Peanut and Pecan Nut Shell Extracts Reduced Disease Incidence and Severity Caused by Grey Mold in Postharvest Strawberries
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Extracts
2.2. Fungal Strain Isolation and Characterization
2.3. Total Phenolic Compounds (TPC) and Antioxidant Activity (AA) of Extracts
2.4. In Vitro Antifungal Assays of Pecan Nut and Peanut Shell Extracts Against B. cinerea
2.5. Ex Vivo Antifungal Assays of Pecan Nut and Peanut Shell Extracts Against B. cinerea on Strawberry Fruits
2.6. Statistical Analysis
3. Results
3.1. Fungal Strain Obtaining
3.2. Extract Yield, Total Phenolic Compounds (TPC), and Antioxidant Activity (AA)
3.3. In Vitro Antifungal Assays of Peanut and Pecan Nut Shell Extracts Against B. cinerea
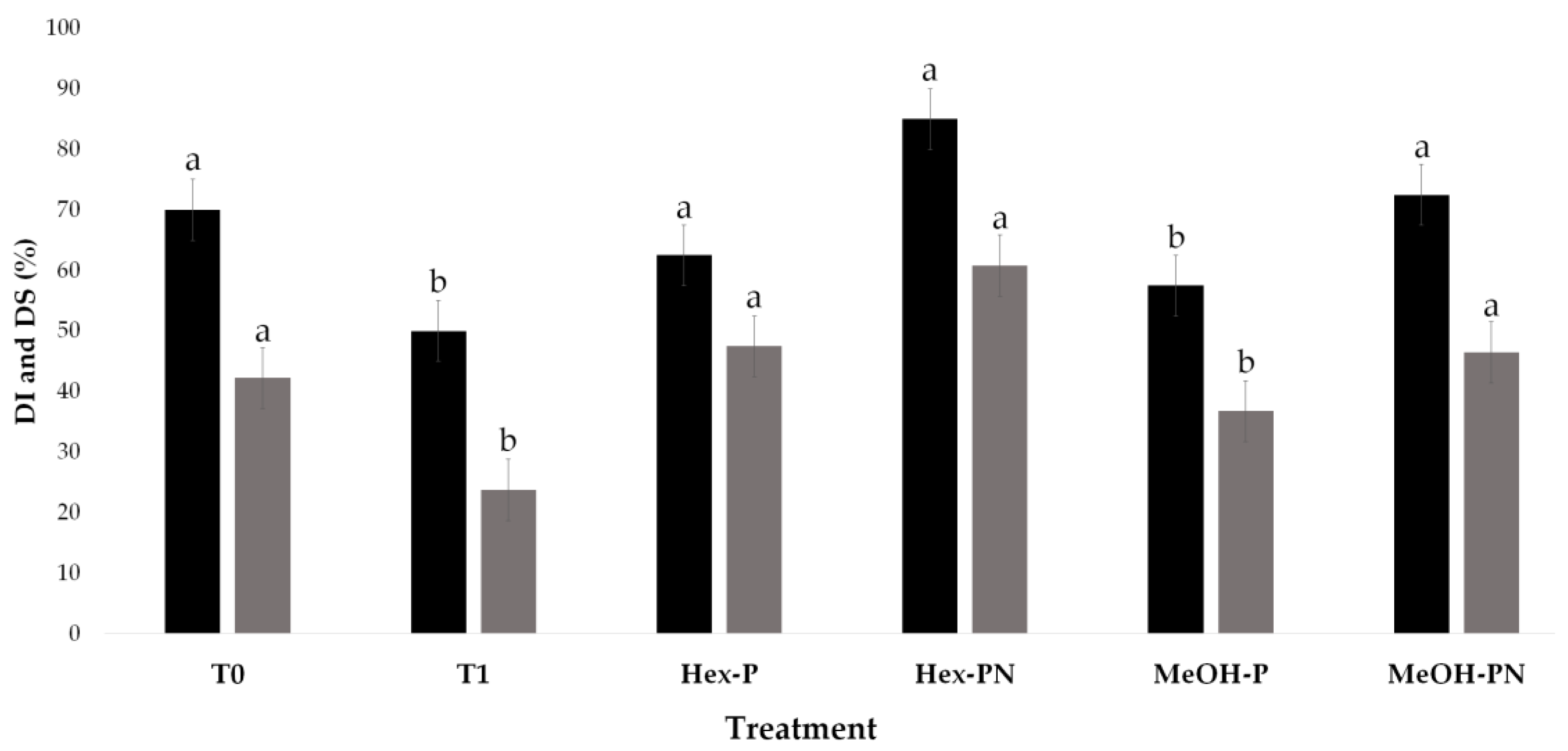
3.4. Ex Vivo Antifungal Assays of Peanut and Pecan Nut Shell Extracts Against B. cinerea on Strawberry Fruits
- Peanut shell extracts, particularly MeOH-P, exhibited superior antifungal activity in reducing mycelial growth in vitro, disease incidence, and disease severity in strawberries infected by B. cinerea, showing similar effectiveness to the synthetic fungicide (T1);
- The pecan nut shell extracts performed less effectively in the ex vivo assays, with lower reductions in both disease incidence and severity compared to peanut shell treatments;
- MeOH-P was the most effective treatment for controlling B. cinerea in strawberries, achieving substantial reductions in disease development, while Hex-P also showed moderate effectiveness.
