% with no significant differences due to the treatments, indicating that tissue water content remained stable across treatments (Table 6).
4. Discussion
The impact of salicylic acid (SA) on plant morphology is complex and appears to depend on multiple interacting factors. In our research, salicylic acid had no marked effects on most morphological parameters. However, the observation that salicylic acid does not impact morphological parameters is not universally supported across all studies. Research findings on the effects of SA on plant morphology vary, indicating that its impact can be context-dependent and influenced by factors such as plant species, developmental stage, environmental conditions, and SA concentration [38]. For instance, in tobacco, 0.1 mM SA reduced shoot growth and leaf epidermal cell size [39]. Research on chickpea plants indicated that SA induced biochemical defense responses, but it did not markedly alter morphological parameters [40]. Exogenous application of SA in Arabidopsis has been shown to inhibit several aspects of growth and development. Applications with 0.02–0.03 mM SA reduced pollen tube length by approximately 25% [41]; the supply to the growth medium at 3–250 µM SA reduced the number of lateral roots and lateral root primordia [42,43,44]; and treatments with 0.1 and 1 mM SA significantly decreased both trichome density and number [45]. Conversely, some studies have reported significant effects of SA on certain morphological traits. For example, a study on bread wheat cultivars demonstrated that SA application under salt stress conditions significantly improved growth parameters, including enhanced seedling growth and physiological attributes [46]. Similarly, research on Dianthus superbus revealed that exogenous SA alleviated the adverse effects of salt stress by activating photosynthesis, promoting plant growth and protecting morphological structures, and enhancing the antioxidant system [47].
Different concentrations and application methods of SA affected the color traits of the poinsettia leaves and bracts. In the ornamental plant industry, visual appeal is paramount. Brighter and more vibrant leaves are often perceived as indicators of plant health and freshness, enhancing marketability. Darker leaves, on the other hand, might suggest aging or stress, potentially reducing consumer appeal [48]. In our study, we found that when salicylic acid was applied by foliar supply, leaves become darker, especially at higher concentrations. At the same time, the root application led to lighter leaves than the control. This statement is partially supported by existing literature. Several studies have reported that foliar application of SA can enhance chlorophyll content, leading to darker green leaves. Foliar application of SA improved chlorophyll content in wheat plants (Triticum aestivum L.), contributing to potentially darker leaves [49]. Furthermore, several studies have shown that foliar application of SA can have a species and dose-dependent protective role against chlorophyll degradation [50,51,52]. For instance, in broccoli, pre-harvest foliar sprays of SA at 0.01% (approximately 0.72 mM) delayed chlorophyll degradation and preserved green color during storage [53,54]. Similarly, in green pepper, pre-harvest foliar application of SA at 0.5 mM enhanced chlorophyll content [55]. Conversely, studies on root application of SA are less prevalent, but some evidence suggests different effects. In Arabidopsis thaliana, low concentrations of SA in the growth medium inhibited root elongation and affected overall plant morphology [56]. Foliar and root applications of SA to tomato seedlings (Solanum lycopersicum Mill.) under salinity stress improved growth parameters [57], though specific impacts on leaf color were not reported.
Green, reddish, and red leaves co-occur in poinsettia. Plant leaves have three pigments: chlorophylls, carotenoids, and anthocyanins [58]. Green leaves are poor in anthocyanin, while progressive anthocyanin accumulation in reddish leaves gradually turns the leaves into red (anthocyanin rich) [59]. The overall perceived color impact of leaves, especially the older ones, was dependent on SA doses. The greater leaf color modification was determined by the 10−4 M SA treatment. Generally, a high concentration of chlorophylls contributes to the green color of leaves, but the accumulation of carotenoids or anthocyanins is responsible for a different color perception [60]. In particular, anthocyanins (responsible for red and blue color) [61] determine a colder color of leaves, while carotenoids (responsible for yellow-orange color) [62] determine a warmer color. Poinsettia leaves treated with 10−4 M SA showed significantly less red and more yellow compared to the control, probably due to promoting yellow pigment or inhibiting red pigment formation. Conversely, supplying the plants with 10−5 M SA led to darker leaves than the control, and less saturated, with some bluer and cooler hue, and slightly less yellow. Given the lack of specific data on leaf color preferences, it is challenging to definitively state whether the market favors poinsettias with dark or light leaves. With an intermediate dose, leaves appeared fresher with a visible and vibrant color change. However, considering that dark green foliage often provides an attractive, striking contrast to brightly red colored bracts, it is plausible that even such a combination is aesthetically appealing. What is better from a marketable point of view depends on the cultivar and bract color, because, in addition to the usual solid red, many new varieties are proposed every year. Bracts can be of various red hues, or white, pink, coral, softly colored blush, or cream. There are also new varieties with yellow bracts (such as the cv Golden Glo) or variegated leaves for which warmer (more yellow-green) or cooler (more blue-green) leaf color tones may enhance their appeal depending on current consumer preferences. Nevertheless, a general decrease in ΔChroma of the youngest leaves, as a function of dose and application mode, suggested a faded or aged color compared to the control, making the plant less visually pleasant. The reduction in ΔChroma observed in our study is supported by the scientific literature. Research indicates that salicylic acid can promote leaf senescence, a process characterized by chlorophyll degradation and reduced pigmentation. This effect is particularly pronounced in younger leaves, where SA application was shown to accelerate aging processes, leading to diminished color intensity [63]. Exogenous application of SA can trigger plant stress responses, resulting in pigment degradation and altered coloration. Such changes can affect the visual appeal of ornamental plants, making them less marketable due to a faded or aged appearance [64].
