Postharvest Quality of Granny Smith Apples: Interplay of Harvest Stage, Storage Duration, and Shelf-Life
Abstract
1. Introduction
2. Materials and Methods
2.1. Apple Variety, Experimental Conditions, and Experimental Design
- Harvest stage: unripe, ripe, and overripe.
- Storage duration: 0, 2, and 4 months.
- Shelf-life period: days 0 (within 24 h of harvesting and removal from storage), 5, 10, and 15.
2.2. Texture Analysis
2.3. Maturity Indices Analysis
2.4. Color Measurement
- Browning Index (Equation (2)), which estimates the purity of brown color in products containing sugar [24]:
- Yellow Index (Equation (4)), which is associated with the Browning Index [25]:
2.5. Starch Grains Quantification
2.6. Raman Microscopy
2.7. Statistical Analysis
3. Results and Discussion
3.1. Textural Parameters
3.2. Maturity Indices
3.3. Colorimetric Parameters
3.4. Starch Grains
3.5. Raman Spectra
- Integration of non-destructive monitoring techniques: The use of hyperspectral imaging or handheld Raman spectroscopy could provide real-time insights into fruit ripening status without damaging the produce.
- Detailed cell wall profiling: Enzymatic activity and pectin solubilization could be monitored to better understand the biochemical basis of firmness loss.
- Varietal comparisons: Expanding the study to include additional apple cultivars with different storage behaviors would enable the development of variety-specific storage protocols.
- Consumer preference assessment: Linking objective texture and flavor parameters with sensory evaluation could help define optimal quality windows from the consumer perspective.
- Storage condition modeling: Predictive modeling based on multivariate data (e.g., PCA, machine learning) could support decision-making in postharvest handling and logistics.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mikus, M.; Galus, S. Extending the Shelf Life of Apples After Harvest Using Edible Coatings as Active Packaging—A Review. Appl. Sci. 2025, 15, 767. [Google Scholar] [CrossRef]
- Volk, G.M.; Bramel, P. Apple Genetic Resources: Diversity and Conservation. In The Apple Genome; Korban, S.S., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 33–45. [Google Scholar]
- Karuppanapandian, T.; Gerber, M.; Viljoen, D.W.; Botes, A.; Moelich, E.I.; Crouch, I.J.; Crouch, E.M. Assessment of Sensory Characteristics of ‘Granny Smith’ Apples Stored in Various Controlled Atmosphere Regimes by a Trained Panel. Acta Hortic. 2022, 1348, 285–290. [Google Scholar] [CrossRef]
- Hussein, Z.; Fawole, O.A.; Opara, U.L. Harvest and Postharvest Factors Affecting Bruise Damage of Fresh Fruits. Hortic. Plant J. 2020, 6, 1–13. [Google Scholar] [CrossRef]
- Donadel, J.Z.; Thewes, F.R.; dos Santos, L.F.; Schultz, E.E.; Berghetti, M.R.P.; Ludwig, V.; Mesadri, J.; Klein, B.; Thewes, F.R.; Schmidt, S.F.P.; et al. Superficial Scald Development in ‘Granny Smith’ and ‘Nicoter’ Apples: The Role of Key Volatile Compounds When Fruit Are Stored under Dynamic Controlled Atmosphere. Food Res. Int. 2023, 173, 113396. [Google Scholar] [CrossRef] [PubMed]
- Gerber, M.; Karuppanapandian, T.; Viljoen, D.W.; Botes, W.J.; Crouch, I.J.; Crouch, E.M. Superficial Scald Induction in ‘Granny Smith’ Apples Subjected to Different Storage Treatments. Acta Hortic. 2023, 1364, 249–256. [Google Scholar] [CrossRef]
- Tomic, N.; Radivojevic, D.; Milivojevic, J.; Djekic, I.; Smigic, N. Effects of 1-Methylcyclopropene and Diphenylamine on Changes in Sensory Properties of ‘Granny Smith’ Apples during Postharvest Storage. Postharvest Biol. Technol. 2016, 112, 233–240. [Google Scholar] [CrossRef]
- Srivastava, S.; Sadistap, S. Non-Destructive Sensing Methods for Quality Assessment of on-Tree Fruits: A Review. J. Food Meas. Charact. 2018, 12, 497–526. [Google Scholar] [CrossRef]
- Streif, J.; Kittemann, D.; Neuwald, D.A.; McCormick, R.; Xuan, H. Pre- and Post-Harvest Managemnet of Fruit Quality, Ripening and Senescence. Acta Hortic. 2010, 877, 55–68. [Google Scholar] [CrossRef]
- Ziv, C.; Fallik, E. Postharvest Storage Techniques and Quality Evaluation of Fruits and Vegetables for Reducing Food Loss. Agronomy 2021, 11, 1133. [Google Scholar] [CrossRef]
- Makule, E.; Dimoso, N.; Tassou, S.A. Precooling and Cold Storage Methods for Fruits and Vegetables in Sub-Saharan Africa—A Review. Horticulturae 2022, 8, 776. [Google Scholar] [CrossRef]
- Prange, R.K.; Wright, A.H. A Review of Storage Temperature Recommendations for Apples and Pears. Foods 2023, 12, 466. [Google Scholar] [CrossRef] [PubMed]
- Shezi, S.; Magwaza, L.S.; Ncama, K. Chapter 3—Importance of Maturity Indexing in Postharvest Management of Selected Subtropical and Temperate Fruits. In Postharvest Management of Fresh Produce Recent Advances; Singh, B.P., Agnihotri, S., Singh, G., Gupta, V.K., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 59–79. [Google Scholar]
- Rizzo, M.; Marcuzzo, M.; Zangari, A.; Gasparetto, A.; Albarelli, A. Fruit Ripeness Classification: A Survey. Artif. Intell. in Agric. 2023, 7, 44–57. [Google Scholar] [CrossRef]
- Yu, J.; Tseng, Y.; Pham, K.; Liu, M.; Beckles, D.M. Starch and Sugars as Determinants of Postharvest Shelf Life and Quality: Some New and Surprising Roles. Curr. Opin. Biotechnol. 2022, 78, 102844. [Google Scholar] [CrossRef] [PubMed]
- Fadon, E.; Rodrigo, J. Combining Histochemical Staining and Image Analysis to Quantify Starch in the Ovary Primordia of Sweet Cherry during Winter Dormancy. J. Vis. Exp. 2019, 145, e58524. [Google Scholar] [CrossRef]
- Monago-Maraña, O.; Wold, J.P.; Remberg, S.F.; Sanden, K.W.; Afseth, N.K. Raman Spectroscopy as a Tool for Characterisation of Quality Parameters in Norwegian Grown Apples during Ripening. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 323, 124903. [Google Scholar] [CrossRef]
- Monago-Maraña, O.; Afseth, N.K.; Knutsen, S.H.; Wubshet, S.G.; Wold, J.P. Quantification of Soluble Solids and Individual Sugars in Apples by Raman Spectroscopy: A Feasibility Study. Postharvest Biol. Technol. 2021, 180, 111620. [Google Scholar] [CrossRef]