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matrose, N.A.; Obikeze, K.; Belay, Z.A.; Caleb, O.J. Plant extracts and other natural compounds as alternatives for post-harvest management of fruit fungal pathogens: A review. Food Biosci. 2021, 41, 100840. [Google Scholar] [CrossRef]
- Savary, S.; Ficke, A.; Aubertot, J.N.; Hollier, C. Crop losses due to diseases and their implications for global food production losses and food security. Food Secur. 2012, 4, 519–537. [Google Scholar] [CrossRef]
- Bernat, M.; Casals, C.; Teixidó, N.; Torres, R.; Carballo, B.C.; Usall, J. Efficacy of environmentally friendly disinfectants against the major postharvest pathogens of stone fruits on plastic and wood surfaces. Food Sci. Technol. Int. 2019, 25, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Romanazzi, G.; Smilanick, J.L.; Feliziani, E.; Droby, S. Integrated management of postharvest gray mold on fruit crops. Postharvest Biol. Technol. 2016, 113, 69–76. [Google Scholar] [CrossRef]
- Choquer, M.; Fournier, E.; Kunz, C.; Levis, C.; Pradier, J.M.; Simon, A.; Viaud, M. Botrytis cinerea virulence factors: New insights into a necrotrophic and polyphageous pathogen. FEMS Microbiol. Lett. 2007, 277, 1–10. [Google Scholar] [CrossRef]
- Bajaj, K.; Adhikary, T.; Gill, P.P.; Kumar, A. Edible coatings enriched with plant-based extracts preserve postharvest quality of fruits: A review. Prog. Org. Coat. 2023, 182, 107669. [Google Scholar] [CrossRef]
- Roidaki, A.; Kollia, E.; Panagopoulou, E.; Chiou, A.; Varzakas, T.; Markaki, P. Super foods and super herbs: Antioxidant and antifungal activity. Curr. Res. Nutr. Food Sci. J. 2016, 4, 138–145. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Ajayi, V.A.; Lateef, A. Biotechnological valorization of agrowastes for circular bioeconomy: Melon seed shell, groundnut shell and groundnut peel. Clean. Circ. Bioecon. 2023, 4, 100039. [Google Scholar] [CrossRef]
- de Prá Andrade, M.; Piazza, D.; Poletto, M. Pecan nutshell: Morphological, chemical and thermal characterization. J. Mater. Res. Technol. 2021, 13, 2229–2238. [Google Scholar] [CrossRef]
- Ngangyo Heya, M.; Romo Hernández, A.L.; Foroughbakhch Pournavab, R.; Ibarra Pintor, L.F.; Díaz-Jiménez, L.; Heya, M.S.; Salas Cruz, L.R.; Carrillo Parra, A. Physicochemical characteristics of biofuel briquettes made from pecan (Carya illinoensis) pericarp wastes of different particle sizes. Molecules 2022, 27, 1035. [Google Scholar] [CrossRef] [PubMed]
- Flores-Estrada, R.A.; Gámez-Meza, N.; Medina-Juárez, L.A.; Castillón-Campaña, L.G.; Molina-Domínguez, C.C.; Rascón-Valenzuela, L.A.; García-Galaz, A. Chemical composition, antioxidant, antimicrobial and antiproliferative activities of wastes from pecan nut [Carya illinoinensis (Wagenh) K. Koch]. Waste Biomass Valori. 2020, 11, 3419–3432. [Google Scholar] [CrossRef]
- Kureck, I.; Policarpi, P.D.; Toaldo, I.M.; Maciel, M.V.; Bordignon-Luiz, M.T.; Barreto, P.L.; Block, J.M. Chemical characterization and release of polyphenols from pecan nut shell [Carya illinoinensis (Wangenh) C. Koch] in zein microparticles for bioactive applications. Plant Foods Hum. Nutr. 2018, 73, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Ozcariz-Fermoselle, M.V.; Fraile-Fabero, R.; Girbés-Juan, T.; Arce-Cervantes, O.; de Rueda-Salgueiro, J.A.; Azul, A.M. Use of lignocellulosic wastes of pecan (Carya illinoinensis) in the cultivation of Ganoderma lucidum. Revista Iberoam. Micol. 2018, 35, 103–109. [Google Scholar] [CrossRef] [PubMed]
