Abstract
Saposhnikovia divaricata (Turcz. ex Ledeb.) Schischk., commonly known as divaricate siler, is a well-known medicinal plant from the Apiaceae family. Its natural habitat is rapidly declining owing to the harvesting of its roots, used as fángfēng in traditional Oriental medicine. This underutilized herb may serve as a valuable source of bioactive phenolic compounds, which can potentially be influenced by salicylic acid (SA) elicitation—a practical method to increase the concentration of valuable substances in plants. A field study showed that foliar application of SA on one-year-old S. divaricata positively influenced the total phenolic content in the herb, with the highest increase observed at 1.0 mM SA. Liquid chromatography–mass spectrometry (LC–MS) data became increasingly complex with rising SA levels, identifying up to 48 compounds, including cinnamoyl quinic acids (CQAs), dihydrofurochromones (DFCs), and flavonol O-glycosides (FOGs), most reported for the first time in this species. The highest concentrations of CQAs, DFCs, and FOGs in plants treated with 1.0 mM SA were 83.14, 3.75, and 60.53 mg/g, respectively, compared to 42.76, 0.95, and 40.73 mg/g in untreated (0.0 mM SA) plants. Nine in vitro antioxidant assays revealed strong radical-scavenging and nitric oxide (NO)- and Fe2+-chelating activities in 1.0 mM SA-treated plants, indicating robust antioxidative properties of the S. divaricata herb. Thus, foliar application of SA considerably enriches the herb with target antioxidants, increasing its medicinal value, which is reflected in the plant’s biological response. This could potentially reduce the overexploitation of natural populations of S. divaricata, helping to preserve this valuable plant.
1. Introduction
Irrational harvesting of plant materials is the primary issue in medicinal plant horticulture, which mainly relies on collecting wild specimens [1,2,3]. The lack of skilled collectors, the pursuit of high profits, and inadequate legislative measures lead to severe consequences not only for local plant populations but sometimes for the entire surrounding ecosystem [4]. Among the species with rapidly shrinking natural habitats owing to unsustainable collection is Saposhnikovia divaricata (Turcz. ex Ledeb.) Schischk. (also known as Ledebouriella divaricata (Turcz. ex Ledeb.) M. Hiroe or Laser divaricatum (Turcz. ex Ledeb.) Thell., Figure 1). This species is typical of the landscapes in Eastern Siberia, the Amur region, Primorye, Mongolia, China, and the Korean Peninsula [5]. The plant grows in meadows, on rocky steppes, on hill slopes, and in thickets of steppe shrubs. It is a perennial monocarpic herb, 40–80 cm tall, with stems branching from the base, which form tumbleweeds [6]. Its leaves are ovate and twice pinnate, while the white flowers are arranged in numerous umbels. Plants older than three years develop strong taproots, which are harvested for medicinal purposes [7]. Because the plant does not produce creeping or adventitious roots to regenerate new individuals, digging up the roots without following sustainable harvesting practices causes rapid population decline and can lead to local extinction [8]. Such activity has already caused a decline of over 60% in natural populations of S. divaricata in the Baikal region, highlighting the urgent need to explore the possibility of harvesting the above-ground parts while preserving the roots [9]. This approach would help conserve natural populations and improve the biodiversity of landscapes where the plants are currently collected.

Figure 1.
Saposhnikovia divaricata (Turcz. ex Ledeb.) Schischk. (divaricate siler) in its natural (a) and cultivated (b) habitat.
The medicinal use of S. divaricata roots originates from the Chinese text Shen Nong’s Materia Medica, where plant extracts were used to treat colds, rheumatism, bone diseases, headaches, and other ailments [6]. Modern research supports these traditional uses, demonstrating that the roots—also known as fángfēng—and their active compounds exhibit anti-inflammatory, analgesic, antitumor, and immunoregulatory effects [10]. However, in Siberian healing practices and among Baikal region lamas, the herb and seeds, referred to as la-la-phud, are used instead of the roots. Decoctions made from these parts are traditionally recommended for treating cold-related stomach diseases [11]. A comparative analysis of the literature reveals an uneven level of research on the chemical composition and biological activity of the underground versus aboveground parts of S. divaricata. Specifically, the roots have been found to contain approximately 200 compounds and exhibit more than 20 different types of biological activities [12]. In contrast, only a few chromones [13], flavonoids, and caffeoylquinic acids [14] have been identified in the herb, and no data are available regarding its biological activity.
Natural populations of medicinal plants do not exhibit consistent biomass renewal of the target species. S. divaricata follows this pattern because sustainable harvesting guidelines allow repeated collection from the same area only once every 3–4 years [9]. Therefore, cultivation in open fields or protected environments is increasingly practiced. S. divaricata grows well in open conditions, yielding substantial amounts of both roots and aerial parts. The mass ratio of underground-to-aboveground organs in 3–4-year-old plants can be as high as 1:(2–3), and in annual plants, it may reach 1:(5–7), suggesting the potential for using this green biomass for beneficial purposes [15]. Studies have shown that the green parts accumulate active chromones, such as 4′-O-glucosyl-5-O-methylvisamminol, at higher levels than the roots, with cimifugin content being similar in both [13]. The herb contains flavonoids and cinnamoyl quinic acids (CQAs) at concentrations several times higher than those found in the roots [14].
An increase in the levels of target beneficial compounds in medicinal plant tissues can be achieved using elicitors—non-toxic substances that influence biosynthesis. This approach sometimes results in a considerable increase in compound concentration and the production of enriched plant biomass with enhanced biological activity [16,17]. Among these, salicylic acid (SA) is the most commonly used and readily available elicitor for application over large, cultivated areas, owing to its eco-friendly nature, cost-effectiveness, and rapid action [18]. A similar approach to enhancing the quality of food and medicinal plants has been proven effective for chia [19], French beans [20], spinach [21], arnica [22], and basil [23], but it has not yet been applied to S. divaricata. Given the widespread use of S. divaricata in traditional medicine, it is important to explore methods for improving the chemical composition of its aerial parts, which may increase its therapeutic efficacy.
