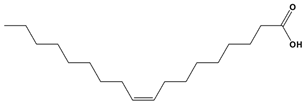
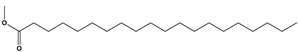
Abstract
The holistic use of Moringa oleifera Lam. seeds is not as popular amongst rural South Africans. This study screened for the phytochemicals, antimicrobial, and antioxidant potentials as well identifying the compounds in the oils of South African Moringa seed oils using cost-effective thin layer chromatography bioautography and dot blot assays, because fewer studies have been conducted using seed samples from this country. The results obtained indicated that the best oil extract yield (24.04%) was obtained for hexane from 60.10 g of powdered seeds. The yield of the other extracts ranged from 6.2 to 9.5%. Positive test results were obtained for terpenoids, steroids, alkaloids, flavonoids, phenols, and tannins, with potentially good antioxidant properties for scavenging free radicals from 2,2-diphenyl-1-picrylhydrazyl (DPPH) and good antimicrobial activity against Acinetobacter baumannii (BAA 747), Escherichia coli (ATCC 25922), Klebsiella pneumoniae (ATCC 27853), and Pseudomonas aeruginosa (ATCC 27853), with the best zone of inhibition of 314.2 mm2 obtained for oil extracted with hexane, followed by dichloromethane, methanol, and acetone oil extracts, respectively. The best minimum inhibitory concentration (MIC) of 0.032 mg/mL against P. aeruginosa was recorded for the hexane oil, compared with ciprofloxacin, which had an MIC of 0.0039 mg/mL against the same pathogen. The identification of the in-oil compounds proposed to mitigate inhibitory activity against the test microbes was carried out through GC-MS analysis matching our results with the GC-MS library. These compounds included ursane-3,16-diol, azetidin-2-one, 1-benzyl-4à-methyl, dibutyl phthalate, 4-methyl-2,4-bis(p-hydroxyphenyl)pent-1-ene, 1H-pyrrole-2,5-dione, 3-ethyl-4-methyl, octopamine rhodoxanthin, 29,30-dinorgammacerane-3,22-diol, 21,21-dimethy, cholan-24-oic acid, 3,7-dioxo, and benzyl alcohol. These are in addition to the stability-indicating marker compounds like oleic acid (54.9%), 9-Octadecenoic acid (z)-, methyl ester (23.3%), n-hexadecanoic acid (9.68%), among others observed over a five year period.
1. Introduction
Moringa oleifera Lam. (Moringaceae) is a perennial plant that can grow up to 9 m tall in a short time. Alternatively called ‘the horseradish tree’ or drumstick, it is native to the Himalayan region of Northwestern India. In 2016, M. oleifera was mainly found in Middle Eastern and Asian countries. However, it has spread to other areas, especially tropical and subtropical lands affected by drought, due to its adaptability [1]. The tree is characterized by a brittle stem and tuberous taproots, which taste like horseradish (hence its common name). The ‘horn of Africa’ countries, mostly located in the eastern part of the continent, as well as Nigeria and South Africa, also commonly use the plant for fencing their compounds. In terms of the impact of geographic biotic and edaphic factors, it is logical to say that one could envision variations in the morphology of M. oleifera seeds. Such variation has been reported for M. oleifera seeds from Mexico, using principal component analysis [2]. We have mentioned in the past the pharmacological and other health benefits derivable from the use of M. oleifera powdered seeds and seed oils, in addition to those meticulously reported by Pandey and co-workers [3] in their review.
Qualitative phytochemical screening is important in plant-based therapy because it confirms the presence or otherwise of various groups of phytochemicals. Such compound families, often present in natural extracts, may exhibit potential activities in mitigating different disease conditions. The biological activities, including but not limited to antimicrobial, antioxidant, and antidiabetic activities, among others, often lead separation chemists to the isolation and purification of known or novel lead compounds that may be useful in the development of new drugs [4]. The extracts are often subjected to chemical tests for individual phytochemical analysis, as described by Balamurugan and co-workers [5]. The in vitro biological testing of medicinal plant extracts is a crucial step-in modern-day drug discovery. Several methods, including agar diffusion, well plate dilution, thin layer chromatography (TLC)–bioautography [6], and minimum inhibitory concentration (MIC) determined by the broth microdilution method, carried out in multi-well microplates [7], are often deployed with a view towards evaluating the biological activities of natural product extracts, including extracts of medicinal plant parts like Moringa oleifera seeds. Usually, the underlying principle for biological activity involves the plant extract interacting with a disease pathogen (bacteria, fungi, virus) or its enzymes to inhibit the growth of the pathogen. A positive test is indicated by colour changes in most cases. Unlike its counterparts from around the world, not much information is available in the literature regarding the qualitative phytochemical screening and biological activities of South African Moringa oleifera seed.
The M. oleifera, in addition to its popular use in water treatment, also finds application in curing eye diseases and headaches. Its other recorded uses involve the management of fevers, snake bites, scorpion stings, and warts. It also treats ulcers, gastritis, skin disorders, bladder infections, scurvy, abdominal tumours, and schistosomes. The phytochemicals responsible include (benzylglucosinate di-oleic-triglyceride Mono-palmitic acid [8]). Apart from the seeds, M. oleifera seed oils have some medicinal uses, amongst them, their use as a purgative, for the alleviation of leprosy, ulcers, treatment of rheumatism, gout, skin pathogens, lupus, and bladder disorders, and phytochemical groups like tocopherols, palmitic, stearic, and arachidic acids are linked to the activity of the seed oils [7].
Antimicrobial resistance is a leading public health challenge globally. Even though there are current efforts, including microbial stewardship, patience adherence and compliance to manage the issue of antimicrobial resistance [9], the World Health Organization still classifies Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp. (ESKAPE) pathogens as critical multidrug-resistant bacteria [10], for which effective therapies are urgently required. To support the search for new anti-microbial agents, medicinal plant research is in the forefront of harnessing active extracts as well as new chemical entities that could serve as new lead compounds for clinical trials and interventions. To this end, this study is aimed at determining the classes of phytochemicals, antibacterials, antioxidants, and the identification of specific in-the-oil compounds that may be responsible for the biological potential of the oil. The stability study of the M. oleifera oils was equally conducted to establish indicators for quality control and commercialization. Findings would contribute to the existing body of knowledge to further add value to the traditional uses and commercialization of Moringa oleifera seed and its seed oils across South Africa and beyond.