- Brookfield CT3 Texture Analyzer. Operating Instructions. Manual No. M08-372-E0315. Available online: https://www.brookfieldengineering.com/-/media/ametekbrookfield/manuals/texture/ct3%20manual%20m08-372-f1116.pdf (accessed on 3 May 2025).
- Parra-Angarita, S.L.; Al Sayed, M.W.; Léonard, A. The Variability of Textural Properties and Drying Characteristics of Dehydrated Sewage Sludge. Clean. Technol. 2025, 7, 31. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; Association of the Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Džamić, M. Praktikum Iz Biohemije; Naučna Knjiga: Beograd, Serbia, 1989. [Google Scholar]
- Minaker, S.; Mason, R.; Chow, D. Optimizing Color Performance of the Ngenuity® 3D Visualization System. Ophthalmol. Sci. 2021, 1, 100054. [Google Scholar] [CrossRef]
- Djekic, I.; Vunduk, J.; Tomašević, I.; Kozarski, M.; Petrovic, P.; Niksic, M.; Pudja, P.; Klaus, A. Application of Quality Function Deployment on Shelf-Life Analysis of Agaricus Bisporus Portobello. LWT 2017, 78, 82–89. [Google Scholar] [CrossRef]
- Al-Hilphy, A.R.; Ali, H.I.; Al-IEssa, S.A.; Gavahian, M.; Mousavi-Khaneghah, A. Assessing Compositional and Quality Parameters of Unconcentrated and Refractive Window Concentrated Milk Based on Color Components. Dairy 2022, 3, 400–412. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Straube, J.; Khanal, B.P.; Knoche, M.; Debener, T. Russeting in Apple Is Initiated after Exposure to Moisture Ends—I. Histological Evidence. Plants 2020, 9, 1293. [Google Scholar] [CrossRef]
- Ding, X.; Zheng, Y.; Jia, R.; Li, X.; Wang, B.; Zhao, Z. Comparison of Fruit Texture and Storage Quality of Four Apple Varieties. Foods 2024, 13, 1563. [Google Scholar] [CrossRef]
- Valenzuela, C.; Aguilera, J.M. Effects of Different Factors on Stickiness of Apple Leathers. J. Food Eng. 2015, 149, 51–60. [Google Scholar] [CrossRef]
- Zdunek, A. Application of Acoustic Emission for Quality Evaluation of Fruits and Vegetables. In Acoustic Emission—Research and Applications; Sikorski, W., Ed.; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar][Green Version]
- Topaiboul, S.; Guo, C.-C.; Gao, R.-H.; Liou, N.-S. The Relation Between Crispness and Texture Properties of Wax Apple. In Conference Mechanics of Biological Systems and Materials, Volume 4. Conference Proceedings of the Society for Experimental Mechanics Series; Barthelat, F., Zavattieri, P., Korach, C., Prorok, B., Grande-Allen, K., Eds.; Springer International Publishing: Charm, Switzerland, 2014; Volume 4, pp. 169–172. [Google Scholar]
- Sabbaghi, H.; Ziaiifar, A.M.; Kashaninejad, M. Textural Profile Analysis (TPA) of Dried Apple Slices Using Infrared Radiation with Intermittent Heating Method. Iran. Food Sci. Technol. Res. J. 2020, 16, 57–72. [Google Scholar]
- Martynenko, A.; Janaszek, M.A. Texture Changes during Drying of Apple Slices. Dry. Technol. 2014, 32, 567–577. [Google Scholar] [CrossRef]
- Leide, J.; de Souza, A.; Papp, I.; Riederer, M. Specific Characteristics of the Apple Fruit Cuticle: Investigation of Early and Late Season Cultivars ‘Prima’ and ‘Florina’ (Malus Domestica Borkh.). Sci. Hortic. 2018, 229, 137–147. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, P.; Wang, X.; Zhu, Z. Degradation of Cell Wall Polysaccharides and Change of Related Enzyme Activities with Fruit Softening in Annona Squamosa during Storage. Postharvest Biol. Technol. 2020, 166, 111203. [Google Scholar] [CrossRef]
- Sredojevic, A.; Radivojevic, D.; Levic, S.M.; Aksic, M.F.; Milivojevic, J.; Djekic, I. Effects of the Fruit Harvest Date and Shelf-Life Nexus of Apples on Different Quality Perspectives. Appl. Sci. 2024, 14, 11737. [Google Scholar] [CrossRef]
- Liu, L.; Lin, H.; Zhou, X.; Zhang, Z.; Zhang, Y.; Deng, S.; Peng, S.; Gong, S.; Guo, S.; Fan, W. Application of Modified Atmosphere Preservation Technology in Cherry Storage: A Review. Agriculture 2025, 15, 462. [Google Scholar] [CrossRef]
- Doerflinger, F.C.; Miller, W.B.; Nock, J.F.; Watkins, C.B. Relationships between Starch Pattern Indices and Starch Concentrations in Four Apple Cultivars. Postharvest Biol. Technol. 2015, 110, 86–95. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, M.; Guo, H.; Yang, W.; Wang, Z.; Cheng, S.; Chen, G. Cuticle Properties, Wax Composition, and Crystal Morphology of Hami Melon Cultivars (Cucumis melo L.) with Differential Resistance to Fruit Softening. Food Chem. 2024, 449, 139234. [Google Scholar] [CrossRef]
- Liu, X.-C.; Tang, Y.-Q.; Li, Y.-C.; Li, S.-J.; Yang, H.-D.; Wan, S.-L.; Wang, Y.-T.; Hu, Z.-D. Identification of Key Sensory and Chemical Factors Determining Flavor Quality of Xinyu Mandarin during Ripening and Storage. Food Chem. X 2024, 22, 101395. [Google Scholar] [CrossRef]
- Li, X.-L.; Su, Q.; Jia, R.-J.; Wang, Z.-D.; Fu, J.-H.; Guo, J.; Yang, H.-J.; Zhao, Z.-Y. Comparison of Cell Wall Changes of Two Different Types of Apple Cultivars during Fruit Development and Ripening. J. Integr. Agric. 2023, 22, 2705–2718. [Google Scholar] [CrossRef]
- Živić, T.; Coelho, A.R.F.; Marques, A.C.; Daccak, D.; Luís, I.C.; Pessoa, C.C.; Lidon, F.; Silva, M.M.; Simões, M.; Legoinha, P. Quality Assessment in Seven Different Apple Varieties Commercialized in Portugal. Emir. J. Food Agric. 2025, 37, 1–8. [Google Scholar] [CrossRef]
- Szalay, L.; Ordidge, M.; Gitta, F.; Hadley, P.; Tóth, M.; Battey, N.H. Grouping of 24 Apple Cultivars on the Basis of Starch Degradation Rate and Their Fruit Pattern. Hortic. Sci. 2013, 40, 93–101. [Google Scholar] [CrossRef]
- Ng, J.K.T.; Schröder, R.; Sutherland, P.W.; Hallett, I.C.; Hall, M.I.; Prakash, R.; Smith, B.G.; Melton, L.D.; Johnston, J.W. Cell Wall Structures Leading to Cultivar Differences in Softening Rates Develop Early during Apple (Malus x Domestica) Fruit Growth. BMC Plant Biol. 2013, 13, 183. [Google Scholar] [CrossRef]
- Cárdenas-Pérez, S.; Chanona-Pérez, J.; Méndez-Méndez, J.V.; Calderón-Domínguez, G.; López-Santiago, R.; Perea-Flores, M.J.; Arzate-Vázquez, I. Evaluation of the Ripening Stages of Apple (Golden Delicious) by Means of Computer Vision System. Biosyst. Eng. 2017, 159, 46–58. [Google Scholar] [CrossRef]
