Tomato Lines Tolerant to Sodium Chloride at Early Growth Stages
Abstract
1. Introduction
2. Materials and Methods
2.1. NaCl Tolerance at Germination Stage
2.2. NaCl Tolerance at the Seedling Stage
2.3. Statistical Analysis
3. Results and Discussion
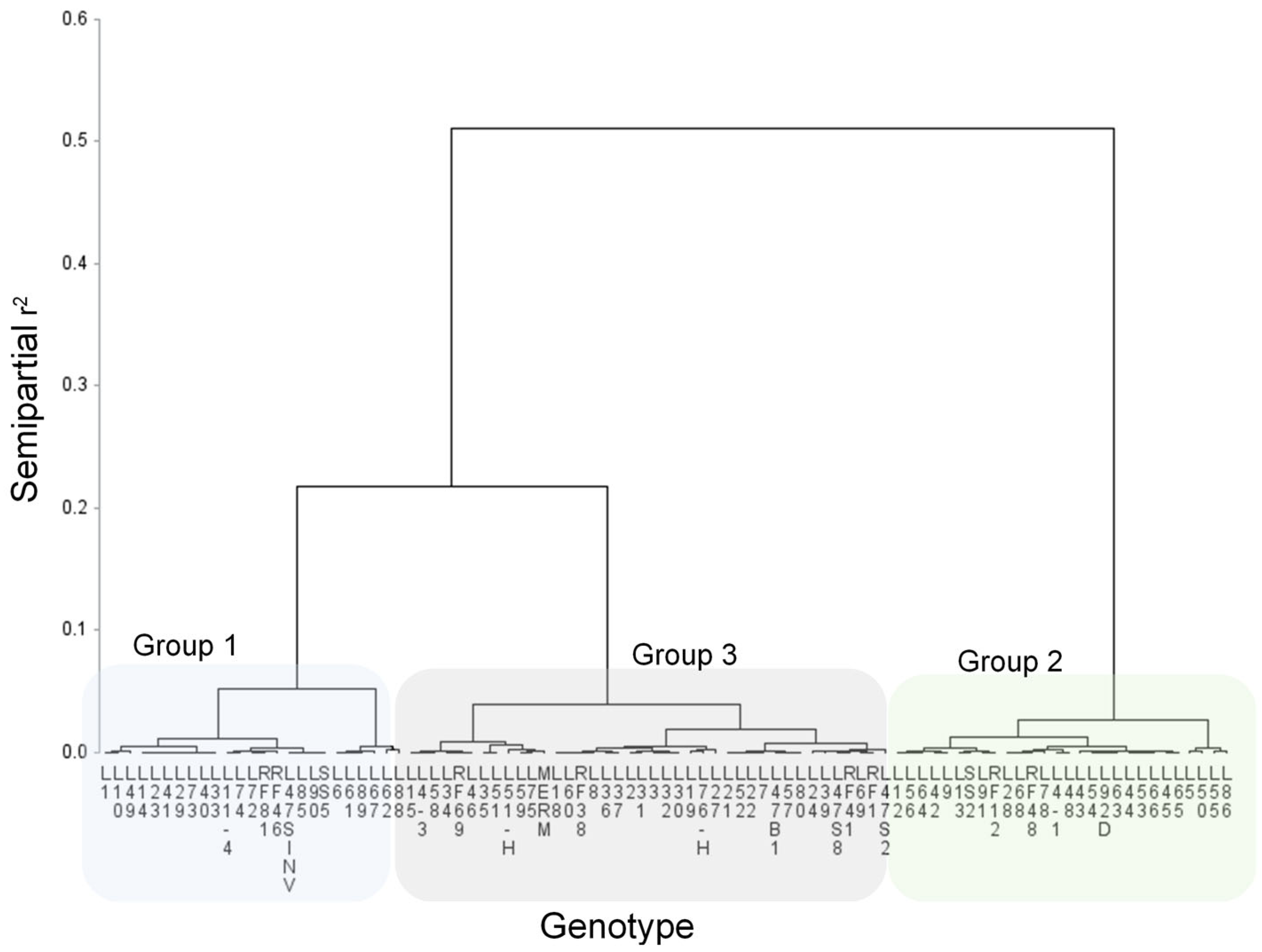
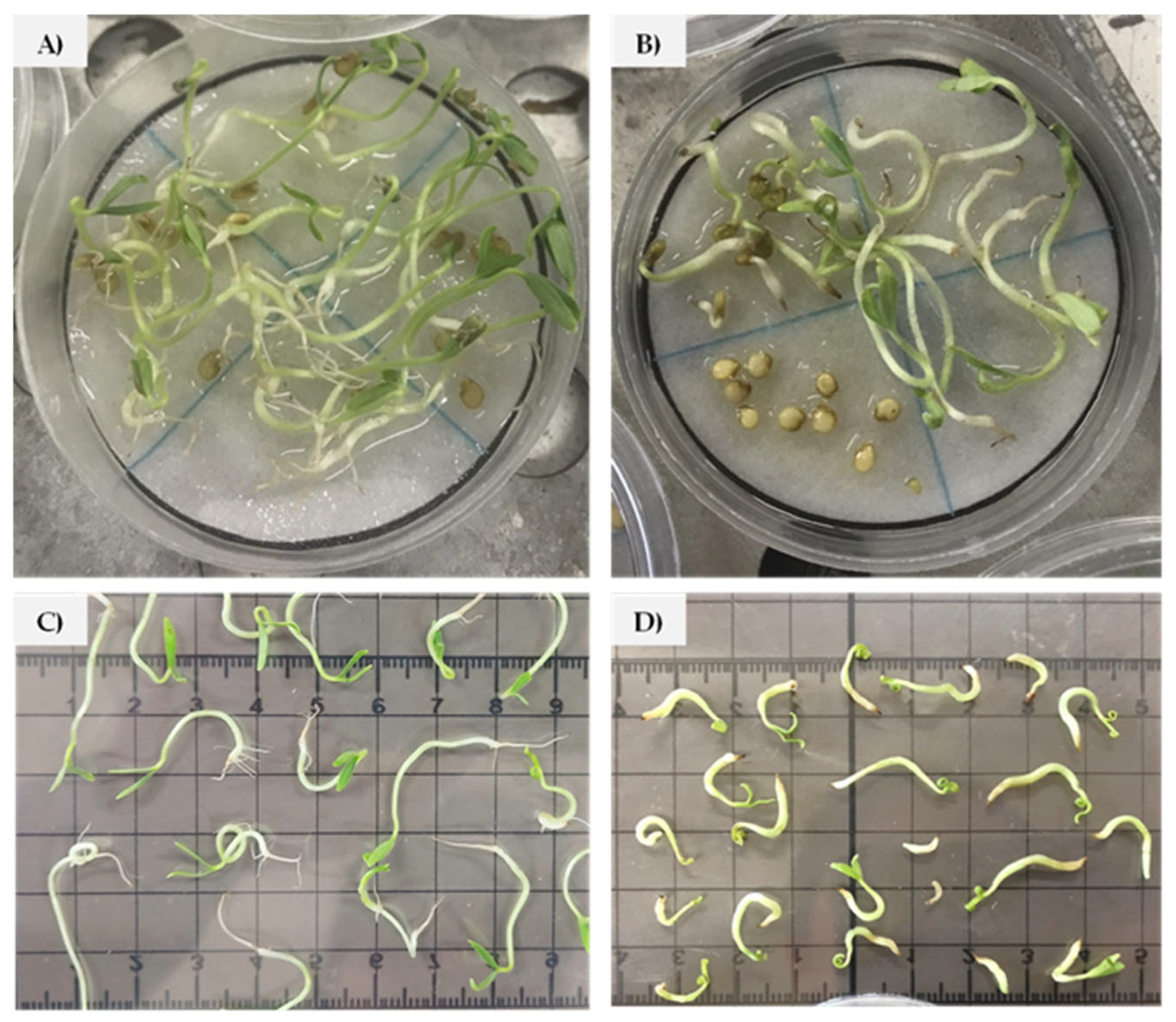
3.1. Salinity Tolerance at Germination Stage
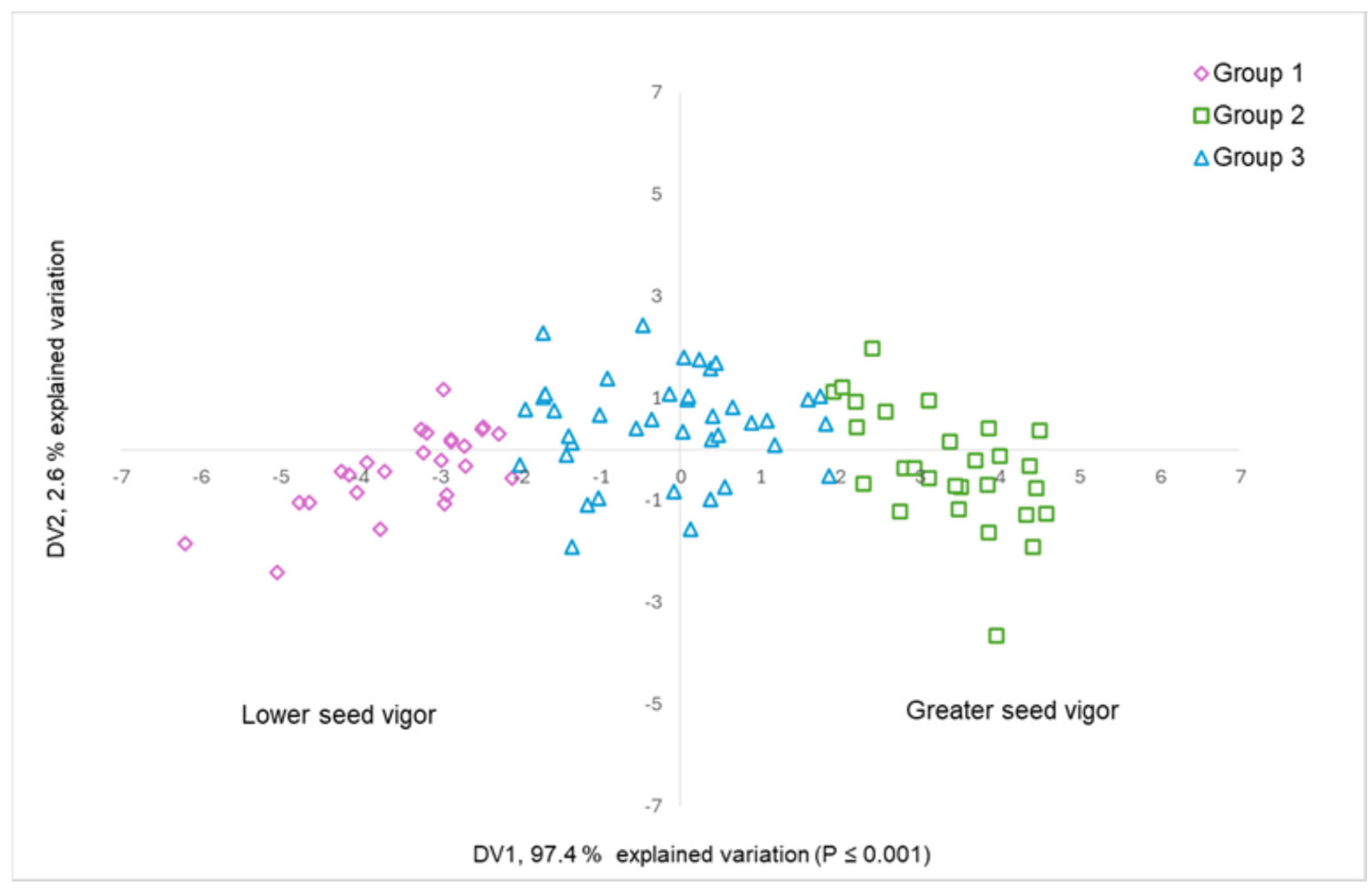
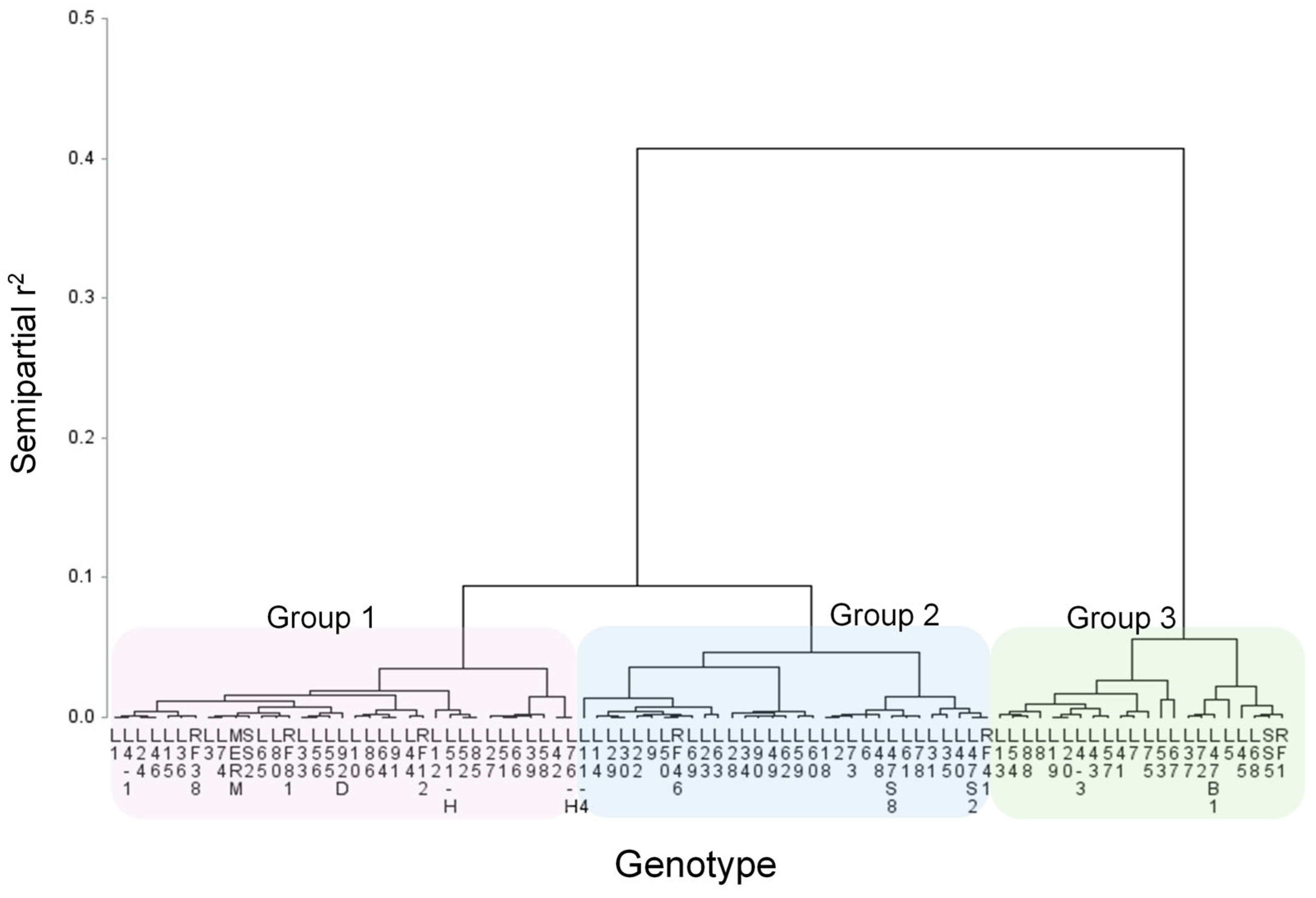
3.2. Salinity Tolerance at Seedling Stage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil salinity under climate change: Challenges for sustainable agriculture and food security. J. Environ. Manag. 2021, 280, 111736. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Hopmans, J.W.; Qureshi, A.S.; Kisekka, I.; Munns, R.; Grattan, S.R.; Rengasamy, P.; Ben-Gal, A.; Assouline, S.; Javaux, M.; Minhas, P.S.; et al. Chapter One—Critical knowledge gaps and research priorities in global soil salinity. Adv. Agron. 2021, 169, 1–191. [Google Scholar] [CrossRef]
- Vargas, R.; Pankova, E.I.; Balyuk, S.A.; Krasilnikov, P.V.; Khasankhanova, G.M. Handbook for Saline Soil Management; Food and Agriculture Organization of the United Nations: Rome, Italy; Lomonosov Moscow State University: Moscow, Russia, 2018. [Google Scholar]
- Uçarlı, C. Effects of Salinity on Seed Germination and Early Seedling Stage. In Abiotic Stress in Plants; Fahad, S., Saud, S., Chen, Y., Wu, C., Wang, D., Eds.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum. 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Secretaría de Agricultura y Desarrollo Rural. Mapa Agrícola de Afectación por Salinidad en México. 2021. Available online: https://www.gob.mx/agricultura/acciones-y-programas/mapa-agricola-de-afectacion-por-salinidad-en-mexico (accessed on 20 July 2024).
- Food and Agriculture Organization of the United (FAOSTAT). Data About Food and Agriculture. 2024. Available online: https://www.fao.org/faostat/en/#data (accessed on 5 July 2024).
- Servicio de Información Agroalimentaria y Pesquera (SIAP). Panorama Agroalimentario 2024. Available online: https://www.gob.mx/agricultura/dgsiap/acciones-y-programas/panorama-agroalimentario-258035 (accessed on 6 April 2024).
- Singh, A. Soil salinity: A global threat to sustainable development. Soil Use and Manag. 2022, 38, 39–67. [Google Scholar] [CrossRef]
- Shams, M.; Khadivi, A. Mechanisms of salinity tolerance and their possible application in the breeding of vegetables. BMC Plant Biol. 2023, 23, 139. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of Plant Responses and Adaptation to Soil Salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
- Adilu, G.S.; Gebre, Y.G. Effect of salinity on seed germination of some tomato (Lycopersicon esculentum Mill.) varieties. J. Aridland Agri. 2021, 7, 76–82. [Google Scholar] [CrossRef]
- Abdel-Farid, I.B.; Marghany, M.R.; Rowezek, M.M.; Sheded, M.G. Effect of Salinity Stress on Growth and Metabolomic Profiling of Cucumis sativus and Solanum lycopersicum. Plants 2020, 9, 1626. [Google Scholar] [CrossRef]