2. Materials and Methods
All chemicals and reagents used in the study were of analytical reagent grade and of the highest quality available, and were purchased from Rochelle Chemicals (Johannesburg, South Africa) or Sigma-Aldrich (Johannesburg, South Africa). These include concentrated nitric acid, hydrochloric acid, sulphuric acid, ferric chloride, the Folin–Ciocalteu Reagent (FCR), sodium carbonate solution (Na2CO3) 20% (w/v), dimethyl sulfoxide (DMSO), micro Propette (20–200 µL), and Propette (10–1000 µL). Distilled water from a purification system was used in all experiments, and chemicals were used as received.
2.1. Plant Material Collection and Preparation
2.1.1. Sample Collection
The South African M. oleifera seed sample was purchased in March 2020 from a farm located at Lebowakgomo in Limpopo, trading under the name Patience Wellness Centre (23°54′30.5″ S 29°26′22.9″ E). The plant was identified by an Indigenous Knowledge System (IKS) Practitioner who owns the farm. Further identification of the seeds was performed by a botanist at the South African National Biodiversity Institute (SANBI), and voucher specimen MOS001 was deposited at the Department of Pharmaceutical Sciences, Sefako Makgatho Health Sciences University, South Africa.
2.1.2. Sample Preparation
The brown shells that covered each seed were manually cracked and each shell was peeled off by hand, to obtain white seeds. The white seeds were ground into fine powder using a grinding machine (Kinematica AG, Lucerne, Switzerland), mortar and pestle, and later a blender. The seeds are known to be very oily, so during the grinding process they became stuck in the machine, causing an oily layer, which made the machine difficult to use and meant we had to clean it after around 5 seeds had been ground. However, by taking them in small portions, all the seeds were ground using the mortar and pestle to obtain a damp powder.
2.1.3. Extraction Method for Moringa oleifera Seeds
Prior to the sequential extraction, 5.0 g of the gum seed resin was extracted using the same extraction protocol used in the preliminary assessment of the phytoconstituents of the plant. Thereafter, sequential extraction of the plant material was conducted using hexane, dichloromethane, acetone, and methanol. This set of solvents was chosen because both polar and non-polar phytocomponents of the oil were targeted. This study deployed the extraction method by Lavenburg and co-workers [11] but with minor modifications. Briefly, 223.5 g of finely powered seed material was weighed using an analytical balance, and the seed resinous material was then transferred into a 1000 mL glass beaker. Then, 500 mL of solvent (hexane) was dispensed using a measuring cylinder and poured into the 1000 mL beaker with the plant material, which was then closed with a parafilm. The mixture was extracted by ultra-sonication (Labotec Pty Ltd., Midrand, South Africa) at a temperature of 25 °C for 30 min, after which the mixture was left to cool for 2 min. The mixture was filtered into an Erlenmeyer flask, using Whatman No. 1 filter paper with a pore size of 40 μm. The extraction process was repeated two more times and the combined filtrate collated. The filtrate mixture was evaporated using a rotary evaporator (Cole Parmer Ltd., Stone-England, UK) connected to a Vacuubrand MZ 2C NT pump (Vacuubrand GmBH + Co Kg, Wertheim, Germany) set at the boiling temperature of the extracting solvents, such that the solvent was evaporated to dryness, to afford an oil solution, which was then transferred into small beakers which were each weighed and labelled. The plant residue from the hexane extract was dried for 45 min, and the same extraction protocol was used to sequentially extract it using dichloromethane (DCM), acetone (ACTN), and methanol (MeOH), based on their respective solvent polarity of least to most polar. Oily extracts from hexane, DCM, ACTN, and MeOH were obtained after filtration and concentration under pressure, with rotary evaporation and air drying. The percentage yield of each of the dry extracts was calculated using Equation (1) below:
2.1.4. Qualitative Phytochemical Screening of M. oleifera Seeds
The qualitative assay of phytochemical compounds was conducted using the methods already established to assess for categories of phytochemical compounds in plant extracts, as described by Balamurugan et al. [5]. The compounds that were evaluated for absence and/or presence included alkaloids, steroids, triterpenoids, tannins, anthraquinones, saponins, glycosides, proteins, flavonoids, and phenols, which have been documented to exert a biological influence on animal tissues. The dry hexane, dichloromethane, acetone, and methanol extracts of M. oleifera leaves obtained were evaluated for their phytochemical content. The standard protocols used, with slight modifications in some experiments, are summarized in Table 1.

Table 1.
Standard protocols used for the qualitative phytochemical analysis of MOS.
2.1.5. Preliminary Thin Layer Chromatography (TLC) Conditions
Thin Layer Chromatography (TLC) silica gel 60 F254 plates were purchased from Sigma-Aldrich (Merck), 290 Concord Road, Billerica, MA, 01821, USA. TLC studies were carried out for all the extracts on aluminum pre-coated silica gel (G) 60 F254 plates. The best resolution of the test extracts was achieved by developing the TLC plates using a mobile phase consisting of different solvent combinations. The solution of the seed extracts was spotted on the TLC plates by using capillary tubes and developed in a TLC chamber using a suitable mobile phase. The developed TLC plates were air-dried and observed under ultraviolet (UV) light. They were later sprayed with MeOH: H2SO4 (v/v), and some were placed on a hot plate for 1 min for the development of colour and to enhance the visibility of the separated bands. The resolution of the phytochemical bands/spots was expressed by the retardation factor (Rf) values, calculated using Equation (2).