As part of an ongoing study on elicitation for medicinal plant improvement [24,25], this paper reports for the first time the effect of foliar application of SA on the phenolic compounds of the S. divaricata herb grown in the Baikal region, analyzed using liquid chromatography–mass spectrometry (LC–MS). Additionally, the antioxidant activity of S. divaricata herb extracts was evaluated using in vitro assays.
2. Materials and Methods
2.1. Seed Material
Saposhnikovia divaricata seeds were collected in 2022 from three Asian locations: Goryachinsk (Siberia, Buryatia Republic, 53°01′17.4″ N 108°18′50.1″ E), Borzya (Far East, Zabaikalskii Krai, 50°18′46.2″ N 116°35′46.3″ E), and Argalan (Mongolia, 47°48′36.8″ N 105°57′54.9″ E). The seeds were authenticated by Prof. N.I. Kashchenko, Doctor of Pharmacy (IGEB SB RAS, Ulan-Ude, Russia), air-dried for two months at 20 °C, and stored at −20 °C before planting.
2.2. Seed Germination and Plant Cultivation
Seeds germination was performed under controlled grow-box conditions (Secret Jardin Hydro Shoot HS480W System, Secret Jardin Agomoon SRL, Manage, Belgium), using Plagron Soil Promix (Plagron, Weert, The Netherlands) as the substrate at 25 °C with 12 h light/12 h dark cycles. Full-spectrum Tallos lamps (Fitovatt, Moscow, Russia) provided a photosynthetic photon flux density (PPFD) [photosynthetically active radiation (PAR)] of 386 μmol/m2/s. This setup was maintained for 4 months [26], after which, seedlings were individually transplanted to an open-field plantation (Experimental Plantation Site No. 07-7j, Mukhorshibir, Republic of Buryatia, Russia; 51°02′46.4″ N, 107°46′52.7″ E, 830 m a.s.l.) without fertilizer application, with water supplied by an automatic drip irrigation system GWB3240 (ELGO, Caesarea, Israel), and where they were grown from June 2023 to June 2024 to reach one year of age. Four experimental groups, each consisting of 40 one-year-old plants from each of the three seed sources, were sprayed with 0.10, 0.50, 1.0, and 2.0 mM concentrations of SA (Sigma-Aldrich, St. Louis, MI, USA) prepared in water containing 0.1% methanol (MeOH). Spraying was performed three times, starting at the full flowering stage (percentage of flowering > 80), with one-week intervals between applications, four times per day at 6 h intervals. The application rate was approximately 60 mL per plant per day. Control plants were sprayed with water containing 0.1% MeOH only (0.0 mM SA). Experimental fields for different levels of SA were located at least 50 m apart to avoid cross-contamination and wind transfer of the elicitor. Two weeks after the third spraying, the entirety of the aerial parts of the herb were harvested, dried in a ventilated oven at 40 °C for 10 days, and stored at 3–4 °C until analysis (Figure 2). The experiment was performed with five biological replicates.

Figure 2.
Samples of S. divaricata herb (Goryachinsk variety) of 0.0 mM SA (a) and 1.0 mM SA (b) foliar application groups after drying.
2.3. Phytochemical Assays
Total phenolic compounds in S. divaricata herb were measured using the spectrophotometric method with the Total Phenolic Content Assay Kit (Zen-Bio, Inc., Durham, UK; cat. No AOX-17), measuring the change in absorbance at 765 nm caused by phenolic compounds reacting with the Folin–Ciocalteu reagent (reference standard gallic acid). Essential oil content was quantified using a steam distillation–extraction assay in a micro steam distillation–extraction apparatus [27]. Total polysaccharide content was determined using the Polysaccharide Content Assay Kit (Profacgen, Shirley, NY, USA; cat. No CASQS-K067M), measuring the change in absorbance at 488 nm caused by carbohydrates reacting with the phenol–sulfuric acid reagent (reference standard glucose).
2.4. Plant Extract Preparation
Plant extract for LC–MS profiling and quantification was prepared from 100 mg of ground plant material (0.125 μm particle size) treated with 10 mL of 80% MeOH by sonication (20 min, 45 °C, ultrasound power 600 W, frequency 50 kHz). The mixture was then centrifuged (9000× g, 20 min), filtered through 0.22 μm syringe filters into a 10 mL measuring flask, and the volume was adjusted to 10 mL with MeOH. The extract was stored at 2 °C before analysis. An internal standard (lupulone, 100 μg/mL in MeOH) was added to the extracts in a 1:1 ratio. Plant extract for the antioxidant study was prepared from 150 g of powdered material extracted with 1.5 L of 80% MeOH using sonication (90 min, 45 °C, ultrasound power 600 W, frequency 50 kHz). The extract was filtered through a cellulose filter and dried under vacuum using a CombiDancer II vacuum evaporator (Hettich AG, Bäch, Switzerland) until completely dry. The extraction yields based on the herb weight were 20.4% for 0.0 mM SA, 20.9% for 0.1 mM SA, 22.8% for 0.5 mM SA, 27.8% for 1.0 mM SA, and 28.0% for 2.0 mM SA.
2.5. High-Performance Chromatography with Photodiode Array and Ion Trap-Time-of-Flight Mass Spectrometry Detection (HPLC-PDA-IT-TOF-MS)
The analysis was performed with an LC-20 Prominence liquid chromatograph (Shimadzu, Columbia, SC, USA) coupled to an SPD-30AM photodiode array detector (PDA; Shimadzu) and an LCMS-9050 ion trap–time-of-flight (IT–TOF) mass spectrometer system (also Shimadzu). Separation was achieved using a Gold-Turbo Basic C18 column (75 mm × 3 mm × 1.8 μm; Dr. Maisch GmbH, Ammerbuch, Germany). The HPLC conditions were as follows: mobile phase consisted of eluent A (0.1% formic acid (HCOOH) in water) and eluent B (0.1% HCOOH in acetonitrile). The gradient program applied was 0–1 min, 2–11% B; 1–9 min, 11–24% B; 9–17 min, 24–32% B; 17–35 min, 32–76% B; and 35–45 min, 76–2% B. The injected volume was 1 µL, the flow rate was set at 1 mL/min, and the column temperature was 25 °C. Absorption spectra were scanned from 200 to 600 nm. MS conditions included electrospray ionization in positive mode; interface temperature set at 300 °C; desolvation line temperature at 250 °C; heating block temperature at 400 °C; nebulizer gas (N2) flow at 3 L/min; heater gas (air) flow at 10 L/min; collision-induced dissociation (CID) gas (Ar) pressure at 270 kPa with an Ar flow rate of 0.3 mL/min; capillary voltage of 3 kV; and mass scan range from m/z 100 to 1900. Metabolite identification was based on retention time, ultraviolet spectra, and mass spectra compared to reference standards, previously isolated compounds, and the literature [14,28,29,30], using LabSolution (Shimadzu, Columbia, SC, USA) workstation software equipped with an internal LC–MS library (Table S1). To quantify compounds 1–48, calibration curves were prepared by creating a series of reference substance dilutions (1–100 μg/mL), each chromatographed three times under the conditions described above [14,15] (Table S2). Using the resulting data, calibration curves plotting “concentration, µg/mL–peak area” were constructed with the Advanced Grapher 2.2 software package (Alentum Software, Inc., New York, NY, USA).