- González-Grande, P.; Suárez, N.; Marín, O. Effect of salinity and seed salt priming on the physiology of adult plants of Solanum lycopersicum cv. ‘Río Grande’. Braz. J. Bot. 2020, 43, 775–787. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef]
- Zörb, C.; Geilfus, C.M.; Dietz, K.J. Salinity and crop yield. Plant Biol. 2019, 21, 31–38. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef]
- Machado, R.; Serralheiro, R. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant Salinity Stress: Many Unanswered Questions Remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Jameel, J.; Anwar, T.; Majeed, S.; Qureshi, H.; Siddiqi, E.H.; Sana, S.; Zaman, W.; Ali, H.M. Effect of salinity on growth and biochemical responses of brinjal varieties: Implications for salt tolerance and antioxidant mechanisms. BMC Plant Biol. 2024, 24, 128. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Wais, A.H.; Ditta, A.; Islam, M.R.; Chowdhury, M.K.; Pramanik, M.H.; Ismaan, H.N.; Soufan, W.; El Sabah, A.; Islam, M.S. Seed Germination and Early Seedling Growth of Sorghum (Sorghum bicolor L. Moench) Genotypes Under Salinity Stress. Pol. Environ. Stud. 2024, 33, 3019–3032. [Google Scholar] [CrossRef]
- Ludwiczak, A.; Osiak, M.; Cárdenas-Pérez, S.; Lubińska-Mielińska, S.; Piernik, A. Osmotic Stress or Ionic Composition: Which Affects the Early Growth of Crop Species More? Agronomy 2021, 11, 435. [Google Scholar] [CrossRef]
- Abbas, A.; Mansha, S.; Waheed, H.; Siddiq, Z.; Hayyat, M.U.; Zhang, Y.-J.; Alwutayd, K. NaCl stress, tissue specific Na+ and K+ up-take and their effect on growth and physiology of Helianthus annuus L. and Solanum lycopersicum L. Sci. Hortic. 2024, 326, 112454. [Google Scholar] [CrossRef]
- Meza, S.L.R.; Egea, I.; Massaretto, I.L.; Morales, B.; Purgatto, E.; Egea-Fernández, J.M.; Bolarin, M.C.; Flores, F.B. Traditional Tomato Varieties Improve Fruit Quality Without Affecting Fruit Yield Under Moderate Salt Stress. Front. Plant Sci. 2020, 11, 587754. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhao, M.; Zhou, S.; Niu, L.; Zhao, L.; Xu, D. Effects of antibiotics on secundary metabolism and oxidative stress in oilseed rape seeds. Env. Sci. Poll. Res. 2024, 31, 27689–27698. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Mbarki, S.; Zivcak, M.; Brestic, M. The Involvement of Different Secondary Metabolites in Salinity Tolerance of Crops. In Salinity Responses and Tolerance in Plants; Kumar, V., Wani, S., Suprasanna, P., Tra, L.S., Eds.; Springer: Cham, Switzerland, 2018; Volume 2, pp. 21–48. [Google Scholar] [CrossRef]
- Ellouzi, H.; Ben Slimene Debez, I.; Amraoui, S.; Rabhi, M.; Hanana, M.; Alyami, N.M.; Debez, A.; Abdelly, C.; Zorrig, W. Effect of seed priming with auxin on ROS detoxification and carbohydrate metabolism and their relationship with germination and early seedling establishment in salt stressed maize. BMC Plant Biol. 2024, 24, 704. [Google Scholar] [CrossRef]
- Seth, R.; Kendurkar, S. In vitro screening: An effective method for evaluation of commercial cultivars of tomato towards salinity stress. Inter. J. Curr. Microbiol. Appl. Sci. 2015, 4, 725–730. [Google Scholar]