2.2. Biological Activity Assay of M. oleifera Seed Extracts
2.2.1. Antioxidant Assay of M. oleifera Seeds Extracts Using a DPPH Assay
Extracts dissolved in their respective solvents were spotted on the silica gel 60 F254 plates and developed in adequate solvent systems. Thereafter, all the plates were sprayed with a methanolic solution of DPPH (2 mg/mL). The antioxidant compounds in the extracts appeared as yellow bands on a light purple background.
2.2.2. In Vitro Qualitative Antimicrobial Assay of M. oleifera Seed Extracts
The four clinical isolate bacterial strains used in this study were Acinetobacter baumannii (BAA 747), Escherichia coli (ATCC 35218), Klebsiella pneumoniae (ATCC 25922), and Pseudomonas aeruginosa (ATCC 27853). Only Gram-negative strains were used in the qualitative assay, because TLC detection was the only expected outcome. These strains were selected from our strain collection, which included Gram-negative bacteria. The methodology outlined by [15] Nonye and Ojiako (2019) was used with slight modification for the qualitative antimicrobial assay. Cultures were incubated at their favourable growth conditions overnight and tested at 37 °C. Overnight cultures grown in Mueller–Hinton (MH) broth of specific profiled A. baumannii, E. coli, K. pneumoniae, and P. aeruginosa strains were used. The growth was adjusted to 0.5 Mac Farhland for all strains. The chromatograms on the developed TLC plates were sprayed with bacterial suspension until wet. This process was carried out in a Laminar flow cabinet Labotec (Midrand, South Africa). Thereafter, the plates were incubated overnight at 37 °C and 100% relative humidity in the dark and then sprayed with a 2 mg/mL solution of p-iodonitrotetrazolium violet (INT) Sigma-Aldrich (Johannesburg, South Africa) for the detection of zones of inhibition [16] and further incubated overnight before being evaluated for bacterial activity.
2.2.3. In Vitro Quantitative Antimicrobial (MIC) Assay of M. oleifera Seed (MOS) Hexane Oil Extract
The isolated Gram-positive and Gram-negative P. aeruginosa (ATCC9721), S. pyrogens (ATCC19615), E. coli (ATCC105363), Bacillus cereus (ATCC14579), and S. aureus (ATCC25923) strains were used because the spectrum of activity of the oil was being investigated based on a microbroth dilution method in 96 multi-well microtiter plates with slight modifications [16]. Each bacterial culture was prepared in Luria Berthani (LB) broth/agar and/or Mueller–Hinton broth (MHB). A 1.0 mg/mL solution of the oil was prepared. Briefly, 30 µL of Mueller–Hinton broth (MHB) was transferred to every well, and 50 µL of the oil (in triplicate) was added to wells in Row A of the microtiter plate, together with the negative (1% dimethyl sulfoxide) and positive controls (ciprofloxacin). Additionally, a blank (sterile MH broth) and standardized bacterium (control) were prepared by transferring 50 µL to the wells, respectively. Two-fold serial dilutions were performed, resulting in decreasing concentrations over the range of 1000 to 1.0 μg/mL. Thereafter, 10 µL of the standardized bacterium was added to the wells of the micro-well plate. After 24 h incubation at 37 °C, 10 μL of resazurin indicator solution (prepared by dissolving a 270 mg tablet in 40 mL of sterile distilled water) was added and incubated for a further 30 min to 1 h, until an optimal colour developed. Bacterial growth inhibition (clear wells, no colour change) was assessed visually and recorded. The MIC was recorded as the lowest concentration of the extract that inhibited bacterial growth.
2.2.4. GC-MS Stability Studies of M. oleifera Hexane Oil
Briefly, 1.0 mg of the oil was dissolved in 1.0 mL of hexane and vortex for 15 s to afford a homogenous solution. A total of 1.0 µL from the stock solution was injected into a split/splitless mode of an Agilent 7890 GC coupled to an Agilent 5977B inert MSD mass spectrometer with a triple axis detector operating in the positive electron ionization (EI) mode. Agilent MSD ChemStation G1701EA, E.02.01.177 (California, USA) was used for the data management. The GC-MS operating conditions are given in Table 2.

Table 2.
GC-MS conditions used for hexane oil analysis.
2.2.5. UV–Vis Spectrophotometer
A spectrophotometer, Nanocolor® uv/vis, Macherey-Nagel GmbH & Co. KG, (Düren Germany) was used. This is a high-precision instrument consisting of reference detection technology (RDT), a halogen lamp (visible range), a Deuterium lamp (UV range), and a monochromator at a wavelength range of 190–110 nm with an operating unit of 10–40 °C at 80% relative humidity. The power supply ranged between 119 and 240 AC and 50/60 Hz. Samples were scanned in less than 1 min, with spectral bands of <2 nm.
2.2.6. TLC Plate UV Visualization Cabinet
The developed TLC plates were visualized under white light, at a short wave (254 nm) and long wave (366 nm). The UV Spectroline lamp model FE (230 volts 50Hz 0.2 Amps) was mounted on a fluorescence analysis cabinet Model CM-10A (Spectronics Corporation, Westbury, NY, USA) TLC viewing chamber. In some instances, though, the plates were visualized by spraying with either 9:1 v/v methanol/H2SO4 solution or 2 mM solution of DPPH dissolved in methanol.
2.2.7. Statistical Analysis
An analysis of variance (ANOVA) single factor test was conducted in Excel, while the average of the replicated data obtained from the MIC analysis was computed, and the results are presented as the mean and standard deviation.
3. Results
3.1. Obtained Masses and Percentage Yields of M. oleifera Seed (MOS) Extracts
Oily hexane, dichloromethane, methanol, and acetone extracts were obtained from the Moringa oleifera seeds. The masses obtained for each extract were 14.40 g (hexane), 2.26 g (dichloromethane), 0.96 g (acetone), and 1.52 g (methanol). The respective percentage yields of each extract, calculated from the starting plant material, are displayed in Table 3. An ANOVA single-factor test with p = 0.159784 (>0.05) indicated no significant difference between the extraction methods and extract yields.