Poinsettia consumer choice focuses primarily on bract color, with red being the dominant option [65], although there is a strong interest in pink, white, and novelty-colored poinsettias [66]. Not all red poinsettias are the same. The bract color can vary widely, ranging from striking red to dark red, bright red, deep red, orange-red, and even to true orange or pink. Based on the information gathered from several studies, Sant’Anna et al. [67] summarized that the color parameters are highly correlated with the pigment content. For instance, the presence of anthocyanins was highly correlated with higher a* values and lower L* values, representing darker shades of red. The lower L* values were also associated with higher contents of polyphenols and antioxidant activity. The application of SA influenced the colorimetric parameters of the first and last bracts of poinsettia plants to a variable extent, with a low impact of SA concentration and a higher effect of the application method. The foliar application resulted in smaller chromatic shifts in the oldest red bracts compared to the control, whereas the root treatment led to a reduction in the red pigmentation and a less saturated color. This may be linked to a hormonal crosstalk mechanism involving SA and jasmonates (JA). JA and methyl jasmonate (MeJA) are known to positively regulate anthocyanin biosynthesis by activating specific transcription factors such as MYB75/PAP1, which, in turn, promote the expression of key structural genes in the flavonoid pathway (e.g., CHS, DFR, ANS, UFGT) [68]. Interference with the JA signaling pathway—whether at the biosynthesis or signaling level—can suppress anthocyanin production. It is well known that salicylic acid induces degradation of activating transcription factors of JA signaling, contributing to the repression of JA-responsive genes [69,70]. In this context, exogenous application of SA has also been shown to inhibit red pigment accumulation in wounded Hippeastrum bulb scales, belonging to flavonoids (including proanthocyanidins), potentially through suppression of jasmonate biosynthesis or action [71], suggesting that SA may act antagonistically to JA in regulating flavonoid metabolism. These findings support the hypothesis that the observed decline in chroma and redness in the first bracts under SA root treatment may derive from downregulation of jasmonate-induced anthocyanin biosynthesis, with effects varying depending on tissue type or developmental stage. While the salicylic acid treatments showed minimal effects on the oldest bracts, they still induced notable changes in the youngest bracts, particularly when applied via foliar spray, leading to darker, less red, and less saturated bracts.
The inverse trend of ∆a* observed between the oldest bracts and the youngest bracts under foliar and root application of SA may be ascribed to a different bract responsiveness, developmental stage, and hormonal distribution and/or interaction patterns.
Anthocyanin accumulation begins at the veraison stage. However, studies have shown that under normal growing conditions, anthocyanin biosynthesis in the skin of fruits begins 2 to 3 weeks before their effect on the color is evident [72]. The oldest bract was already partially developed or mature at the time of the treatments. The youngest bract likely developed after or during the period between the two treatments. Older bracts likely had an already established pigment profile and may have exhibited limited responsiveness to foliar SA application. Younger bracts, forming during the treatment period, were more exposed during their development to the full hormonal influence, especially from foliar SA, which acts more locally. The SA root application works systemically; the plant absorbs it slowly and spreads throughout the plant via the vascular system. This means it may reach the older bracts (closer to the base) sooner or in higher concentration. So, it is likely that new tissues (younger bracts) showed the accumulated effect of SA during their formation (especially from foliar application), while old tissues (older bracts) reflected only residual or systemic effects, possibly more pronounced with root application.
The foliar application was more effective on the youngest bract than root application, even in brightness (L*) and yellow (b*) color component and chroma. Assuming a medium-dark red-orange color reference for the control, a simultaneous decrease in L*, a*, and b* values generally indicates a color shift toward cooler, brownish-red tones, approaching crimson or bluish-red. This often enhances the perceived intensity and purity of red. The change in b*, especially in foliar-treated plants, implies that the yellow component of the color varies significantly. This result suggests that salicylic acid also influenced the synthesis or stability of the yellow pigments (such as carotenoids). Several factors, including the age of the plant, the season, microbial attack, cropping, radiation, competition, and nutritional status, have been proven to influence the secondary metabolite profile in higher plants [73]. Carotenoid content is also influenced by diverse environmental disorders like drought and temperature [74]. In other studies, the consequences of altitude and temperature on the carotenoid content of saffron were observed [75,76]. While multiple studies have demonstrated that SA regulates anthocyanin accumulation in various plant species [77,78,79], the impact of SA on carotenoid biosynthesis appears to be variable, more dependent on dose, plant species, and environmental conditions [80,81]. Our results are supported by Vithana et al. [82], who observed that preharvest spray application of 1 mM SA effectively reduced L* and b* in blood orange. SA influenced pigment and provitamin A content of Ficus deltoidea leaves in a concentration-dependent manner [83]. Although the foliar treatment led to a darker, duller last bract appearance with significantly reduced chroma and higher ΔE values (key indicators of visual differences), none of the plants were deemed to be of lower quality. This means that SA treatments could be used to modulate a more orange color in the cream varieties and a cooler color in the red varieties.