- Maguire, J.D. Speed of germination-aid in selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Cadahia López, C. Fertirrigación: Cultivos Hortícolas y Ornamentales, 2nd ed.; Mundiprensa: Barcelona, Spain, 2000. [Google Scholar]
- Gower, J.C. A Comparison of Some Methods of Cluster Analysis. Biometrics. 1967, 23, 623. [Google Scholar] [CrossRef]
- Ward, J.H. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Hotelling, H. A Generalized T Test and Measure of Multivariate Dispersion. In Proceedings of the Second Berkeley Symposium on Mathematical Statistics and Probability; Neyman, J., Ed.; University of California Press: Berkeley, CA, USA, 1951; pp. 23–42. [Google Scholar] [CrossRef]
- Johnson, D.E. Applied Multivariate Methods for Data Analysts; Brooks Cole Publishing Company: Pacific Grove, MA, USA, 1998. [Google Scholar]
- Fadhil, A.A.; Swaid, S.Y.; Mohammed, S.J.; Al-Abboodi, A. Impact of Salinity on Tomato Seedling Development: A Comparative Study of Germination and Growth Dynamics in Different Cultivars. J. Chem. Health Risks 2024, 14, 183–190. [Google Scholar] [CrossRef]
- Shanika, M.M.A.N.; Seran, T.H. Impact of salinity on seed germination and seedling performance of tomato (Solanum lycopersicum L.) cv KC-1. J. Sci. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- López-Méndez, A.G.; Rodríguez-Pérez, J.E.; Mascorro-Gallardo, J.O.; Sahagún-Castellanos, J.; Lobato-Ortiz, R. Sodium Chloride Tolerance during Germination and Seedling Stages of Tomato (Solanum lycopersicum L.) Lines Native to Mexico. Horticulturae 2024, 10, 466. [Google Scholar] [CrossRef]
- de Oliveira, L.B.; de Oliveira, L.B. Effects of water salinization on tomato seedlings (Solanum lycopersicum). Int. J. Adv. Eng. Res. Sci. 2023, 10, 015–021. [Google Scholar] [CrossRef]
- Ruiz Espinoza, H.F.; Villalpando Gutiérrez, R.L.; Murillo Amador, B.; Beltrán Morales, F.A.; Hernández Montiel, L.G. Respuesta diferencial a la salinidad de genotipos de tomate (Lycopersicon esculentum Mill.) en primeras etapas fenológicas. Terra Latinoam. 2014, 32, 311–323. [Google Scholar]
- Mwando, E.; Angessa, T.T.; Han, Y.; Zhou, G.; Li, C. Quantitative Trait Loci Mapping for Vigour and Survival Traits of Barley Seedlings after Germinating under Salinity Stress. Agronomy 2021, 11, 103. [Google Scholar] [CrossRef]
- Sané, A.K.; Diallo, B.; Kane, A.; Sagna, M.; Sané, D.; Sy, M.O. In Vitro Germination and Early Vegetative Growth of Five Tomato (Solanum lycopersicum L.) Varieties under Salt Stress Conditions. Am. J. Plant Sci. 2021, 12, 796–817. [Google Scholar] [CrossRef]
- Chakma, P.; Hossain, M.M.; Rabbani, M.G. Effects of salinity stress on seed germination and seedling growth of tomato. J. Bangladesh Agri. Univ. 2019, 17, 490–499. [Google Scholar] [CrossRef]
- Mustafa, A.A.; Abass, M.H.; Awad, K.M. Responses of three tomato (Lycopersicon esculentum L.) varieties to different salinity levels. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 277–291. [Google Scholar]