Table 3.
Percentage yield of the four oily extracts of M. oleifera seeds.
A column plot of the mass and percentage yield of the four extracts (figure not displaed) briefly visualizes the observation that the hexane extract had the highest yield, followed by dichloromethane, methanol, and acetone, with the lowest yield.
3.2. Qualitative Phytochemical Analysis of MOS Oils
We herein report on the class of phytochemicals present in M. oleifera seed oils of the South African ecotype (Table 4). These were preliminary results; the reader may find a detailed chemical characterization of South African Moringa seed oils in our earlier work [17]. These compounds belong to phytochemical groups such as sterols, carboxylic acids, steroids, and alkaloids, among other classes of compounds that have been reported for MOS from other countries and thoroughly reviewed by Özcan [18], highlighting the fact that MOS oils contain tocopherols, sterol 24-methylenecholesterol, campesterol, campestanol, 7-campestanol, stigmasterol, clerosterol, stigmastanol, sterols and other steroids, 7-avenasterol, 5-avenasterol, isoavenasterol, and 7,14-stigmastanol; its fatty acids including oleic, palmitic, stearic, behenic, and arachidic acids [19].

Table 4.
Phytochemicals present/absent in the extracts of Moringa oleifera seed oils.
3.3. Preliminary TLC-Bioautography Dot Blot Antioxidant Activity and Chemical Profiling of MOS Extracts
The 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) free radical method is an antioxidant assay based on electron transfer that produces a violet solution in methanol. DPPH solution in methanol was used to spray the TLC plates spotted with the solution of the different extracts, and they were then left in a dark cupboard for 30 min to dry (Figure 1).
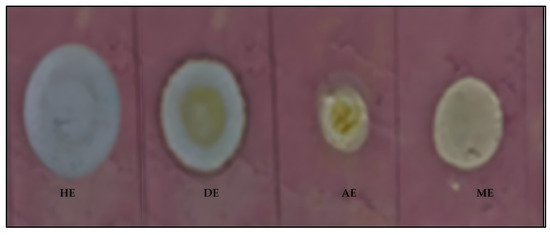
Figure 1.
TLC-dot blot revealing the antioxidant activity of the five (hexane, dichloromethane, acetone, methanol, and water) oil extracts of Moringa oleifera seed oils. HE = hexane extract, DE = dichloromethane extract, AE = acetone extract, and ME = methanol extract.
As evident in Figure 1, the four MOS oil extracts (hexane, dichloromethane, acetone, and methanol) indicated potential for antioxidant or free radical scavenging activities. This was a possibility, judging by the creamy to white coloration against the DPPH purple background on the TLC plate.
To further investigate the exact compound bands that might possess antioxidant activity, a selection of solvents and their combinations were tried as mobile phase to resolve the four extracts into the mixture of compound bands via TLC analysis. It was borne in mind that oils are usually not easy to resolve by TLC due to poor capillary action and solubility problems of oils in most organic solvents. After trying several solvent systems, success was achieved with good separation of the oil extracts (Figure 2) when the TLC plate spotted with the extracts was developed using n-hexane/diethyl ether (8:2 v/v). The different compound bands obtained after the good resolution are displayed in Table 5. The hexane, dichloromethane, acetone, and methanol extracts indicated 4, 5, 2, and 2 compound bands, respectively, at Rf range of 0.05–0.88.
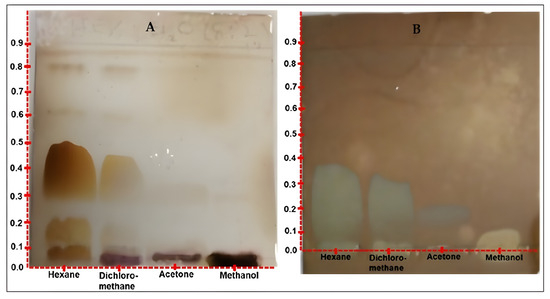
Figure 2.
(A) TLC chromatogram indicating a relatively better resolution of the 4 oil extracts. Plate developed in n-hexane/diethyl ether (8:2 v/v) and derivatized with methanol/H2SO4 (9:1 v/v), and (B) TLC chromatogram revealing compounds with antioxidant potentials. Plate sprayed with 2.0 mM solution of DPPH/MeOH.

Table 5.
Compound bands present in the oil extracts of Moringa oleifera seeds as revealed by TLC analysis.
A different TLC analysis of the four extracts was carried out and the plate sprayed with 0.4 mM of DPPH solution. The result revealed that out of the six mixtures of compound bands on the TLC plate (Figure 2A), only one compound displayed free radical scavenging potential activity (Figure 2B). This compound band was smeared with an Rf of 0.2, with the lowest concentration in the acetone extract, and an Rf of 0.35, for the highest concentration in the hexane extract.
3.4. In Vitro Qualitative Antimicrobial Activity of MOS Oil Extracts by TLC-Bioautography
This report forms part of the antimicrobial study of South African Moringa oleifera seeds’ four oily extracts—hexane, dichloromethane, acetone, and methanol—against four clinically isolated bacterial strains. The TLC-bioautographic dot-blot assay (Figure 3) revealed positive antimicrobial activity of the four extracts against all the test organisms.
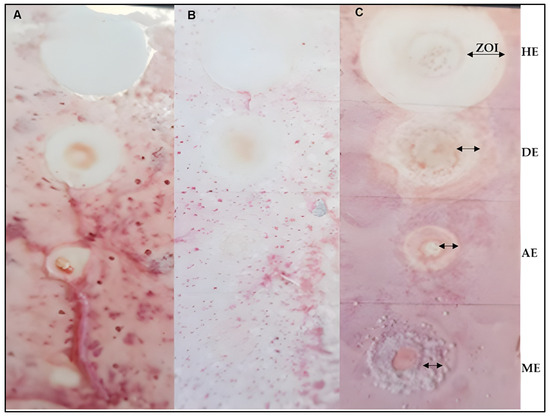
Figure 3.
