Salt Stress-Induced Ascorbic Acid Accumulation and Its Trade-Off with Mannan Content in Tomato
Abstract
1. Introduction
2. Materials and Methods
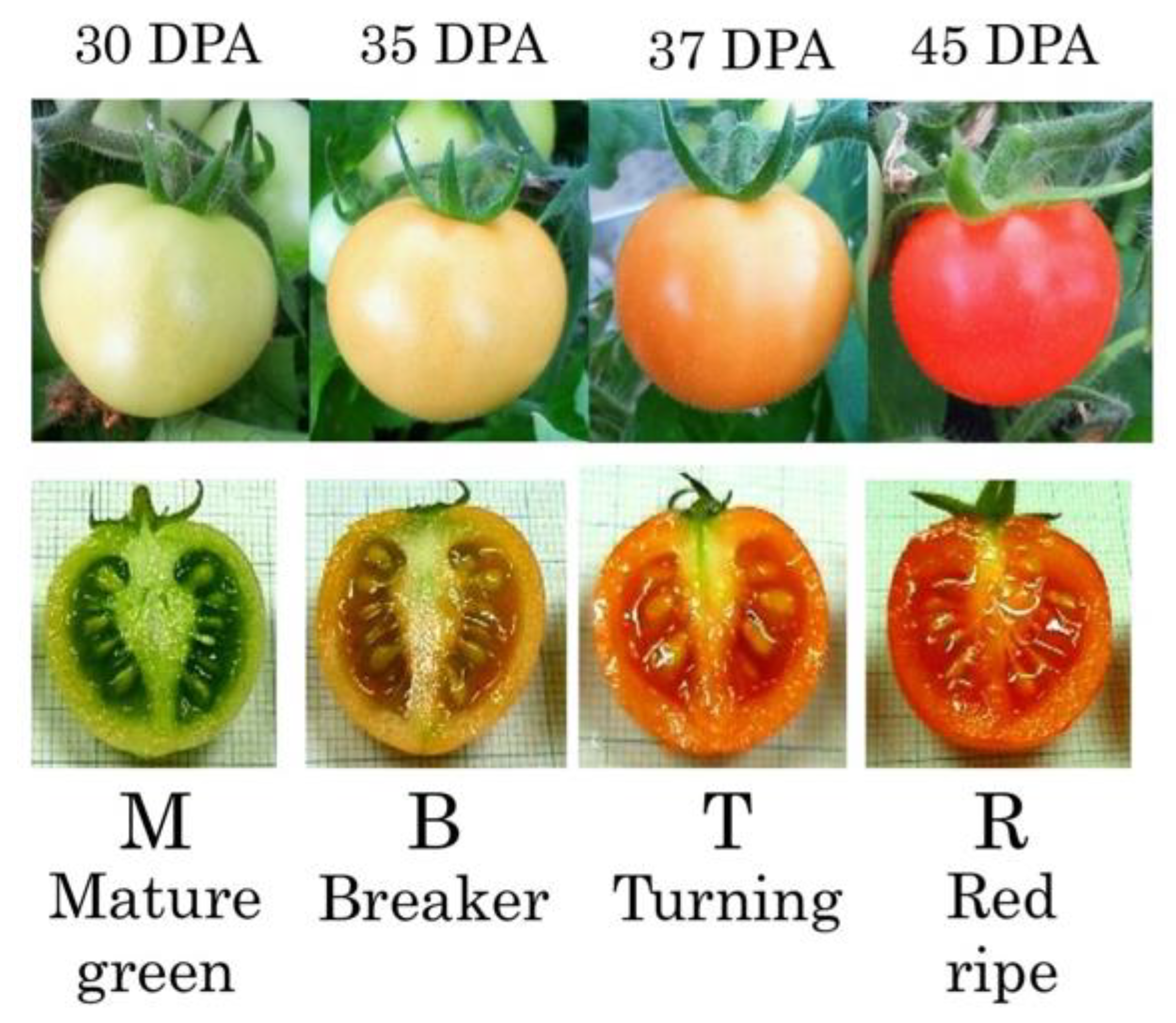
2.1. Plant Materials and Growth Conditions
2.2. Extraction of Cell Walls
2.3. Analysis of Mannan and Cell Wall Sugar Components by Gas Chromatography
2.4. Expression Analysis of Mannan Synthase Gene and Ascorbic Acid Synthase Gene in Tomato Fruit Under Salinity Conditions
2.5. Measurement of Ascorbic Acid Content Under Salt Stress with Addition of Mannose
2.6. Measurement of Germination Rate
2.7. Statistical Analysis
3. Results
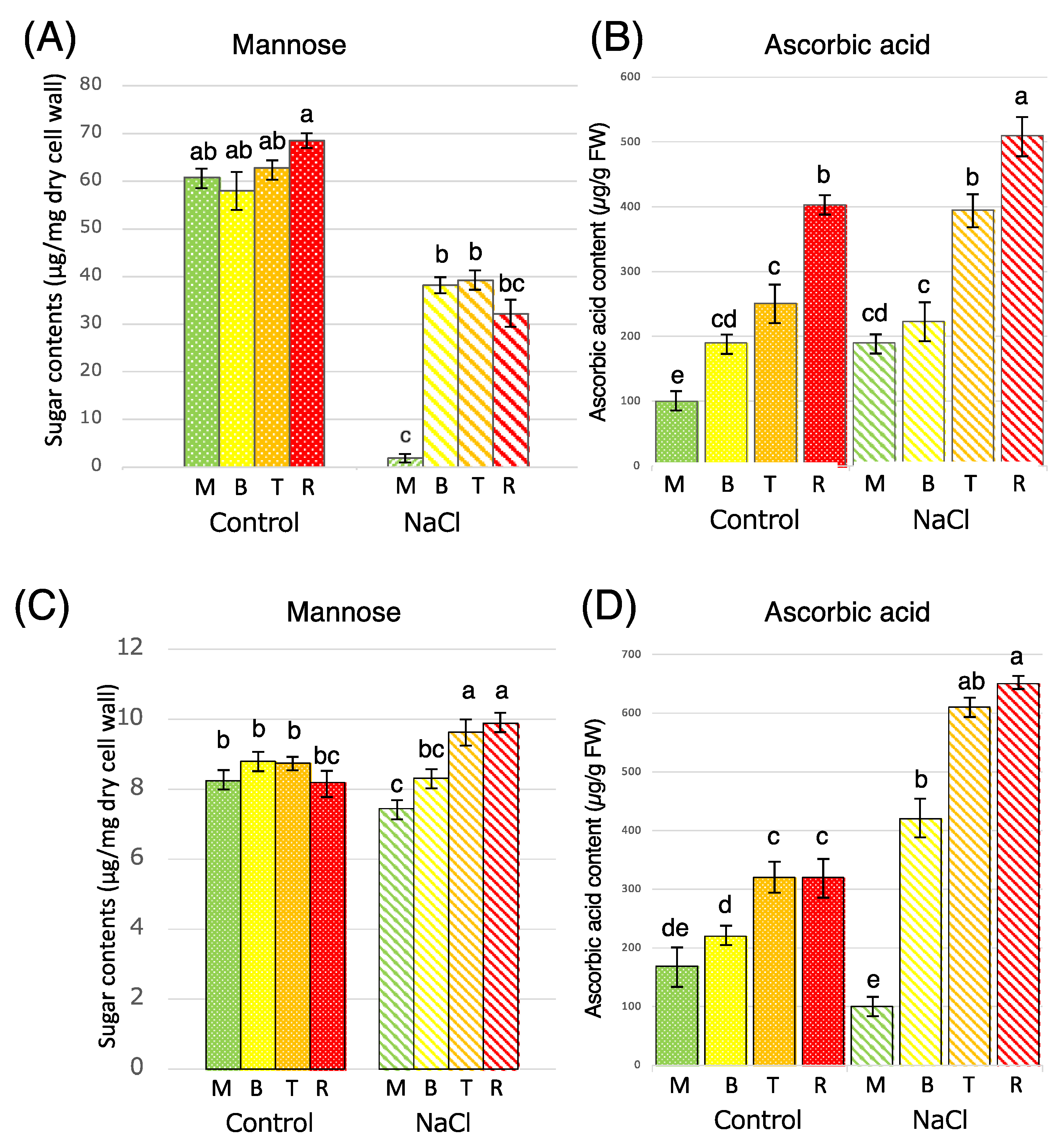
3.1. Measurement of Mannose and Ascorbic Acid Content in Tomato Fruit Under Salt Stress Conditions
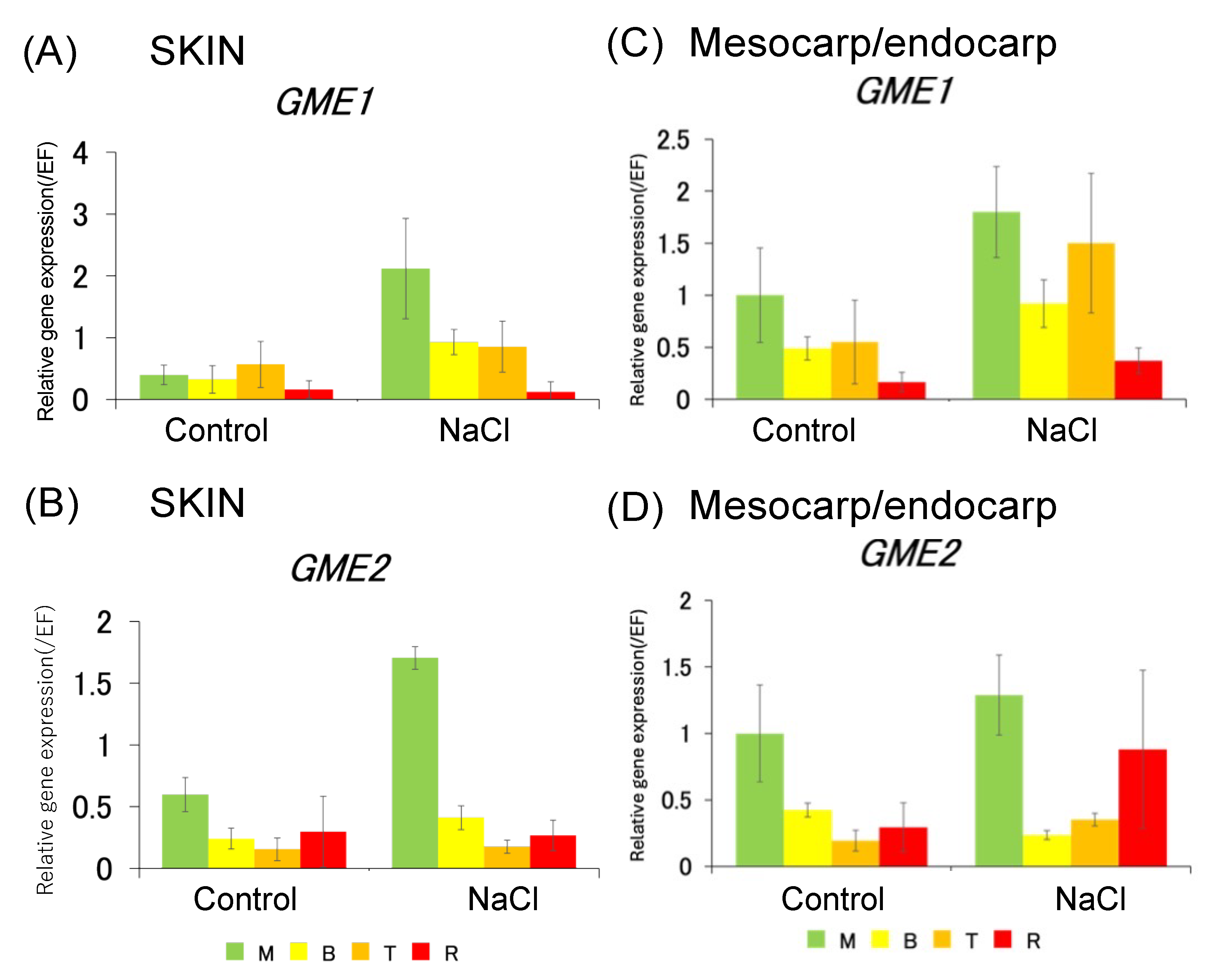
3.2. Expression Analysis of Mannan Synthase Gene and Ascorbic Acid Synthase Gene in Tomato Fruit Under Salt Stress Conditions
3.3. Changes in Ascorbic Acid Content Under Salt Stress Conditions with Added Mannose
3.4. Measurement of Ascorbic Acid Content Under Salt Stress Conditions with Added Mannose
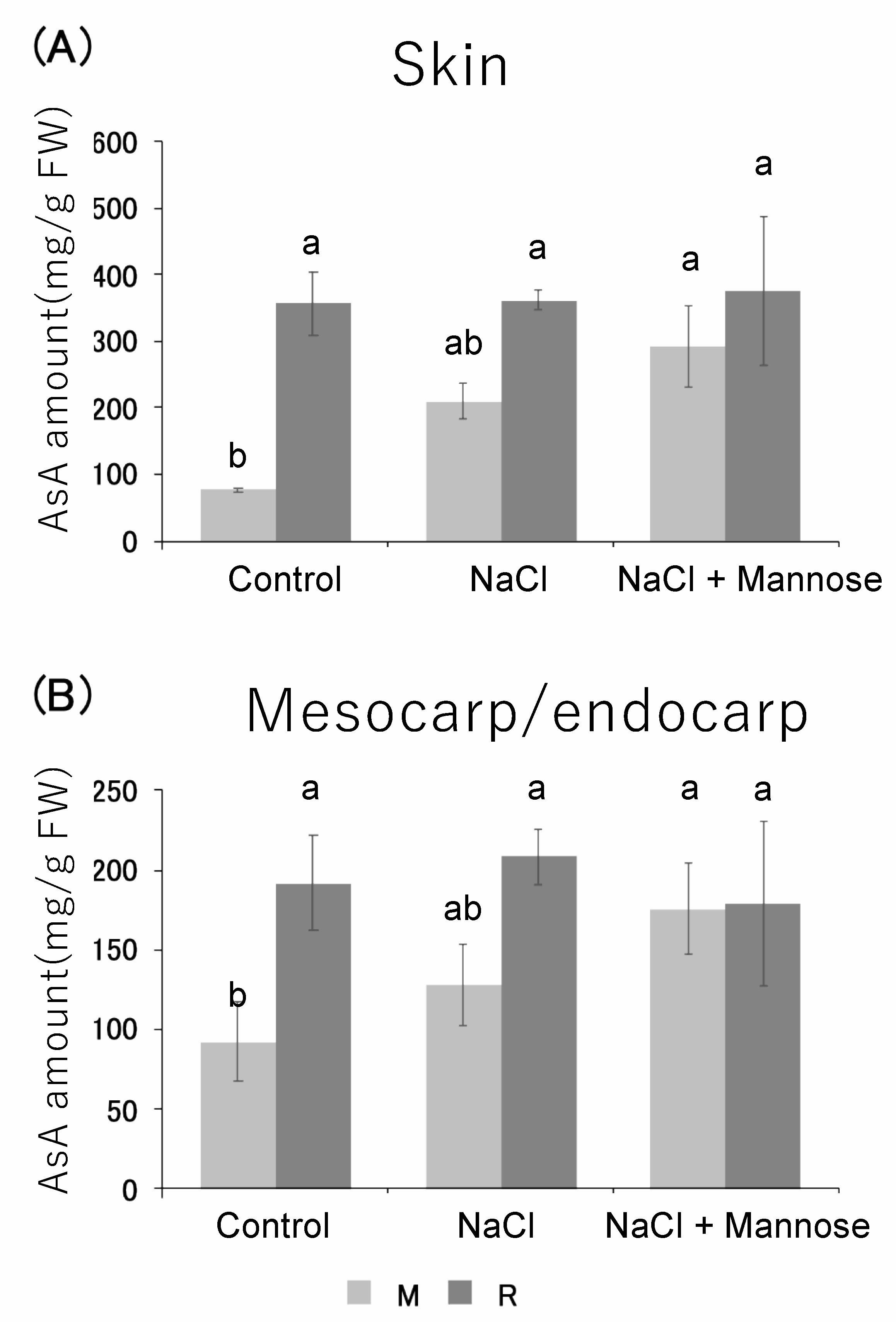
3.5. Expression Analysis of Ascorbic Acid Synthase Gene in Skin and Pericarp
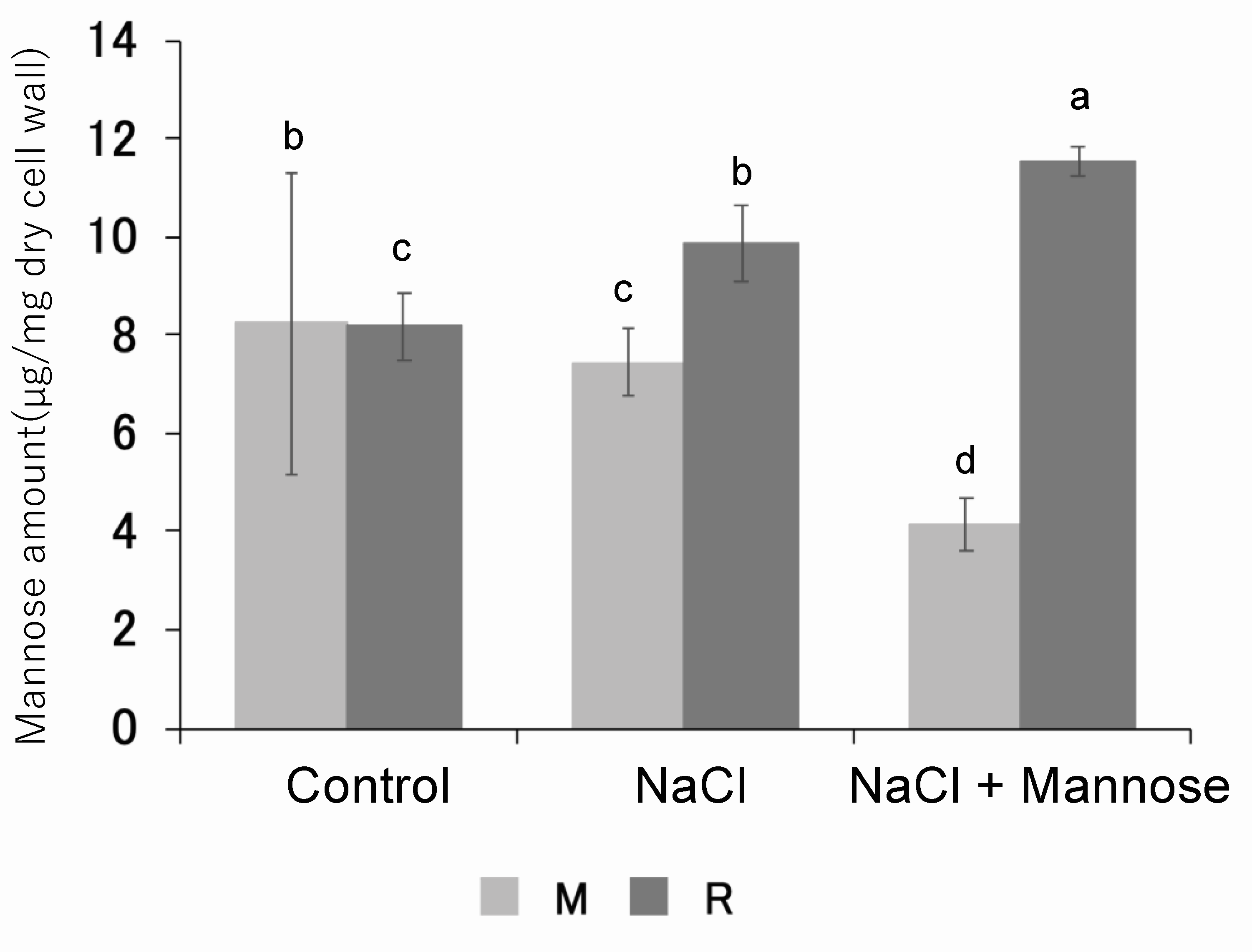