In our research, SA significantly affected the canopy. Different studies underlined that the impact of SA on plant morphology, including canopy area, is complex and influenced by multiple factors. Its effects can vary depending on concentration, application method, and environmental conditions across different plant species. Foliar applications of SA on Aconitum napellus under chromium stress led to significant improvements in growth parameters such as shoot length, root length, and biomass, suggesting a potential positive impact on overall plant development and canopy area [84]. Ortega [85] found that balancing disease resistance and plant growth when increasing SA levels can be challenging. High SA concentrations, while enhancing disease resistance, were associated with suppressed plant growth, indicating that high levels of SA might negatively affect morphological and growth parameters such as canopy area. A significant improvement of the canopy area with foliar low dose of SA and its reduction when increasing the SA concentration was also found in our trial.
The salicylic acid positively influenced bract surface development, which is visually appealing and commercially valuable. While SA is recognized for its role in plant growth and development, specific studies directly examining its impact on poinsettia bract development are limited. SA treatments can increase the rooting percentages of poinsettia cuttings [86]. Enhanced root growth could influence overall plant growth, indirectly affecting bract development. However, we have observed no significant effect on root biomass attributable to the mode of application (root or foliar). Additionally, SA has been shown to regulate cell division and expansion, key processes in plant organ development. SA plays a crucial role in these processes, suggesting that it could influence the development of various plant structures, including red bracts [38]. While direct evidence is scarce, these findings imply that SA at moderate doses may positively affect surface bract development, possibly through its influence on overall plant growth and organ development. The observed effects on canopy area at low SA doses may be partially ascribed to enhanced CO2 assimilation and stomatal regulation, processes known to be modulated by SA in other species [87].
A higher fresh and dry weight of the red bracts was observed as an effect of the intermediate treatments, while SA at higher doses had a detrimental effect, as confirmed by other studies [47,88,89,90]. Root treatment was less effective in altering bract dry matter accumulation compared to high-concentration foliar application, possibly due to a more gradual and systemic uptake of SA through the root system and reduced local stress in young foliar tissues. Root-applied SA improved photosynthetic activity, cell membrane stability, and overall plant health of tomato plants [91]. Root application of SA allows a more gradual and systemic uptake, improving plant growth. Foliar applications of SA can sometimes trigger local stress responses, potentially leading to oxidative stress and interference with normal physiological processes. In contrast, root applications have been associated with improved nutrient uptake and reduced stress markers. A study on lettuce under cadmium stress demonstrated that root-applied SA alleviated growth inhibition more effectively than foliar application [92].
5. Conclusions
This study demonstrated that salicylic acid exerts dose- and method-dependent effects on key ornamental traits of poinsettia. While most morphological traits remained unaffected, SA treatments significantly influenced the colorimetric characteristics of leaves and red bracts, notably impacting on brightness, chroma, and red tone. Foliar application of high SA concentrations (10−3 M) consistently reduced visual quality, leading to darker, less saturated bracts and reduced canopy development and bract dry weight. In contrast, moderate doses (10−4 M), especially when applied to the root, improved bract surface and color saturation without compromising plant growth. The findings emphasize the importance of selecting suitable concentrations and application methods when using SA as a biostimulant in floriculture to prevent undesirable effects on pigmentation and marketability. Carefully calibrated salicylic acid treatments can serve as effective and practical tools for enhancing visual quality and supporting the transition toward more sustainable, low-input ornamental crop production systems.
Author Contributions
Conceptualization, A.M. (Alessandro Miceli), F.V. and A.M. (Alessandra Moncada); Data curation, S.C.; Formal analysis, A.E.; Investigation, A.E., A.M. (Alessandro Miceli), F.V., S.C. and A.M. (Alessandra Moncada); Methodology, A.E., A.M. (Alessandro Miceli), F.V., S.C. and A.M. (Alessandra Moncada); Writing—original draft, A.E., F.V. and A.M. (Alessandra Moncada); Writing—review and editing, A.E. and A.M. (Alessandro Miceli). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in tables and figures.
