Response of Shoot Growth to Ecological Factors Highlights a Synergistic Relationship Between Yield and Catechin Accumulation in Tea Plant (Camellia sinensis L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Environmental Conditions and Manipulation Methods
2.3. Determination of Catechin Content
2.4. Measurement of Growth Indexes
2.5. RNA Extraction and qRT-PCR
2.6. Data Analysis
3. Results
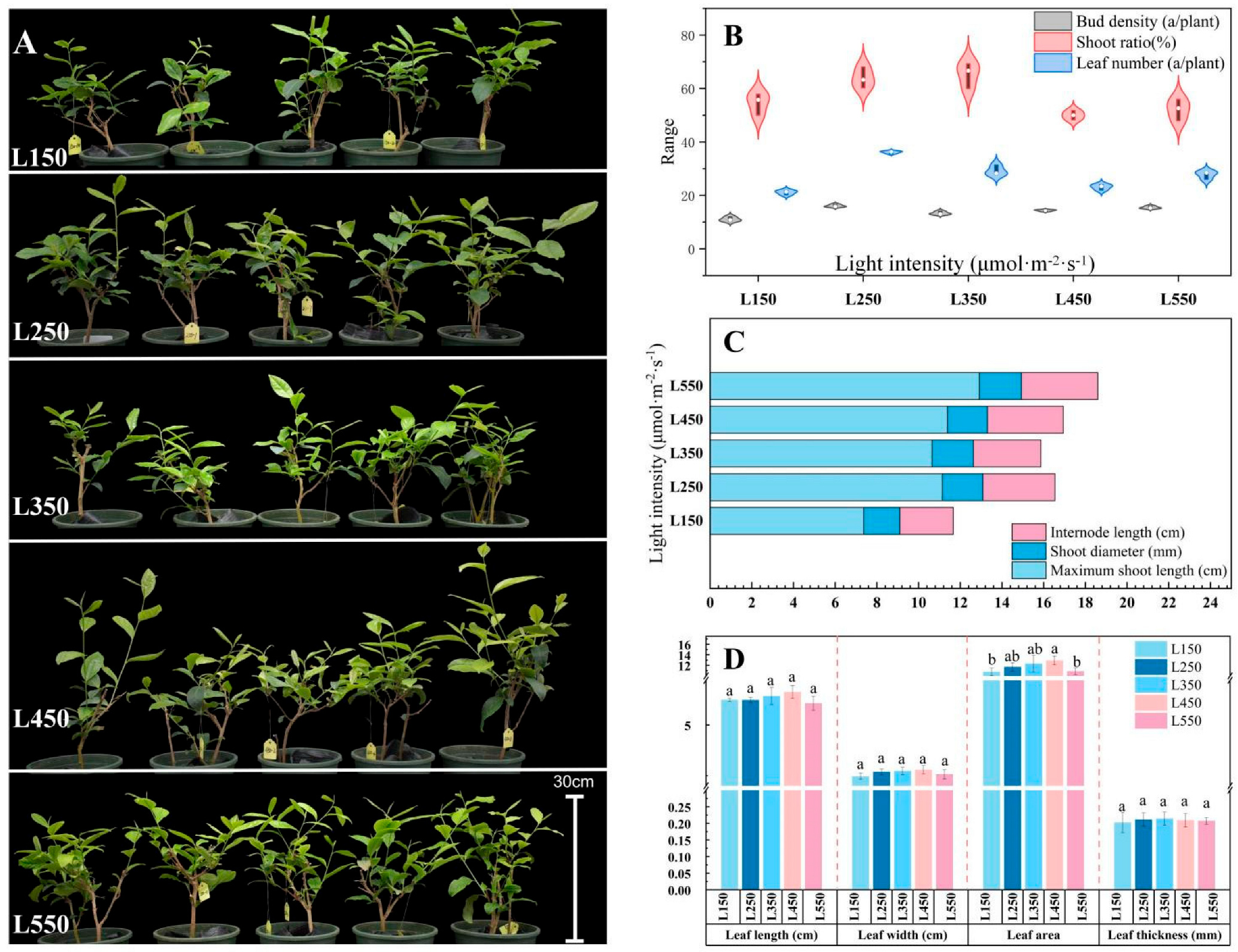
3.1. Growth Patterns of Tea Shoots in Response to Individual Changes in Ecological Factors
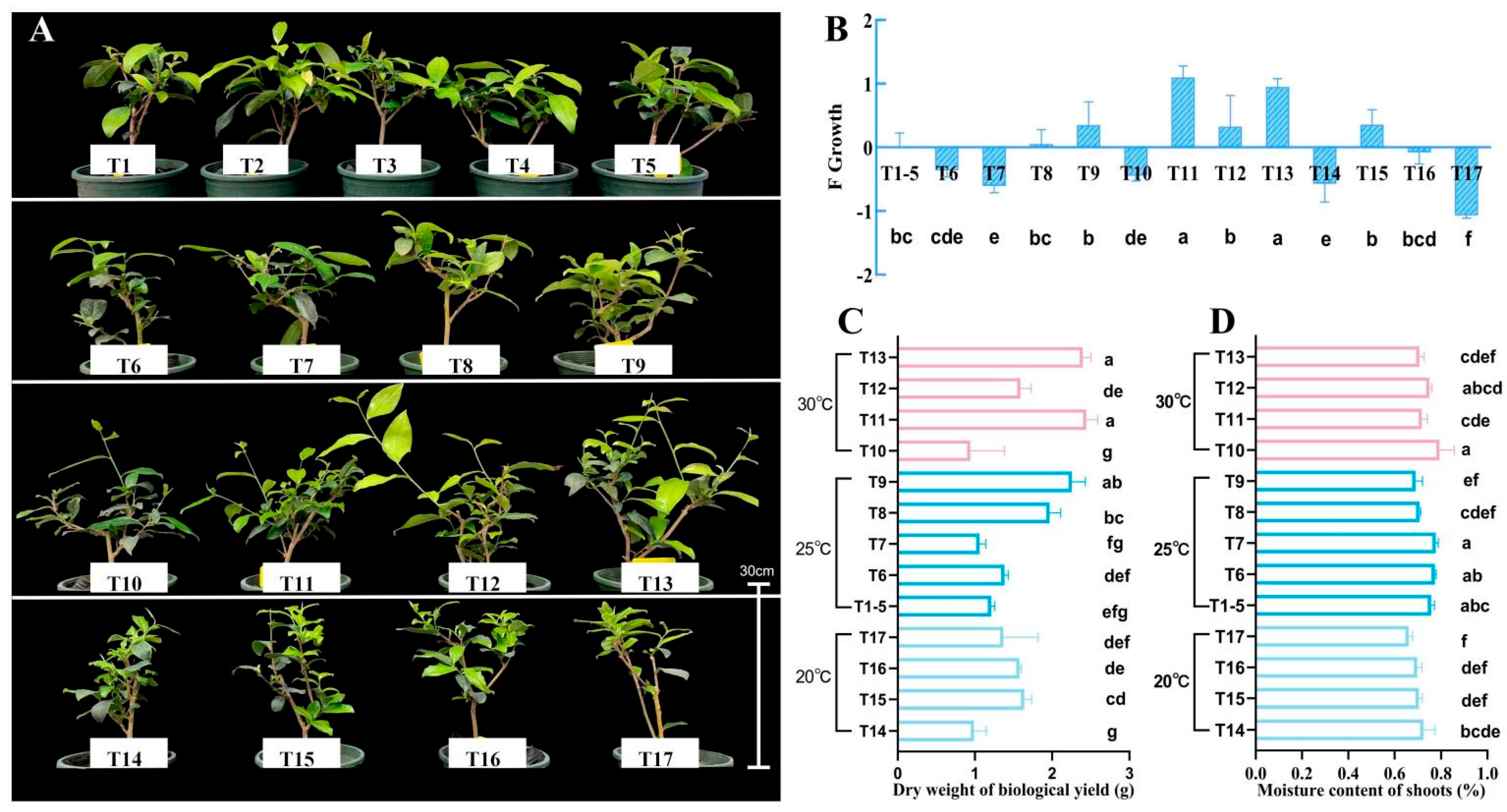
3.2. Response of Tea Shoot Growth to the Interactive Changes in Ecological Factors
3.3. Gene Responses in Shoot Growth to Ecological Factor Changes
3.4. Relationship Between Shoot Growth and Catechin Content in Response to Environmental Conditions
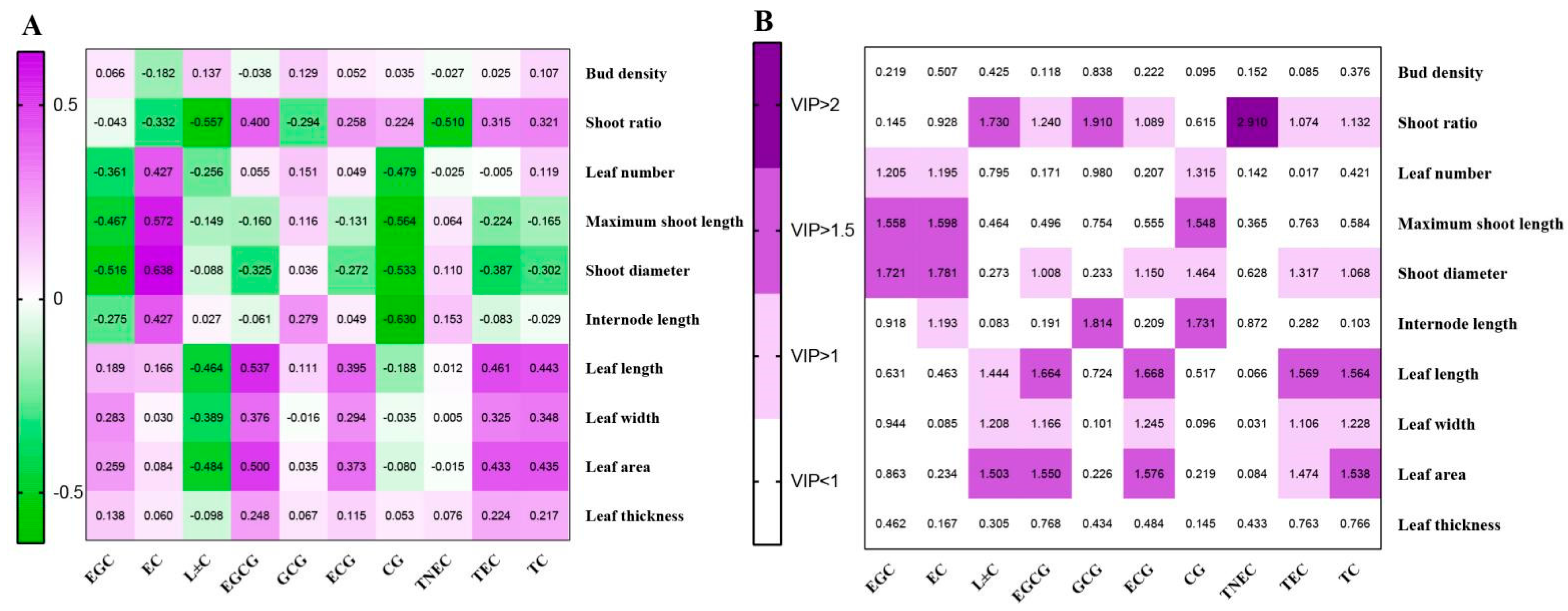
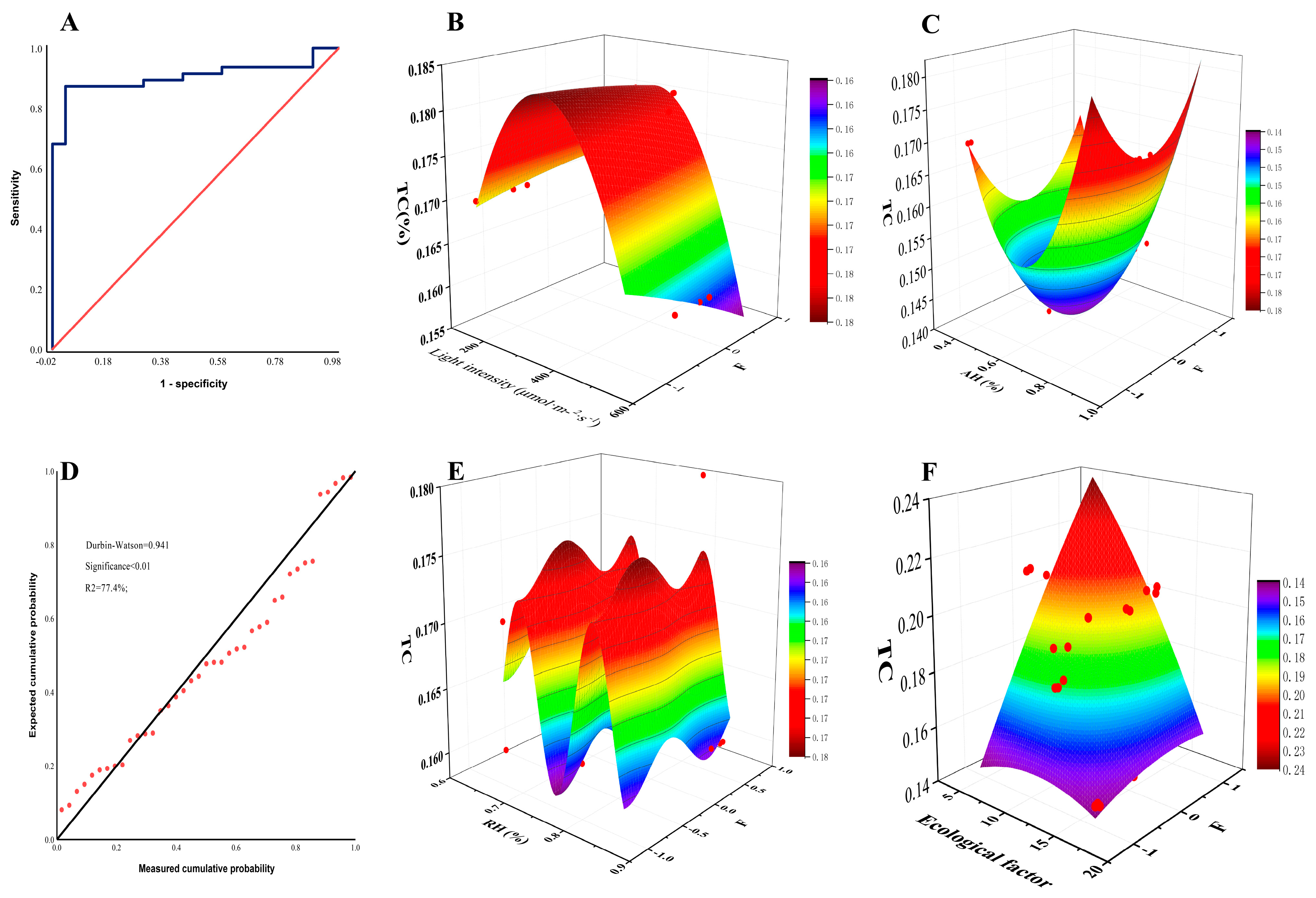
3.4.1. Correlation Analysis and Partial Least Squares Analysis
3.4.2. Regression Analysis and Surface Fitting
3.5. Molecular Mechanisms of Shoot Growth Mediating Catechin Accumulation in Tea Plants
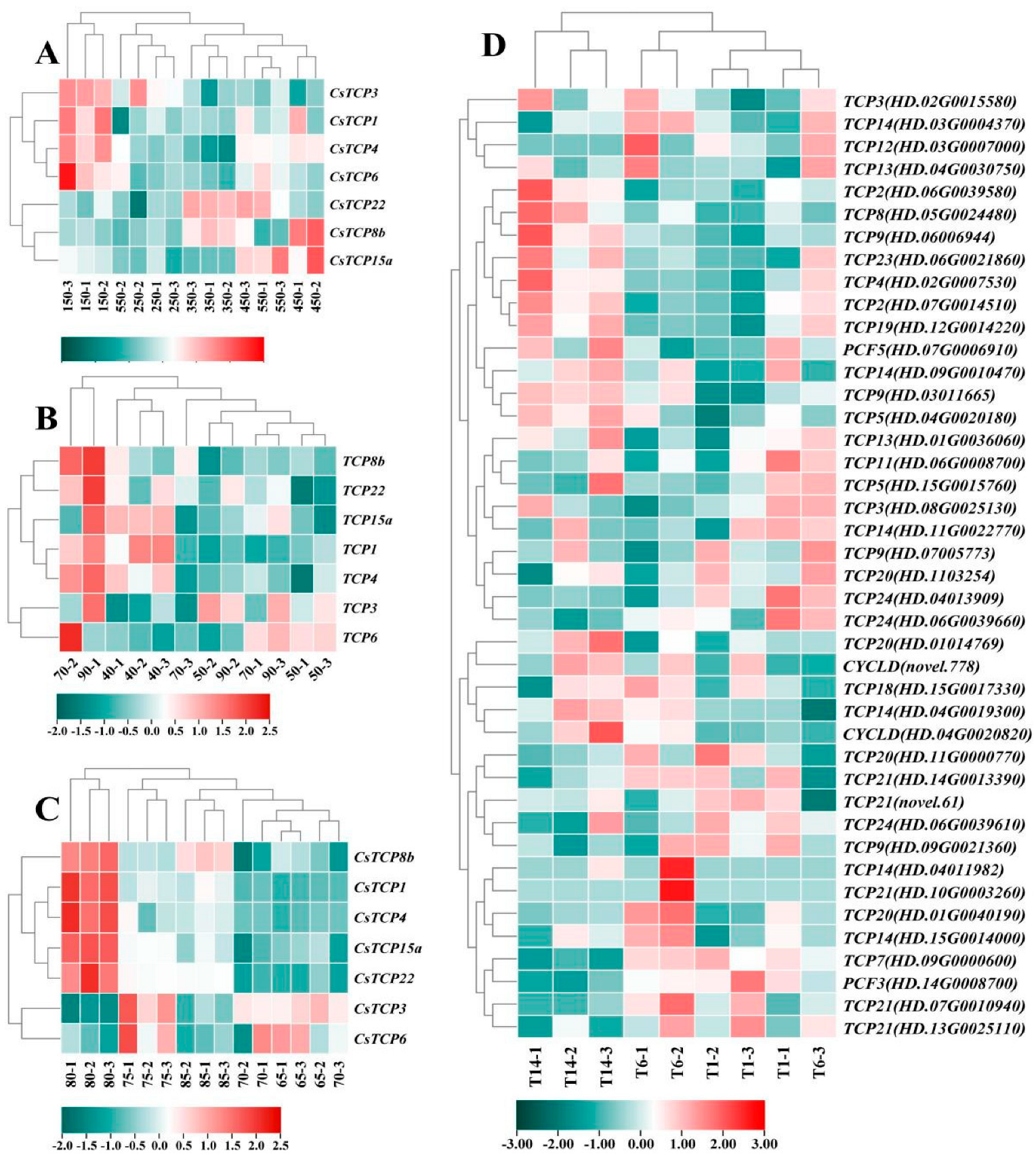
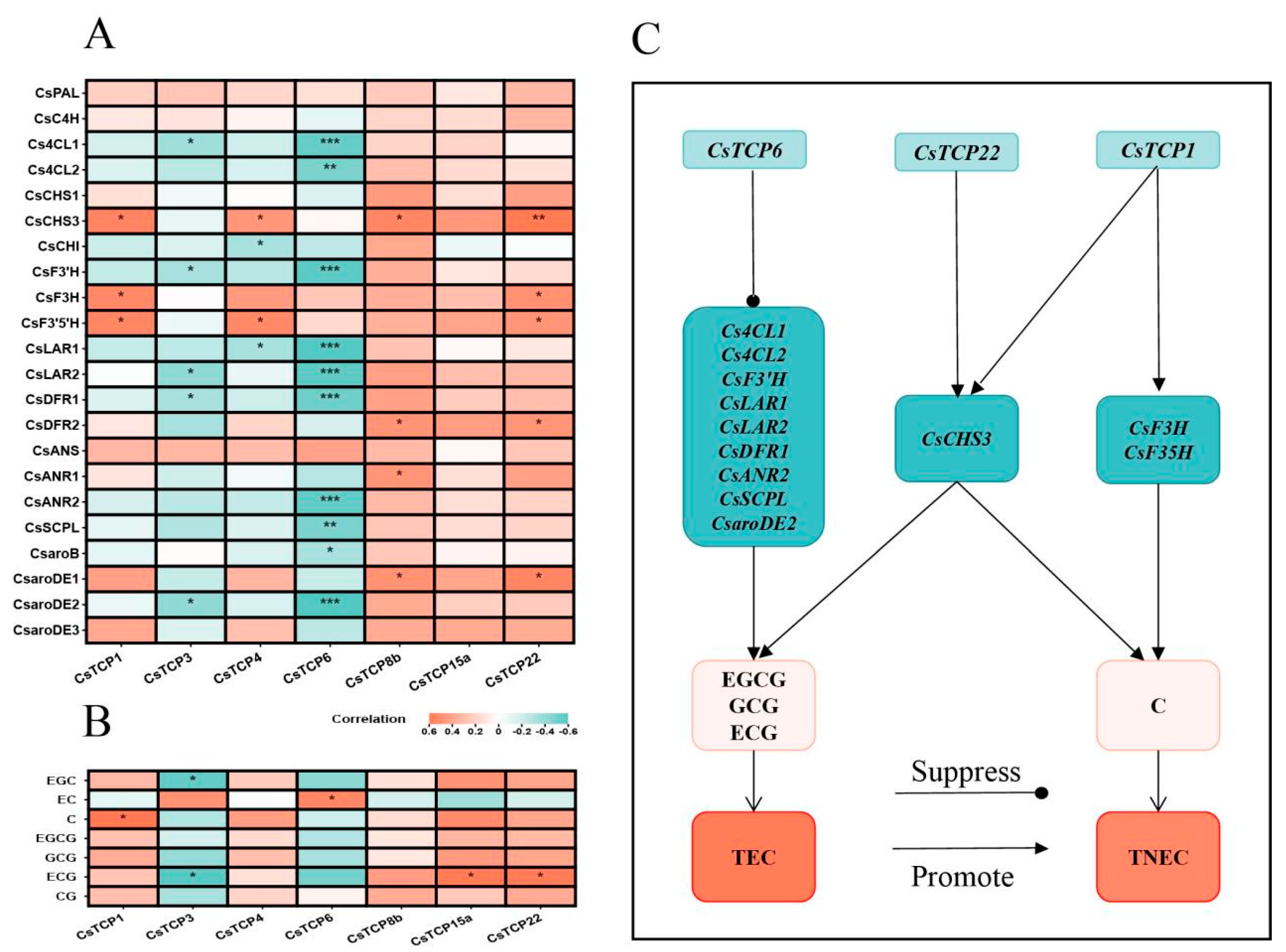
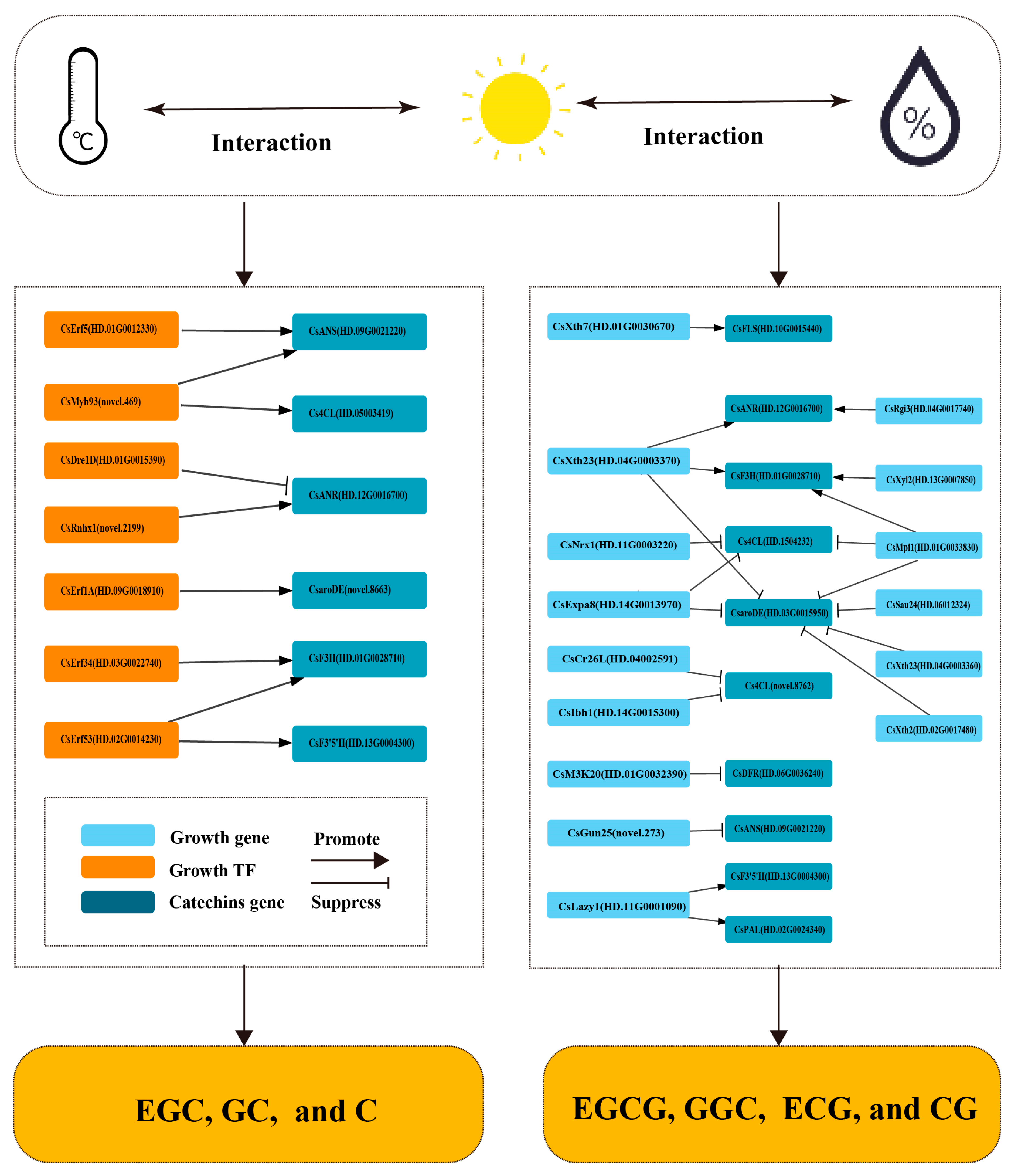
3.5.1. Co-Expression of Growth Transcription Factors and Catechin-Related Genes Under Individual Changes in Ecological Factors
3.5.2. Co-Expression of Growth Transcription Factors and Catechin-Related Genes Under Interactive Changes in Ecological Factors
4. Discussion
4.1. Ecological Conditions for Enhancing Tea Growth and Yield
4.2. Synchronous Relationship Between Catechin Biosynthesis and Shoot Growth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EGCG | Epigallocatechin gallate |
| CG | Catechin gallate |
| GCG | Gallocatechin gallate |
| ECG | Epicatechin gallate |
| TC | Total catechins |
| TNEC | Total non-esterified catechins |
| TEC | Total esterified catechins |
| PCA | Principal component analysis |
| PLS | Partial Least Squares |
| ARF | Auxin response factor |
| ERF | Ethylene response factor |
| RH | Relative humidity of substrate |
| AH | Relative humidity of air |
References
- Liao, Q.; Liang, P.-X.; Xing, Y.; Yao, Z.-F.; Chen, J.-P.; Pan, L.-P.; Deng, Y.-Q.; Liu, Y.-X.; Huang, D.-L. Optimizing Selenium Application for Enhanced Quality and Nutritional Value of Spring Tea (Camellia sinensis). Horticulturae 2025, 11, 423. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agricultural Organization of the United Nations. Available online: http://www.fao.org/faostat (accessed on 23 March 2025).