2.6. Antioxidant Assays
The radical-scavenging activity of S. divaricata herb extracts was evaluated using microplate spectrophotometric assays with 2,2-diphenyl-1-picrylhydrazyl or DPPH• radicals (100 μg/mL radical solution in MeOH, detection wavelength 520 nm, incubation time 15 min) [31], 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) or ABTS•+ cation radicals (7 mM radical solution in 2.45 mM potassium persulphate, detection wavelength 734 nm, incubation time 20 min) [31], N,N-dimethyl-p-phenylenediamine dihydrochloride or DMPD• radicals (1 mM radical solution in 0.1 M acetate buffer, pH 5.25, detection wavelength 505 nm, incubation time 20 min) [32], hydroxyl or OH• radicals (6 mM pyrogallol solution in 0.05 M Tris-HCl buffer, pH 8.2, detection wavelength 325 nm, incubation time 5 min) [31], and superoxide or O2•− anion radicals (deoxyribose 2.8 mM, H2O2 3.6 mM, and 5.0 mM FeCl3 solution in 0.2 M phosphate buffer, pH 7.4, detection wavelength 530 nm, incubation time 20 min) [31]. The inactivation of chlorine radicals (Cl•) and bromine radicals (Br•) was assessed by coulometric titration using electrogenerated bromine radicals and Potentiostat Expert-006 (Econics Expert Ltd., Moscow, Russia) with a four-electrode two-compartment electrochemical cell [33]. Ferrous (II) chelating activity (FeCA) was measured by spectrophotometric o-phenanthroline method assay (FeSO4 22.24 mg/mL solution in phosphate-buffered saline, pH 6.0, detection wavelength 500 nm, incubation time 20 min) [34]. Trolox was used as the reference standard. Each analysis was performed in quintuplicate, and the results are presented as the mean ± standard deviation (S.D.).
2.7. Statistical Analysis
Statistical analyses were performed using one-way analysis of variance and Statistica software (ver. 12.6, Dell, Texas, TX, USA), with mean differences assessed by Fisher’s least significant difference (LSD) test at α = 0.05. Statistical significance was set at p < 0.05. The results are expressed as means ± S.D. Linear regression analysis and calibration curve construction were performed using Advanced Grapher 2.2 (Alentum Software, Inc., Ramat-Gan, Israel).
3. Results and Discussion
3.1. Effect of Foliar Salicylic Acid (SA) Application on the General Phytochemical Composition S. divaricata Herb
A preliminary field experiment with one-year-old S. divaricata from three coenopopulations in the Asian region demonstrated that seeds from different parental sources influenced the chemical composition of subsequent generations. Even when controlling for substrate (soil) and environmental conditions (temperature, insolation, and humidity), variations in the content of key phytocomponents in the S. divaricata herb were observed. The Goryachinsk variety (Siberia) exhibited the highest levels of phenolic compounds (82.75 mg/g) and polysaccharides (45.63 mg/g). In contrast, seeds from the Argalan variety (Mongolia) produced plants with the lowest phenolic compound (37.59 mg/g) and polysaccharide (24.81 mg/g) content among those studied but with the highest essential oil content (5.9 mg/g) (Table 1; 0.0 mM SA). The Borzya variety (Far East) showed intermediate levels across all three groups of compounds.

Table 1.
Total content of phenolic compounds, essential oil, and polysaccharides in herb of 3 varieties of S. divaricata after 0.0–2.0 mM SA treatment, mg/g dry plant weight ± SD.
Owing to the observed differences, plants grown from seeds of all three populations were subjected to foliar treatment with SA at four concentrations (0.1–2.0 mM). Phenolic compounds were the only group to respond to SA elicitation by increasing in all three varieties, with the highest increase at 1.0 mM SA: Goryachinsk variety 82.75 mg/g→158.42 mg/g (+91.4%), Borzya variety 45.63 mg/g→65.14 mg/g (+42.8%), and Argalan variety 37.59 mg/g→48.73 mg/g (+29.6%).
This indicates that the Goryachinsk variety has a greater biosynthetic potential to accumulate phenolic compounds after SA treatment. Interestingly, essential oil concentrations in the herb decreased across all three groups after SA treatment, with the lowest levels observed at 2.0 mM SA: Goryachinsk variety 3.7 mg/g→2.5 mg/g (−32.4%), Borzya variety 4.2 mg/g→2.9 mg/g (−31.0%), and Argalan variety 5.9 mg/g→3.0 mg/g (−49.2%). This demonstrates a negative effect of SA on the synthesis of volatile (terpene) compounds in S. divaricata. Additionally, no significant changes were observed in the content of herbal polysaccharides in any variety.