- De La Rosa, L.A.; Alvarez-Parrilla, E.; Shahidi, F. Phenolic compounds and antioxidant activity of kernels and shells of Mexican pecan (Carya illinoinensis). J. Agric. Food Chem. 2011, 59, 152–162. [Google Scholar] [CrossRef]
- Stafne, E.T.; Rohla, C.T.; Carroll, B.L. Pecan shell mulch impact on ‘Loring’peach tree establishment and first harvest. Horttechnology 2009, 19, 775–780. [Google Scholar] [CrossRef]
- Morgan, C.; Poor, C.; Giudice, B.; Bibb, J. Agricultural byproducts as amendments in bioretention soils for metal and nutrient removal. J. Environ. Eng. 2020, 146, 04020029. [Google Scholar] [CrossRef]
- Aguayo-Villarreal, I.A.; Ramírez-Montoya, L.A.; Hernández-Montoya, V.; Bonilla-Petriciolet, A.; Montes-Morán, M.A.; Ramírez-López, E.M. Sorption mechanism of anionic dyes on pecan nut shells (Carya illinoinensis) using batch and continuous systems. Ind. Crops Prod. 2013, 48, 89–97. [Google Scholar] [CrossRef]
- Hernández-Montoya, V.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Montes-Morán, M.A.; Pérez-Cruz, M.A. Role of the pericarp of Carya illinoinensis as biosorbent and as precursor of activated carbon for the removal of lead and acid blue 25 in aqueous solutions. J. Anal. Appl. Pyrolysis 2011, 92, 143–151. [Google Scholar] [CrossRef]
- Garcia-Larez, F.L.; Esquer, J.; Guzmán, H.; Zepeda-Quintana, D.S.; Moreno-Vásquez, M.J.; Rodríguez-Félix, F.; Del Toro-Sánchez, C.L.; López-Corona, B.E.; Tapia-Hernández, J.A. Effect of Ultrasound-Assisted Extraction (UAE) parameters on the recovery of polyphenols from pecan nutshell waste biomass and its antioxidant activity. Biomass Convers. Biorefin. 2024, 15, 10977–10995. [Google Scholar] [CrossRef]
- Dunford, N.T.; Gumus, Z.P.; Gur, C.S. Chemical composition and antioxidant properties of pecan shell water extracts. Antioxidants 2022, 11, 1127. [Google Scholar] [CrossRef] [PubMed]
- Arciello, A.; Panzella, L.; Dell’Olmo, E.; Abdalrazeq, M.; Moccia, F.; Gaglione, R.; Salazar, S.A.; Napolitano, A.; Mariniello, L.; Giosafatto, C.V. Development and characterization of antimicrobial and antioxidant whey protein-based films functionalized with pecan (Carya illinoinensis) nut shell extract. Food Packag. Shelf Life 2021, 29, 100710. [Google Scholar] [CrossRef]
- Moccia, F.; Agustin-Salazar, S.; Berg, A.L.; Setaro, B.; Micillo, R.; Pizzo, E.; Weber, F.; Gamez-Meza, N.; Schieber, A.; Cerruti, P.; et al. Pecan (Carya illinoinensis (Wagenh.) K. Koch) nut shell as an accessible polyphenol source for active packaging and food colorant stabilization. ACS Sustain. Chem. Eng. 2020, 8, 6700–6712. [Google Scholar] [CrossRef] [PubMed]
- Neira-Vielma, A.A.; Meléndez-Ortiz, H.I.; García-López, J.I.; Sanchez-Valdes, S.; Cruz-Hernández, M.A.; Rodríguez-González, J.G.; Ramírez-Barrón, S.N. Green synthesis of silver nanoparticles using pecan nut (Carya illinoinensis) shell extracts and evaluation of their antimicrobial activity. Antibiotics 2022, 11, 1150. [Google Scholar] [CrossRef]
- Sahar, J.D.; Hoorieh, D.; Farzaneh, N.; Malak, H. Characterization and the evaluation of antimicrobial activities of silver nanoparticles biosynthesized from Carya illinoinensis leaf extract. Heliyon 2020, 6, e03624. [Google Scholar]
- Cepeda-Siller, M.; García-Calvario, J.M.; Hernández-Juárez, A.; Ochoa-Fuentes, Y.M.; Garrido-Cruz, F.; Cerna-Chávez, E.; Dávila-Medina, M.D. Toxicity of Carya illinoinensis (Fágales: Junglandaceae) extracts against Meloidogyne incógnita (Tylenchida: Heteroderidae) in tomato. Ecosis. Recur. Agropec. 2018, 5, 143–148. [Google Scholar]