3.6. Measurement of Mannan Content in Skin and Pericarp of Mannose-Supplemented Tomato Fruits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AsA | Ascorbic Acid |
| GME | GDP-Mannose Epimerase |
| CSLA | Cellulose Synthase-like A |
| ROS | Reactive Oxygen Species |
References
- Fujita, M.; Fujita, Y.; Noutoshi, Y.; Takahashi, F.; Narusaka, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Crosstalk between Abiotic and Biotic Stress Responses: A Current View from the Points of Convergence in the Stress Signaling Networks. Curr. Opin. Plant Biol. 2006, 9, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Bolarin, M.C.; Estañ, M.T.; Caro, M.; Romero-Aranda, R.; Cuartero, J. Relationship between Tomato Fruit Growth and Fruit Osmotic Potential under Salinity. Plant Sci. 2001, 160, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Gama, P.B.S.; Inanaga, S.; Tanaka, K.; Nakazawa, R. Physiological Response of Common Bean (Phaseolus Vulgaris L.) Seedlings to Salinity Stress. Afr. J. Biotechnol. 2007, 6, 79–88. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, M.; Shibli, R.; Ajlouni, M.; Nimri, L. Tomato Root and Shoot Responses to Salt Stress under Different Levels of Phosphorus Nutrition. J. Plant Nutr. 1998, 21, 1667–1680. [Google Scholar] [CrossRef]