Antimicrobial TLC-dot blot assay of MOS extracts indicating activity against E. coli (A), K. pneumonia (B), and A. baumannii (C) clinical organisms.  ZOI = Zone of inhibition, HE = Hexane extract, DE = dichloromethane extract (ZOI: 314.2 mm), AE = acetone extract, and ME = methanol extract. The plate for P. aeruginosa is not pictured because it broke accidentally.
ZOI = Zone of inhibition, HE = Hexane extract, DE = dichloromethane extract (ZOI: 314.2 mm), AE = acetone extract, and ME = methanol extract. The plate for P. aeruginosa is not pictured because it broke accidentally.
 ZOI = Zone of inhibition, HE = Hexane extract, DE = dichloromethane extract (ZOI: 314.2 mm), AE = acetone extract, and ME = methanol extract. The plate for P. aeruginosa is not pictured because it broke accidentally.
ZOI = Zone of inhibition, HE = Hexane extract, DE = dichloromethane extract (ZOI: 314.2 mm), AE = acetone extract, and ME = methanol extract. The plate for P. aeruginosa is not pictured because it broke accidentally.
Overall, Table 6 summarizes the antibacterial activity of the South African Moringa oleifera seed oils hexane, dichloromethane, acetone, and methanol. All the extracts were active against A. baumannii, K. pneumonia, E. coli, and P. aeruginosa, which are implicated in some human infectious diseases [20].

Table 6.
Activity of MOS oil extracts against four clinical isolate bacteria strains, as determined by TLC-bioautography dot blot assay.
3.5. In Vitro Quantitative Antimicrobial Activity of MOS Hexane Oil Extracts and In-Oil Compound Identification by GC-MS Analysis
The antibacterial activity of the hexane oil against two Gram-negative (Pseudomonas aeruginosa and Escherichia coli) and three Gram-positive (Streptococcus pyogenes, Bacillus cereus, and Staphylococcus aureus) bacteria was undertaken (Table 6). We investigated this oil, out of the four oily extracts, because hexane is popular in the extraction of edible and other oils [11]. In addition, our study indicated that the hexane oil gave the highest yield and exhibited good qualitative antimicrobial activity across all the test pathogens (Table 7). A p-value of 0.64 (>0.05) indicated no significant difference in the biological activities of the four extracts. The identification of the in-oil compounds proposed to mitigate the antimicrobial effects of the oil was carried out through GC-MS analysis matching our results with GC-MS library. The names and the structures of selected identified compounds are listed in Figure 4.

Table 7.
Minimum inhibitory concentration (MIC in mg/mL) for the hexane extract (oil).
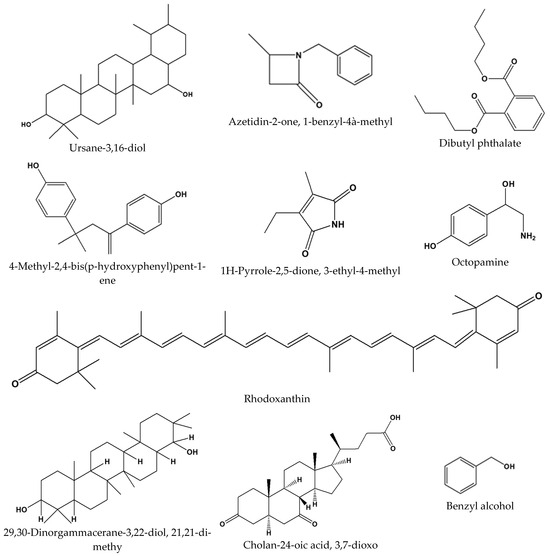
Figure 4.
Selected compounds detected in South African Moringa oleifera Lam. seed oils proposed to be responsible for the antimicrobial potentials of oils against World Health Organization (WHO) Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter (ESKAPE) pathogens.
3.6. GC-MS Satiability Studies of Hexane Oil
The same GC-MS condition used to analyze the hexane oil in 2020 was repeated and used for the hexane oil analysis in 2025. The results obtained affirm to the stability of the oil over a five year period. The chromatogram of the hexane oil analyzed in 2025 is displayed in Figure 5. Even though there were apparent meagre physical discrepancies in the peaks resolution, the annotation of the peaks from the 2025 analyzed samples afforded the same marker compounds that were identified in 2020. Amongst these marker compounds are oleic acid (54.9%), 9-octadecenoic acid (Z)-, methyl ester (23.3%), n-hexadecanoic acid (9.68%), among others (Table 8), through marker compound content observed over a five-year period.

Figure 5.
GC-MS chromatogram indicating the stability of the MOS oil.

Table 8.
Structures of stability-indicating marker compounds observed over a five-year period (2020–2025).
4. Discussion
4.1. Obtained Masses and Percentage Yields of Moringa oleifera Seed (MOS) Extracts
The results found were expected because oils are more soluble in non-polar solvents than they are in polar solvents. The highest yield, exhibited by hexane over dichloromethane, was an indication that the oils are more soluble in hexane than in dichloromethane, even though both are non-polar solvents [21].
4.1.1. Qualitative Phytochemical Analysis of MOS Oils
The Moringa oleifera phytochemical profile from our study indicated the presence of phytochemicals in the Moringa seed oils. Terpenoids and steroids have a stronger presence, which makes sense, as this group of phytochemicals are present mostly in vegetable oils. There were also flavonoids and phenols detected in the hexane and DCM extract, and less of these phytochemicals in the polar acetone and methanol extracts. The detection of these polar phytochemicals in non-polar hexane and dichloromethane extracts seems to contradict the like-dissolves-like paradigm. However, our results are not an isolated case, as other authors have reported similar results in the past [22]. Thus, one could propose that such extracts may possess the antimicrobial and antioxidant activities that are typical of such phytochemical groups. Phenolic glycosides were present only in the hexane and DCM, and there was no presence of tannins; but just traces of saponins.