- Garrido, C.F.; Cepeda, S.M.; Hernández, C.F.; Ochoa, F.Y.; Cerna, C.E.; Morales, A.D. Efectividad biológica de extractos de Carya illinoensis, para el control de Meloidogyne incognita. Rev. Mex. Cienc. Agríc. 2014, 5, 1317–1323. [Google Scholar]
- Lujan, P.A.; Dura, S.; Guzman, I.; Steiner, R.; Sanogo, S. Comparison of proanthocyanidin and phenolic rich extracts derived from pecan shell and husk as elicitors of induced resistance against Phytophthora capsici on Chile pepper. Plant Health Prog. 2023, 24, 369–374. [Google Scholar] [CrossRef]
- Kharel, K.; Kraśniewska, K.; Gniewosz, M.; Prinyawiwatkul, W.; Fontenot, K.; Adhikari, A. Antimicrobial screening of pecan shell extract and efficacy of pecan shell extract-pullulan coating against Listeria monocytogenes, Salmonella enterica, and Staphylococcus aureus on blueberries. Heliyon 2024, 10, e29610. [Google Scholar] [CrossRef]
- Yemmireddy, V.K.; Cason, C.; Moreira, J.; Adhikari, A. Effect of pecan variety and the method of extraction on the antimicrobial activity of pecan shell extracts against different foodborne pathogens and their efficacy on food matrices. Food Cont. 2020, 112, 107098. [Google Scholar] [CrossRef]
- Caxambu, S.; Biondo, E.; Kolchinski, E.M.; Padilha, R.L.; Brandelli, A.; Sant’Anna, V. Evaluation of the antimicrobial activity of pecan nut [Carya illinoinensis (Wangenh) C. Koch] shell aqueous extract on minimally processed lettuce leaves. Food Sci. Technol. 2016, 36, 42–45. [Google Scholar] [CrossRef]
- do Prado, A.C.; da Silva, H.S.; da Silveira, S.M.; Barreto, P.; Vieira, C.R.; Maraschin, M.; Salvador Ferreira, S.R.; Block, J.M. Effect of the extraction process on the phenolic compounds profile and the antioxidant and antimicrobial activity of extracts of pecan nut [Carya illinoinensis (Wangenh) C. Koch] shell. Ind. Crop. Prod. 2014, 52, 552–561. [Google Scholar] [CrossRef]
- Babu, D.; Crandall, P.G.; Johnson, C.L.; O’Bryan, C.A.; Ricke, S.C. Efficacy of antimicrobials extracted from organic pecan shell for inhibiting the growth of Listeria spp. J. Food Sci. 2013, 78, 1899–1903. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Castillo, F.D.; Castillo-Reyes, F.; Gallegos-Morales, G.; Rodríguez-Herrera, R.; Aguilar-González, C.N. Lippia graveolens and Carya illinoensis organic extracts and their in vitro effect against Rhizoctonia Solani Kuhn. Am. J. Agric. Biol. Sci. 2010, 5, 380–384. [Google Scholar] [CrossRef][Green Version]
- Osorio, E.; Flores, M.; Hernández, D.; Ventura, J.; Rodríguez, R.; Aguilar, C.N. Biological efficiency of polyphenolic extracts from pecan nuts shell (Carya Illinoensis), pomegranate husk (Punica granatum) and creosote bush leaves (Larrea tridentata Cov.) against plant pathogenic fungi. Ind. Crops Prod. 2010, 31, 153–157. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, J.; Du, F. Potential use of peanut by-products in food processing: A review. J. Food Sci. Technol. 2012, 49, 521–529. [Google Scholar] [CrossRef]
- Mohd Zaini, N.A.; Azizan, N.A.; Abd Rahim, M.H.; Jamaludin, A.A.; Raposo, A.; Raseetha, S.; Zandonadi, R.P.; BinMowyna, M.N.; Raheem, D.; Lho, L.H.; et al. A narrative action on the battle against hunger using mushroom, peanut, and soybean-based wastes. Front. Public Health 2023, 11, 1175509. [Google Scholar] [CrossRef]