Acknowledgments
The authors extend their gratitude to the Romano brothers, owners of the Conca d’Oro Nursery, for generously providing the propagation material and for making their facilities and technical resources available for the execution of this trial.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Krug, B.A.; Burnett, S.E.; Dennis, J.H.; Lopez, R.G. Growers Look at Operating a Sustainable Greenhouse. GMPro 2008, 28, 43–45. [Google Scholar]
- Miceli, A.; Moncada, A.; Vetrano, F.; Esposito, A. Response of Tagetes patula L. and Ageratum houstonianum Mill. to Microbial Biostimulant Inoculation and Organic Fertilization. Agronomy 2023, 13, 2522. [Google Scholar] [CrossRef]
- Xia, L.; Hao, W.; Qin, J.; Ji, F.; Yue, X. Carbon Emission Reduction and Promotion Policies Considering Social Preferences and Consumers’ Low-Carbon Awareness in the Cap-and-Trade System. J. Clean. Prod. 2018, 195, 1105–1124. [Google Scholar] [CrossRef]
- Choi, S.; Feinberg, R.A. The LOHAS (Lifestyle of Health and Sustainability) Scale Development and Validation. Sustainability 2021, 13, 1598. [Google Scholar] [CrossRef]
- Wani, M.A.; Nazki, I.T.; Din, A.; Iqbal, S.; Wani, S.A.; Khan, F.U. Neelofar Floriculture Sustainability Initiative: The Dawn of New Era. Sustain. Agric. Rev. 2018, 27, 91–127. [Google Scholar]
- Lopez, R.G.; Burnett, S.E.; Dennis, J.H.; Krug, B.A. 8 Steps to Take to Become Sustainable. GMPro 2008, 28, 50. [Google Scholar]
- Barreiro-Hurlé, J.; Espinosa-Goded, M.; Dupraz, P. Does Intensity of Change Matter? Factors Affecting Adoption of Agri-Environmental Schemes in Spain. J. Environ. Plan. Manag. 2010, 53, 891–905. [Google Scholar] [CrossRef]
- Martin, S.W.; Roberts, R.K.; Larkin, S.L.; Larson, J.A.; Paxton, K.W.; English, B.C.; Marra, M.C.; Reeves, J.M. A Binary Logit Estimation of Factors Affecting Adoption of GPS Guidance Systems by Cotton Producers. J. Agric. Appl. Econ. 2008, 40, 345–355. [Google Scholar]
- Gillespie, J.; Lewis, D. Processor Willingness to Adopt a Crawfish Peeling Machine: An Application of Technology Adoption under Uncertainty. J. Agric. Appl. Econ. 2008, 40, 369–383. [Google Scholar] [CrossRef][Green Version]
- Paudel, K.P.; Gauthier, W.M.; Westra, J.V.; Hall, L.M. Factors Influencing and Steps Leading to the Adoption of Best Management Practices by Louisiana Dairy Farmers. J. Agric. Appl. Econ. 2008, 40, 203–222. [Google Scholar] [CrossRef]
- D’souza, G.; Cyphers, D.; Phipps, T. Factors Affecting the Adoption of Sustainable Agricultural Practices. Agric. Resour. Econ. Rev. 1993, 22, 159–165. [Google Scholar] [CrossRef]
- Kumar, M. Plant Growth Regulators and Their Implication in Ornamental Horticulture: An Overview. Int. J. Agric. Environ. Biotechnol. 2021, 14, 417–445. [Google Scholar] [CrossRef]
- Mejri, S.; Ghinet, A.; Magnin-Robert, M.; Randoux, B.; Abuhaie, C.-M.; Tisserant, B.; Gautret, P.; Rigo, B.; Halama, P.; Reignault, P. New Plant Immunity Elicitors from a Sugar Beet Byproduct Protect Wheat against Zymoseptoria Tritici. Sci. Rep. 2023, 13, 90. [Google Scholar] [CrossRef]
- Imandi, S.; Reddy, G.V.S. Studies on the Effect of Plant Growth Regulators on Vegetative Growth, Flowering, Yield and Shelf Life of the Marigold Cv. Siracole. Int. J. Agric. Sci. Res. (IJASR) 2017, 7, 65–70. [Google Scholar]
- Islam, M.K.; Khorsheduzzaman, A.K.M.; Rahman, M.L.; Moniruzzanan, M.; Talukder, M.B.; Rahim, M.A. Effect of Growth Regulators on Plant Emergence, Growth and Flower Production of Gladiolus. Bangladesh J. Agric. Sci. 2012, 37, 17–21. [Google Scholar]
- Jadhav, P.B.; Mangave, B.D.; Singh, A.; Dekhane, S.S.; Patel, D.J. Effect of Plant Growth Regulators and Natural Growth Substances on Growth and Flowering of Gladiolus Cv. Am. Beauty. Int. J. Curr. Res. 2015, 7, 17674–17676. [Google Scholar]
- Kaushik, H.; Kumar, J.; Singh, J.P.; Singh, R.K.; Rajbeer, R.; Kumar, S. Effect of GA3 and Biofertilizers on Growth and Flowering in Gladiolus (Gladiolus floribundus L.) Cv. American Beauty. Adv. Res. J. Crop Improv. 2016, 7, 52–55. [Google Scholar] [CrossRef]
- Abdolmaleki, M.; Khosh-khui, M.; Eshghi, S.; Ramezanian, A. Improvement in Vase Life of Cut Rose Cv “Dolce Vita” by Preharvest Foliar Application of Calcium Chloride and Salicylic Acid. Int. J. Hortic. Sci. Technol. 2015, 2, 55–66. [Google Scholar]