4.1.2. TLC-Bioautography Dot Blot Antioxidant Activity and Chemical Profiling of MOS Extracts
The acetone extract displayed the faintest cream coloration against the DPPH, indicating that it should exhibit the least antioxidant activity, while the n-hexane extract should exhibit the most antioxidant activity of all the extracts, based on the same reason. While the dot blot assay was not detailed enough in terms of revealing the exact compounds that are responsible for the biological activity of a plant extract under investigation, a TLC-bioautography is a more feasible approach [23].
To further investigate the exact compound bands that might possess antioxidant activity, a selection of solvents and their combinations were tried to resolve the four extracts into the mixture of compound bands via TLC analysis. Due to the colourless nature of the oils, the different bands representing the mixture of compounds were not visible to the naked eye nor under ultraviolet lights at 256 and 366 nm post development of the TLC plates. However, the bands were made visible after the developed plate was derivatized with methanol/H2SO4 (9:1 v/v). As revealed in Section 3.2 and Section 3.4, the dichloromethane extract of South African Moringa oleifera seed oil had a slightly greater mixture of compounds than the hexane extract, with both having totals of six and five mixture compounds bands, respectively. Both the acetone and the methanol extracts indicated just two compounds, and those few compounds were associated with the mobile phase choice used for the analysis of the extracts by TLC.
It must be clarified here that, unlike the dot blot assay, the TLC-bioautography is a better approach in drug discovery research because the antioxidant displayed compound can be targeted, isolated, purified, and elucidated as a potentially new antioxidant compound if it is different from those that are known prior to these studies. Its presence supports the traditional use of South African Moringa oleifera seeds and their oils in the management of oxidative stress-related diseases. It should also be pointed out that other antioxidant compounds were also noticed at the base of the TLC plate and may be better resolved with improved solvent system combinations.
4.1.3. In Vitro Qualitative Antimicrobial Activity of MOS Oil Extracts by TLC-Bioautography
As alluded to in Section 3.4, the entire TLC plate (c) was coated with the bacterial solution, and the zone of inhibition (ZOI) of each extract, which signifies activity, decreased in terms of activity from hexane (with the most activity) down to the acetone extract, with the least activity. This observation is in support of the result obtained for the antioxidant activity of the extracts because the hexane extracts also indicated more of the antioxidant compound with respect to the other extracts.
Several methods, including disc and well diffusion, have previously been used to determine the antimicrobial activity of different Moringa parts [6,7]. Minimum inhibitory as well as minimum bactericidal concentrations have been reported. However, the advantage of the TLC-dot blot and TLC-bioautography methods used in this study lies in the fact that the potentially active compounds are localized and visualized on the TLC plate [23]. The isolation and purification of such active compounds are easily targeted. Moringa oleifera seeds from countries including India [22], Nigeria [24], and Thailand [25], to mention just a few, have been reported for their antimicrobial activities. Based on the observed zones of inhibition (ZOI), one can highlight here that the hexane extract was most active, with ZOI = 314.2 mm. This was followed by dichloromethane, acetone, and lastly methanol extracts. The general observed trend in the antimicrobial activity of MOS decreased as the polarity of the solvent used for extraction increased. The results obtained from this study are in slight variance with those reported for MOS oils from other countries, including in [26,27].
4.1.4. In Vitro Quantitative Antimicrobial Activity of MOS Hexane Oil Extracts
The MOS hexane oil exhibited good to significant antimicrobial inhibitory activities, with the best recorded for P. aeruginosa compared to ciprofloxacin. This is because, according to Gibbons [28], 0.064 mg/mL MIC for a natural product should be considered significant to mitigate pathogenic effects on host cells. The observation that a Gram-negative (P. aeruginosa) bacterial strain is the most susceptible to the seed oil extract is in line with the available literature [29]. Other MIC values of the MOS hexane oil extract are displayed in Table 5.
The results of this study support the claim that terpenoids and steroids inherently express a stronger presence in the seeds of South African Moringa oleifera seed oils. This speaks logically to the presence of this group of phytochemicals appearing mostly in oils. The detection of terpenoids and steroids, which share structural similarities, in the Moringa oleifera seed oils from South Africa sheds new light on the potential use of the plant oils in the management of diseases, because terpenoids are known to possess antitumor, anti-inflammatory, antibacterial, antiviral, and antimalarial effects; promote transdermal absorption; prevent and treat cardiovascular diseases; and possess hypoglycaemic activities. In addition, previous studies have also found that terpenoids have many potential applications, such as insect resistance, immunoregulation, antioxidation and antiaging effects, and neuroprotection. Terpenoids have a complex structure with diverse effects and different mechanisms of action [30]. In a like manner, flavonoids in the hexane and DCM extract might only sound fictitious from a polarity perspective of this group of phytochemicals. We are of the opinion that the glycosylated forms of the flavonoids are the phytochemicals detected in the seed oil investigated. One can then underscore the potential of using such extracts traditionally to manage microbial infections and oxidative stress-related diseases. Over and above these considerations, all the phytochemicals have their unique and broad-spectrum health benefits that humans and other domestic animals can tap from South African Moringa oleifera seed oils. The bactericidal effect of the flavonoids, terpenoids, or alkaloids could be a function of the inhibition of ATPase activity, blocking of bacterial DNA synthesis, inhibition of biofilm formation, resistance to the quorum sensing effect, and the impact on membrane integrity or permeability. Moreover, enzyme modification, plasmid curing, and the drug efflux pump are the other mechanisms of action of phytochemicals to inhibit bacterial activity [27].