- Gunathilaka, R.P.D.; Smart, J.C.R.; Fleming, C.M. The impact of changing climate on perennial crops: The case of tea production in Sri Lanka. Clim. Chang. 2017, 140, 577–592. [Google Scholar] [CrossRef]
- Wijeratne, M.A.; Anandacoomaraswamy, A.; Amarathunga, M.K.S.L.D.; Ratnasiri, J.; Kalra, N. Assessment of impact of climate change on productivity of tea (Camellia sinensis L.) plantations in Sri Lanka. J. Natl. Sci. Found. Sri Lanka 2007, 35, 119–126. [Google Scholar] [CrossRef]
- Tim, B.; Michal, K.; Christoph, M.; Sibyll, S.; Yvonne, J. First process-based simulations of climate change impacts on global tea production indicate large effects in the World’s major producer countries. Environ. Res. Lett. 2020, 15, e034023. [Google Scholar] [CrossRef]
- Rigden, A.J.; Ongoma, V.; Huybers, P. Kenyan tea is made with heat and water: How will climate change influence its yield? Environ. Res. Lett. 2020, 15, e044003. [Google Scholar] [CrossRef]
- Raj, E.E.; Ramesh, K.V.; Rajkumar, R. Modelling the impact of agrometeorological variables on regional tea yield variability in South Indian tea-growing regions: 1981–2015. Cogent Food Agric. 2019, 5, e1581457. [Google Scholar] [CrossRef]
- Duncan, J.M.A.; Saikia, S.D.; Gupta, N.; Biggs, E.M. Observing climate impacts on tea yield in Assam, India. Appl. Geogr. 2016, 77, 64–71. [Google Scholar] [CrossRef]
- Chen, H.; Liu, C.J.; Liu, C.F.; Hu, C.Y.; Hsiao, M.C.; Chiou, M.T.; Su, Y.S.; Tsai, H.T. A Growth Model to Estimate Shoot Weights and Leaf Numbers in Tea. Agron. J. 2019, 111, 2255–2262. [Google Scholar] [CrossRef]
- Lou, W.; Sun, K.; Zhao, Y.; Deng, S.; Zhou, Z. Impact of climate change on inter-annual variation in tea plant output in Zhejiang, China. Int. J. Climatol. 2020, 41, 479–490. [Google Scholar] [CrossRef]
- Jayasinghe, S.L.; Kumar, L. Potential Impact of the Current and Future Climate on the Yield, Quality, and Climate Suitability for Tea Camellia sinensis (L.) O. Kuntze: A Systematic Review. Agronomy 2021, 11, 619. [Google Scholar] [CrossRef]