- Kassebi, S.; Farkas, C.; Székely, L.; Géczy, A.; Korzenszky, P. Late Shelf Life Saturation of Golden Delicious Apple Parameters: TSS, Weight, and Colorimetry. Appl. Sci. 2023, 13, 159. [Google Scholar] [CrossRef]
- Mehdi, A.; Sohail, A.; Mazahir, M.; Abbasi, K.S.; Amir, R.M.; Ali, W.; Asim, M. 40. Assessment of Physico-Chemical and Phyto-Chemical Properties of Six Apple Varities Cultivated in District Nagar and Hunza Gilgit Baltistan, Pakistan. Pure Appl. Biol. 2020, 9, 1627–1636. [Google Scholar] [CrossRef]
- Ahmad, F.; Zaidi, S.; Arshad, M. Postharvest Quality Assessment of Apple during Storage at Ambient Temperature. Heliyon 2021, 7, e07714. [Google Scholar] [CrossRef]
- Sadat Razavi, M.; Golmohammadi, A.; Nematollahzadeh, A.; Ghanbari, A.; Davari, M.; Rovera, C.; Carullo, D.; Farris, S. Impact of Bacterial Cellulose Nanocrystals-Gelatin/Cinnamon Essential Oil Emulsion Coatings on the Quality Attributes of ‘Red Delicious’ Apples. Coatings 2022, 12, 741. [Google Scholar] [CrossRef]
- Ali, M.A.; Raza, H.; Khan, M.A.; Hussain, M. Effect of Different Periods of Ambient Storage on Chemical Composition of Apple Fruit. Int. J. Agric. Biol. 2004, 6, 568–571. [Google Scholar]
- Poirier, B.C.; Mattheis, J.P.; Rudell, D.R. Extending ‘Granny Smith’Apple Superficial Scald Control Following Long-Term Ultra-Low Oxygen Controlled Atmosphere Storage. Postharvest Biol. Technol. 2020, 161, 111062. [Google Scholar] [CrossRef]
- Lurie, S.; Watkins, C.B. Superficial Scald, Its Etiology and Control. Postharvest Biol. Technol. 2012, 65, 44–60. [Google Scholar] [CrossRef]
- Marc, M.; Cournol, M.; Hanteville, S.; Poisson, A.-S.; Guillou, M.-C.; Pelletier, S.; Laurens, F.; Tessier, C.; Coureau, C.; Renou, J.-P. Pre-Harvest Climate and Post-Harvest Acclimation to Cold Prevent from Superficial Scald Development in Granny Smith Apples. Sci. Rep. 2020, 10, 6180. [Google Scholar] [CrossRef]
- Tijero, V.; Girardi, F.; Botton, A. Fruit Development and Primary Metabolism in Apple. Agronomy 2021, 11, 1160. [Google Scholar] [CrossRef]
- Brust, H.; Orzechowski, S.; Fettke, J. Starch and Glycogen Analyses: Methods and Techniques. Biomolecules 2020, 10, 1020. [Google Scholar] [CrossRef]
- Hanrahan, I.; Galeni, M. Developing a Cv.‘WA 38’ Starch Scale for the Washington State Apple Industry. Available online: https://treefruit.wsu.edu/developing-a-cv-wa-38-starch-scale-for-the-washington-state-apple-industry/ (accessed on 6 July 2025).
- Veleșcu, I.D.; Rațu, R.N.; Arsenoaia, V.-N.; Roșca, R.; Cârlescu, P.M.; Țenu, I. Research on the Process of Convective Drying of Apples and Apricots Using an Original Drying Installation. Agriculture 2023, 13, 820. [Google Scholar] [CrossRef]
- Gorfer, L.M.; Vestrucci, L.; Grigoletto, V.; Lazazzara, V.; Zanella, A.; Robatscher, P.; Scampicchio, M.; Oberhuber, M. Chlorophyll Breakdown during Fruit Ripening: Qualitative Analysis of Phyllobilins in the Peel of Apples (Malus domestica Borkh.) Cv.‘Gala’during Different Shelf Life Stages. Food Res. Int. 2022, 162, 112061. [Google Scholar] [CrossRef]
- Hirschler, R. Whiteness, Yellowness, and Browning in Food Colorimetry: A Critical Review. In Color in Food: Technological and Psychophysical Aspects; Caivano, J.L., del Pilar Buera, M., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 93–104. [Google Scholar]
- Subhashree, S.N.; Sunoj, S.; Xue, J.; Bora, G.C. Quantification of Browning in Apples Using Colour and Textural Features by Image Analysis. Food Qual. Saf. 2017, 1, 221–226. [Google Scholar] [CrossRef]
- Shimizu, T.; Okada, K.; Moriya, S.; Komori, S.; Abe, K. A High-Throughput Color Measurement System for Evaluating Flesh Browning in Apples. J. Am. Soc. Hortic. Sci. 2021, 146, 241–251. [Google Scholar] [CrossRef]
- Łysiak, G.; Kurlus, R.; Zydlik, Z.; Walkowiak-Tomczak, D. Apple Skin Colour Changes during Harvest as an Indicator of Maturity. Acta Sci. Pol. Hortorum Cultus 2014, 13, 71–83. [Google Scholar]
- Brookfield, P.; Murphy, P.; Harker, R.; MacRae, E. Starch Degradation and Starch Pattern Indices; Interpretation and Relationship to Maturity. Postharvest Biol. Technol. 1997, 11, 23–30. [Google Scholar] [CrossRef]
- Doerflinger, F.C.; Miller, W.B.; Nock, J.F.; Watkins, C.B. Variations in Zonal Fruit Starch Concentrations of Apples–a Developmental Phenomenon or an Indication of Ripening? Hortic. Res. 2015, 2, 15047. [Google Scholar] [CrossRef]
- Lee, J.; Mattheis, J.P.; Rudell, D.R. Fruit Size Affects Physiological Attributes and Storage Disorders in Cold-Stored ‘Royal Gala’Apples. HortScience 2013, 48, 1518–1524. [Google Scholar] [CrossRef]
- Li, X.; Peng, W.; Zhang, M.; Zhao, Q.; Fang, Y.; Sun, X.; Ma, T. Mechanisms of Texture and Cell Microstructure Changes during Post-Ripening of ‘Cuixiang’Kiwifruit. Postharvest Biol. Technol. 2024, 207, 112596. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Li, Y.; Lv, Y.; Zhao, J.; Li, H.; Zhang, B.; Zhang, M.; Tian, J.; Li, X. Epigenomic Mechanism Regulating the Quality and Ripeness of Apple Fruit with Differing Harvest Maturity. Physiol. Plant 2024, 176, e14278. [Google Scholar] [CrossRef]
- Li, R.; Rosado-Souza, L.; Sampathkumar, A.; Fernie, A.R. The Relationship between Cell Wall and Postharvest Physiological Deterioration of Fresh Produce. Plant Physiol. Biochem. 2024, 210, 108568. [Google Scholar] [CrossRef]
- Kniese, J.; Race, A.M.; Schmidt, H. Classification of Cereal Flour Species Using Raman Spectroscopy in Combination with Spectra Quality Control and Multivariate Statistical Analysis. J. Cereal Sci. 2021, 101, 103299. [Google Scholar] [CrossRef]


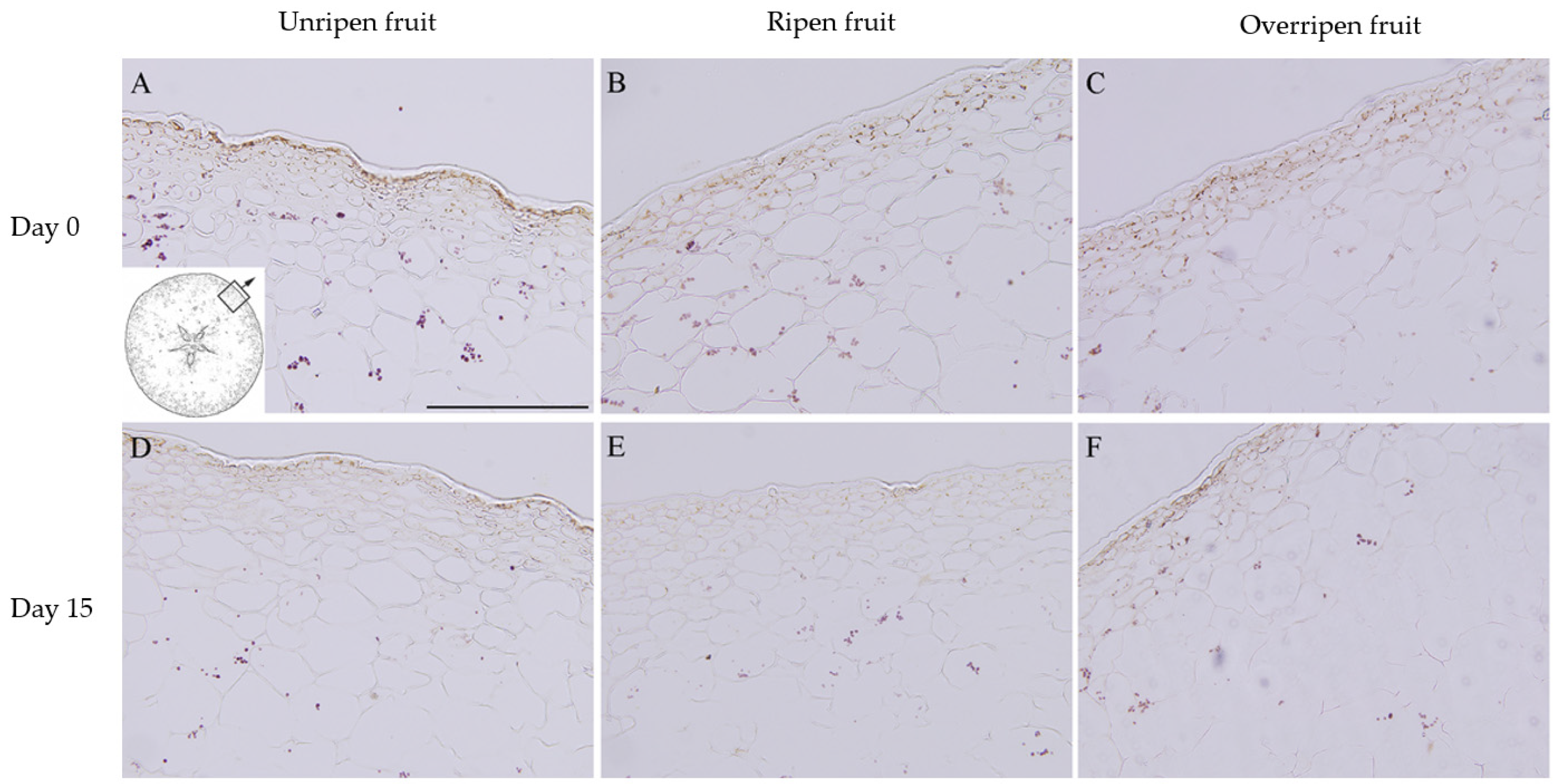
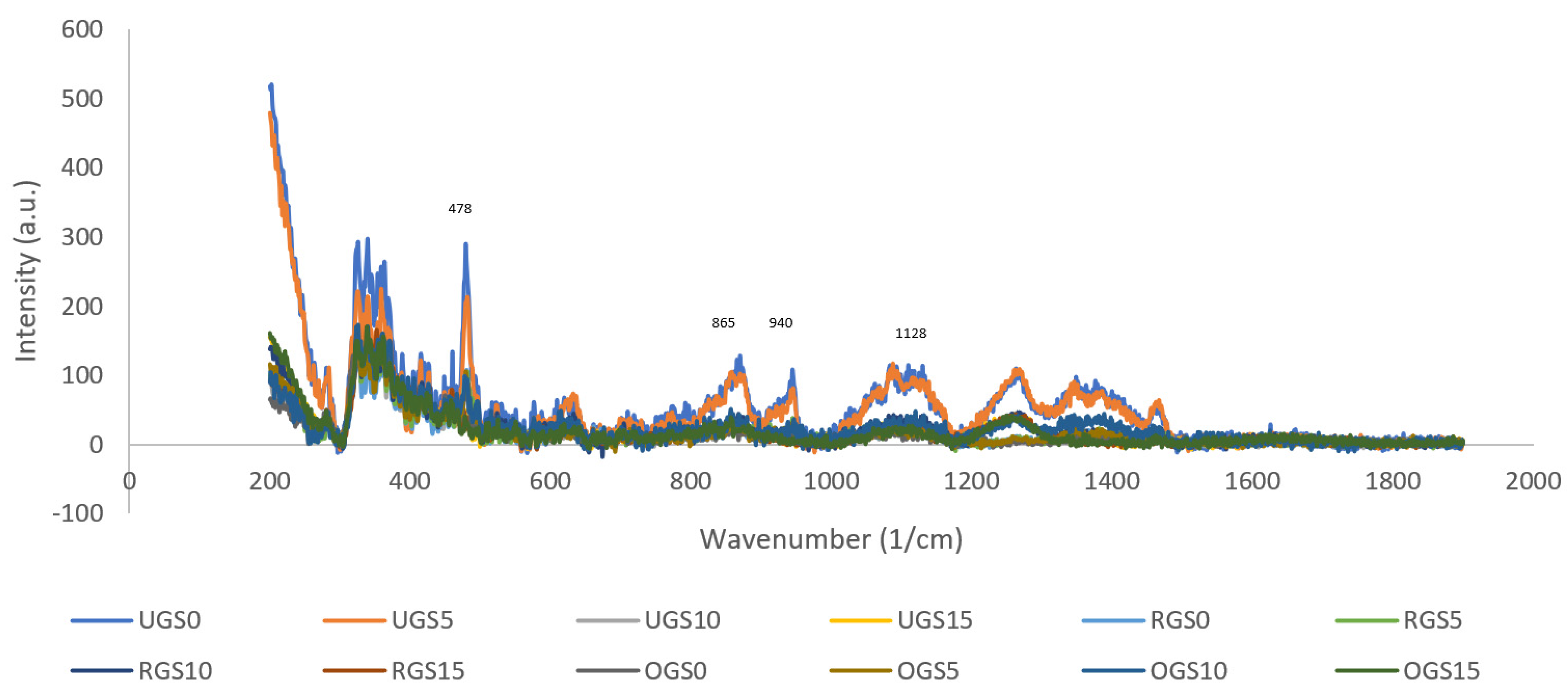

| Textural Parameters | General Description | Unit |
|---|---|---|
| Hardness cycle 1 | The peak force recorded during the first compression cycle; represents the firmness of the sample. | N |
| Deformation at hardness | The displacement at which the maximum force (hardness) occurs during the first cycle; reflects the sample’s resistance to deformation. | mm |