With a good MIC against P. aeruginosa obtained from the hexane oil, compared with the ciprofloxacin standard, this study suggests that South African Moringa oleifera hexane oil extract can serve as an alternative to current antibiotics that are resisted by P. aeruginosa, a non-fermentative Gram-negative opportunistic pathogen frequently encountered in difficult-to-treat hospital-acquired infections and wastewaters [31]. With an MIC of 0.25 mg/mL, which is considered effective against E. coli, S. pyrogens, B. cereus, and S. aureus, MOS hexane oil is a significant natural product in the management of infectious diseases caused by these pathogens. This significant spotlight on MOS hexane oil’s inhibitory activity is due to the fact that P. aeruginosa, E. coli, S. pyrogens, B. cereus, and S. aureus all belong to the World Health Organization’s ‘ESKAPE’ (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) global pathogen list, with the highest “priority status” in terms of the threats that these pathogens pose to humans with regard to resistance to currently used antibiotics [32]. The potential multidrug-resistance mechanisms of the ESKAPE pathogens would include drug inactivation, which involves irreversible cleavage catalyzed by an enzyme, the reduced accumulation of the drug either due to reduced permeability or by increased efflux of the drug, and modification of the target site where the antibiotic may act. The ESKAPE pathogens could equally form biofilms that physically prevent the immune response cells of the host or antibiotics from acting to inhibit the pathogen [33].
In our recent publication [17], we notified the scientific community of the specific compounds that are present in the oils, and this study herein reports on the phytochemical, antioxidant, and antimicrobial potentials of the oils. The compounds in the oil include but are not limited to cis-13-octadeaconic acid, cis-vaccenic acid, an isomer of oleic acid, 9-octadecanoic acid-(z)-methyl ester, 2,21-Dipyridylamine, and 29,30-Dinorgammacerane-3,22-diol, 21,21-dimethyl-, (3α, 8α, 9α, 13α, 14α, 17α, 18α, 22α). Based on the phytochemical class in Table 4, steroids, terpenoids, phenols, flavonoids, and alkaloids were predominantly present in the oils. The selected compounds from this phytochemical class detected in the oils, some of which we have reported on earlier, are displayed in Figure 4. We strongly suggest that these compounds mitigate and may justify the antimicrobial and antioxidant activities of the South African Moringa oleifera seed oils.
4.1.5. GC-MS Stability Studies of Hexane Oil
This study is crucial for the development of plant-based products and commercialization. Taking this into account for Moringa oils if formulated to products including emulsions, lotions among others, the results of the current study encourages the formulation and commercialization of such products. Moringa oil, like other oils loaded with alginate, zeolite, chitisan, or liposome, is a promising drug delivery system.
In particular, the microemulsion consisting of water-in-oil, oil-in-water or bicontinuous type is able to break the epidermal skin layer or barrier [34]. This speaks to Moringa oils as a suitable alternative for transdermal drug delivery that has the potential to increase absorption of the drug as well as its bioavailability [35]. Combining the aforementioned good quality attributes with the antimicrobial activities of the Moringa oil that is reported in the study, places the oil as a double-barrel drug–drug delivery system.
However, standard storage conditions were not ensured for oil used in the study because we never anticipated formulating commercial products at this stage of our research on the Moringa and its medicinally useful parts. The poor storage conditions, we assume, are responsible for the difference in the percentage amount of the markers we reported in 2022 [17] compared to that in the current study. By and large, formulating and commercializing various pharmaceutical products containing Moringa oil as the pharmaceutical ingredient is worth venturing into.
5. Conclusions
Moringa oleifera seeds are popular in South Africa as a water purification agent. Whereas we first shed light on the potential of the seed oils to be used as a cooking oil in a paper we published in September 2022, the current study findings are in strong support of the use of the seed oils as an antidote against free radicals that may cause oxidative stress-related diseases. In addition, the oils also indicate potential in the management of microbial infections that are caused by clinical isolates, including Acinetobacter baumannii (BAA 747), Escherichia coli (ATCC 25922), Klebsiella pneumoniae (ATCC 27853), and Pseudomonas aeruginosa (ATCC 278534). We intend to disseminate the valuable information contained in this study to the farmers whose samples we purchased for the study, by physically visiting them and giving them a presentation. By so doing, the use of the seeds and/or their oil will go beyond water purification. Future work especially on the quantitative antioxidant-using robust assays are suggested.
Author Contributions
K.B., conceptualization; M.M., formal investigations—extractions and qualitative antioxidant and antimicrobial assays; K.B., quantitative antimicrobial assays; K.B., GC-MS compound identification; K.B., writing of original manuscript; K.B., revision of the original manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors would like to thank the Sefako Makgatho Health Science University, School of Pharmacy for the financial support towards the article processing charges. The authors declare that they have not used Artificial Intelligence (AI) tools in the creation of this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Moringa oleifera Seeds and Oil: Characteristics and Uses for Human Health. Int. J. Mol. Sci. 2016, 12, 2141. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hernández, R.; Hernández-Rodríguez, M.; CruzMonterrosa, R.G.; Díaz-Ramírez, M. Morphological characterization of Moringa oleifera seeds from different crops of Mexico. Agro Product. 2021, 14, 3–15. [Google Scholar] [CrossRef]