- Scholberg, J.M.S.; Locascio, S.J. Growth Response of Snap Bean and Tomato as Affected by Salinity and Irrigation Method. HortScience 1999, 34, 259–264. [Google Scholar] [CrossRef]
- Magán, J.J.; Gallardo, M.; Thompson, R.B.; Lorenzo, P. Effects of Salinity on Fruit Yield and Quality of Tomato Grown in Soil-Less Culture in Greenhouses in Mediterranean Climatic Conditions. Agric. Water Manag. 2008, 95, 1041–1055. [Google Scholar] [CrossRef]
- Ho, L.C. A Cellular Hypothesis for the Induction of Blossom-End Rot in Tomato Fruit. Ann. Bot. 2005, 95, 571–581. [Google Scholar] [CrossRef]
- Saito, T.; Matsukura, C.; Sugiyama, M.; Watahiki, A.; Ohshima, I.; Iijima, Y.; Konishi, C.; Fujii, T.; Inai, S.; Fukuda, N.; et al. Screening for γ-Aminobutyric Acid (GABA)-Rich Tomato Varieties. J. Jpn. Soc. Hortic. Sci. 2008, 77, 242–250. [Google Scholar] [CrossRef]
- Yin, Y.-G.; Kobayashi, Y.; Sanuki, A.; Kondo, S.; Fukuda, N.; Ezura, H.; Sugaya, S.; Matsukura, C. Salinity Induces Carbohydrate Accumulation and Sugar-Regulated Starch Biosynthetic Genes in Tomato (Solanum Lycopersicum L. cv. ‘Micro-Tom’) Fruits in an ABA- and Osmotic Stress-Independent Manner. J. Exp. Bot. 2010, 61, 563–574. [Google Scholar] [CrossRef]