- Sun, L.; Li, X.; Shen, J.; Wang, S.; Xu, X.; Fan, K.; Wang, Y.; Bi, C.; Ding, Z. Optimizing tea plantation through rapeseed intercropping: Ecological and pest-resistant benefits. Ind. Crop. Prod. 2025, 227, e120821. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, B. Application of Apriori Algorithm in Meteorological Disaster Information Mining. In Cooperative Design, Visualization, and Engineering, Proceedings of the 15th International Conference, CDVE 2018, Hangzhou, China, 21–24 October 2018; Springer International Publishing: Cham, Switzerland, 2018; pp. 258–261. [Google Scholar] [CrossRef]
- Edirisinghe, J.C.; Ranjan, H.; Herath, H.M.L.K.; Jayasinghe-Mudalige, U.K.; Wijeratne, M.; Kuruppu, V.; Jayathilake, C.; Wijesuriya, W.; Somarathna, K.; Karunaratne, S. Impact of climate on tea yield: An empirical investigation from Sri Lanka. J. Natl. Sci. Found. Sri Lanka 2024, 52, 183–190. [Google Scholar] [CrossRef]
- Rebecca, B.; Sean, C.; Bruce, A.; Selena, A.; Timothy, G.; Albert, R.; John, S.; Wenyan, H.; Matt, H.; Colin, O. Association between Empirically Estimated Monsoon Dynamics and Other Weather Factors and Historical Tea Yields in China: Results from a Yield Response Model. Climate 2016, 4, 20. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Chen, C.-T.; Li, R.-Y.; Lu, Y.-H.; Chiang, L.-C. Climate risk analysis of low-altitude tea gardens in central Taiwan using a Bayesian network. Environ. Monit. Assess. 2024, 196, e809. [Google Scholar] [CrossRef]
- Chang, C.-L.; Huang, C.-C.; Chen, H.-W. Design and Implementation of Artificial Intelligence of Things for Tea (Camellia sinensis L.) Grown in a Plant Factory. Agronomy 2022, 12, 2384. [Google Scholar] [CrossRef]
- Zhiwei, T.; Wei, M.; Qichang, Y.; Famin, D. Application status and challenges of machine vision in plant factory—A review. Inf. Process. Agric. 2021, 9, 195–211. [Google Scholar] [CrossRef]
- Kaya, C. Intelligent Environmental Control in Plant Factories: Integrating Sensors, Automation, and AI for Optimal Crop Production. Food Energy Secur. 2025, 14, e70026. [Google Scholar] [CrossRef]
- Cai, W.; Bu, K.; Zha, L.; Zhang, J.; Lai, D.; Bao, H. Energy consumption of plant factory with artificial light: Challenges and opportunities. Renew. Sustain. Energy Rev. 2024, 210, e115235. [Google Scholar] [CrossRef]
- Azad, M.O.K.; Gruda, N.S.; Naznin, M.T. Energy Efficiency of Glasshouses and Plant Factories for Sustainable Urban Farming in the Desert Southwest of the United States of America. Horticulturae 2024, 10, 1055. [Google Scholar] [CrossRef]
- Graamans, L.; Baeza, E.; Andy, V.D.D.; Tsafaras, I.; Stanghellini, C. Plant factories versus greenhouses: Comparison of resource use efficiency. Agric. Syst. 2018, 160, 31–43. [Google Scholar] [CrossRef]