- Gao, A.X.; Xiao, J.; Xia, T.C.; Dong, T.T.; Tsim, K.W. The extract of peanut shells enhances neurite outgrowth of neuronal cells: Recycling of agricultural waste for the development of nutraceutical products. J. Funct. Foods 2022, 91, 105023. [Google Scholar] [CrossRef]
- Meng, W.; Shi, J.; Zhang, X.; Lian, H.; Wang, Q.; Peng, Y. Effects of peanut shell and skin extracts on the antioxidant ability, physical and structure properties of starch-chitosan active packaging films. Int. J. Biol. Macromol. 2020, 152, 137–146. [Google Scholar] [CrossRef]
- Adhikari, B.; Dhungana, S.K.; Ali, M.W.; Adhikari, A.; Kim, I.D.; Shin, D.H. Antioxidant activities, polyphenol, flavonoid, and amino acid contents in peanut shell. J. Saudi Soc. Agric. Sci. 2019, 18, 437–442. [Google Scholar] [CrossRef]
- Oulidi, O.; Nakkabi, A.; Boukhlifi, F.; Fahim, M.; Lgaz, H.; Alrashdi, A.A.; Elmoualij, N. Peanut shell from agricultural wastes as a sustainable filler for polyamide biocomposites fabrication. J. King Saud Univ. Sci. 2022, 34, 102148. [Google Scholar] [CrossRef]
- Kim, M.; Jung, J.M.; Jung, S.; Kim, J.; Bhatnagar, A.; Tsang, Y.F.; Lin, K.A.; Kwon, E.E. Biochar as a catalyst in the production of syngas and biodiesel from peanut waste. Int. J. Ener. Res. 2022, 46, 19287–19299. [Google Scholar] [CrossRef]
- Kim, M.; Lee, D.J.; Jung, S.; Chang, S.X.; Lin, K.Y.; Bhatnagar, A.; Kwon, E.E.; Tsang, Y.F. Valorization of peanut wastes into a catalyst in the production of biodiesel. Int. J. Ener. Res. 2022, 46, 1299–1312. [Google Scholar] [CrossRef]
- Tsade, H.; Murthy, H.A.; Muniswamy, D. Bio-sorbents from agricultural wastes for eradication of heavy metals: A review. J. Mat. Environ. Sci. 2020, 11, 1719–1735. [Google Scholar]
- Shruthi, K.M.; Pavithra, M.P. A study on utilization of groundnut shell as biosorbent for heavy metals removal. Int. J. Eng. Technol. 2018, 4, 411–415. [Google Scholar]
- Oliveira, F.D.; Paula, J.H.; Freitas, O.M.; Figueiredo, S.A. Copper and lead removal by peanut hulls: Equilibrium and kinetic studies. Desalination 2009, 248, 931–940. [Google Scholar] [CrossRef][Green Version]
- Salem, M.A.; Aborehab, N.M.; Al-Karmalawy, A.A.; Fernie, A.R.; Alseekh, S.; Ezzat, S.M. Potential valorization of edible nuts by-products: Exploring the immune-modulatory and antioxidants effects of selected nut shells extracts in relation to their metabolic profiles. Antioxidants 2022, 11, 462. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, M.; He, L.; Fu, P.; Wang, L.; Zhou, J. Optimization of microwave-assisted enzymatic extraction of polyphenols from waste peanut shells and evaluation of its antioxidant and antibacterial activities in vitro. Food Bioprod. Proces. 2013, 91, 158–168. [Google Scholar] [CrossRef]
- Terea, H.; Selloum, D.; Rebiai, A.; Bouafia, A.; Ben Mya, O. Preparation and characterization of cellulose/ZnO nanoparticles extracted from peanut shells: Effects on antibacterial and antifungal activities. Biomass Convers. Biorefin. 2024, 14, 19489–19500. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Plexida, S.; Pereira, C.; Dias, M.I.; Calhelha, R.; Chrysargyris, A.; Tzortzakis, N.; Petrović, J.; Soković, M.D.; et al. The sustainable use of cotton, hazelnut and ground peanut waste in vegetable crop production. Sustainability 2020, 12, 8511. [Google Scholar] [CrossRef]
- Kyei, S.K.; Akaranta, O.; Darko, G. Synthesis, characterization and antimicrobial activity of peanut skin extract-azo-compounds. Sci. Afr. 2020, 8, e00406. [Google Scholar] [CrossRef]