- Pandey, A.; Pandey, R.D.; Tripathi, P.K.; Gupta, P.P. Moringa Oleifera Lam. Sahijan—A Plant with a Plethora of Diverse Therapeutic Benefits: An Updated Retrospection. Med. Aromat. Plants 2011, 1, 1–8. [Google Scholar] [CrossRef]
- Savithramma, N.; Rao, M.L.; Ankanna, S. Preliminary Phytochemical Screening of Some Important Medicinal Plants. Int. J. Ayurvedic Herb. Med. 2012, 2, 139–145. [Google Scholar]
- Balamurugan, V.; Fatima, S.; Velurajan, S. A guide to phytochemical analysis. Int. J. Adv. Res. Innov. Ideas Educ. 2019, 5, 236–245. [Google Scholar]
- Choma, I.M.; Wioleta, J. TLC-direct bioautography as a high throughput method for detection of antimicrobials in plants. Chromatography 2021, 2, 225–238. [Google Scholar] [CrossRef]
- Anwar, F.; Bhanger, M.I. Analytical characterization of Moringa oleifera seeds, part-I. The antibiotic compound and its deactivation in aqueous solution. J. Sci. 2003, 5, 6558–6563. [Google Scholar]
- Padayachee, B.; Baijnath, H. An updated comprehensive review of the medicinal, phytochemical and pharmacological properties of Moringa oleifera. S. Afr. J. Bot. 2020, 129, 304–316. [Google Scholar] [CrossRef]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miller, W.R.; Arias, C.A. ESKAPE pathogens: Antimicrobial resistance, epidemiology, clinical impact and therapeutics. Nat. Rev. Microbiol. 2024, 22, 598–616. [Google Scholar] [CrossRef] [PubMed]
- Lavenburg, V.M.; Rosentrater, K.A.; Jung, S. Extraction Methods of Oils and Phytochemicals from Seeds and Their Environmental and Economic Impacts. Processes 2021, 9, 1839. [Google Scholar] [CrossRef]
- Jaradat, N.; Hussen, F.; Ali, A.A. Preliminary phytochemical screening, quantitative estimation of total flavonoids, total phenols and antioxidant activity of Ephedra alata Decne. J. Mater. Environ. Sci. 2015, 6, 1771–1778. [Google Scholar]
- Asha, S.; Asha, A.; Rajeshkumar, S. Evaluation of phytochemical constituents and antimicrobial activity of silver nanoparticle synthesized ipomoea nil against selected pathogens. Asian J. Pharm. Clin. Res. 2017, 10, 183–187. [Google Scholar] [CrossRef]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G. Phytochemical Screening and Extraction: A review. Int. Pharm. Sci. 2011, 1, 98–106. [Google Scholar][Green Version]
- Nonye, O.E. Phytochemical Analysis and Antimicrobial Screening Of Moringa Oleifera Leaves Extract. Int. J. Eng. Sci. 2014, 3, 32–35. [Google Scholar][Green Version]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Bassey, K.; Mabowe, M.; Mothibe, M.; Witika, B.A. Chemical Characterization and Nutritional Markers of South African Moringa oleifera Seed Oils. Molecules 2022, 27, 574. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.M. Moringa spp: Composition and bioactive properties. S. Afr. J. Bot. 2020, 29, 25–31. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2011, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Karaiskos, I.; Lagou, S.; Pontikis, K.; Rapti, V.; Poulakou, G. The ‘old’ and the ‘new’ Antibiotics for the MDR Gram-negative pathogens: For whom, when, and how. Front. Public. Health 2019, 7, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.P.J.; Prasad, S.R.; Banerjee, R.; Agarwal, D.K.; Kulkarni, K.S.; Ramesh, K.V. Green solvents and technologies for oil extraction from oil seeds. Chem. Cent. J. 2017, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Priya, V.; Abiramasundari, P.; Devi, S.G.; Jeyanthi, G.P. Antibacterial activity of the leaves, bark, seed and flesh of Moringa oleifera. Int. J. Pharm. Sci. Res. 2011, 2, 2045–2049. [Google Scholar]
- Wang, M.; Zhang, Y.; Wang, R.; Wang, Z.; Yang, B.; Kuang, H. An Evolving Technology That Integrates Classical Methods with Continuous Technological Developments: Thin-Layer Chromatography Bioautography. Molecules 2021, 26, 4647. [Google Scholar] [CrossRef] [PubMed]
- Mbata, C.A.; Adebayo, A.O.; Chinyere, N.; Cecilia, D. Antimicrobial Activity of the Leaf and Seed Extracts of Moringa Oleifera on Some Bacteria Isolates. Int. J. Med. Sci. Clin. Res. 2015, 3, 3904–3912. [Google Scholar]
- Ruttarattanamongkol, K.; Petrasch, A. Antimicrobial activities of Moringa oleifera seed and seed oil residue and oxidative stability of its cold pressed oil compared with extra virgin olive oil. Songklanakarin J. Sci. Technol. 2015, 37, 587–594. [Google Scholar]
- Lalas, S.; Gortzi, O.; Athanasiadis, V.; Tsaknis, J.; Chinou, I. Determination of antimicrobial activity and resistance to oxidation of Moringa peregrina seed oil. Molecules 2012, 17, 2330–2334. [Google Scholar] [CrossRef] [PubMed]
- Abdulrasheed, M.; Ibrahim, I.H.; Mubarak, M.A.; Umar, F.A. Comparison of antimicrobial activity of seed oil of garlic and Moringa oleifera against some food-borne microorganisms. Bayero J. Pure Appl. Sci. 2015, 8, 196–201. [Google Scholar] [CrossRef]
- Gibbons, S. Anti-staphylococcal plant natural products. Nat. Prod. Rep. 2004, 2, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Van, L.T.; Hagiu, I.; Popovici, A.; Marinescu, F.; Gheorghe, I.; Curutiu, C.; Ditu, L.M.; Holban, A.-M.; Sesan, T.E.; Lazar, V. Antimicrobial Efficiency of Some Essential Oils in Antibiotic-Resistant Pseudomonas aeruginosa Isolates. Plants 2022, 11, 2003. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, Y.; Tian, W.; Cui, X.; Tu, P.; Li, J.; Shi, S.; Liu, X. Biosynthesis Investigations of Terpenoid, Alkaloid, and Flavonoid Antimicrobial Agents Derived from Medicinal Plants. Antibiotics 2022, 11, 1380. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in pharmacological activities of terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X20903555. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Chen, M.; Duan, Z.; Zou, Y.; He, Y.; Zeng, F.; Yuan, Y.; Fu, T.; Tu, H.; Li, R.; et al. Fabrication, charaterization, and bioactivity of self-assessmbled carrier-free colloidal dispersion from citrus Lemon ‘Rosso’ essential oil and tea polyphenols. Food Chem. 2024, 457, 140058. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dou, J.; Wang, Y.; Mu, G.; Zhang, X. Advances in plant essential oils and drug delivery systems for skincare. Front. Pharmacology 2025, 16, 1578280. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).