- Rajendran, K.; Tester, M.; Roy, S.J. Quantifying the three main components of salinity tolerance in cereals. Plant Cell Environ. 2009, 32, 237–249. [Google Scholar] [CrossRef]
- Long, Y.; Chen, J.; Zeng, F.; Wu, S. An activatable NIR-II fluorescent probe for tracking heavy-metal ion and high-level salt-induced oxidative stress in plant sprouts. Aggregate 2023, 4, e288. [Google Scholar] [CrossRef]
- Kaneko, M.; Itoh, H.; Ueguchi-Tanaka, M.; Ashikari, M.; Matsuoka, M. The α-Amylase Induction in Endosperm during Rice Seed Germination Is Caused by Gibberellin Synthesized in Epithelium. Plant Physiol. 2002, 128, 1264–1270. [Google Scholar] [CrossRef]
- Zhang, P.; Senge, M.; Dai, Y. Effects of salinity stress on growth, yield, fruit quality and water use efficiency of tomato under hydroponics system. Rev. Agric. Sci. 2016, 4, 46–55. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Feng, X.; Hussain, T.; Guo, K.; Liu, X. Nonuniform salinity regulates leaf characteristics and improve photosynthesis of cherry tomatoes under high salinity. Environ. Exp. Bot. 2024, 217, 105565. [Google Scholar] [CrossRef]
- Nawaz, A.; Amjad, M.; Afzal, I.; Aslam Pervez, M. Effect of halopriming on germination and seedling vigor of tomato. Afr. J. Agric. Res. 2011, 6, 3551–3559. [Google Scholar]
- Sanjuan-Lara, F.; Ramírez-Vallejo, P.; Sánchez-García, P.; Sandoval-Villa, M.; Livera-Muñoz, M.; Carrillo-Rodríguez, J.C.; Perales-Segovia, C. Tolerancia de líneas nativas de tomate (Solanum lycopersicum L.) a la salinidad con NaCl. Interciencia 2015, 40, 704–709. [Google Scholar]
- Bogoutdinova, L.R.; Khaliluev, M.R.; Chaban, I.A.; Gulevich, A.A.; Shelepova, O.V.; Baranova, E.N. Salt Tolerance Assessment of Different Tomato Varieties at the Seedling Stage. Horticulturae 2024, 10, 598. [Google Scholar] [CrossRef]
- Akram, M.; Yasin Ashraf, M.; Ahmad, R.; Waraich, E.A.; Iqbal, J.; Mohsan, M. Screening for salt tolerance in maize (Zea mays L.) hybrids at an early seedling stage. Pak. J. Bot. 2010, 42, 141–154. [Google Scholar]
- Rodríguez-Delfín, A.; Posadas, A.; Quiroz, R. Rendimiento y absorción de algunos nutrimentos en plantas de camote cultivadas con estrés hídrico y salino. Rev. Chapingo Ser. Hortic. 2014, 20, 19–28. [Google Scholar] [CrossRef]
- Babu, M.A.; Singh, D.; Gothandam, K.M. The effect of salinity on growth, hormones and mineral elements in leaf and fruit of tomato cultivar PKM1. J. Anim. Plant Sci. 2012, 22, 159–164. [Google Scholar]
- Saha, J.; Brauer, E.K.; Sengupta, A.; Popescu, S.C.; Gupta, K.; Gupta, B. Polyamines as redox homeostasis regulators during salt stress in plants. Front. Environ. Sci. 2015, 3, 21. [Google Scholar] [CrossRef]
- Sabzmeydani, E.; Sedaghathoor, S.; Hashemabadi, D. Salinity response of Kentucky bluegrass (Poa pratensis L.) as influenced by salicylic acid and progesterone. Rev. Chapingo Ser. Hortic. 2019, 26, 49–63. [Google Scholar] [CrossRef]
- Parra-Terraza, S.; Angulo-Castro, A.; Sánchez-Peña, P.; Valdéz-Torres, J.B.; Rubio-Carrasco, W. Effect of Cl- and Na+ ratios in nutrient solutions on tomato (Solanum lycopersicum L.) yield in a hydroponic system. Rev. Chapingo Ser. Hortic. 2022, 28, 67–78. [Google Scholar] [CrossRef]