- Velmurugan, P.; Sivakumar, S.; Young-Chae, S.; Seong-Ho, J.; Pyoung-In, Y.; Jeong-Min, S.; Sung-Chul, H. Synthesis and characterization comparison of peanut shell extract silver nanoparticles with commercial silver nanoparticles and their antifungal activity. J. Ind. Eng. Chem. 2015, 31, 51–54. [Google Scholar] [CrossRef]
- Rivilli, P.L.; Alarcón, R.; Isasmendi, G.L.; Pérez, J.D. Stepwise isothermal fast pyrolysis (SIFP). Part II. SIFP of peanut shells-Antifungal properties of phenolic fractions. BioResources 2012, 7, 0112–0117. [Google Scholar] [CrossRef]
- Di Liberto, M.G.; Stegmayer, M.I.; Svetaz, L.A.; Derita, M.G. Evaluation of Argentinean medicinal plants and isolation of their bioactive compounds as an alternative for the control of postharvest fruits phytopathogenic fungi. Rev. Bras. Farmacog. 2019, 29, 686–688. [Google Scholar] [CrossRef]
- Álvarez, N.H.; Stegmayer, M.I.; Seimandi, G.M.; Pensiero, J.F.; Zabala, J.M.; Favaro, M.A.; Derita, M.G. Natural products obtained from Argentinean native plants are fungicidal against citrus postharvest diseases. Horticulturae 2023, 9, 562. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal Barcording Consortium. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. CLSI Standard M38, 3rd ed.; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2017; pp. 1–35. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin Ciocalteu reagent. Method Enzymol. 1999, 299, 152–178. [Google Scholar]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef]
- Magaldi, S.; Mata-Essayag, S.; De Capriles, C.H.; Pérez, C.; Colella, M.T.; Olaizola, C.; Ontiveros, Y. Well diffusion for antifungal susceptibility testing. Int. J. Infect. Dis. 2004, 8, 39–45. [Google Scholar] [CrossRef]
- Stegmayer, M.I.; Fernández, L.N.; Álvarez, N.H.; Seimandi, G.M.; Reutemann, A.G.; Derita, M.G. In vitro antifungal screening of Argentine native or naturalized plants against the phytopathogen Monilinia fructicola. Comb. Chem. High Throughput Screen. 2022, 25, 1158–1166. [Google Scholar]
- Di Liberto, M.G.; Seimandi, G.M.; Fernández, L.N.; Ruiz, V.E.; Svetaz, L.A.; Derita, M.G. Botanical control of citrus green mold and peach brown rot on fruits assays using a Persicaria acuminata phytochemically characterized extract. Plants 2021, 10, 425. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.; Mohamed, M.T.; Yusoff, S.F.; Hata, E.M.; Tajidin, N.E. Selecting antagonistic yeast for postharvest biocontrol of Colletotrichum gloeosporioides in papaya fruit and possible mechanisms involved. Agronomy 2021, 11, 760. [Google Scholar] [CrossRef]
- Romanazzi, G.; Nigro, F.; Ippolito, A.; Salerno, M. Effect of short hypobaric treatments on postharvest rots of sweet cherries, strawberries and table grapes. Postharvest Biol. Technol. 2001, 22, 1–6. [Google Scholar] [CrossRef]
- Singh, V.; Deverall, B.J. Bacillus subtilis as a control agent against fungal pathogens of citrus fruit. Trans. Br. Mycol. Soc. 1984, 83, 487–490. [Google Scholar] [CrossRef]
- Favaro, M.A.; Roeschlin, R.A.; Ribero, G.G.; Maumary, R.L.; Fernández, L.N.; Lutz, A.; Sillon, M.; Rista, L.M.; Marano, M.R.; Gariglio, N.F. Relationship between copper content in orange leaves, bacterial biofilm formation and citrus canker disease control after different copper treatment. Crop Prot. 2019, 92, 182–189. [Google Scholar] [CrossRef]
- Ngolong Ngea, G.L.; Qian, X.; Yang, Q.; Dhanasekaran, S.; Ianiri, G.; Ballester, A.R.; Zhang, X.; Castoria, R.; Zhang, H. Securing fruit production: Opportunities from the elucidation of the molecular mechanisms of postharvest fungal infections. Comp. Rev. Food Sci. Food Saf. 2021, 20, 2508–2533. [Google Scholar] [CrossRef]