| Total work cycle 1 | The area under the force–time curve during the first cycle; represents the total energy required to deform the sample in that cycle. | mJ |
| Load at target | The force measured at a predefined deformation distance; indicates the resistance of the fruit tissue at a standardized penetration depth. | N |
| Deformation at target | The actual distance at which the target force is reached or maintained; used to quantify tissue yield characteristics. | mm |
| Adhesiveness | The negative force area following the first compression, representing the work required to overcome attractive forces between the sample and the probe during probe withdrawal. | mJ |
| Quantity of fractures | The number of discrete fracture events (peaks) observed during the test; associated with crispness or fracture behavior of the fruit. | - |
| Fracturability | The force at the first significant fracture event, usually the first drop after initial peak; associated with the brittleness or initial cracking of the surface. | N |
| 1st fracture work performed | The energy (area under the curve) expended up to the first fracture event; indicates how much work is needed to initiate breakdown of tissue structure. | mJ |
| 1st fracture deformation | The deformation at which the first fracture occurs; gives insight into the elastic properties of the tissue before breaking. | mm |
| Hardness work cycle 2 | The area under the curve of the second compression cycle, reflecting the sample’s resistance after deformation. | mJ |
| Cohesiveness | The ratio of the area under the second compression cycle to the first one. Indicates how well the fruit withstands a second deformation relative to the first—internal bonding strength. | - |
| Springiness | The distance the sample recovers in height between the end of the first and the start of the second compression; reflects the elastic recovery of the sample. | Mm |
| Gumminess | A derived parameter calculated as Hardness × Cohesiveness. Reflects the energy required to disintegrate a semisolid food. | N |
| Chewiness index | A compound parameter calculated as Hardness × Cohesiveness × Springiness. Reflects the energy needed to chew a solid sample until it is ready for swallowing. | e |
| Average peak load | The mean value of all force peaks observed during the compression; gives an average measure of internal structural resistance across the test. | N |
| HC1 | DH | DT | AD | FR | COH | SPR | GUM | CHI | |
|---|---|---|---|---|---|---|---|---|---|
| Harvest stage (H) | |||||||||
| Unripe | 3.71 ± 4.32 | 9.87 ± 0.19 b | 9.93 ± 0.02 | 0.82 ± 0.79 | 45.70 ± 10.62 | 0.02 ± 0.01 | 3.22 ± 2.68 | 0.17 ± 0.23 a | 0.05 ± 0.12 |
| Ripe | 3.55 ± 3.63 | 9.92 ± 0.14 ab | 9.93 ± 0.02 | 0.88 ± 0.83 | 43.08 ± 8.95 | 0.02 ± 0.02 | 2.96 ± 2.95 | 0.10 ± 0.17 b | 0.04 ± 0.08 |
| Overripe | 3.05 ± 4.97 | 9.94 ± 0.08 a | 9.92 ± 0.01 | 0.91 ± 0.76 | 42.81 ± 9.04 | 0.02 ± 0.02 | 2.87 ± 2.18 | 0.09 ± 0.13 b | 0.04 ± 0.06 |
| Storage duration (S) | |||||||||
| 0 month | 4.80 ± 4.00 a | 9.90 ± 0.16 | 9.93 ± 0.02 | 0.86 ± 0.58 | 46.36 ± 9.65 a | 0.02 ± 0.02 | 3.62 ± 2.78 a | 0.20 ± 0.23 a | 0.08 ± 0.12 a |
| 2 months | 3.24 ± 5.24 ab | 9.91 ± 0.14 | 9.93 ± 0.02 | 0.87 ± 0.88 | 44.25 ± 7.80 ab | 0.02 ± 0.02 | 3.00 ± 2.36 ab | 0.09 ± 0.15 b | 0.03 ± 0.05 b |
| 4 months | 2.27 ± 3.16 b | 9.92 ± 0.13 | 9.93 ± 0.01 | 0.88 ± 0.89 | 40.98 ± 10.53 b | 0.02 ± 0.02 | 2.43 ± 2.58 b | 0.08 ± 0.14 b | 0.03 ± 0.07 b |
| Shelf-life period (D) | |||||||||
| Day 0 | 2.94 ± 3.46 | 9.88 ± 0.17 | 9.92 ± 0.01 | 0.84 ± 0.72 | 44.76 ± 10.31 | 0.02 ± 0.02 | 3.32 ± 3.08 | 0.15 ± 0.24 | 0.06 ± 0.15 |
| Day 5 | 4.25 ± 6.29 | 9.90 ± 0.17 | 9.93 ± 0.02 | 0.85 ± 0.82 | 44.61 ± 8.87 | 0.02 ± 0.02 | 3.20 ± 2.41 | 0.13 ± 0.17 | 0.05 ± 0.07 |
| Day 10 | 3.90 ± 3.87 | 9.93 ± 0.11 | 9.92 ± 0.02 | 0.88 ± 0.78 | 44.10 ± 10.52 | 0.02 ± 0.02 | 2.85 ± 2.84 | 0.11 ± 0.13 | 0.04 ± 0.07 |
| Day 15 | 2.67 ± 2.80 | 9.94 ± 0.11 | 9.93 ± 0.02 | 0.90 ± 0.86 | 41.98 ± 8.63 | 0.02 ± 0.01 | 2.69 ± 2.03 | 0.10 ± 0.19 | 0.03 ± 0.05 |
| H × S × D | |||||||||
| Unripe × 0 month × Day 0 | 5.57 ± 6.96 | 9.75 ± 0.28 | 9.92 ± 0.01 | 0.78 ± 0.54 | 48.93 ± 12.00 | 0.03 ± 0.01 | 5.09 ± 3.06 | 0.56 ± 0.40 | 0.24 ± 0.35 |
| Unripe × 0 month × Day 5 | 7.76 ± 7.48 | 9.82 ± 0.26 | 9.95 ± 0.05 | 1.10 ± 0.33 | 52.44 ± 9.22 | 0.03 ± 0.01 | 2.71 ± 2.18 | 0.35 ± 0.29 | 0.08 ± 0.06 |
| Unripe × 0 month × Day 10 | 5.38 ± 2.56 | 9.95 ± 0.03 | 9.92 ± 0.00 | 1.28 ± 0.41 | 49.44 ± 13.07 | 0.03 ± 0.02 | 4.91 ± 3.58 | 0.21 ± 0.14 | 0.10 ± 0.13 |
| Unripe × 0 month × Day 15 | 1.99 ± 1.60 | 9.89 ± 0.16 | 9.93 ± 0.03 | 0.50 ± 0.52 | 46.56 ± 8.73 | 0.03 ± 0.01 | 3.11 ± 1.95 | 0.05 ± 0.07 | 0.03 ± 0.05 |
| Unripe × 2 months × Day 0 | 5.62 ± 5.45 | 9.91 ± 0.12 | 9.93 ± 0.02 | 1.44 ± 0.97 | 51.24 ± 4.99 | 0.02 ± 0.01 | 1.89 ± 1.53 | 0.29 ± 0.28 | 0.02 ± 0.02 |
| Unripe × 2 months × Day 5 | 3.34 ± 1.12 | 9.97 ± 0.04 | 9.91 ± 0.01 | 2.12 ± 1.01 | 41.76 ± 11.18 | 0.02 ± 0.02 | 4.52 ± 3.33 | 0.07 ± 0.08 | 0.06 ± 0.08 |
| Unripe × 2 months × Day 10 | 4.69 ± 5.59 | 9.96 ± 0.04 | 9.93 ± 0.03 | 0.84 ± 0.89 | 45.63 ± 6.12 | 0.03 ± 0.02 | 3.54 ± 2.93 | 0.14 ± 0.10 | 0.05 ± 0.07 |
| Unripe × 2 months × Day 15 | 1.33 ± 0.98 | 9.82 ± 0.22 | 9.93 ± 0.01 | 0.22 ± 0.16 | 43.62 ± 8.39 | 0.02 ± 0.01 | 3.23 ± 3.00 | 0.03 ± 0.03 | 0.01 ± 0.01 |
| Unripe × 4 months × Day 0 | 0.90 ± 0.26 | 9.78 ± 0.30 | 9.92 ± 0.01 | 0.26 ± 0.15 | 38.99 ± 5.72 | 0.01 ± 0.00 | 1.33 ± 0.60 | 0.09 ± 0.10 | 0.00 ± 0.00 |
| Unripe × 4 months × Day 5 | 1.37 ± 0.72 | 9.94 ± 0.08 | 9.93 ± 0.01 | 0.70 ± 0.79 | 50.36 ± 11.23 | 0.02 ± 0.01 | 3.74 ± 2.08 | 0.03 ± 0.03 | 0.01 ± 0.02 |
| Unripe × 4 months × Day 10 | 3.47 ± 5.72 | 9.77 ± 0.28 | 9.92 ± 0.00 | 0.56 ± 0.42 | 37.75 ± 13.63 | 0.02 ± 0.01 | 2.96 ± 3.93 | 0.20 ± 0.24 | 0.01 ± 0.02 |
| Unripe × 4 months × Day 15 | 3.14 ± 1.61 | 9.93 ± 0.08 | 9.92 ± 0.01 | 0.08 ± 0.08 | 41.63 ± 15.84 | 0.01 ± 0.01 | 1.59 ± 1.81 | 0.05 ± 0.04 | 0.03 ± 0.03 |