- Ezura, H.; Hiwasa-Tanase, K. Fruit Development. In Plant Developmental Biology—Biotechnological Perspectives; Pua, E.C., Davey, M.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 301–318. ISBN 978-3-642-02300-2. [Google Scholar]
- Gillaspy, G.; Ben-David, H.; Gruissem, W. Fruits: A Developmental Perspective. Plant Cell 1993, 5, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Terao, A.; Hyodo, H.; Satoh, S.; Iwai, H. Changes in the Distribution of Cell Wall Polysaccharides in Early Fruit Pericarp and Ovule, from Fruit Set to Early Fruit Development, in Tomato (Solanum Lycopersicum). J. Plant Res. 2013, 126, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, H.; Terao, A.; Furukawa, J.; Sakamoto, N.; Yurimoto, H.; Satoh, S.; Iwai, H. Tissue Specific Localization of Pectin–Ca2+ Cross-Linkages and Pectin Methyl-Esterification during Fruit Ripening in Tomato (Solanum Lycopersicum). PLoS ONE 2013, 8, e78949. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.J. The Role of Cell Wall Hydrolases in Fruit Softening. In Horticultural Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1983; pp. 169–219. ISBN 978-1-118-06072-8. [Google Scholar]
- Seymour, G.B.; Colquhoun, I.J.; Dupont, M.S.; Parsley, K.R.; Selvendran, R.R. Composition and Structural Features of Cell Wall Polysaccharides from Tomato Fruits. Phytochemistry 1990, 29, 725–731. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, P.; Chen, F.; Lai, S.; Yu, H.; Yang, H. Effects of Calcium and Pectin Methylesterase on Quality Attributes and Pectin Morphology of Jujube Fruit under Vacuum Impregnation during Storage. Food Chem. 2019, 289, 40–48. [Google Scholar] [CrossRef]
- Moreira, L.R.S.; Filho, E.X.F. An Overview of Mannan Structure and Mannan-Degrading Enzyme Systems. Appl. Microbiol. Biotechnol. 2008, 79, 165–178. [Google Scholar] [CrossRef]
- Schröder, R.; Wegrzyn, T.F.; Sharma, N.N.; Atkinson, R.G. LeMAN4 Endo-Beta-Mannanase from Ripe Tomato Fruit Can Act as a Mannan Transglycosylase or Hydrolase. Planta 2006, 224, 1091–1102. [Google Scholar] [CrossRef]
- Ishida, K.; Ohba, Y.; Yoshimi, Y.; Wilson, L.F.L.; Echevarría-Poza, A.; Yu, L.; Iwai, H.; Dupree, P. Differing Structures of Galactoglucomannan in Eudicots and Non-Eudicot Angiosperms. PLoS ONE 2023, 18, e0289581. [Google Scholar] [CrossRef]
- Wheeler, G.L.; Jones, M.A.; Smirnoff, N. The Biosynthetic Pathway of Vitamin C in Higher Plants. Nature 1998, 393, 365–369. [Google Scholar] [CrossRef]
- Hu, T.; Ye, J.; Tao, P.; Li, H.; Zhang, J.; Zhang, Y.; Ye, Z. The Tomato HD-Zip I Transcription Factor SlHZ24 Modulates Ascorbate Accumulation through Positive Regulation of the d-Mannose/l-Galactose Pathway. Plant J. 2016, 85, 16–29. [Google Scholar] [CrossRef]