- Yoneda, Y. Optimization Process of Plant Growth Environment for Improving Content Compounds Using Physiological and Genetic Information in a Closed-type Plant Factory. Jpn. Agric. Res. Q. 2021, 55, 201–208. [Google Scholar] [CrossRef]
- Miyauchi, S.; Yuki, T.; Fuji, H.; Kojima, K.; Yonetani, T.; Tomio, A.; Bamba, T.; Fukusaki, E. High-quality green tea leaf production by artificial cultivation under growth chamber conditions considering amino acids profile. J. Biosci. Bioeng. 2014, 118, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, S.; Yonetani, T.; Yuki, T.; Tomio, A.; Bamba, T.; Fukusaki, E. Quality evaluation of green tea leaf cultured under artificial light condition using gas chromatography/mass spectrometry. J. Biosci. Bioeng. 2016, 123, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Tukhvatshin, M.; Peng, Q.; Zhao, X.; Liu, J.; Xiang, P.; Lin, J. Identifying meteorological factors influencing catechin biosynthesis and optimizing cultivation conditions of tea plant (Camellia sinensis). Front. Plant Sci. 2025, 16, e1532880. [Google Scholar] [CrossRef]
- Xiang, P.; Zhu, Q.; Zhang, L.; Xu, P.; Liu, L.; Li, Y.; Cheng, B.; Wang, X.; Liu, J.; Shi, Y.; et al. Integrative analyses of transcriptome and metabolome reveal comprehensive mechanisms of Epigallocatechin-3-gallate (EGCG) biosynthesis in response to ecological factors in tea plant (Camellia sinensis). Food Res. Int. 2023, 166, e112591. [Google Scholar] [CrossRef]
- Omae, H.; Takeda, Y. Modeling Winter Dormancy of Tea Buds and Simulation in Southern Japan. Jpn. Agric. Res. Q. 2003, 37, 189–194. [Google Scholar] [CrossRef]
- Carr, M.K.V. The role of water in the growth of the tea (Camellia sinensis) crop: A synthesis of research in eastern africa. 1. water relations. Exp. Agric. 2010, 46, 327–349. [Google Scholar] [CrossRef]
- Benti, T.; Debela, A.; Bekele, Y.; Suleman, S. Effect of seasonal variation on yield and leaf quality of tea clone (Camellia sinensis (L.) O. Kuntze) in South West Ethiopia. Heliyon 2023, 9, e14051. [Google Scholar] [CrossRef]
- De Costa, W.A.J.M.; Mohotti, A.J.; Wijeratne, M.A. Ecophysiology of tea. Braz. J. Plant Physiol. 2007, 19, 299–332. [Google Scholar] [CrossRef]
- Sano, T.; Horie, H.; Matsunaga, A.; Hirono, Y. Effect of shading intensity on morphological and color traits and on chemical components of new tea (Camellia sinensis L.) shoots under direct covering cultivation. J. Sci. Food Agric. 2018, 98, 5666–5676. [Google Scholar] [CrossRef]