- Pérez-Alfocea, F.; Albacete, A.; Ghanem, M.E.; Dodd, I.C. Hormonal regulation of source—Sink relations to maintain crop productivity under salinity: A case study of root-to-shoot signalling in tomato. Funct. Plant Biol. 2010, 37, 592. [Google Scholar] [CrossRef]
- Roy, S.J.; Negrão, S.; Tester, M. Salt resistant crop plants. Curr. Opin. Biotechnol. 2014, 26, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Pailles, Y.; Awlia, M.; Julkowska, M.; Passone, L.; Zemmouri, K.; Negrão, S.; Schmöckel, S.M.; Tester, M. Diverse Traits Contribute to Salinity Tolerance of Wild Tomato Seedlings from the Galapagos Islands. Plant Physiol. 2020, 182, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Solanki, P.; Narayan, M.; Meena, S.S.; Srivastava, R.K. Floating raft wastewater treatment system: A review. J. Pure App. Microbiol. 2017, 11, 1113–1116. [Google Scholar] [CrossRef]
- Yokoi, S.; Quintero, F.J.; Cubero, B.; Ruiz, M.T.; Bressan, R.A.; Hasegawa, P.M.; Pardo, J.M. Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. Plant J. 2002, 30, 529–539. [Google Scholar] [CrossRef]
- Wu, X.; Shu, S.; Wang, Y.; Yuan, R.; Guo, S. Exogenous putrescine alleviates photoinhibition caused by salt stress through cooperation with cyclic electron flow in cucumber. Photosynth. Res. 2019, 141, 303–314. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Chen, H.; Huang, J.; Zhang, Y.; Qi, M.; Liu, Y.; Li, T. Photosynthetic Response Mechanism of Soil Salinity-Induced Cross-Tolerance to Subsequent Drought Stress in Tomato Plants. Plants 2020, 9, 363. [Google Scholar] [CrossRef]
- Akram, S.; Ghaffar, M.; Wadood, A.; Shokat, S.; Hameed, A.; Waheed, M.Q.; Arif, M.A.R. A GBS-based genome-wide association study reveals the genetic basis of salinity tolerance at the seedling stage in bread wheat (Triticum aestivum L.). Front. Genet. 2022, 13, 997901. [Google Scholar] [CrossRef]
- Hichri, I.; Muhovski, Y.; Clippe, A.; Žižková, E.; Dobrev, P.I.; Motyka, V.; Lutts, S. SlDREB2, a tomato dehydration-responsive element-binding 2 transcription factor, mediates salt stress tolerance in tomato and Arabidopsis. Plant Cell Ennviron. 2015, 39, 62–79. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, R.; Yang, X.; Ju, Q.; Li, W.; Lü, S.; Tran, L.S.P.; Xu, J. The R2R3-MYB transcription factor AtMYB49 modulates salt tolerance in Arabidopsis by modulating the cuticle formation and antioxidant defence. Plant Cell Ennviron. 2020, 43, 1925–1943. [Google Scholar] [CrossRef]
- Dogan, M.; Tipirdamaz, R.; Demir, Y. Salt resistance of tomato species grown in sand culture. Plant Soil Environ. 2010, 56, 499–507. [Google Scholar] [CrossRef]
- Khalid, M.F.; Huda, S.; Yong, M.; Li, L.H.; Chen, Z.H.; Ahmed, T. Alleviation of drought and salt stress in vegetables: Crop responses and mitigation strategies. Plant Growth Regul. 2023, 99, 177–194. [Google Scholar] [CrossRef]

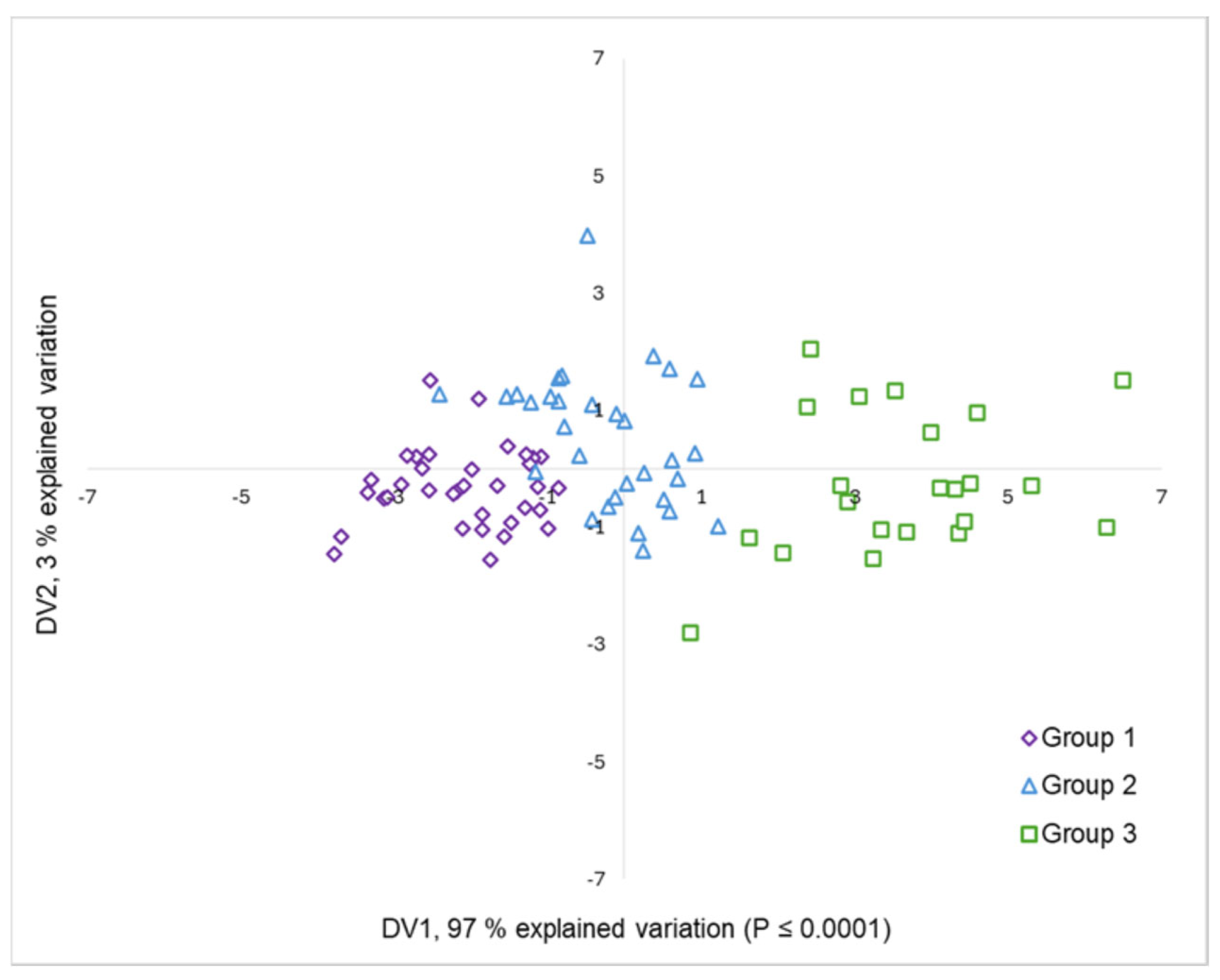

| Variable | Eigenvectors | Discriminant Functions | ||
|---|---|---|---|---|