| Ripe × 0 month × Day 0 | 2.79 ± 1.96 | 9.91 ± 0.18 | 9.92 ± 0.01 | 1.12 ± 0.56 | 40.55 ± 9.27 | 0.02 ± 0.01 | 3.86 ± 4.86 | 0.06 ± 0.11 | 0.04 ± 0.07 |
| Ripe × 0 month × Day 5 | 6.38 ± 4.65 | 9.85 ± 0.24 | 9.94 ± 0.03 | 0.34 ± 0.45 | 45.13 ± 9.30 | 0.03 ± 0.03 | 2.52 ± 2.46 | 0.22 ± 0.21 | 0.06 ± 0.06 |
| Ripe × 0 month × Day 10 | 7.75 ± 3.95 | 9.97 ± 0.04 | 9.92 ± 0.02 | 1.14 ± 0.57 | 41.62 ± 4.14 | 0.02 ± 0.02 | 3.24 ± 3.96 | 0.17 ± 0.12 | 0.07 ± 0.09 |
| Ripe × 0 month × Day 15 | 3.42 ± 1.95 | 9.95 ± 0.05 | 9.92 ± 0.01 | 0.84 ± 0.92 | 46.97 ± 7.73 | 0.02 ± 0.01 | 3.14 ± 1.01 | 0.12 ± 0.09 | 0.06 ± 0.06 |
| Ripe × 2 months × Day 0 | 3.26 ± 2.63 | 9.93 ± 0.04 | 9.92 ± 0.01 | 1.12 ± 0.84 | 49.66 ± 5.17 | 0.01 ± 0.01 | 1.59 ± 0.67 | 0.03 ± 0.05 | 0.01 ± 0.01 |
| Ripe × 2 months × Day 5 | 1.80 ± 1.32 | 9.74 ± 0.32 | 9.92 ± 0.00 | 0.24 ± 0.09 | 39.52 ± 5.57 | 0.03 ± 0.04 | 2.99 ± 3.91 | 0.09 ± 0.16 | 0.04 ± 0.09 |
| Ripe × 2 months × Day 10 | 2.20 ± 2.25 | 9.95 ± 0.04 | 9.92 ± 0.01 | 0.62 ± 0.83 | 40.11 ± 6.11 | 0.01 ± 0.00 | 1.93 ± 1.45 | 0.03 ± 0.03 | 0.00 ± 0.01 |
| Ripe × 2 months × Day 15 | 1.71 ± 0.97 | 9.97 ± 0.05 | 9.93 ± 0.01 | 0.26 ± 0.24 | 44.85 ± 9.48 | 0.02 ± 0.01 | 3.45 ± 2.14 | 0.15 ± 0.29 | 0.04 ± 0.06 |
| Ripe × 4 months × Day 0 | 2.08 ± 1.76 | 9.96 ± 0.04 | 9.93 ± 0.02 | 1.06 ± 0.93 | 43.34 ± 16.88 | 0.03 ± 0.03 | 4.66 ± 4.42 | 0.12 ± 0.20 | 0.10 ± 0.21 |
| Ripe × 4 months × Day 5 | 3.78 ± 4.57 | 9.94 ± 0.06 | 9.92 ± 0.02 | 1.28 ± 1.11 | 39.16 ± 7.19 | 0.01 ± 0.01 | 3.84 ± 3.25 | 0.06 ± 0.06 | 0.01 ± 0.01 |
| Ripe × 4 months × Day 10 | 2.81 ± 4.49 | 9.91 ± 0.07 | 9.92 ± 0.01 | 0.68 ± 1.30 | 44.75 ± 14.92 | 0.01 ± 0.00 | 1.27 ± 0.97 | 0.04 ± 0.07 | 0.00 ± 0.01 |
| Ripe × 4 months × Day 15 | 4.67 ± 6.48 | 9.97 ± 0.05 | 9.94 ± 0.03 | 1.86 ± 0.26 | 41.27 ± 6.44 | 0.02 ± 0.02 | 3.08 ± 3.84 | 0.17 ± 0.35 | 0.06 ± 0.10 |
| Overripe × 0 month × Day 0 | 3.13 ± 2.07 | 9.90 ± 0.18 | 9.92 ± 0.00 | 0.72 ± 0.43 | 39.34 ± 12.39 | 0.03 ± 0.01 | 6.88 ± 2.27 | 0.12 ± 0.11 | 0.07 ± 0.06 |
| Overripe × 0 month × Day 5 | 3.29 ± 2.21 | 9.92 ± 0.11 | 9.92 ± 0.01 | 0.76 ± 0.62 | 48.20 ± 4.11 | 0.02 ± 0.00 | 3.86 ± 1.02 | 0.22 ± 0.19 | 0.13 ± 0.09 |
| Overripe × 0 month × Day 10 | 5.24 ± 2.96 | 9.95 ± 0.03 | 9.92 ± 0.02 | 1.22 ± 0.61 | 57.21 ± 3.76 | 0.01 ± 0.01 | 1.63 ± 0.79 | 0.07 ± 0.08 | 0.01 ± 0.02 |
| Overripe × 0 month × Day 15 | 4.93 ± 3.11 | 9.97 ± 0.04 | 9.93 ± 0.01 | 0.52 ± 0.28 | 39.87 ± 7.61 | 0.02 ± 0.01 | 2.43 ± 1.44 | 0.23 ± 0.30 | 0.04 ± 0.03 |
| Overripe × 2 months × Day 0 | 2.21 ± 2.25 | 9.85 ± 0.12 | 9.92 ± 0.01 | 0.78 ± 0.92 | 51.07 ± 7.88 | 0.02 ± 0.01 | 3.24 ± 1.65 | 0.05 ± 0.05 | 0.02 ± 0.02 |
| Overripe × 2 months × Day 5 | 9.14 ± 15.70 | 9.97 ± 0.05 | 9.92 ± 0.01 | 0.40 ± 0.39 | 39.25 ± 7.09 | 0.03 ± 0.02 | 2.25 ± 1.48 | 0.08 ± 0.09 | 0.05 ± 0.07 |
| Overripe × 2 months × Day 10 | 1.91 ± 1.20 | 9.91 ± 0.08 | 9.93 ± 0.01 | 0.68 ± 0.88 | 46.96 ± 4.36 | 0.03 ± 0.03 | 4.60 ± 3.20 | 0.09 ± 0.12 | 0.05 ± 0.07 |
| Overripe × 2 months × Day 15 | 1.69 ± 1.63 | 9.95 ± 0.09 | 9.94 ± 0.03 | 1.74 ± 0.46 | 37.31 ± 2.57 | 0.02 ± 0.03 | 2.79 ± 0.47 | 0.07 ± 0.13 | 0.03 ± 0.05 |
| Overripe × 4 months × Day 0 | 0.85 ± 0.42 | 9.95 ± 0.04 | 9.92 ± 0.00 | 0.32 ± 0.31 | 39.70 ± 8.09 | 0.02 ± 0.01 | 1.36 ± 1.54 | 0.03 ± 0.03 | 0.01 ± 0.01 |
| Overripe × 4 months × Day 5 | 1.39 ± 0.88 | 9.94 ± 0.05 | 9.92 ± 0.01 | 0.74 ± 0.56 | 45.65 ± 6.43 | 0.03 ± 0.02 | 2.42 ± 1.43 | 0.05 ± 0.06 | 0.03 ± 0.04 |
| Overripe × 4 months × Day 10 | 1.63 ± 1.39 | 9.97 ± 0.04 | 9.93 ± 0.01 | 0.92 ± 0.96 | 33.39 ± 5.26 | 0.02 ± 0.02 | 1.57 ± 2.05 | 0.06 ± 0.08 | 0.02 ± 0.04 |
| Overripe × 4 months × Day 15 | 1.14 ± 0.67 | 9.98 ± 0.04 | 9.93 ± 0.01 | 2.12 ± 0.53 | 35.74 ± 3.64 | 0.02 ± 0.01 | 1.40 ± 1.03 | 0.03 ± 0.03 | 0.02 ± 0.03 |
| Significance | |||||||||
| H | n.s. | * | n.s. | n.s. | n.s. | n.s. | n.s. | * | n.s. |
| S | ** | n.s. | n.s. | n.s. | ** | n.s. | * | *** | ** |
| D | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| H × S | n.s. | n.s. | n.s. | *** | n.s. | n.s. | n.s. | n.s. | n.s. |
| H × D | n.s. | n.s. | n.s. | *** | n.s. | n.s. | n.s. | ** | n.s. |
| S × D | n.s. | n.s. | * | ** | ** | n.s. | n.s. | n.s. | n.s. |
| H × S × D | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| TWC1 | LT | DT | AD | FWP | APL | |
|---|---|---|---|---|---|---|
| Harvest stage (H) | ||||||
| Unripe | 117.19 ± 19.84 a | 9.84 ± 2.14 a | 9.91 ± 0.03 | 1.95 ± 0.56 | 15.09 ± 32.07 | 26.44 ± 4.37 |
| Ripe | 115.63 ± 17.02 a | 9.56 ± 1.72 a | 9.90 ± 0.06 | 1.90 ± 0.63 | 15.08 ± 37.02 | 26.69 ± 4.59 |
| Overripe | 108.70 ± 22.78 b | 8.83 ± 2.04 b | 9.91 ± 0.04 | 2.05 ± 0.80 | 18.76 ± 38.74 | 25.93 ± 4.60 |
| Storage duration (S) | ||||||
| 0 month | 134.03 ± 10.82 a | 11.53 ± 1.32 a | 9.89 ± 0.08 b | 1.74 ± 0.46 b | 9.26 ± 32.26 | 27.51 ± 4.48 a |
| 2 months | 110.06 ± 14.74 b | 8.86 ± 1.34 b | 9.91 ± 0.01 a | 2.02 ± 0.73 ab | 17.75 ± 36.59 | 26.34 ± 4.29 ab |
| 4 months | 97.43 ± 14.37 c | 7.85 ± 1.17 c | 9.91 ± 0.01 a | 2.13 ± 0.73 a | 21.91 ± 38.01 | 25.21 ± 4.52 b |
| Shelf-life period (D) | ||||||
| Day 0 | 120.68 ± 18.81 a | 10.06 ± 1.95 a | 9.89 ± 0.07 b | 1.96 ± 0.85 | 23.33 ± 43.73 | 26.83 ± 3.73 ab |
| Day 5 | 120.06 ± 16.81 a | 9.97 ± 1.96 a | 9.92 ± 0.01 a | 2.00 ± 0.67 | 23.99 ± 44.76 | 28.00 ± 3.70 a |
| Day 10 | 108.21 ± 22.88 b | 9.02 ± 2.04 b | 9.90 ± 0.06 ab | 2.03 ± 0.57 | 7.82 ± 17.71 | 25.08 ± 5.02 b |
| Day 15 | 106.41 ± 18.22 b | 8.60 ± 1.74 b | 9.91 ± 0.01 a | 1.87 ± 0.55 | 10.09 ± 28.07 | 25.52 ± 4.94 b |
| H × S × D | ||||||
| Unripe × 0 month × Day 0 | 141.34 ± 7.57 | 12.82 ± 0.82 | 9.86 ± 0.12 | 1.74 ± 0.30 | 0.34 ± 0.11 | 28.62 ± 3.43 |
| Unripe × 0 month × Day 5 | 146.86 ± 5.54 | 13.81 ± 0.88 | 9.91 ± 0.01 | 2.10 ± 0.48 | 0.36 ± 0.13 | 26.19 ± 5.29 |
| Unripe × 0 month × Day 10 | 131.54 ± 8.09 | 10.94 ± 0.72 | 9.91 ± 0.00 | 1.80 ± 0.52 | 5.42 ± 11.45 | 26.99 ± 4.21 |
| Unripe × 0 month × Day 15 | 126.66 ± 8.21 | 10.86 ± 0.63 | 9.91 ± 0.00 | 1.54 ± 0.40 | 0.32 ± 0.11 | 27.71 ± 6.51 |
| Unripe × 2 months × Day 0 | 128.72 ± 10.67 | 10.46 ± 0.46 | 9.91 ± 0.00 | 1.88 ± 0.53 | 6.10 ± 12.97 | 25.53 ± 3.21 |