It has been previously demonstrated that SA elicitation can increase various phenolic contents in valuable Apiaceae plants. For example, Visnaga daucoides Gaertn. (Ammi visnaga (L.) Lam.), also known as the toothpick plant or toothpick weed, showed the highest accumulation of γ-pyrones (78.28 mg/g), including spasmolytic furanochromones khellin and visnagin, in response to 2.0 mM SA irrigation [35]. Similarly, Apium graveolens L. (celery) more efficiently stored phthalides with COX-2 selective inhibitor activity after treatment with 1–10 mM SA, reaching 15.2 mg/g of senkyunolide A [36]. Additionally, the phenolic compound concentration in Coriandrum sativum L. (coriander) foliage increased from 14.8 to 16.6 mg/g after foliar application of SA [37]. In this regard, the observed accumulation of phenolic compounds in the S. divaricata herb aligns with the general trend that is typical of the Apiaceae family as a whole.
3.2. Phenolic Compounds Profile of SA-Treated S. divaricata Herb
The data demonstrated that the phenolic compounds in the S. divaricata herb were highly responsive to SA treatment, highlighting the importance of studying the composition of these metabolites. There are two main reasons for this analysis: (1) a targeted investigation of the phenolic metabolomic profile is needed because it has not been previously explored and is essential for understanding the plant’s biochemistry, and (2) for identifying how the chemical composition changes under SA influence is essential to determine the most sensitive compounds that respond to elicitation. The study focused on the Goryachinsk variety seeds, owing to their highest phenolic content and the strongest response to SA treatment. High-performance liquid chromatography with photodiode array and ion trap–time-of-flight mass spectrometry (HPLC–PDA–IT–TOF–MS) effectively accomplished the task, enabling the detection and identification of 48 compounds in total (Figure 3, Table 2).
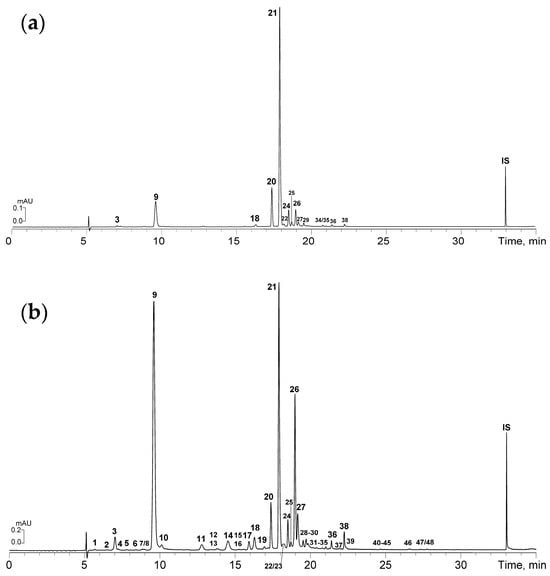
Figure 3.
HPLC-PDA chromatograms of S. divaricata herb (Goryachinsk variety) with the lowest phenolic level (control group, 0.0 mM SA, (a) and the highest phenolic level (1.0 mM SA, (b) at 320 nm. Compounds 1–48 description shown in Table 2. IS–internal standard (lupulone).

Table 2.
Retention time (tR), molecular formula (MF), MS data, UV pattern (UVP), and identification level (IL) of compounds 1–48 found in S. divaricata herb.
The first clear difference between the S. divaricata samples before and after SA treatment was the number of detected compounds. In the untreated herb with zero SA, 16 compounds were found, while after treatment with 1–2 mM SA, this number increased 3-fold to 48. Without foliar SA application, the natural metabolites included mono- and di-caffeoylquinic acids (3, 9, 26, 27), 5-O-trans-feruloyl-quinic acid (18), two DFCs—prim-O-glycosylcimifugin (17) and divarichromone B (29)—and nine FOGs, such as rutin (20), isoquercitrin (21), nicotiflorin (22), astragalin (24), isorhamnetin 3-O-glucoside (25), kaempferol 3-O-(2″-O-p-coumaroyl)-rhamnoside (36), kaempferol 3-O-(2″,3″-di-O-p-coumaroyl)-rhamnoside (38), and compounds 34 and 35, tentatively identified as flavonols. The latter flavonoids are isomers showing typical quercetin p-coumarate derivative UV patterns and mass spectral signals at m/z 739, 593, 447, and 301, characteristic of quercetin O-desoxyhexoside di-O-p-coumarates [30]. Previous studies on S. divaricata metabolites commonly reported compounds 3, 9, 17, 20, 21, 24–26, and 29 [6], whereas compounds 18, 22, 27, 34–36, and 38 represent newly identified phenolics in this species.
Minimal treatment with 0.1 mM SA led to changes in the HPLC profile of the extracts, revealing the presence of 5-O-cis-feruloyl-quinic acid (19), narcissin (23), and afzelin (28), all new compounds for the plant. Increasing the SA concentration to 0.5 mM allowed the detection of three additional phenolics—3-O-trans-caffeoyl-quinic acid (10), 5-O-cis-caffeoyl-quinic acid (11), and 5-O-trans-p-coumaroyl-quinic acid (14)—bringing the total number of identified compounds to 22.
The most pronounced changes in the HPLC profile of S. divaricata were observed after treatment with 1 mM and 2 mM SA. The metabolites produced include well-known phytophenolics such as CQAs, including 1-O-trans-caffeoyl-quinic acid (1), 3-O-trans-p-coumaroyl-quinic acid (15), and 4-O-trans-p-coumaroyl-quinic acid (8); the DFC divarichromone A (16); and flavonoids manghaslin (4), clitorin (5), and typhaneoside (6). Tentatively identified compounds include caffeoyl-quinic acid 2, cimifugin di-O-hexoside-O-pentoside 7 (m/z 761 [M–H]−→MS2 629, 467, 305), cimifugin di-O-hexosides 12 and 13 (m/z 629 [M–H]−→MS2 467, 305), quercetin O-hexoside O-p-coumarates 30 and 31 (m/z 609 [M–H]−→MS2 463, 301), quercetin O-desoxyhexoside O-p-coumarates 32 and 33 (m/z 593 [M–H]−→MS2 447, 301), kaempferol O-desoxyhexoside O-p-coumarate 37 (m/z 577 [M–H]−→MS2 431, 285), kaempferol O-desoxyhexoside di-O-p-coumarate 39 (m/z 723 [M–H]−→MS2 577, 431, 285), isorhamnetin O-desoxyhexoside O-p-coumarates 40 and 41 (m/z 607 [M–H]−→MS2 461, 315), isorhamnetin O-desoxyhexoside di-O-p-coumarates 42 and 44 (m/z 753 [M–H]−→MS2 607, 461, 315), kaempferol O-desoxyhexoside tri-O-p-coumarates 43, 45 and 48 (m/z 869 [M–H]−→MS2 723, 577, 431, 285), and isorhamnetin O-desoxyhexoside tri-O-p-coumarates 46 and 47 (m/z 899 [M–H]−→MS2 753, 607, 461, 315). Except for divarichromone A, all other compounds were identified in S. divaricata for the first time, bringing the total number of newly identified compounds in this species to 38.