- Bahrami-Rad, S. Effect of light intensity on photosynthesis and antioxidant defense in boron deficient tea plants. Acta Biol. Szeged. 2011, 55, 265–272. [Google Scholar]
- Fang, Z.-T.; Jin, J.; Ye, Y.; He, W.-Z.; Shu, Z.-F.; Shao, J.-N.; Fu, Z.-S.; Lu, J.-L.; Ye, J.-H. Effects of Different Shading Treatments on the Biomass and Transcriptome Profiles of Tea Leaves (Camellia sinensis L.) and the Regulatory Effect on Phytohormone Biosynthesis. Front. Plant Sci. 2022, 13, 909765. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Li, C.; Nie, J.; Shao, S.; Rogers, K.M.; Zhang, Y.; Li, Z.; Yuan, Y. Stable isotope and photosynthetic response of tea grown under different temperature and light conditions. Food Chem. 2022, 368, 130771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, K.; Tang, Q.; Zeng, L.; Wu, Z. Light Intensity Regulates Low-Temperature Adaptability of Tea Plant through ROS Stress and Developmental Programs. Int. J. Mol. Sci. 2023, 24, 9852. [Google Scholar] [CrossRef]
- Lin, S.K.; Lin, J.; Liu, Q.L.; Ai, Y.F.; Ke, Y.Q.; Chen, C.; Zhang, Z.Y.; He, H. Time-course of photosynthesis and non-structural carbon compounds in the leaves of tea plants (Camellia sinensis L.) in response to deficit irrigation. Agric. Water Manag. 2014, 144, 98–106. [Google Scholar] [CrossRef]
- Cheruiyot, E.K.; Mumera, L.M.; Ng'Etich, W.K.; Hassanali, A.; Wachira, F. Polyphenols as Potential Indicators for Drought Tolerance in Tea (Camellia sinensis L.). J. Agric. Chem. Soc. Jpn. 2007, 71, 2190–2197. [Google Scholar] [CrossRef]
- Ng’etich, W.K.; Stephens, W. Responses of tea to environment in kenya. 1. genotype × environment interactions for total dry matter production and yield. Exp. Agric. 2001, 37, 333–342. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Y.; Zhang, Y.; Dong, Y.; Liu, Y.; Liu, L.; Wan, S.; He, J.; Yu, Y. Accumulation of Galactinol and ABA Is Involved in Exogenous EBR-Induced Drought Tolerance in Tea Plants. J. Agric. Food Chem. 2022, 70, 13391–13403. [Google Scholar] [CrossRef]
- Dai, F.; Rong, Z.; Wu, Q.; Fathi Abd_Allah, E.; Liu, C.; Liu, S. Mycorrhiza improves plant growth and photosynthetic characteristics of tea plants in response to drought stress. Biocell 2022, 46, 1339–1346. [Google Scholar] [CrossRef]