| Unripe × 2 months × Day 5 | 112.80 ± 6.48 | 9.97 ± 0.46 | 9.92 ± 0.01 | 2.26 ± 1.10 | 4.60 ± 9.62 | 25.71 ± 1.76 |
| Unripe × 2 months × Day 10 | 102.88 ± 23.61 | 8.78 ± 1.46 | 9.91 ± 0.00 | 1.74 ± 0.30 | 6.52 ± 13.91 | 22.51 ± 6.84 |
| Unripe × 2 months × Day 15 | 102.56 ± 11.35 | 8.69 ± 1.11 | 9.91 ± 0.00 | 1.86 ± 0.38 | 4.20 ± 8.67 | 23.72 ± 5.72 |
| Unripe × 4 months × Day 0 | 111.82 ± 5.83 | 8.92 ± 0.42 | 9.91 ± 0.00 | 1.86 ± 0.48 | 65.44 ± 59.62 | 28.12 ± 2.42 |
| Unripe × 4 months × Day 5 | 107.48 ± 16.13 | 8.09 ± 1.09 | 9.92 ± 0.01 | 2.20 ± 0.47 | 67.50 ± 48.16 | 28.74 ± 3.69 |
| Unripe × 4 months × Day 10 | 100.16 ± 8.59 | 7.87 ± 0.43 | 9.92 ± 0.01 | 2.46 ± 0.74 | 12.86 ± 17.02 | 26.93 ± 3.92 |
| Unripe × 4 months × Day 15 | 93.48 ± 15.55 | 6.90 ± 1.39 | 9.91 ± 0.01 | 1.98 ± 0.43 | 7.40 ± 15.76 | 26.53 ± 2.12 |
| Ripe × 0 month × Day 0 | 133.56 ± 7.18 | 12.19 ± 1.41 | 9.86 ± 0.12 | 1.24 ± 0.38 | 0.34 ± 0.13 | 26.80 ± 6.24 |
| Ripe × 0 month × Day 5 | 129.06 ± 10.45 | 11.19 ± 0.93 | 9.91 ± 0.00 | 2.18 ± 0.58 | 23.72 ± 52.31 | 25.74 ± 2.25 |
| Ripe × 0 month × Day 10 | 139.40 ± 10.24 | 11.67 ± 0.75 | 9.81 ± 0.14 | 1.56 ± 0.23 | 0.30 ± 0.07 | 28.34 ± 6.10 |
| Ripe × 0 month × Day 15 | 126.64 ± 3.39 | 10.46 ± 0.47 | 9.91 ± 0.00 | 1.76 ± 0.25 | 0.34 ± 0.11 | 27.10 ± 4.93 |
| Ripe × 2 months × Day 0 | 124.18 ± 10.89 | 10.43 ± 0.79 | 9.91 ± 0.01 | 1.44 ± 0.75 | 69.90 ± 63.41 | 24.72 ± 6.16 |
| Ripe × 2 months × Day 5 | 119.08 ± 9.85 | 9.47 ± 0.80 | 9.92 ± 0.01 | 1.86 ± 0.79 | 22.42 ± 49.24 | 29.18 ± 3.40 |
| Ripe × 2 months × Day 10 | 101.02 ± 10.91 | 8.25 ± 0.86 | 9.92 ± 0.01 | 2.14 ± 0.24 | 25.78 ± 42.85 | 27.21 ± 2.83 |
| Ripe × 2 months × Day 15 | 107.88 ± 7.59 | 7.88 ± 0.47 | 9.91 ± 0.00 | 2.04 ± 0.17 | 0.26 ± 0.05 | 28.20 ± 2.77 |
| Ripe × 4 months × Day 0 | 100.60 ± 8.84 | 8.17 ± 1.29 | 9.91 ± 0.00 | 2.32 ± 0.55 | 6.46 ± 13.66 | 25.98 ± 2.04 |
| Ripe × 4 months × Day 5 | 111.72 ± 14.22 | 8.41 ± 1.28 | 9.92 ± 0.01 | 1.88 ± 1.02 | 30.68 ± 54.05 | 29.87 ± 4.60 |
| Ripe × 4 months × Day 10 | 99.44 ± 7.06 | 8.50 ± 0.60 | 9.91 ± 0.00 | 2.18 ± 0.77 | 0.38 ± 0.11 | 25.08 ± 3.42 |
| Ripe × 4 months × Day 15 | 94.96 ± 13.36 | 8.14 ± 1.05 | 9.91 ± 0.00 | 2.14 ± 0.72 | 0.34 ± 0.05 | 22.10 ± 6.25 |
| Overripe × 0 month × Day 0 | 141.90 ± 14.12 | 11.08 ± 1.63 | 9.83 ± 0.12 | 1.68 ± 0.26 | 0.28 ± 0.04 | 28.12 ± 3.57 |
| Overripe × 0 month × Day 5 | 137.36 ± 6.21 | 11.23 ± 1.25 | 9.91 ± 0.01 | 1.60 ± 0.40 | 54.98 ± 75.02 | 31.89 ± 2.50 |
| Overripe × 0 month × Day 10 | 131.76 ± 14.36 | 11.70 ± 1.30 | 9.91 ± 0.00 | 1.88 ± 0.58 | 0.34 ± 0.11 | 26.72 ± 3.97 |
| Overripe × 0 month × Day 15 | 122.32 ± 5.67 | 10.35 ± 0.39 | 9.92 ± 0.01 | 1.78 ± 0.55 | 24.34 ± 53.70 | 25.94 ± 4.18 |
| Overripe × 2 months × Day 0 | 110.60 ± 12.94 | 8.45 ± 1.43 | 9.91 ± 0.00 | 2.76 ± 1.60 | 48.82 ± 65.05 | 28.45 ± 1.30 |
| Overripe × 2 months × Day 5 | 115.66 ± 8.90 | 9.29 ± 0.73 | 9.91 ± 0.01 | 2.02 ± 0.48 | 5.66 ± 11.87 | 28.92 ± 1.36 |
| Overripe × 2 months × Day 10 | 91.92 ± 7.16 | 7.11 ± 0.90 | 9.92 ± 0.01 | 2.26 ± 0.71 | 18.38 ± 11.61 | 23.62 ± 4.49 |
| Overripe × 2 months × Day 15 | 103.44 ± 11.28 | 7.52 ± 0.69 | 9.91 ± 0.00 | 2.00 ± 0.35 | 0.38 ± 0.08 | 28.28 ± 3.85 |
| Overripe × 4 months × Day 0 | 93.44 ± 3.97 | 8.04 ± 0.53 | 9.91 ± 0.00 | 2.68 ± 0.97 | 12.28 ± 16.44 | 25.11 ± 2.43 |
| Overripe × 4 months × Day 5 | 100.52 ± 4.66 | 8.27 ± 0.73 | 9.91 ± 0.01 | 1.94 ± 0.67 | 6.02 ± 12.62 | 25.75 ± 3.06 |
| Overripe × 4 months × Day 10 | 75.74 ± 3.66 | 6.36 ± 0.47 | 9.92 ± 0.01 | 2.22 ± 0.54 | 0.36 ± 0.09 | 18.28 ± 2.21 |
| Overripe × 4 months × Day 15 | 79.78 ± 13.18 | 6.57 ± 1.42 | 9.92 ± 0.01 | 1.72 ± 1.22 | 53.22 ± 47.23 | 20.07 ± 0.92 |
| Significance | ||||||
| H | *** | *** | n.s. | n.s. | n.s. | n.s. |
| S | *** | *** | *** | ** | n.s. | ** |
| D | *** | *** | * | n.s. | n.s. | ** |
| H × S | * | n.s. | n.s. | n.s. | ** | *** |
| H × D | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| S × D | * | n.s. | * | n.s. | * | n.s. |
| H × S × D | n.s. | ** | n.s. | n.s. | * | n.s. |
| FIRM | SSC | SPI | TS | RS | pH | TA | Scald | |
|---|---|---|---|---|---|---|---|---|
| Harvest stage (H) | ||||||||
| Unripe | 7.30 ± 0.79 a | 10.53 ± 0.75 b | 5.29 ± 1.49 c | 6.74 ± 0.48 b | 5.90 ± 0.54 b | 3.18 ± 0.16 c | 0.56 ± 0.10 a | 3.40 ± 3.01 a |
| Ripe | 7.15 ± 0.82 b | 10.58 ± 0.60 b | 6.17 ± 1.74 b | 6.76 ± 0.43 b | 5.93 ± 0.47 b | 3.24 ± 0.17 b | 0.53 ± 0.09 b | 1.88 ± 2.46 b |
| Overripe | 6.78 ± 0.89 c | 10.92 ± 0.56 a | 7.38 ± 1.31 a | 7.00 ± 0.39 a | 6.19 ± 0.46 a | 3.28 ± 0.18 a | 0.48 ± 0.07 c | 0.67 ± 1.15 c |
| Storage duration (S) | ||||||||
| 0 month | 7.71 ± 0.51 a | 10.18 ± 0.51 c | 6.28 ± 1.73 | 6.54 ± 0.36 c | 5.63 ± 0.35 c | 3.04 ± 0.05 c | 0.61 ± 0.08 a | - |
| 2 months | 7.24 ± 0.75 a | 10.54 ± 0.42 b | - | 6.74 ± 0.30 b | 5.87 ± 0.31 b | 3.25 ± 0.08 b | 0.48 ± 0.07 b | 0.38 ± 0.96 |
| 4 months | 6.28 ± 0.58 b | 11.31 ± 0.48 a | - | 7.21 ± 0.37 a | 6.51 ± 0.37 a | 3.41 ± 0.11 a | 0.47 ± 0.06 b | 3.58 ± 2.71 |
| Shelf-life period (D) | ||||||||
| Day 0 | 7.44 ± 0.73 a | 10.51 ± 0.81 b | 4.61 ± 1.09 d | 6.71 ± 0.51 a | 5.88 ± 0.57 b | 3.19 ± 0.15 d | 0.58 ± 0.08 a | 0.63 ± 1.14 c |
| Day 5 | 7.13 ± 0.77 b | 10.71 ± 0.62 ab | 5.67 ± 1.46 c | 6.87 ± 0.48 ab | 6.05 ± 0.55 a | 3.22 ± 0.17 c | 0.52 ± 0.08 b | 1.97 ± 2.69 b |
| Day 10 | 6.95 ± 0.92 bc | 10.65 ± 0.54 ab | 6.83 ± 1.10 b | 6.81 ± 0.34 ab | 5.96 ± 0.39 ab | 3.25 ± 0.17 b | 0.51 ± 0.09 b | 2.58 ± 2.76 ab |
| Day 15 | 6.78 ± 0.89 c | 10.83 ± 0.65 a | 8.00 ± 1.08 a | 6.94 ± 0.43 c | 6.12 ± 0.48 a | 3.29 ± 0.19 a | 0.47 ± 0.08 c | 2.74 ± 2.87 a |
| H × S × D | ||||||||
| Unripe × 0 month × Day 0 | 7.88 ± 0.62 | 9.47 ± 0.31 | 3.83 ± 0.41 | 6.07 ± 0.23 | 5.13 ± 0.22 | 2.97 ± 0.02 | 0.74 ± 0.02 | - |
| Unripe × 0 month × Day 5 | 7.83 ± 0.50 | 10.30 ± 0.10 | 4.33 ± 0.52 | 6.36 ± 0.05 | 5.46 ± 0.08 | 3.01 ± 0.02 | 0.66 ± 0.02 | - |
| Unripe × 0 month × Day 10 | 7.78 ± 0.43 | 9.83 ± 0.35 | 5.67 ± 0.82 | 6.47 ± 0.29 | 5.60 ± 0.30 | 3.00 ± 0.02 | 0.69 ± 0.06 | - |