- Staroń, P.; Pszczółka, K.; Chwastowski, J.; Banach, M. Sorption behavior of Arachis hypogaea shells against Ag+ ions and assessment of antimicrobial properties of the product. Environ. Sci. Pollut. Res. 2020, 27, 19530–19542. [Google Scholar] [CrossRef]
- Samir, R.M.; Osman, A.; El-Sayed, A.I.; Algaby, A.M. Physicochemical properties and antimicrobial effects of roselle corolla, onion peels and peanut skins anthocyanins. Zagazig J. Agric. Res. 2019, 46, 769–781. [Google Scholar]
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Devi, S.I.; Pooja Vashista, P.V.; Sharma, C.B. Purification to homogeneity and characterization of two antifungal proteins from the roots of Arachis hypogaea L. Natl. Acad. Sci. Lett. 2005, 28, 21–28. [Google Scholar]
- Sobolev, V.S.; Khan, S.I.; Tabanca, N.; Wedge, D.E.; Manly, S.P.; Cutler, S.J.; Coy, M.R.; Becnel, J.J.; Neff, S.A.; Gloer, J.B. Biological activity of peanut (Arachis hypogaea) phytoalexins and selected natural and synthetic stilbenoids. J. Agric. Food Chem. 2011, 59, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Ng, T.B. Hypogin, a novel antifungal peptide from peanuts with sequence similarity to peanut allergen. J. Pept. Res. 2001, 57, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Gupta, A.D.; Prasad, R. Arabinogalactan protein from Arachis hypogaea: Role as carrier in drug-formulations. Int. J. Pharm. 2007, 333, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; de Figueiredo Furtado, G.; Lopes Cunha, R. Bioaccessibility of lipophilic compounds vehiculated in emulsions: Choice of lipids and emulsifiers. J. Agric. Food Chem. 2019, 67, 13–18. [Google Scholar] [CrossRef]
- O’Driscoll, C.M.; Griffin, B.T. Biopharmaceutical challenges associated with drugs with low aqueous solubility—The potential impact of lipid-based formulations. Adv. Drug Deliv. Rev. 2008, 60, 617–624. [Google Scholar] [CrossRef]
- Bromilow, R.H.; Evans, A.A.; Nicholls, P.H. The influence of lipophilicity and formulation on the distribution of pesticides in laboratoty-scale sediment/water systems. Pest Manag. Sci. 2003, 59, 238–244. [Google Scholar] [CrossRef]
- Tucuch-Pérez, M.A.; Arredondo-Valdés, R.; Hernández-Castillo, F.D. Antifungal activity of phytochemical compounds of extracts from Mexican semi-desert plants against Fusarium oxysporum from tomato by microdilution in plate method. Nova Sci. 2020, 12, 25. [Google Scholar] [CrossRef]
- Mirahmadi, S.F.; Norouzi, R. Chemical composition, phenolic content, free radical scavenging and antifungal activities of Achillea biebersteinii. Food Biosci. 2017, 18, 53–59. [Google Scholar] [CrossRef]
- Arumugam, M.; Manikandan, D.B.; Sridhar, A.; Palaniyappan, S.; Jayaraman, S.; Ramasamy, T. GC–MS based metabolomics strategy for cost-effective valorization of agricultural waste: Groundnut shell extracts and their biological inhibitory potential. Waste Biomass Valori. 2022, 13, 4179–4209. [Google Scholar] [CrossRef]
- Abdelshafy, A.M.; Belwal, T.; Liang, Z.; Wang, L.; Li, D.; Luo, Z.; Li, L. A comprehensive review on phenolic compounds from edible mushrooms: Occurrence, biological activity, application and future prospective. Crit. Rev. Food Sci. Nutr. 2022, 62, 6204–6224. [Google Scholar] [CrossRef]