| DV1 | DV2 | DV1 | DV2 | |
| Total dry matter (TDM) | 0.98 | −0.12 | 1.56 | −2.39 |
| Germination percentage (GP) | 0.96 | 0.25 | 0.37 | 2.24 |
| Plumule length (PL) | 0.77 | 0.21 | −0.08 | 0.93 |
| Radicle length (RL) | 0.49 | −0.22 | −0.20 | −0.24 |
| Germination rate index (GRI) | 0.96 | 0.05 | 1.09 | −0.42 |
| NaCl (mM) | GP (%) | GRI | PL (cm) | RL (cm) | TDM (mg) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 80.62 | a | 6.84 | a | 3.40 | a | 1.08 | a | 0.028 | a |
| 80 | 50.87 | b | 2.04 | b | 1.94 | b | 0.58 | b | 0.015 | b |
| % Reduction | 37 | 70 | 43 | 47 | 44 | |||||
| HSD | 6.24 | 0.59 | 0.46 | 0.17 | 0.0029 |
| NaCl (mM) | GRO | GP (%) | GRI | PL (cm) | RL (cm) | TDM (mg) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 80.37 | b | 6.44 | b | 3.32 | a | 1.00 | a | 0.03 | a |
| 80 | 1 | 30.26 | d | 1.06 | e | 1.33 | d | 0.44 | d | 0.01 | d |
| % Reduction | 62 | 84 | 60 | 57 | 69 | ||||||
| 0 | 2 | 86.37 | a | 7.85 | a | 3.40 | a | 1.06 | a | 0.03 | a |
| 80 | 2 | 75.07 | b | 3.38 | c | 2.52 | b | 0.73 | b | 0.02 | b |
| % Reduction | 13 | 57 | 26 | 31 | 14 | ||||||
| 0 | 3 | 76.82 | b | 6.41 | b | 3.46 | a | 1.14 | a | 0.03 | a |
| 80 | 3 | 48.38 | c | 1.78 | d | 1.96 | c | 0.57 | c | 0.01 | c |
| % Reduction | 37 | 72 | 44 | 50 | 48 | ||||||
| HSD | 3.05 | 0.17 | 0.13 | 0.07 | 0.0012 |
| NaCl (mM) | GEN | GRO | GP (%) | GRI | RL (cm) | PL (cm) | TDM (mg) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | L4-1 | 2 | 96 | a | 10.2 | a | 1.34 | a | 3.71 | a | 0.0258 | a |
| 80 | L4-1 | 2 | 84 | a | 5.1 | b | 1.11 | a | 3.10 | a | 0.0252 | a |
| % Reduction | 13 | 50 | 17 | 17 | 2 | |||||||
| 0 | L45 | 2 | 94 | a | 8.0 | a | 0.90 | a | 3.37 | a | 0.0306 | a |
| 80 | L45 | 2 | 80 | a | 3.5 | b | 0.66 | a | 2.63 | a | 0.0312 | a |
| % Reduction | 15 | 56 | 27 | 22 | 102 | |||||||
| 0 | L5 | 2 | 100 | a | 9.7 | a | 0.84 | a | 2.70 | a | 0.0299 | a |
| 80 | L5 | 2 | 94 | a | 4.6 | b | 0.68 | a | 2.19 | a | 0.0300 | a |
| % Reduction | 6 | 53 | 19 | 19 | 103 | |||||||
| 0 | L9 | 2 | 92 | a | 7.6 | a | 1.20 | a | 3.50 | a | 0.0328 | a |
| 80 | L9 | 2 | 78 | a | 3.3 | b | 0.77 | a | 2.57 | a | 0.0240 | a |
| % Reduction | 15 | 57 | 36 | 27 | 27 | |||||||
| 0 | L92D | 2 | 98 | a | 9.9 | a | 0.94 | a | 3.15 | a | 0.0214 | a |
| 80 | L92D | 2 | 91 | a | 4.1 | b | 0.54 | a | 2.16 | a | 0.0193 | a |
| % Reduction | 7 | 59 | 43 | 32 | 10 | |||||||
| 0 | L29 | 1 | 94 | a | 8.7 | a | 1.41 | a | 3.35 | a | 0.0403 | a |
| 80 | L29 | 1 | 36 | b | 1.3 | b | 0.43 | b | 1.59 | b | 0.0126 | b |
| % Reduction | 62 | 84 | 70 | 53 | 69 | |||||||
| 0 | L33 | 1 | 88 | a | 6.5 | a | 1.16 | a | 3.24 | a | 0.0402 | a |
| 80 | L33 | 1 | 38 | b | 1.4 | b | 0.42 | a | 1.56 | b | 0.0131 | b |
| % Reduction | 57 | 79 | 64 | 52 | 67 | |||||||
| 0 | L40 | 1 | 80 | a | 7.0 | a | 0.94 | a | 4.13 | a | 0.0256 | a |
| 80 | L40 | 1 | 27 | b | 1.0 | b | 0.33 | a | 1.64 | b | 0.0086 | b |
| % Reduction | 66 | 86 | 65 | 60 | 66 | |||||||
| 0 | L41 | 1 | 98 | a | 7.1 | a | 1.22 | a | 3.44 | a | 0.0354 | a |
| 80 | L41 | 1 | 38 | b | 1.2 | b | 0.55 | a | 1.28 | b | 0.0102 | b |