- Wu, Z.-J.; Wang, W.-L.; Zhuang, J. TCP family genes control leaf development and its responses to hormonal stimuli in tea plant Camellia sinensis (L.) O. Kuntze. Plant Growth Regul. 2017, 83, 43–53. [Google Scholar] [CrossRef]
- Ashihara, H.; Deng, W.-W.; Mullen, W.; Crozier, A. Distribution and biosynthesis of flavan-3-ols in Camellia sinensis seedlings and expression of genes encoding biosynthetic enzymes. Phytochemistry 2010, 71, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, Y.; Li, W.; Zhao, L.; Meng, F.; Wang, Y.; Tan, H.; Yang, H.; Wei, C.; Wan, X.; et al. Tissue-Specific, Development-Dependent Phenolic Compounds Accumulation Profile and Gene Expression Pattern in Tea Plant Camellia sinensis. PLoS ONE 2013, 8, e62315. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Dong, X.; Wang, Y.; Maker, G.; Agarwal, M.; Ding, Z. Tea-Soybean Intercropping Improves Tea Quality and Nutrition Uptake by Inducing Changes of Rhizosphere Bacterial Communities. Microorganisms 2022, 10, 2149. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Li, X.; Liu, A.; Chen, S. Physiological and Defense Responses of Tea Plants to Elevated CO2: A Review. Front. Plant Sci. 2020, 11, e305. [Google Scholar] [CrossRef]
- Li, X.; Li, M.-H.; Deng, W.-W.; Ahammed, G.J.; Wei, J.-P.; Yan, P.; Zhang, L.-P.; Fu, J.-Y.; Han, W.-Y. Exogenous melatonin improves tea quality under moderate high temperatures by increasing epigallocatechin-3-gallate and theanine biosynthesis in Camellia sinensis L. J. Plant Physiol. 2020, 253, e153273. [Google Scholar] [CrossRef]
- Chen, Y.M.; Tsao, T.M.; Liu, C.C.; Lin, K.C.; Wang, M.K. Aluminium and nutrients induce changes in the profiles of phenolic substances in tea plants (Camellia sinensis). J. Sci. Food Agric. 2011, 91, 1111–1117. [Google Scholar] [CrossRef]
- Cheruiyot, E.K.; Mumera, L.M.; Ngetich, W.K.; Hassanali, A.; Wachira, F.; Wanyoko, J.K. Shoot epicatechin and epigallocatechin contents respond to water stress in tea Camellia sinensis (L.) O. Kuntze. Biosci. Biotechnol. Biochem. 2008, 72, 1219–1226. [Google Scholar] [CrossRef][Green Version]
- Ryu, H.W.; Yuk, H.J.; An, J.H.; Kim, D.-Y.; Song, H.-H.; Oh, S.-R. Comparison of secondary metabolite changes in Camellia sinensis leaves depending on the growth stage. Food Control 2017, 73, 916–921. [Google Scholar] [CrossRef]
- Jeon, D.B.; Hong, Y.S.; Lee, G.H.; Park, Y.M.; Lee, C.M.; Nho, E.Y.; Choi, J.Y.; Jamila, N.; Khan, N.; Kim, K.S. Determination of volatile organic compounds, catechins, caffeine and theanine in Jukro tea at three growth stages by chromatographic and spectrometric methods. Food Chem. 2017, 219, 443–452. [Google Scholar] [CrossRef]