- Hilbig, J.; Alves, V.R.; Müller, C.M.; Micke, G.A.; Vitali, L.; Pedrosa, R.C.; Block, J.M. Ultrasonic-assisted extraction combined with sample preparation and analysis using LC-ESI-MS/MS allowed the identification of 24 new phenolic compounds in pecan nut shell [Carya illinoinensis (Wangenh) C. Koch] extracts. Food Res. Int. 2018, 106, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Oulahal, N.; Degraeve, P. Phenolic-rich plant extracts with antimicrobial activity: An alternative to food preservatives and biocides? Front. Microbiol. 2022, 12, 753518. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.; Barros, L.; Ferreira, I.C. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.P.; Pechina, B.D.; Sarkis, J.R. A comprehensive approach to pecan nut valorization: Extraction and characterization of soluble and insoluble-bound phenolics. J. Am. Oil Chem. Soc. 2022, 99, 843–854. [Google Scholar] [CrossRef]
- Cason, C.; Yemmireddy, V.K.; Moreira, J.; Adhikari, A. Antioxidant properties of pecan shell bioactive components of different cultivars and extraction methods. Foods 2021, 10, 713. [Google Scholar] [CrossRef]
- do Prado, A.C.; Aragão, A.M.; Fettand, R.; Block, J.M. Antioxidant properties of pecan nut [Carya illinoinensis (Wangenh.) C. Koch] shell infusion. Grasas Aceites 2009, 60, 330–335. [Google Scholar] [CrossRef]
- Porto, L.C.; da Silva, J.; Ferraz, A.D.; Corrêa, D.S.; dos Santos, M.S.; Porto, C.D.; Picada, J.N. Evaluation of acute and subacute toxicity and mutagenic activity of the aqueous extract of pecan shells [Carya illinoinensis (Wangenh.) K. Koch]. Food Chem. Toxicol. 2013, 59, 579–585. [Google Scholar] [CrossRef]
- Gao, F.; Ye, H.; Yu, Y.; Zhang, T.; Deng, X. Lack of toxicological effect through mutagenicity test of polyphenol extracts from peanut shells. Food Chem. 2011, 129, 920–924. [Google Scholar] [CrossRef]


| Extract | Yield (%) | TPC (mg GAE/g) | AA (%) | |
|---|---|---|---|---|
| Peanut shells | Hex-P | 0.72 | 131.6 ± 3.8 | 42.01 ± 3.5 |
| MeOH-P | 4.5 | |||
| Pecan nut shells | Hex-PN | 0.84 | 438.7 ± 7.7 | 58.9 ± 3.7 |
| MeOH-PN | 24.35 |
| Extract | Concentration Extract (ppm) | MGI (%) |
|---|---|---|
| T0 | 0 ± 0 a | |
| T1 | 100 | 100 ± 0 b |
| Hex-P | 1000 | 0 ± 0 a |
| 2000 | 6.7 ± 5.7 a | |
| MeOH-P | 1000 | 0 ± 0 a |
| 2000 | 100 ± 0 b | |
| Hex-PN | 1000 | 66.7 ± 9.5 b |
| 2000 | 93.4 ± 6.5 b | |
| MeOH-PN | 1000 | 0 ± 0 a |
| 2000 | 46.7 ± 10.4 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seimandi, G.M.; Fernández, L.N.; Ruiz, V.E.; Favaro, M.A.; Derita, M.G. Peanut and Pecan Nut Shell Extracts Reduced Disease Incidence and Severity Caused by Grey Mold in Postharvest Strawberries. Horticulturae 2025, 11, 690. https://doi.org/10.3390/horticulturae11060690
Seimandi GM, Fernández LN, Ruiz VE, Favaro MA, Derita MG. Peanut and Pecan Nut Shell Extracts Reduced Disease Incidence and Severity Caused by Grey Mold in Postharvest Strawberries. Horticulturae. 2025; 11(6):690. https://doi.org/10.3390/horticulturae11060690
Chicago/Turabian StyleSeimandi, Gisela M., Laura N. Fernández, Verónica E. Ruiz, María A. Favaro, and Marcos G. Derita. 2025. "Peanut and Pecan Nut Shell Extracts Reduced Disease Incidence and Severity Caused by Grey Mold in Postharvest Strawberries" Horticulturae 11, no. 6: 690. https://doi.org/10.3390/horticulturae11060690
APA StyleSeimandi, G. M., Fernández, L. N., Ruiz, V. E., Favaro, M. A., & Derita, M. G. (2025). Peanut and Pecan Nut Shell Extracts Reduced Disease Incidence and Severity Caused by Grey Mold in Postharvest Strawberries. Horticulturae, 11(6), 690. https://doi.org/10.3390/horticulturae11060690