- Chaudhary, A.; Mishra, A.; Bola, P.K.; Nagar, K.K.; Chaudhary, P. Effect of Foliar Application of Zinc and Salicylic Acid on Flowering and Yield of African Marigold Cv. Pusa Narangi Gainda. HortFlora Res. Spectr. 2015, 4, 351–355. [Google Scholar]
- Gerailoo, S.; Ghasemnezhad, M.; Shiri, M.A. Effect of Short Time Treatment of Salicylic Acid in Delaying Flowers Senescence in Cut Rose (Rosa hybrida) Cv. Yellow Island. J. Plant Res. (Iran. J. Biol.) 2014, 27, 299–309. [Google Scholar]
- Mansouri, H. Salicylic Acid and Sodium Nitroprusside Improve Postharvest Life of Chrysanthemums. Sci. Hortic. 2012, 145, 29–33. [Google Scholar] [CrossRef]
- Akram, A.; Asghar, M.A.; Younis, A.; Akbar, A.F.; Talha, M.; Farooq, A.; Akhtar, G.; Shafiqe, M.; Mushtaq, M.Z. Foliar Application of Salicylic Acid and Its Impact on Pre and Post-Harvest Attributes of Antirrhinum majus L. J. Pure Appl. Agric. 2022, 7, 1–11. [Google Scholar]
- Choudhary, A.; Mishra, A.; Bola, P.K.; Moond, S.K.; Dhayal, M. Effect of Foliar Application of Zinc and Salicylic Acid on Growth, Flowering and Chemical Constitute of African Marigold Cv. Pusa Narangi Gainda (Targets erecta L.). J. Appl. Nat. Sci. 2016, 8, 1467–1470. [Google Scholar] [CrossRef]
- Jahanbazi, T.; Mortezaienejad, F.; Jafararpoor, M. Impact of Salicylic Acid and Jasmonic Acid on Keeping Quality of Rose (Cv.‘Angelina’) Flower. J. Nov. Appl. Sci. 2014, 3, 1328–1335. [Google Scholar]
- Abdou, M.H.; El-Sayed, A.A.; Attia, F.A.; Khalil, A.R. Effect of Compost, Salicylic and Ascorbic Acids Treatments on Vegetative Growth and Flowering of Gladiolus grandiflorus Cv. White Prosperity. Sci. J. Flowers Ornam. Plants 2014, 1, 223–231. [Google Scholar] [CrossRef]
- Devarakonda, S.; Madhumathi, C.; Lakshmi, L.M.; Bhaskar, V.V.; Umamahesh, V.; Rajasekharam, T.; Reddy, M. Effect of Plant Elicitors on Growth, Yield and Quality of Papaya (Carica papaya). Curr. Hortic. 2020, 8, 23–28. [Google Scholar] [CrossRef]
- Kazemi, M. Foliar Application of Salicylic Acid and Methyl Jasmonate on Yield, Yield Components and Chemical Properties of Tomato. Jordan J. Agric. Sci. 2014, 10, 771–778. [Google Scholar] [CrossRef]
- Navarro, C. Poinsettia Growers in Mexico Expanding Domestic Sales, Still Not Allowed to Export to US Market. Lat. Am. Digit. Beat 2010, 078088. [Google Scholar]
- Taylor, J.M.; Lopez, R.G.; Currey, C.J.; Janick, J. The Poinsettia: History and Transformation. Chron. Hortic. 2011, 51, 23–28. [Google Scholar]
- Elwan, M.W.M.; El-Hamahmy, M.A.M. Improved Productivity and Quality Associated with Salicylic Acid Application in Greenhouse Pepper. Sci. Hortic. 2009, 122, 521–526. [Google Scholar] [CrossRef]
- Choudhary, A.; Mishra, A.; Nagar, K.K.; Meena, R.R. Foliar Application of Zinc and Salicylic Acid on African Marigold. J. Crop Weed 2016, 12, 107–111. [Google Scholar]
- Sonneveld, C.; Voogt, W.; Sonneveld, C.; Voogt, W. Nutrient Solutions for Soilless Cultures. In Plant Nutrition of Greenhouse Crops; Springer: Berlin/Heidelberg, Germany, 2009; pp. 257–275. [Google Scholar]
- Bakker, J.; Bridle, P.; Timberlake, C.F. Tristimulus Measurements (CIELAB 76) of Port Wine Colour. Vitis 1986, 25, 67–78. [Google Scholar]
- McLellan, M.R.; Lind, L.R.; Kime, R.W. Hue Angle Determinations and Statistical Analysis for Multiquadrant Hunter L, a, b Data. J. Food Qual. 1995, 18, 235–240. [Google Scholar] [CrossRef]
- Shamey, R. CIE 1994 (ΔL* ΔC*ab ΔH*ab). In Encyclopedia of Color Science and Technology; Shamey, R., Ed.; Springer International Publishing: Cham, Switzerland, 2023; p. 174. ISBN 978-3-030-89862-5. [Google Scholar]
- Shamey, R. Hue Difference, Delta H. In Encyclopedia of Color Science and Technology; Shamey, R., Ed.; Springer International Publishing: Cham, Switzerland, 2023; p. 906. ISBN 978-3-030-89862-5. [Google Scholar]
- Easlon, H.M.; Bloom, A.J. Easy Leaf Area: Automated Digital Image Analysis for Rapid and Accurate Measurement of Leaf Area. Appl. Plant Sci. 2014, 2, 1400033. [Google Scholar] [CrossRef]
- Li, A.; Sun, X.; Liu, L. Action of Salicylic Acid on Plant Growth. Front. Plant Sci. 2022, 13, 878076. [Google Scholar] [CrossRef]