- Zeng, L.; Zhou, X.; Liao, Y.; Yang, Z. Roles of specialized metabolites in biological function and environmental adaptability of tea plant (Camellia sinensis) as a metabolite studying model. J. Adv. Res. 2021, 34, 159–171. [Google Scholar] [CrossRef]
- Yu, S.; Li, P.; Zhao, X.; Tan, M.; Ahmad, M.Z.; Xu, Y.; Tadege, M.; Zhao, J. CsTCPs regulate shoot tip development and catechin biosynthesis in tea plant (Camellia sinensis). Hortic. Res. 2021, 8, e104. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yan, J.; Miu, Y.; Huang, R.; Wei, H.; Wang, L.; Zhang, C.; Yuan, L.; Tong, H. The spatiotemporal variations of L-glutamic acid and catechins during the development of etiolated tea leaves in ‘Huangjinye’. Sci. Hortic. 2024, 328, e112888. [Google Scholar] [CrossRef]
- Xiang, P.; Wilson, I.W.; Huang, J.; Zhu, Q.; Tan, M.; Lu, J.; Liu, J.; Gao, S.; Zheng, S.; Lin, D.; et al. Co-regulation of catechins biosynthesis responses to temperature changes by shoot growth and catechin related gene expression in tea plants (Camellia sinensis L.). J. Hortic. Sci. Biotechnol. 2021, 96, 228–238. [Google Scholar] [CrossRef]
- Li, S.; Zachgo, S. TCP3 interacts with R2R3-MYB proteins, promotes flavonoid biosynthesis and negatively regulates the auxin response in Arabidopsis thaliana. Plant J. 2013, 76, 901–913. [Google Scholar] [CrossRef]
- Viola, I.L.; Camoirano, A.; Gonzalez, D.H. Redox-Dependent Modulation of Anthocyanin Biosynthesis by the TCP Transcription Factor TCP15 during Exposure to High Light Intensity Conditions in Arabidopsis. Plant Physiol. 2016, 170, 74–85. [Google Scholar] [CrossRef]
- Mei, X.; Wan, S.; Lin, C.; Zhou, C.; Hu, L.; Deng, C.; Zhang, L. Integration of Metabolome and Transcriptome Reveals the Relationship of Benzenoid-Phenylpropanoid Pigment and Aroma in Purple Tea Flowers. Front. Plant Sci. 2021, 12, e762330. [Google Scholar] [CrossRef]
- Hur, Y.-S.; Oh, J.; Kim, N.; Kim, S.; Son, O.; Kim, J.; Um, J.-H.; Ji, Z.; Kim, M.-h.; Ko, J.-H.; et al. Arabidopsis transcription factor TCP13 promotes shade avoidance syndrome-like responses by directly targeting a subset of shade-responsive gene promoters. J. Exp. Bot. 2024, 75, 241–257. [Google Scholar] [CrossRef]