| % Reduction | 61 | 83 | 55 | 63 | 71 | |||||||
| 0 | L73 | 1 | 94 | a | 8.4 | a | 1.52 | a | 3.81 | a | 0.0416 | a |
| 80 | L73 | 1 | 36 | b | 1.1 | b | 0.45 | b | 1.755 | b | 0.0124 | b |
| % Reduction | 62 | 87 | 70 | 54 | 70 | |||||||
| HSD | 34 | 1.8 | 0.78 | 1.47 | 0.0133 |
| Variable | Eigenvectors | Discriminant Functions | ||
|---|---|---|---|---|
| VD1 | VD2 | VD1 | VD2 | |
| Seedling height (SH) | 0.28 | 0.62 | 0.36 | 0.74 |
| Root length (RL) | 0.07 | 0.23 | −0.29 | 0.09 |
| Root dry matter (RDM) | 0.25 | 0.62 | −0.07 | 0.93 |
| Aerial dry matter (ADM) | 0.97 | −0.11 | −1.76 | 1.83 |
| Total dry matter (TDM) | 0.98 | −0.08 | 4.18 | −2.30 |
| Leaf area (LA) | 0.62 | 0.31 | 0.02 | 0.05 |
| NaCl (mM) | SH (cm) | RL (cm) | RDM (g) | ADM (g) | TDM (g) | LA (cm2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 18.3 | a | 44.0 | a | 0.2 | a | 2.1 | a | 2.3 | a | 702.2 | a |
| 150 | 10.2 | b | 38.0 | a | 0.5 | a | 1.8 | a | 2.3 | a | 348.5 | b |
| % Reduction | 44 | 14 | −187 | 13 | 0 | 50 | ||||||
| HSD | 7.5 | 6.7 | 0.4 | 1.2 | 1.6 | 248.9 | ||||||
| CV | 12.3 | 12.5 | 34.1 | 37.5 | 35.3 | 21.0 |
| NaCl (mM) | GRO | SH (cm) | RL (cm) | RDM (g) | ADM (g) | TDM (g) | LA (cm2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 19.5 | a | 44.9 | a | 0.19 | c | 2.65 | a | 2.85 | a | 771.5 | a |
| 150 | 1 | 10.3 | d | 38.1 | b | 0.51 | a | 1.84 | bc | 2.35 | b | 351.7 | de |
| % reduction | 47 | 15 | −163 | 31 | 17 | 54 | |||||||
| 0 | 2 | 18.4 | b | 43.7 | a | 0.16 | c | 2.07 | b | 2.24 | b | 718.8 | b |
| 150 | 2 | 10.5 | d | 38.3 | b | 0.50 | a | 1.89 | bc | 2.39 | b | 366.3 | d |
| % reduction | 43 | 13 | −201 | 9 | −7 | 49 | |||||||
| 0 | 3 | 16.0 | c | 42.9 | a | 0.13 | c | 1.15 | d | 1.29 | c | 559.1 | c |
| 150 | 3 | 9.4 | e | 37.1 | b | 0.42 | b | 1.64 | c | 2.07 | b | 317.0 | e |
| % reduction | 42 | 14 | −219 | −42 | −61 | 43 | |||||||
| HSD | 6.42 | 1.97 | 0.062 | 0.28 | 0.31 | 40.16 |
| NaCl | GEN | SH (cm) | RL (cm) | RDM (g) | ADM (g) | TDM (g) | LA (cm2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | L13 | 18.3 | a | 50.5 | a | 0.109 | a | 0.99 | a | 1.10 | a | 518.7 | a |
| 150 | L13 | 9.4 | b | 41.0 | a | 0.449 | a | 1.42 | a | 1.87 | a | 316.2 | a |
| % reduction | 49 | 19 | −313 | −44 | −70 | 39 | |||||||
| 0 | L20 | 14.7 | a | 36.8 | a | 0.129 | a | 1.01 | a | 1.14 | a | 683.0 | a |
| 150 | L20 | 8.8 | a | 36.2 | a | 0.357 | a | 1.41 | a | 1.77 | a | 340.3 | a |
| % reduction | 40 | 2 | −176 | −39 | −55 | 50 | |||||||
| 0 | L43 | 14.5 | a | 40.8 | a | 0.090 | a | 0.68 | a | 0.77 | a | 455.7 | a |
| 150 | L43 | 8.9 | a | 34.3 | a | 0.269 | a | 1.18 | a | 1.45 | a | 244.2 | a |
| % reduction | 39 | 16 | −198 | −72 | −87 | 46 | |||||||
| 0 | L5 | 10.2 | a | 46.3 | a | 0.111 | a | 0.76 | a | 0.87 | a | 344.4 | a |
| 150 | L5 | 8.4 | a | 33.7 | a | 0.204 | a | 1.06 | a | 1.27 | a | 205.0 | a |
| % reduction | 18 | 27 | −84 | −40 | −45 | 40 | |||||||
| 0 | L75 | 15.1 | a | 37.8 | a | 0.085 | a | 0.84 | a | 0.93 | a | 409.1 | a |