| Unripe × 0 month × Day 15 | 7.54 ± 0.31 | 10.27 ± 0.12 | 7.33 ± 0.52 | 6.66 ± 0.12 | 5.81 ± 0.13 | 3.08 ± 0.05 | 0.54 ± 0.03 | - |
| Unripe × 2 months × Day 0 | 8.35 ± 0.38 | 10.40 ± 0.10 | - | 6.75 ± 0.10 | 5.84 ± 0.11 | 3.05 ± 0.03 | 0.60 ± 0.03 | 0.00 ± 0.00 |
| Unripe × 2 months × Day 5 | 7.65 ± 0.19 | 9.90 ± 0.10 | - | 6.43 ± 0.09 | 5.51 ± 0.08 | 3.15 ± 0.07 | 0.48 ± 0.03 | 0.00 ± 0.00 |
| Unripe × 2 months × Day 10 | 7.56 ± 0.28 | 10.60 ± 0.53 | - | 6.79 ± 0.32 | 5.85 ± 0.31 | 3.31 ± 0.02 | 0.51 ± 0.01 | 1.33 ± 1.03 |
| Unripe × 2 months × Day 15 | 7.21 ± 0.30 | 10.13 ± 0.15 | - | 6.36 ± 0.04 | 5.51 ± 0.04 | 3.33 ± 0.03 | 0.44 ± 0.01 | 2.75 ± 1.08 |
| Unripe × 4 months × Day 0 | 7.00 ± 0.50 | 11.17 ± 0.55 | - | 7.09 ± 0.41 | 6.34 ± 0.40 | 3.27 ± 0.01 | 0.55 ± 0.04 | 2.58 ± 1.28 |
| Unripe × 4 months × Day 5 | 6.57 ± 0.30 | 11.43 ± 0.15 | - | 7.30 ± 0.11 | 6.56 ± 0.10 | 3.26 ± 0.01 | 0.56 ± 0.04 | 6.67 ± 1.75 |
| Unripe × 4 months × Day 10 | 6.11 ± 0.70 | 10.80 ± 0.10 | - | 6.84 ± 0.09 | 6.12 ± 0.08 | 3.30 ± 0.01 | 0.46 ± 0.03 | 7.00 ± 1.10 |
| Unripe × 4 months × Day 15 | 6.19 ± 0.44 | 12.07 ± 0.29 | - | 7.74 ± 0.17 | 7.02 ± 0.18 | 3.46 ± 0.01 | 0.44 ± 0.02 | 6.83 ± 0.75 |
| Ripe × 0 month × Day 0 | 7.39 ± 0.53 | 9.27 ± 0.25 | 4.00 ± 0.00 | 6.06 ± 0.07 | 5.19 ± 0.10 | 3.03 ± 0.01 | 0.67 ± 0.05 | - |
| Ripe × 0 month × Day 5 | 7.58 ± 0.21 | 9.97 ± 0.15 | 5.67 ± 1.63 | 6.35 ± 0.15 | 5.50 ± 0.14 | 3.01 ± 0.04 | 0.64 ± 0.03 | - |
| Ripe × 0 month × Day 10 | 7.81 ± 0.47 | 10.23 ± 0.25 | 7.67 ± 0.82 | 6.40 ± 0.18 | 5.46 ± 0.19 | 3.05 ± 0.03 | 0.62 ± 0.03 | - |
| Ripe × 0 month × Day 15 | 8.15 ± 0.69 | 10.93 ± 0.35 | 7.33 ± 0.52 | 7.10 ± 0.29 | 6.15 ± 0.28 | 3.06 ± 0.04 | 0.60 ± 0.02 | - |
| Ripe × 2 months × Day 0 | 7.89 ± 0.41 | 10.47 ± 0.12 | - | 6.63 ± 0.14 | 5.75 ± 0.15 | 3.27 ± 0.02 | 0.55 ± 0.04 | 0.00 ± 0.00 |
| Ripe × 2 months × Day 5 | 7.93 ± 0.59 | 10.60 ± 0.17 | - | 6.70 ± 0.13 | 5.81 ± 0.10 | 3.31 ± 0.04 | 0.46 ± 0.02 | 0.50 ± 1.22 |
| Ripe × 2 months × Day 10 | 7.20 ± 0.34 | 10.40 ± 0.26 | - | 6.66 ± 0.18 | 5.73 ± 0.16 | 3.30 ± 0.04 | 0.41 ± 0.02 | 0.00 ± 0.00 |
| Ripe × 2 months × Day 15 | 6.44 ± 0.44 | 10.83 ± 0.32 | - | 6.94 ± 0.23 | 6.12 ± 0.22 | 3.27 ± 0.02 | 0.48 ± 0.01 | 0.00 ± 0.00 |
| Ripe × 4 months × Day 0 | 6.47 ± 0.36 | 10.50 ± 0.10 | - | 6.63 ± 0.07 | 5.93 ± 0.08 | 3.25 ± 0.02 | 0.56 ± 0.01 | 0.92 ± 1.07 |
| Ripe × 4 months × Day 5 | 6.36 ± 0.40 | 11.27 ± 0.31 | - | 7.47 ± 0.48 | 6.76 ± 0.47 | 3.41 ± 0.03 | 0.45 ± 0.04 | 4.00 ± 0.63 |
| Ripe × 4 months × Day 10 | 6.31 ± 0.45 | 11.37 ± 0.31 | - | 7.24 ± 0.22 | 6.39 ± 0.16 | 3.47 ± 0.01 | 0.47 ± 0.01 | 4.08 ± 2.20 |
| Ripe × 4 months × Day 15 | 6.28 ± 0.36 | 11.07 ± 0.15 | - | 6.97 ± 0.19 | 6.33 ± 0.15 | 3.51 ± 0.01 | 0.42 ± 0.02 | 5.50 ± 2.43 |
| Overripe × 0 month × Day 0 | 7.87 ± 0.51 | 10.47 ± 0.15 | 6.00 ± 0.63 | 6.46 ± 0.16 | 5.53 ± 0.15 | 3.15 ± 0.03 | 0.52 ± 0.06 | - |
| Overripe × 0 month × Day 5 | 7.60 ± 0.40 | 10.53 ± 0.21 | 7.00 ± 0.00 | 6.96 ± 0.24 | 5.99 ± 0.23 | 3.06 ± 0.05 | 0.52 ± 0.06 | - |
| Overripe × 0 month × Day 10 | 7.72 ± 0.66 | 10.63 ± 0.06 | 7.17 ± 0.41 | 6.96 ± 0.09 | 6.04 ± 0.10 | 3.07 ± 0.02 | 0.52 ± 0.02 | - |
| Overripe × 0 month × Day 15 | 7.41 ± 0.58 | 10.23 ± 0.35 | 9.33 ± 0.52 | 6.64 ± 0.25 | 5.69 ± 0.25 | 3.03 ± 0.03 | 0.57 ± 0.02 | - |
| Overripe × 2 months × Day 0 | 7.23 ± 0.71 | 11.03 ± 0.12 | - | 7.11 ± 0.14 | 6.27 ± 0.13 | 3.25 ± 0.01 | 0.55 ± 0.01 | 0.00 ± 0.00 |
| Overripe × 2 months × Day 5 | 6.58 ± 0.26 | 10.97 ± 0.67 | - | 7.01 ± 0.54 | 6.19 ± 0.52 | 3.23 ± 0.03 | 0.49 ± 0.01 | 0.00 ± 0.00 |
| Overripe × 2 months × Day 10 | 6.51 ± 0.60 | 10.57 ± 0.23 | - | 6.67 ± 0.16 | 5.88 ± 0.15 | 3.26 ± 0.02 | 0.48 ± 0.01 | 0.00 ± 0.00 |
| Overripe × 2 months × Day 15 | 6.31 ± 0.22 | 10.63 ± 0.60 | - | 6.87 ± 0.48 | 5.97 ± 0.45 | 3.30 ± 0.02 | 0.36 ± 0.03 | 0.00 ± 0.00 |
| Overripe × 4 months × Day 0 | 6.96 ± 0.29 | 11.87 ± 0.42 | - | 7.58 ± 0.29 | 6.91 ± 0.28 | 3.47 ± 0.03 | 0.48 ± 0.01 | 0.25 ± 0.27 |
| Overripe × 4 months × Day 5 | 6.07 ± 0.37 | 11.40 ± 0.44 | - | 7.26 ± 0.32 | 6.63 ± 0.31 | 3.50 ± 0.06 | 0.44 ± 0.04 | 0.67 ± 0.68 |
| Overripe × 4 months × Day 10 | 5.57 ± 0.36 | 11.40 ± 0.30 | - | 7.25 ± 0.23 | 6.60 ± 0.22 | 3.49 ± 0.01 | 0.41 ± 0.01 | 3.08 ± 1.36 |
| Overripe × 4 months × Day 15 | 5.53 ± 0.37 | 11.33 ± 0.15 | - | 7.20 ± 0.15 | 6.53 ± 0.17 | 3.53 ± 0.03 | 0.38 ± 0.03 | 1.33 ± 0.52 |
| Significance | ||||||||
| H | *** | *** | *** | *** | *** | *** | *** | *** |
| S | *** | *** | - | *** | *** | *** | *** | - |
| D | *** | ** | *** | ** | *** | *** | *** | *** |
| H × S | *** | ** | - | n.s. | n.s. | *** | *** | *** |
| H × D | * | *** | *** | ** | *** | *** | *** | *** |
| S × D | *** | ** | - | *** | *** | *** | ** | *** |
| H × S × D | ** | *** | - | *** | *** | *** | *** | *** |
| L* | a* | b* | BI | YI | ΔE | |
|---|---|---|---|---|---|---|
| Harvest stage (H) | ||||||
| Unripe | 61.57 ± 6.07 a | −5.56 ± 5.13 a | 32.05 ± 4.01 a | 62.99 ± 9.93 b | 74.34 ± 5.64 ab | 6.56 ± 5.49 b |
| Ripe | 62.66 ± 5.51 a | −6.42 ± 4.03 b | 33.09 ± 3.39 b | 63.66 ± 11.45 ab | 75.66 ± 7.28 b | 4.95 ± 4.75 a |
| Overripe | 64.16 ± 4.71 b | −5.68 ± 3.32 a | 32.64 ± 2.85 ab | 60.99 ± 9.23 a | 72.93 ± 7.01 a | 5.78 ± 3.37 ab |
| Storage duration (S) | ||||||
| 0 month | 62.82 ± 4.55 b | −9.48 ± 1.11 a | 31.96 ± 2.35 a | 55.48 ± 6.93 a | 72.93 ± 6.15 a | 4.07 ± 3.28 a |
| 2 months | 65.62 ± 4.14 c | −6.02 ± 2.34 b | 34.15 ± 2.65 b | 62.83 ± 8.32 b | 74.59 ± 6.81 ab | 5.71 ± 3.39 b |
| 4 months | 59.95 ± 6.22 a | −2.16 ± 4.51 c | 31.66 ± 4.48 a | 69.23 ± 10.37 c | 75.41 ± 7.08 b | 7.51 ± 6.10 c |
| Shelf-life period (D) | ||||||
| Day 0 | 62.22 ± 3.62 a | −7.73 ± 2.28 a | 35.52 ± 2.41 | 60.31 ± 7.13 a | 74.80 ± 5.85 | 2.81 ± 2.95 a |
| Day 5 | 62.01 ± 5.02 a | −6.18 ± 3.91 b | 32.01 ± 3.62 | 61.40 ± 8.98 ab | 73.80 ± 6.58 | 5.49 ± 4.34 b |
| Day 10 | 63.72 ± 6.60 b | −5.18 ± 4.91 c | 32.92 ± 3.71 | 63.48 ± 11.57 bc | 74.05 ± 7.05 | 7.53 ± 4.62 c |
| Day 15 | 63.23 ± 6.34 ab | −4.45 ± 4.63 c | 32.91 ± 3.90 | 65.00 ± 12.13 c | 74.59 ± 7.49 | 7.21 ± 4.93 c |