Whether the newly discovered compounds are natural metabolites present only in trace amounts below the detection limit remains to be established through further research. It is known that exogenous SA affects the production of certain phenolic compounds, causing their accumulation [18]. In S. divaricata, SA may change biosynthetic pathways, leading to the emergence of compound groups not previously identified in the plant. This is supported by the detection of flavonol triglycosides 4–6 and acylated FOGs 30–48, which had not been reported before. Although the exact nature of this phenomenon is unclear, it can be concluded that elicitation positively affects the metabolome by increasing its complexity, thereby increasing the potential biological effects of the plant extracts.
3.3. Content of Cinnamoyl Quinic Acids, Dihydrofurochromones, and Flavonol O-Glycosides in SA-Treated S. divaricata Herb
All 48 identified compounds, including CQAs, DFCs, and FOGs, were quantitatively analyzed. Both compound groups and individual substances showed a gradual accumulation in S. divaricata herb as SA treatment increased from 0.0 to 1.0 mM, with a slight decrease or, less frequently, a slight increase at 2.0 mM SA (Table 3). The total caffeoylquinic acid (CQA) content in untreated plants was 41.47 mg/g, of which 5-O-trans-caffeoyl-quinic acid accounted for 26.92 mg/g (~65% of total CQA). This was followed by 3,5-di-O-caffeoyl-quinic acid (8.77 mg/g), 4-O-trans-caffeoyl-quinic acid (3.03 mg/g), and 4,5-di-O-caffeoyl-quinic acid (2.75 mg/g).

Table 3.
Content of compounds 1–48 in S. divaricata herb (Goryachinsk variety) after 0.0–2.0 mM SA treatment, mg/g dry plant weight ± SD.
Foliar application of 0.1 mM SA led to a 2.2% increase in total CQA, while 0.5 mM SA resulted in a 14.9% increase compared to 0.0 mM SA. The total CQA reached its peak at 1.0 mM SA with 75.42 mg/g, representing an 81.9% increase. In plants treated with 1.0 mM SA, the content of the four main CQAs was 48.10 mg/g for 5-O-trans-caffeoyl-quinic acid, 12.63 mg/g for 3,5-di-O-caffeoyl-quinic acid, 7.39 mg/g for 4-O-trans-caffeoyl-quinic acid, and 5.70 mg/g for 4,5-di-O-caffeoyl-quinic acid.
p-Coumaroyl quinic acids were absent or present only in trace amounts in the 0.0 and 0.1 mM SA groups but increased from 0.57 to 2.00 mg/g between 0.5 and 2.0 mM SA treatments, with 5-O-trans-p-coumaroyl-quinic acid being the predominant compound (0.52–1.70 mg/g). The only feruloyl quinic acid detected, 5-O-trans-feruloyl-quinic acid, exhibited a 4.4-fold increase, rising from 1.29 mg/g at 0.0 mM SA to 5.73 mg/g at 1.0 mM SA. CQAs are known dietary polyphenols with established antioxidant, cardioprotective, anti-inflammatory, and neuroprotective activities [38]. The total CQA content in S. divaricata herb can reach 83.14 mg/g, comparable to levels found in espresso coffee [39] and cloudy apple juice [40], both known sources of food phenolics.
The level of DFCs in untreated plants was 0.95 mg/g, mainly owing to divarichromone B. Treatment with 1.0–2.0 mM SA increased the total DFC content to 3.84–3.85 mg/g, representing a 304–305% increase compared to 0.0 mM SA. This included divarichromone B (2.18 mg/g) and prim-O-glycosylcimifugin (1.57–1.60 mg/g). Because chromones are active compounds and chemical markers of S. divaricata roots [10,12], the observed increase in these target compounds suggests potential for using the herb as a medicinal raw material and as a substitute for the roots. The level of prim-O-glycosylcimifugin in S. divaricata herb treated with 1–2 mM SA matches that of first-grade cultivated roots [41], supporting the possibility of using the herb for medicinal purposes.
In the S. divaricata herb, the initial flavonoid composition was 32.69 mg/g quercetins, 6.95 mg/g kaempferols, and 1.09 mg/g isorhamnetins, contributing to a total flavonoid concentration of 40.73 mg/g. Quercetin derivatives constituted approximately four-fifths of all flavonoids, with isoquercitrin (27.70 mg/g) and rutin (4.96 mg/g) being the most prevalent. Elicitation with 1.0 mM SA led to a considerable increase in isoquercitrin content, reaching 32.63 mg/g (+17.8% compared to 0.0 mM SA), and rutin, which increased to 10.82 mg/g (+118.2%). The quercetin content reached 43.88 mg/g. Astragalin and kaempferol 3-O-(2″,3″-di-O-p-coumaroyl)-rhamnoside dominated the kaempferol composition; their levels fluctuated between 3.38–4.12 mg/g and 2.08–4.27 mg/g, respectively, in the 0.0–2.0 mM SA treatment range. Concentrations of other kaempferol derivatives remained below 1.7 mg/g in all SA-treated groups. Isorhamnetins constituted the least abundant flavonoid fraction. In the 0.0 mM SA group, only isorhamnetin 3-O-glucoside was detected; narcissin subsequently appeared in the 0.1 mM SA group, with additional minor compounds observed in trace amounts after 1–2 mM SA treatment. Despite their comparatively lower concentrations, these isorhamnetin compounds demonstrated a considerable increase in the 1.0 mM SA group, with isorhamnetin 3-O-glucoside reaching 2.50 mg/g and narcissin 1.76 mg/g. This indicates that all identified flavonoids accumulated in S. divaricata herb tissues in response to SA elicitation. The total flavonoid content in 1.0 mM SA-treated S. divaricata herb reached 60.53 mg/g, classifying the plant as an accumulator.