| 150 | L75 | 10.1 | b | 34.8 | a | 0.369 | a | 1.46 | a | 1.83 | a | 277.8 | a |
| % reduction | 33 | 8 | −334 | −74 | −97 | 32 | |||||||
| 0 | L41 | 15.2 | a | 37.8 | a | 0.091 | a | 0.95 | a | 1.04 | a | 420.6 | a |
| 150 | L41 | 8.8 | b | 39.5 | a | 0.369 | a | 1.43 | a | 1.80 | a | 279.0 | a |
| % reduction | 42 | −5 | −305 | −51 | −73 | 34 | |||||||
| 0 | L8 | 14.1 | a | 46.5 | a | 0.1 | a | 0.8 | a | 0.9 | a | 387.6 | a |
| 150 | L8 | 7.6 | b | 44.0 | a | 0.4 | a | 1.2 | a | 1.5 | a | 280.1 | a |
| % Reduction | 46 | 5 | −186 | −49 | −68 | 28 | |||||||
| 0 | L67 | 8.0 | a | 26.3 | a | 0.0 | a | 0.2 | a | 0.3 | a | 130.5 | a |
| 150 | L67 | 4.4 | b | 27.8 | a | 0.1 | a | 0.4 | a | 0.5 | a | 114.6 | a |
| % Reduction | 44 | −6 | −150 | −79 | −89 | 12 | |||||||
| 0 | L4-3 | 18.6 | a | 33.0 | a | 0.1 | a | 1.0 | a | 1.1 | a | 487.4 | a |
| 150 | L4-3 | 8.6 | b | 32.3 | a | 0.3 | a | 1.4 | a | 1.8 | a | 252.9 | b |
| % reduction | 53 | 2 | −196 | −40 | −55 | 48 | |||||||
| 0 | L53 | 13.7 | a | 44.3 | a | 0.2 | a | 0.9 | a | 1.0 | a | 343.1 | a |
| 150 | L53 | 8.3 | b | 31.2 | a | 0.3 | a | 1.8 | a | 2.2 | a | 230.3 | a |
| % reduction | 39 | 30 | −103 | −107 | −107 | 33 |
| Germination | Tolerant Group 2 | Intermediate Tolerance Group 3 | Susceptible Group 1 | |
|---|---|---|---|---|
| Seedling | ||||
| Tolerant Group 3 | L5, L13, L28, L43, L53, L54, L68 | L4-3, L8, L19, L35, L37, L47B1, L57, L75, RF1 | L7, L41, L67, L72, L88, SS5 | |
| Intermediate tolerance Group 2 | L9, L45, L48, L63, L78 | L2, L18, L22, L30, L31, L34, L47S2, L58, L59, L60, L69 | L10, 11-4, L14, L23, L29, L40, L47S8, L49, L61, L62, L73, L90, RF46 | |
| Susceptible Group 1 | L4-1, L12, L42, L44, L50, L55, L56, L64, L65, L66, L86, L91, L92D, RF12, SS2 | L3, L15, L24, L27, L36, L39, L46, L51, L51H, L52, L76H, L80, MERM, RF38, RF41 | L1, L6, L33, L74, L85, RF81 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deanda-Tovar, A.A.; Rodríguez-Pérez, J.E.; Sahagún-Castellanos, J.; Colinas-y-León, M.T.B.; Pérez-Rodríguez, P.; Paredes-Cervantes, A.E. Tomato Lines Tolerant to Sodium Chloride at Early Growth Stages. Horticulturae 2025, 11, 532. https://doi.org/10.3390/horticulturae11050532
Deanda-Tovar AA, Rodríguez-Pérez JE, Sahagún-Castellanos J, Colinas-y-León MTB, Pérez-Rodríguez P, Paredes-Cervantes AE. Tomato Lines Tolerant to Sodium Chloride at Early Growth Stages. Horticulturae. 2025; 11(5):532. https://doi.org/10.3390/horticulturae11050532
Chicago/Turabian StyleDeanda-Tovar, Alma Aurora, Juan Enrique Rodríguez-Pérez, Jaime Sahagún-Castellanos, María Teresa Beryl Colinas-y-León, Paulino Pérez-Rodríguez, and Ana Elizabeth Paredes-Cervantes. 2025. "Tomato Lines Tolerant to Sodium Chloride at Early Growth Stages" Horticulturae 11, no. 5: 532. https://doi.org/10.3390/horticulturae11050532
APA StyleDeanda-Tovar, A. A., Rodríguez-Pérez, J. E., Sahagún-Castellanos, J., Colinas-y-León, M. T. B., Pérez-Rodríguez, P., & Paredes-Cervantes, A. E. (2025). Tomato Lines Tolerant to Sodium Chloride at Early Growth Stages. Horticulturae, 11(5), 532. https://doi.org/10.3390/horticulturae11050532