- Zheng, X.; Gong, M.; Zhang, Q.; Tan, H.; Li, L.; Tang, Y.; Li, Z.; Peng, M.; Deng, W. Metabolism and Regulation of Ascorbic Acid in Fruits. Plants 2022, 11, 1602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Senge, M.; Dai, Y. Effects of Salinity Stress on Growth, Yield, Fruit Quality and Water Use Efficiency of Tomato Under Hydroponics System. Rev. Agric. Sci. 2016, 4, 46–55. [Google Scholar] [CrossRef]
- Voxeur, A.; Gilbert, L.; Rihouey, C.; Driouich, A.; Rothan, C.; Baldet, P.; Lerouge, P. Silencing of the GDP-D-Mannose 3,5-Epimerase Affects the Structure and Cross-Linking of the Pectic Polysaccharide Rhamnogalacturonan II and Plant Growth in Tomato. J. Biol. Chem. 2011, 286, 8014–8020. [Google Scholar] [CrossRef] [PubMed]
- Carpita, N.C.; Gibeaut, D.M. Structural Models of Primary Cell Walls in Flowering Plants: Consistency of Molecular Structure with the Physical Properties of the Walls during Growth. Plant J. Cell Mol. Biol. 1993, 3, 1–30. [Google Scholar] [CrossRef]
- Iwai, H. Virtual issue: Cell Wall Functions in Plant Growth and Environmental Responses. J. Plant Res. 2021, 134, 1155–1158. [Google Scholar] [CrossRef]