- Dat, J.F.; Lopez-Delgado, H.; Foyer, C.H.; Scott, I.M. Effects of Salicylic Acid on Oxidative Stress and Thermotolerance in Tobacco. J. Plant Physiol. 2000, 156, 659–665. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; War, M.Y.; Ignacimuthu, S. Role of Salicylic Acid in Induction of Plant Defense System in Chickpea (Cicer arietinum L.). Plant Signal Behav. 2011, 6, 1787–1792. [Google Scholar] [CrossRef]
- Rong, D.; Luo, N.; Mollet, J.C.; Liu, X.; Yang, Z. Salicylic Acid Regulates Pollen Tip Growth through an NPR3/NPR4-Independent Pathway. Mol. Plant 2016, 9, 1478–1491. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Abas, M.; Verstraeten, I.; Glanc, M.; Molnár, G.; Hajný, J.; Lasák, P.; Petřík, I.; Russinova, E.; Petrášek, J. Salicylic Acid Targets Protein Phosphatase 2A to Attenuate Growth in Plants. Curr. Biol. 2020, 30, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Armengot, L.; Marquès-Bueno, M.M.; Soria-Garcia, A.; Müller, M.; Munné-Bosch, S.; Martínez, M.C. Functional Interplay between Protein Kinase CK 2 and Salicylic Acid Sustains PIN Transcriptional Expression and Root Development. Plant J. 2014, 78, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, T.; Groot, E.P.; Kazantsev, F.V.; Teale, W.; Omelyanchuk, N.; Kovrizhnykh, V.; Palme, K.; Mironova, V. V Salicylic Acid Affects Root Meristem Patterning via Auxin Distribution in a Concentration-Dependent Manner. Plant Physiol. 2019, 180, 1725–1739. [Google Scholar] [CrossRef]
- Traw, M.B.; Bergelson, J. Interactive Effects of Jasmonic Acid, Salicylic Acid, and Gibberellin on Induction of Trichomes in Arabidopsis. Plant Physiol. 2003, 133, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Abdi, N.; Van Biljon, A.; Steyn, C.; Labuschagne, M.T. Salicylic Acid Improves Growth and Physiological Attributes and Salt Tolerance Differentially in Two Bread Wheat Cultivars. Plants 2022, 11, 1853. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zheng, J.; Zhang, X.; Hu, Q.; Qian, R. Salicylic Acid Alleviates the Adverse Effects of Salt Stress on Dianthus superbus (Caryophyllaceae) by Activating Photosynthesis, Protecting Morphological Structure, and Enhancing the Antioxidant System. Front. Plant Sci. 2017, 8, 600. [Google Scholar] [CrossRef]
- Owen, W.G.; Lopez, R.G. Geranium and Purple Fountain Grass Leaf Pigmentation Is Influenced by End-of-Production Supplemental Lighting with Red and Blue Light-Emitting Diodes. HortScience 2017, 52, 236–244. [Google Scholar] [CrossRef]
- Fairoj, S.A.; Islam, M.M.; Islam, M.A.; Zaman, E.; Momtaz, M.B.; Hossain, M.S.; Jahan, N.A.; Shams, S.-N.-U.; Urmi, T.A.; Rasel, M.A. Salicylic Acid Improves Agro-Morphology, Yield and Ion Accumulation of Two Wheat (Triticum aestivum L.) Genotypes by Ameliorating the Impact of Salt Stress. Agronomy 2022, 13, 25. [Google Scholar] [CrossRef]
- Mahajan, M.; Nazir, F.; Jahan, B.; Siddiqui, M.H.; Iqbal, N.; Khan, M.I.R. Salicylic Acid Mitigates Arsenic Stress in Rice (Oryza sativa) via Modulation of Nitrogen–Sulfur Assimilation, Ethylene Biosynthesis, and Defense Systems. Agriculture 2023, 13, 1293. [Google Scholar] [CrossRef]
- Zahra, S.; Amin, B.; Ali, V.S.M.; Ali, Y.; Mehdi, Y. The Salicylic Acid Effect on the Tomato (Lycopersicum esculentum Mill.) Sugar, Protein and Proline Contents under Salinity Stress (NaCl). J. Biophys. Struct. Biol. 2010, 2, 35–41. [Google Scholar]
- Çag, S.; Cevahir-Öz, G.; Sarsag, M.; Gören-Saglam, N. Effect of Salicylic Acid on Pigment, Protein Content and Peroxidase Activity in Excised Sunflower Cotyledons. Pak. J. Bot. 2009, 41, 2297–2303. [Google Scholar]
- Rastegar, S.; Shojaie, A.; Koy, R.A.M. Foliar Application of Salicylic Acid and Calcium Chloride Delays the Loss of Chlorophyll and Preserves the Quality of Broccoli during Storage. J. Food Biochem. 2022, 46, e14154. [Google Scholar] [CrossRef]
- Youssef, S.M.; López-Orenes, A.; Ferrer, M.A.; Calderón, A.A. Foliar Application of Salicylic Acid Enhances the Endogenous Antioxidant and Hormone Systems and Attenuates the Adverse Effects of Salt Stress on Growth and Yield of French Bean Plants. Horticulturae 2023, 9, 75. [Google Scholar] [CrossRef]