- Li, N.-N.; Lu, J.-L.; Li, Q.-S.; Zheng, X.-Q.; Wang, X.-C.; Wang, L.; Wang, Y.-C.; Ding, C.-Q.; Liang, Y.-R.; Yang, Y.-J. Dissection of Chemical Composition and Associated Gene Expression in the Pigment-Deficient Tea Cultivar ‘Xiaoxueya’ Reveals an Albino Phenotype and Metabolite Formation. Front. Plant Sci. 2019, 10, e1543. [Google Scholar] [CrossRef]
- Li, D.; Tang, X.; Dong, Y.; Wang, Y.; Shi, S.; Li, S.; Liu, Y.; Ge, H.; Chen, H. Comparative genomic investigation of TCP gene family in eggplant (Solanum melongena L.) and expression analysis under divergent treatments. Plant Cell Rep. 2022, 41, 2213–2228. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, S.; Zhou, C.; Chen, L.; Zaripov, T.; Zhan, D.; Weng, J.; Lin, Y.; Lai, Z.; Guo, Y. Integrated Transcriptome, microRNA, and Phytochemical Analyses Reveal Roles of Phytohormone Signal Transduction and ABC Transporters in Flavor Formation of Oolong Tea (Camellia sinensis) during Solar Withering. J.-Agric. Food Chem. 2020, 68, 12749–12767. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, P.; Zhu, Q.; Tukhvatshin, M.; Cheng, B.; Tan, M.; Liu, J.; Huang, J.; Hu, Y.; Shi, Y.; Wu, L.; et al. Response of Shoot Growth to Ecological Factors Highlights a Synergistic Relationship Between Yield and Catechin Accumulation in Tea Plant (Camellia sinensis L.). Horticulturae 2025, 11, 624. https://doi.org/10.3390/horticulturae11060624
Xiang P, Zhu Q, Tukhvatshin M, Cheng B, Tan M, Liu J, Huang J, Hu Y, Shi Y, Wu L, et al. Response of Shoot Growth to Ecological Factors Highlights a Synergistic Relationship Between Yield and Catechin Accumulation in Tea Plant (Camellia sinensis L.). Horticulturae. 2025; 11(6):624. https://doi.org/10.3390/horticulturae11060624
Chicago/Turabian StyleXiang, Ping, Qiufang Zhu, Marat Tukhvatshin, Bosi Cheng, Meng Tan, Jianghong Liu, Jiaxin Huang, Yunfei Hu, Yutao Shi, Liangyu Wu, and et al. 2025. "Response of Shoot Growth to Ecological Factors Highlights a Synergistic Relationship Between Yield and Catechin Accumulation in Tea Plant (Camellia sinensis L.)" Horticulturae 11, no. 6: 624. https://doi.org/10.3390/horticulturae11060624
APA StyleXiang, P., Zhu, Q., Tukhvatshin, M., Cheng, B., Tan, M., Liu, J., Huang, J., Hu, Y., Shi, Y., Wu, L., & Lin, J. (2025). Response of Shoot Growth to Ecological Factors Highlights a Synergistic Relationship Between Yield and Catechin Accumulation in Tea Plant (Camellia sinensis L.). Horticulturae, 11(6), 624. https://doi.org/10.3390/horticulturae11060624