| H × S × D | ||||||
| Unripe × 0 month × Day 0 | 61.02 ± 3.22 | −9.82 ± 0.35 | 31.94 ± 1.46 | 57.05 ± 4.86 | 74.89 ± 4.03 | 3.55 ± 1.78 |
| Unripe × 0 month × Day 5 | 61.03 ± 2.30 | −9.36 ± 1.11 | 31.70 ± 1.64 | 56.86 ± 4.01 | 74.22 ± 2.83 | 3.15 ± 1.83 |
| Unripe × 0 month × Day 10 | 60.16 ± 2.21 | −9.44 ± 0.78 | 31.36 ± 2.10 | 56.82 ± 3.83 | 74.42 ± 3.49 | 3.58 ± 2.42 |
| Unripe × 0 month × Day 15 | 62.60 ± 2.33 | −9.39 ± 0.45 | 31.08 ± 1.01 | 53.16 ± 3.98 | 71.04 ± 3.78 | 2.88 ± 1.49 |
| Unripe × 2 months × Day 0 | 63.76 ± 3.82 | −8.06 ± 0.84 | 32.76 ± 2.07 | 58.40 ± 5.55 | 73.53 ± 4.68 | 3.86 ± 2.10 |
| Unripe × 2 months × Day 5 | 65.05 ± 2.53 | −7.82 ± 0.84 | 34.93 ± 1.95 | 63.33 ± 6.97 | 76.84 ± 5.65 | 4.42 ± 1.66 |
| Unripe × 2 months × Day 10 | 65.08 ± 3.64 | −6.64 ± 1.61 | 35.06 ± 1.75 | 65.43 ± 7.42 | 77.23 ± 6.50 | 5.38 ± 2.04 |
| Unripe × 2 months × Day 15 | 68.72 ± 2.38 | −4.74 ± 1.38 | 37.43 ± 1.71 | 69.10 ± 5.85 | 77.88 ± 4.12 | 8.85 ± 2.12 |
| Unripe × 4 months × Day 0 | 60.90 ± 5.13 | −5.28 ± 3.47 | 30.19 ± 3.21 | 58.67 ± 6.40 | 70.85 ± 5.29 | 6.12 ± 4.20 |
| Unripe × 4 months × Day 5 | 56.01 ± 8.72 | 1.44 ± 5.14 | 27.12 ± 4.51 | 66.56 ± 8.38 | 69.15 ± 3.30 | 13.07 ± 7.62 |
| Unripe × 4 months × Day 10 | 57.46 ± 8.77 | 0.95 ± 5.50 | 30.10 ± 5.81 | 73.53 ± 13.09 | 74.87 ± 8.85 | 12.44 ± 6.61 |
| Unripe × 4 months × Day 15 | 57.05 ± 8.22 | 1.47 ± 5.36 | 30.89 ± 5.65 | 76.97 ± 9.71 | 77.11 ± 6.25 | 11.40 ± 8.05 |
| Ripe × 0 month × Day 0 | 62.59 ± 3.58 | −9.59 ± 0.86 | 33.13 ± 2.41 | 59.48 ± 9.11 | 75.99 ± 8.41 | 0.00 ± 0.00 |
| Ripe × 0 month × Day 5 | 63.23 ± 3.07 | −9.33 ± 0.85 | 30.45 ± 2.40 | 51.11 ± 7.70 | 69.05 ± 7.47 | 4.33 ± 1.95 |
| Ripe × 0 month × Day 10 | 73.87 ± 2.92 | −11.53 ± 0.59 | 35.89 ± 2.20 | 50.83 ± 5.89 | 69.52 ± 5.36 | 12.02 ± 2.70 |
| Ripe × 0 month × Day 15 | 60.41 ± 2.34 | −9.39 ± 0.49 | 32.14 ± 1.70 | 59.37 ± 7.22 | 76.18 ± 6.03 | 3.53 ± 1.16 |
| Ripe × 2 months × Day 0 | 63.09 ± 2.91 | −8.29 ± 0.42 | 32.09 ± 2.63 | 57.07 ± 7.52 | 72.73 ± 6.07 | 0.00 ± 0.00 |
| Ripe × 2 months × Day 5 | 63.25 ± 2.86 | −7.02 ± 1.73 | 34.02 ± 2.03 | 64.15 ± 6.87 | 76.93 ± 4.74 | 4.03 ± 1.85 |
| Ripe × 2 months × Day 10 | 63.07 ± 4.03 | −5.61 ± 1.36 | 33.22 ± 2.22 | 64.16 ± 7.46 | 75.44 ± 5.86 | 5.25 ± 1.46 |
| Ripe × 2 months × Day 15 | 65.55 ± 2.82 | −4.97 ± 2.02 | 35.97 ± 2.31 | 69.48 ± 8.13 | 78.53 ± 6.08 | 6.61 ± 2.20 |
| Ripe × 4 months × Day 0 | 60.80 ± 2.71 | −5.79 ± 2.15 | 33.15 ± 2.23 | 66.82 ± 2.99 | 77.88 ± 3.92 | 0.00 ± 0.00 |
| Ripe × 4 months × Day 5 | 59.90 ± 4.92 | −4.32 ± 2.47 | 31.75 ± 4.54 | 66.44 ± 10.43 | 75.70 ± 9.26 | 5.84 ± 4.36 |
| Ripe × 4 months × Day 10 | 60.21 ± 6.02 | −1.51 ± 4.41 | 34.00 ± 4.57 | 76.97 ± 7.41 | 80.56 ± 6.34 | 8.05 ± 5.20 |
| Ripe × 4 months × Day 15 | 55.91 ± 5.30 | 0.32 ± 4.82 | 31.22 ± 5.22 | 78.07 ± 12.78 | 79.38 ± 8.33 | 9.70 ± 6.82 |
| Overripe × 0 month × Day 0 | 60.52 ± 2.48 | −9.28 ± 1.55 | 31.93 ± 1.82 | 58.62 ± 6.77 | 75.49 ± 5.29 | 3.54 ± 2.14 |
| Overripe × 0 month × Day 5 | 61.64 ± 3.27 | −9.20 ± 0.57 | 31.59 ± 2.64 | 56.53 ± 8.83 | 73.48 ± 7.85 | 3.93 ± 2.22 |
| Overripe × 0 month × Day 10 | 63.37 ± 3.17 | −8.87 ± 1.31 | 31.71 ± 1.66 | 54.89 ± 6.46 | 71.67 ± 5.26 | 3.59 ± 1.98 |
| Overripe × 0 month × Day 15 | 63.46 ± 4.13 | −8.55 ± 1.07 | 30.60 ± 2.18 | 52.22 ± 6.67 | 69.15 ± 6.69 | 4.72 ± 2.77 |
| Overripe × 2 months × Day 0 | 63.86 ± 3.47 | −7.73 ± 0.99 | 34.11 ± 1.58 | 62.75 ± 6.08 | 76.49 ± 5.21 | 4.02 ± 1.81 |
| Overripe × 2 months × Day 5 | 66.03 ± 3.29 | −5.79 ± 1.18 | 34.19 ± 1.86 | 62.69 ± 7.34 | 74.19 ± 6.23 | 5.57 ± 1.67 |
| Overripe × 2 months × Day 10 | 67.84 ± 4.37 | −2.79 ± 2.82 | 32.03 ± 2.94 | 58.55 ± 10.43 | 67.62 ± 6.73 | 9.06 ± 1.93 |
| Overripe × 2 months × Day 15 | 72.08 ± 3.30 | −2.77 ± 1.05 | 33.96 ± 3.39 | 58.90 ± 10.45 | 67.64 ± 9.19 | 11.44 ± 2.41 |
| Overripe × 4 months × Day 0 | 63.47 ± 3.51 | −5.75 ± 1.45 | 33.34 ± 2.23 | 63.95 ± 8.16 | 75.31 ± 7.06 | 4.23 ± 2.77 |
| Overripe × 4 months × Day 5 | 61.93 ± 4.16 | −4.24 ± 1.38 | 32.36 ± 3.44 | 64.89 ± 7.42 | 74.61 ± 5.68 | 5.03 ± 2.84 |
| Overripe × 4 months × Day 10 | 62.46 ± 5.77 | −1.15 ± 3.07 | 32.92 ± 4.59 | 70.16 ± 8.59 | 75.16 ± 6.24 | 8.41 ± 3.63 |
| Overripe × 4 months × Day 15 | 63.26 ± 2.97 | −2.08 ± 1.86 | 32.93 ± 2.96 | 67.73 ± 7.42 | 74.41 ± 6.38 | 5.81 ± 2.43 |
| Significance | ||||||
| H | *** | ** | * | * | *** | *** |
| S | *** | *** | *** | *** | ** | *** |
| D | ** | *** | n.s. | *** | n.s. | *** |
| H × S | *** | *** | *** | n.s. | *** | *** |
| H × D | *** | n.s. | * | *** | *** | *** |
| S × D | *** | *** | *** | *** | ** | *** |
| H × S × D | *** | ** | n.s. | n.s. | n.s. | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sredojevic, A.; Radivojevic, D.; Levic, S.M.; Fotiric Aksic, M.; Milivojevic, J.; Djordjevic, M.; Spasojevic, S.; Djekic, I. Postharvest Quality of Granny Smith Apples: Interplay of Harvest Stage, Storage Duration, and Shelf-Life. Horticulturae 2025, 11, 868. https://doi.org/10.3390/horticulturae11080868
Sredojevic A, Radivojevic D, Levic SM, Fotiric Aksic M, Milivojevic J, Djordjevic M, Spasojevic S, Djekic I. Postharvest Quality of Granny Smith Apples: Interplay of Harvest Stage, Storage Duration, and Shelf-Life. Horticulturae. 2025; 11(8):868. https://doi.org/10.3390/horticulturae11080868
Chicago/Turabian StyleSredojevic, Ana, Dragan Radivojevic, Steva M. Levic, Milica Fotiric Aksic, Jasminka Milivojevic, Milena Djordjevic, Slavica Spasojevic, and Ilija Djekic. 2025. "Postharvest Quality of Granny Smith Apples: Interplay of Harvest Stage, Storage Duration, and Shelf-Life" Horticulturae 11, no. 8: 868. https://doi.org/10.3390/horticulturae11080868
APA StyleSredojevic, A., Radivojevic, D., Levic, S. M., Fotiric Aksic, M., Milivojevic, J., Djordjevic, M., Spasojevic, S., & Djekic, I. (2025). Postharvest Quality of Granny Smith Apples: Interplay of Harvest Stage, Storage Duration, and Shelf-Life. Horticulturae, 11(8), 868. https://doi.org/10.3390/horticulturae11080868