3.4. Antioxidant Potential of SA-Treated S. divaricata Herb
The presence of phenolic compounds in plant extracts directly determines their antioxidant activity, with the manifestation of this activity being proportional to their concentration. In its natural state, the S. divaricata herb can exhibit a total phenolic content of up to 84.44 mg/g, a level indicative of considerable antioxidant potential. Furthermore, SA elicitation leads to an even greater accumulation of CQAs, DFCs, and FOGs, totaling up to 147.12 mg/g, each capable of exhibiting biological activity. Although numerous previous studies have reported the antioxidant activity of S. divaricata root drugs, attributing it to various phenolic compounds such as chromones [42], coumarins [43], and flavonoids [44,45], alongside polysaccharides [46] and essential oils [47], the absence of data on antioxidant activity in the S. divaricata herb itself warrants investigation.
Three established antiradical assays, namely 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) cation radical (ABTS•+), and N,N-dimethyl-p-phenylenediamine dihydrochloride radical (DMPD•), revealed high scavenging activity in untreated S. divaricata herb (0.0 mM SA). Its IC50 values were 8.63 μg/mL (DPPH•), 4.63 μg/mL (ABTS•+), and 57.63 μg/mL (DMPD•), performing comparably to or better than the Trolox reference (10.08, 4.58, and 55.69 μg/mL, respectively) (Table 4). SA treatment further improved this activity, with the 1.0 mM SA group exhibiting maximal potential, achieving IC50 values of 5.24 μg/mL (DPPH•), 2.35 μg/mL (ABTS•+), and 35.29 μg/mL (DMPD•).

Table 4.
Antioxidant parameters of extracts from S. divaricata herb (Goryachinsk variety) after 0.0–2.0 mM SA treatment ± SD.
Natural radicals, such as the hydroxyl radical (OH•) and superoxide radical (O2•−), were scavenged by S. divaricata herb extracts, with IC50 values of 15.43 μg/mL (OH•) and 110.47 μg/mL (O2•−) for the 0.0 mM SA group, and 8.29 μg/mL (OH•) and 62.42 μg/mL (O2•−) for the 1.0 mM SA group. In comparison, Trolox showed IC50 values of 15.29 μg/mL for OH• and 105.11 μg/mL for O2•−, indicating that the extracts demonstrated high radical-scavenging activity. Inorganic radicals such as the chlorine radical (Cl•) and bromine radical (Br•) were inactivated by S. divaricata herb extracts less effectively than by Trolox. However, the trend of increasing activity—from the 0.0 mM SA group (Cl•: 453.4 mg Trolox/g; Br•: 386.4 mg Trolox/g) to the 0.1 mM SA group (Cl•: 783.3 mg Trolox/g; Br•: 704.2 mg Trolox/g)—remained consistent. The nitric oxide (NO•)-trapping ability of the 0.0 mM SA group (IC50 = 1.14 mg/mL) was comparable to that of Trolox (IC50 = 0.83 mg/mL) and further improved in the 1.0 mM SA group (IC50 = 2.29 mg/mL). A similar pattern was observed for Fe2+-chelating activity, which increased from 1.82 mM Fe2+ ions/g in the 0.0 mM SA group to 5.27 mM Fe2+ ions/g in the 1.0 mM SA group.
The expression of all evaluated antioxidant activity indicators demonstrated that SA elicitation improved the biopotential of the S. divaricata herb, with the highest values observed in the 1.0 mM SA treatment group. A similar trend has been reported in various food and medicinal plants. For example, elicitation of chia sprouts (Salvia hispanica L.) with 1.0–2.0 mM SA led to increased total flavonoid content, improved ABTS and DPPH in vitro values, and enhanced antioxidant parameters in rats with obesity-related oxidative stress [19]. Foliar application of 50–100 mM SA increased the levels of total soluble phenols, flavonoids, flavanols, and hydroxycinnamic acids in French bean (Phaseolus vulgaris L. cv. Saxa), along with increases in ABTS, DPPH, and FRAP antioxidant indicators [20]. Similarly, elicitation with 0.01–1 mg/mL SA improved the total phenolics, flavonoids, antioxidant enzyme activity, ABTS, and FRAP values in spinach (Spinacia oleracea L.) [21] and arnica (Arnica montana L.) [22], contributing to improved chemical composition and bioactivity of the herbal parts. Holy basil (Ocimum sanctum L.) also responded to foliar spraying with 0.1–1.5 mM SA by increasing the levels of eugenol, total phenolics, total flavonoids, and DPPH activity [23].
4. Conclusions
One-year-old plants of Saposhnikovia divaricata were subjected to foliar treatment with 0.1–2.0 mM SA in a pioneering field study aimed at evaluating the influence of elicitors on the species’ metabolite profile and bioactivity. The SA application increased the content of phenolic compounds and antioxidant potential in the herb. Specifically, SA treatment increased both the diversity and concentration of CQAs, DFCs, and FOGs, with the most pronounced effects observed after 1.0 mM SA application. The key compounds showing significant increases included 5-O-trans-caffeoylquinic acid, divarichromone B, prim-O-glycosylcimifugin, isoquercitrin, and rutin. The increase in phenolic compound concentration led to improved antioxidant activity, including radical scavenging, NO inactivation, and Fe2+-chelating capacity, of S. divaricata herb extracts. These findings indicate that elicitation with 1.0 mM SA is an effective strategy for improving the beneficial chemobiological properties of S. divaricata, a well-known medicinal plant.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae11080895/s1: Table S1: Reference standards used for HPLC profiling and quantification; Table S2: Regression equations, correlation coefficients, standard deviation, limits of detection, limits of quantification, and linear ranges for reference standards.
Author Contributions
Conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing—original draft preparation, writing—review and editing, visualization, supervision, project administration, funding acquisition, D.N.O., N.I.K., and N.K.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education and Science of Russia, grant numbers 121030100227-7; FSRG-2023-0027.