- Dobón-Suárez, A.; Giménez, M.J.; García-Pastor, M.E.; Zapata, P.J. Salicylic Acid Foliar Application Increases Crop Yield and Quality Parameters of Green Pepper Fruit during Postharvest Storage. Agronomy 2021, 11, 2263. [Google Scholar] [CrossRef]
- Li, S.; Nayar, S.; Jia, H.; Kapoor, S.; Wu, J.; Yukawa, Y. The Arabidopsis Hypoxia Inducible AtR8 Long Non-Coding RNA Also Contributes to Plant Defense and Root Elongation Coordinating with WRKY Genes under Low Levels of Salicylic Acid. Noncoding RNA 2020, 6, 8. [Google Scholar] [CrossRef]
- Souri, M.K.; Tohidloo, G. Effectiveness of Different Methods of Salicylic Acid Application on Growth Characteristics of Tomato Seedlings under Salinity. Chem. Biol. Technol. Agric. 2019, 6, 26. [Google Scholar] [CrossRef]
- Feild, T.S.; Lee, D.W.; Holbrook, N.M. Why Leaves Turn Red in Autumn. The Role of Anthocyanins in Senescing Leaves of Red-Osier Dogwood. Plant Physiol. 2001, 127, 566–574. [Google Scholar] [CrossRef]
- Moustaka, J.; Panteris, E.; Adamakis, I.-D.S.; Tanou, G.; Giannakoula, A.; Eleftheriou, E.P.; Moustakas, M. High Anthocyanin Accumulation in Poinsettia Leaves Is Accompanied by Thylakoid Membrane Unstacking, Acting as a Photoprotective Mechanism, to Prevent ROS Formation. Environ. Exp. Bot. 2018, 154, 44–55. [Google Scholar] [CrossRef]
- Carlson, J.E.; Holsinger, K.E. Natural Selection on Inflorescence Color Polymorphisms in Wild Protea Populations: The Role of Pollinators, Seed Predators, and Intertrait Correlations. Am. J. Bot. 2010, 97, 934–944. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemistry, Cell Biology, and Biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids as Natural Functional Pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Yu, X.; Xu, Y.; Yan, S. Salicylic Acid and Ethylene Coordinately Promote Leaf Senescence. J. Integr. Plant Biol. 2021, 63, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kohli, S.K.; Khanna, K.; Ramakrishnan, M.; Kumar, V.; Bhardwaj, R.; Brestic, M.; Skalicky, M.; Landi, M.; Zheng, B. Salicylic Acid: A Phenolic Molecule with Multiple Roles in Salt-Stressed Plants. J. Plant Growth Regul. 2023, 42, 4581–4605. [Google Scholar] [CrossRef]
- Barrett, J. Consumer Poinsettia Preference. L Gard. Retail. 2005, 4, 1–4. [Google Scholar]
- Posadas, B.C.; Coker, C.E.H.; Jackson, C.; Knight, P.R.; DelPrince, J.M.; Langlois, S.A.; Ryals, J.B. Online Survey of Consumer Preferences for Poinsettia Cultivars. Horticulturae 2023, 9, 449. [Google Scholar] [CrossRef]
- Sant’Anna, V.; Gurak, P.D.; Marczak, L.D.F.; Tessaro, I.C. Tracking Bioactive Compounds with Colour Changes in Foods—A Review. Dye. Pigment. 2013, 98, 601–608. [Google Scholar] [CrossRef]
- Shan, X.; Zhang, Y.; Peng, W.; Wang, Z.; Xie, D. Molecular Mechanism for Jasmonate-Induction of Anthocyanin Accumulation in Arabidopsis. J. Exp. Bot. 2009, 60, 3849–3860. [Google Scholar] [CrossRef] [PubMed]
- Caarls, L.; Pieterse, C.M.J.; Van Wees, S.C.M. How Salicylic Acid Takes Transcriptional Control over Jasmonic Acid Signaling. Front. Plant Sci. 2015, 6, 170. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.G.; Ellis, C.; Devoto, A. The Jasmonate Signal Pathway. Plant Cell 2002, 14, S153–S164. [Google Scholar] [CrossRef] [PubMed]
- Treutter, D. Significance of Flavonoids in Plant Resistance and Enhancement of Their Biosynthesis. Plant Biol. 2005, 7, 581–591. [Google Scholar] [CrossRef]
- Darne, G. New Hypotheses on Anthocyanin Biosynthesis in Berries and Leaves of the Grapevine. Vitis 1993, 32, 77–85. [Google Scholar]
- Harborne, J.B.; Marby, H.; Marby, T.J. The Flavonoids; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 1489929096. [Google Scholar]
- Bouvier, F.; Backhaus, R.A.; Camara, B. Induction and Control of Chromoplast-Specific Carotenoid Genes by Oxidative Stress. J. Biol. Chem. 1998, 273, 30651–30659. [Google Scholar] [CrossRef]
- Zarinkamar, F.; Tajik, S.; Soleimanpour, S. Effects of Altitude on Anatomy and Concentration of Crocin, Picrocrocin and Safranal in ‘Crocus sativus’ L. Aust. J. Crop Sci. 2011, 5, 831–838. [Google Scholar]
- Ammanullah, M.M.; Sekar, S.; Vicent, S. Plant Growth Substances in Crop Production. Asian J. Plant Sci. 2010, 9, 215–222. [Google Scholar] [CrossRef]