Data Availability Statement
The original contributions presented in this study are included in the article and supplementary material. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) cation radical |
| CQA | Caffeoyl quinic acids |
| DMPD | N,N-Dimethyl-p-phenylenediamine dihydrochloride radical |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl radical |
| FeCA | Ferrous ions chelating activity |
| FRAP | Ferric reducing antioxidant power |
| HPLC-PDA-IT-TOF-MS | High-performance chromatography with photodiode array and ion trap-time-of-flight mass spectrometry detection |
| SA | Salicylic acid |
References
- Ónodi, G.; Kröel-Dulay, G.; Kovács-Láng, E. Comparing the accuracy of three non-destructive methods in estimating aboveground plant biomass. Commun. Ecol. 2017, 18, 56–62. [Google Scholar] [CrossRef]
- Buxbaum, N.; Lieth, J.H.; Earles, M. Non-destructive plant biomass monitoring with high spatio-temporal resolution via proximal RGB-D imagery and end-to-end deep learning. Front. Plant Sci. 2022, 13, 758818. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.C.; Sessa, E.; Paton, A. Guidelines for the effective and ethical sampling of herbaria. Nat. Ecol. Evol. 2025, 9, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Jeltsch, F.; Moloney, K.A.; Schurr, F.M.; Köchy, M.; Schwager, M. The state of plant population modelling in light of environmental change. Perspect. Plant Ecol. Evol. Syst. 2007, 9, 171–189. [Google Scholar] [CrossRef]
- Chen, B.; Zou, H.; Zhang, B.; Zhang, X.; Jin, X.; Wang, C.; Zhang, X. Distribution pattern and change prediction of Saposhnikovia divaricata suitable area in China under climate change. Ecol. Indicat. 2022, 143, 109311. [Google Scholar] [CrossRef]
- Gao, J.W.; Zhan, Y.; Wang, Y.H. Advances in phytochemistry and modern pharmacology of Saposhnikovia divaricata (Turcz.) Schischk. Chin. J. Integr. Med. 2023, 29, 1033–1044. [Google Scholar] [CrossRef]
- Li, D.; Yang, C.; Yao, R.; Ma, L. Origin identification of Saposhnikovia divaricata by CNN embedded with the hierarchical residual connection block. Agronomy 2023, 13, 1199. [Google Scholar] [CrossRef]
- Han, Z.; Cui, Y.; Wang, Y.; Wang, Y.; Sun, Z.; Han, M.; Yang, L. Effect of rhizospheric fungus on biological control of root rot (Fusarium equiseti) disease of Saposhnikovia divaricata. Agronomy 2022, 12, 2906. [Google Scholar] [CrossRef]
- Shishmarev, V.M.; Shichmareva, T.M. Recourses of Medicinal Plants of Transbaikalia; BSC SD RAS: Ulan-Ude, Russia, 2017; pp. 32–104. [Google Scholar]
- Kreiner, J.; Pang, E.; Lenon, G.B.; Yang, A.W.H. Saposhnikoviae divaricata: A phytochemical, pharmacological, and pharmacokinetic review. Chin. J. Nat. Med. 2017, 15, 255–264. [Google Scholar] [CrossRef]
- Aseeva, T.A. Tibetan Medicine of Buryats; SO RAN: Novosibirsk, Russia, 2008; pp. 143–154. [Google Scholar]
- Chen, Y.; Xu, Z.; Gao, S.; Zhang, T.; Chen, T. Quality evaluation of Saposhnikovia divaricata (Turcz.) Schischk from different origins based on HPLC fingerprint and chemometrics. J. Chem. 2022, 2022, 1155650. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, T.; Chen, C.; Xu, Z.; Liu, C. Transcriptomics explores the potential of flavonoid in non-medicinal parts of Saposhnikovia divaricata (Turcz.) Schischk. Front. Plant Sci. 2023, 14, 1067920. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Shishmareva, T.M.; Shishmarev, V.M. New dihydrofurochromones from Saposhnikovia divaricata. Chem. Nat. Compd. 2025, 61, 557–559. [Google Scholar] [CrossRef]
- Shishmarev, V.M.; Shichmareva, T.M.; Aseeva, T.A. Recommendations for Introduction of Medicinal Plants in Buryatia Republic; BSC SD RAS: Ulan-Ude, Russia, 2018; pp. 28–95. [Google Scholar]
- Kandoudi, W.; Radácsi, P.; Gosztola, B.; Zámboriné Németh, É. Elicitation of medicinal plants in vivo—Is it a realistic tool? The effect of methyl jasmonate and salicylic acid on Lamiaceae species. Horticulturae 2022, 8, 5. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Ali, B. Salicylic acid: An efficient elicitor of secondary metabolite production in plants. Biocatal. Agricult. Biotechnol. 2021, 31, 101884. [Google Scholar] [CrossRef]
- Gómez-Velázquez, H.D.J.; Aparicio-Fernández, X.; Reynoso-Camacho, R. Chia sprouts elicitation with salicylic acid and hydrogen peroxide to improve their phenolic content, antioxidant capacities in vitro and the antioxidant status in obese rats. Plant Foods Hum. Nutr. 2021, 76, 363–370. [Google Scholar] [CrossRef]
- Youssef, S.M.; López-Orenes, A.; Ferrer, M.A.; Calderón, A.A. Foliar application of salicylic acid enhances the endogenous antioxidant and hormone systems and attenuates the adverse effects of salt stress on growth and yield of French bean plants. Horticulturae 2023, 9, 75. [Google Scholar] [CrossRef]
- Singh, S. Salicylic acid elicitation improves antioxidant activity of spinach leaves by increasing phenolic content and enzyme levels. Food Chem. Adv. 2023, 2, 100156. [Google Scholar] [CrossRef]
- Petrova, M.; Geneva, M.; Trendafilova, A.; Miladinova-Georgieva, K.; Dimitrova, L.; Sichanova, M.; Nikolova, M.; Ivanova, V.; Dimitrova, M.; Sozoniuk, M. Antioxidant capacity and accumulation of aaffeoylquinic acids in Arnica montana L. in vitro shoots after elicitation with yeast extract or salicylic acid. Plants 2025, 14, 967. [Google Scholar] [CrossRef]