- Li, Z.; Ma, N.; Sun, P.; Zhang, F.; Li, L.; Li, H.; Zhang, S.; Wang, X.; You, C.; Zhang, Z. Fungal Invasion-induced Accumulation of Salicylic Acid Promotes Anthocyanin Biosynthesis through MdNPR1-MdTGA2. 2 Module in Apple Fruits. Plant J. 2024, 119, 1859–1879. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Zapata, P.J.; Castillo, S.; Martínez-Romero, D.; Valero, D.; Serrano, M.; Guillén, F. Preharvest Salicylate Treatments Enhance Antioxidant Compounds, Color and Crop Yield in Low Pigmented-Table Grape Cultivars and Preserve Quality Traits during Storage. Antioxidants 2020, 9, 832. [Google Scholar] [CrossRef]
- Shi, L.; Li, X.; Fu, Y.; Li, C. Environmental Stimuli and Phytohormones in Anthocyanin Biosynthesis: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 16415. [Google Scholar] [CrossRef]
- Xiang, S.; Qiu, X.; Yan, X.; Ruan, R.; Cheng, P. Salicylic Acid Improved the Growth of Dunaliella salina and Increased the Proportion of 9-Cis-β-Carotene Isomers. Mar. Drugs 2025, 23, 18. [Google Scholar] [CrossRef] [PubMed]
- Benitez-García, I.; Vanegas-Espinoza, P.; Paredes-Lopez, O.; Villar-Martínez, A. Salicilic Acid Effect on Carotenoid Production and Carotenogenic Gene Expression of in Vitro Culture of Marigold. J. Chem. Biol. Phys. Sci. 2014, 30, 74. [Google Scholar]
- Vithana, M.D.K.; Singh, Z.; Ul Hasan, M. Pre-and Post-Harvest Elicitation with Methyl Jasmonate and Salicylic Acid Followed by Cold Storage Synergistically Improves Red Colour Development and Health-Promoting Compounds in Blood Oranges. J. Plant Growth Regul. 2024, 43, 1657–1671. [Google Scholar] [CrossRef]
- Ismail, A.; Shahidan, N.; Mat, N.; Othman, R. Effect of Salicylic Acid on Carotenoids and Chlorophyll Content in Mas Cotek (Ficus deltoidea Jack Var. Trengganuensis) Leaves and Its Retinol Activity Equivalents (RAE). J. Pharm. Nutr. Sci. 2020, 10, 25–33. [Google Scholar] [CrossRef]
- Ramzan, M.; Javed, T.; Hassan, A.; Ahmed, M.Z.; Ashraf, H.; Shah, A.A.; Iftikhar, M.; El-Sheikh, M.A.; Raja, V. Protective Effects of the Exogenous Application of Salicylic Acid and Chitosan on Chromium-Induced Photosynthetic Capacity and Osmotic Adjustment in Aconitum Napellus. BMC Plant Biol. 2024, 24, 933. [Google Scholar] [CrossRef]
- Ortega, M.A.; Celoy, R.M.; Chacon, F.; Yuan, Y.; Xue, L.-J.; Pandey, S.P.; Drowns, M.R.; Kvitko, B.H.; Tsai, C.-J. Altering Cold-Regulated Gene Expression Decouples the Salicylic Acid–Growth Trade-off in Arabidopsis. Plant Cell 2024, 36, 4293–4308. [Google Scholar] [CrossRef] [PubMed]
- Sardoei, A.S.; Fahraji, S.S.; Ghasemi, H. Effect of Salicylic Acid on Rooting of Poinsettia (Euphorbia pulcherrima). Int. J. Adv. Biol. Biomed. Res. 2014, 2, 1883–1886. [Google Scholar]
- Horváth, E.; Pál, M.; Szalai, G.; Páldi, E.; Janda, T. Exogenous 4-Hydroxybenzoic Acid and Salicylic Acid Modulate the Effect of Short-Term Drought and Freezing Stress on Wheat Plants. Biol. Plant 2007, 51, 480–487. [Google Scholar] [CrossRef]
- Hayat, S.; Fariduddin, Q.; Ali, B.; Ahmad, A. Effect of Salicylic Acid on Growth and Enzyme Activities of Wheat Seedlings. Acta Agron. Hung. 2005, 53, 433–437. [Google Scholar] [CrossRef]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic Acid beyond Defence: Its Role in Plant Growth and Development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef]
- Phong Lam, V.; Loi, D.N.; Shin, J.; Mi, L.K.; Park, J. Optimization of Salicylic Acid Concentrations for Increasing Antioxidant Enzymes and Bioactive Compounds of Agastache Rugosa in a Plant Factory. PLoS ONE 2024, 19, e0306340. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.; Senaratna, T.; Sivasithamparam, K. Salicylic Acid Induces Salinity Tolerance in Tomato (Lycopersicon esculentum Cv. Roma): Associated Changes in Gas Exchange, Water Relations and Membrane Stabilisation. Plant Growth Regul. 2006, 49, 77–83. [Google Scholar]
- Tang, W.; Liang, L.; Xie, Y.; Li, X.; Lin, L.; Huang, Z.; Sun, B.; Sun, G.; Tu, L.; Li, H. Foliar Application of Salicylic Acid Inhibits the Cadmium Uptake and Accumulation in Lettuce (Lactuca sativa L.). Front. Plant Sci. 2023, 14, 1200106. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).