- Rithichai, P.; Jirakiattikul, Y.; Nambuddee, R.; Itharat, A. Effect of salicylic acid foliar application on bioactive compounds and antioxidant activity in holy basil (Ocimum sanctum L.). Int. J. Agron. 2024, 2024, 8159886. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K. New flavonoids of the genus Scutellaria. II. Baicalein and wogonin glycosides from S. baicalensis. Chem. Nat. Compd. 2024, 60, 229–234. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Taraskin, V.V.; Chirikova, N.K. Methyl jasmonate elicitation enhances the biosynthesis of coumarin derivatives in Phlojodicarpus sibiricus. Russ. J. Plant Physiol. 2025, 72, 104. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K. Phenolic compounds of six unexplored Asteraceae species from Asia: Comparison of wild and cultivated plants. Horticulturae 2024, 10, 486. [Google Scholar] [CrossRef]
- Godefroot, M.; Sandra, P.; Verzele, M. New method for quantitative essential oil analysis. J. Chromatogr. A 1981, 203, 325–335. [Google Scholar] [CrossRef]
- Lin, L.; Harnly, J.M. Identification of hydroxycinnamoylquinic acids of arnica flowers and burdock roots using a standardized LC-DAD-ESI/MS profiling method. J. Agricult. Food Chem. 2008, 56, 10105–10114. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Akobirshoeva, A.; Zilfikarov, I.N.; Vennos, C. Isorhamnetin and quercetin derivatives as anti-acetylcholinesterase principles of marigold (Calendula officinalis) flowers and preparations. Int. J. Molec. Sci. 2017, 18, 1685. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chemposov, V.V.; Chirikova, N.K. Metabolites of prickly rose: Chemodiversity and digestive-enzyme-inhibiting potential of Rosa acicularis and the main ellagitannin rugosin D. Plants 2021, 10, 2525. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K.; Vasilieva, A.G.; Fedorov, I.A. LC-MS profile, gastrointestinal and gut microbiota stability and antioxidant activity of Rhodiola rosea herb metabolites: A comparative study with subterranean organs. Antioxidants 2020, 9, 526. [Google Scholar] [CrossRef]
- Fogliano, V.; Verde, V.; Randazzo, G.; Ritieni, A. Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J. Agricult. Food Chem. 1999, 47, 1035–1040. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Vasilieva, A.G.; Chirikova, N.K. Fragaria viridis fruit metabolites: Variation of LC-MS profile and antioxidant potential during ripening and storage. Pharmaceuticals 2020, 13, 262. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K. A novel HPLC-assisted method for investigation of the Fe2+-chelating activity of flavonoids and plant extracts. Molecules 2014, 19, 18296–18316. [Google Scholar] [CrossRef]
- Osama, S.; El Sherei, M.; Al-Mahdy, D.A.; Bishr, M.; Salama, O. Effect of Salicylic acid foliar spraying on growth parameters, γ-pyrones, phenolic content and radical scavenging activity of drought stressed Ammi visnaga L. plant. Ind Crops Prod. 2019, 134, 1–10. [Google Scholar] [CrossRef]
- Rico-Chávez, A.K.; Pérez-Ramírez, I.F.; Escobar-Ortíz, A.; Feregrino-Pérez, A.A.; Torres-Pacheco, I.; Guevara-González, R.G. Hormetic elicitation of phthalides in celery seeds (Apium graveolens L. var dulce) and its effect on seedling development. Ind Crops Prod. 2023, 202, 117022. [Google Scholar] [CrossRef]
- Afshari, M.; Pazoki, A.; Sadeghipour, O. Biochemical changes of coriander (Coriandrum sativum L.) plants under drought stress and foliar application of salicylic acid and silicon nanoparticles. J. Med. Plants By-prod. 2023, 3, 197–207. [Google Scholar] [CrossRef]
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef] [PubMed]
- Crozier, T.W.M.; Stalmach, A.; Lean, M.E.J.; Crozier, A. Espresso coffees, caffeine and chlorogenic acid intake: Potential health implications. Food Funct. 2012, 3, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Kahle, K.; Huemmer, W.; Scheppach, W.; Erk, T.; Richling, E. Polyphenols are intensively metabolized in the human gastrointestinal tract after apple juice consumption. J. Agric. Food Chem. 2007, 55, 10605–10614. [Google Scholar] [CrossRef]
- Lyu, L.; Li, X.; Zang, E.; Yan, Y.; Yang, M.; Wang, W.; Zhang, C.; Li, M. Specification and grade of Saposhnikoviae Radix (Saposhnikovia divaricata). Chin. Herb. Med. 2022, 14, 543–553. [Google Scholar] [CrossRef]
- Li, L.; Gui, Y.G.; Shi, D.F. Anti-oxidant activities of chromones from Saposhnikovia divaricata. Lishizhen Med. Mater. Med. Res. 2010, 21, 2135–2137. [Google Scholar]
- Wang, C.C.; Chen, L.G.; Yang, L.L. Inducible nitric oxide synthase inhibitor of the Chinese herb Ⅰ. Saposhnikovia divaricata (Turcz.) Schischk. Cancer Lett. 1999, 145, 151–157. [Google Scholar] [CrossRef]
- Tai, J.; Cheung, S. Anti-proliferative and antioxidant activities of Saposhnikovia divaricata. Oncol. Rep. 2007, 18, 227–234. [Google Scholar] [CrossRef][Green Version]
- Kim, M.; Seo, K.S.; Yun, W. Antimicrobial and antioxidant activity of Saposhnikovia divaricata, Peucedanum japonicum and Glehnia littoralis. Ind. J. Pharm. Sci. 2018, 8, 560–565. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Tian, Y.J.; Zhang, J. Studies on the antioxidative activity of polysaccharides from Radix Saposhnikoviae. J. Chin. Med. Mater. 2008, 31, 268–272. [Google Scholar]
- Li, B.; Yang, Z.; Mao, F. Phytochemical profile and biological activities of the essential oils in the aerial part and root of Saposhnikovia divaricata. Sci. Rep. 2023, 13, 8672. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).