- Zushi, K.; Matsuzoe, N.; Kitano, M. Developmental and Tissue-Specific Changes in Oxidative Parameters and Antioxidant Systems in Tomato Fruits Grown Under Salt Stress. Sci. Hortic. 2009, 122, 362–368. [Google Scholar] [CrossRef]
- Soyama, K.; Yano, A.; Miyakoshi, A.; Itano, M.; Sugiyama, H.; Iwai, H. Regulation of Cell Wall Remodeling is an Important Factor in the Reduction of Tomato Fruit Size Immediately after Fruit Set Induced by Salinity Conditions. Hortic. J. 2024, 93, 397–405. [Google Scholar] [CrossRef]
- Dumville, J.C.; Fry, S.C. Solubilisation of Tomato Fruit Pectins by Ascorbate: A Possible Non-Enzymic Mechanism of Fruit Softening. Planta 2003, 217, 951–961. [Google Scholar] [CrossRef]
- Ford, C.M.; Sweetman, C.; Fry, S.C. Ascorbate Degradation: Pathways, Products, and Possibilities. J. Exp. Bot. 2024, 75, 2733–2739. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasegawa, C.; Yamada, K.; Hoyano, N.; Sano, M.; Soyama, K.; Iwai, H. Salt Stress-Induced Ascorbic Acid Accumulation and Its Trade-Off with Mannan Content in Tomato. Horticulturae 2025, 11, 400. https://doi.org/10.3390/horticulturae11040400
Hasegawa C, Yamada K, Hoyano N, Sano M, Soyama K, Iwai H. Salt Stress-Induced Ascorbic Acid Accumulation and Its Trade-Off with Mannan Content in Tomato. Horticulturae. 2025; 11(4):400. https://doi.org/10.3390/horticulturae11040400
Chicago/Turabian StyleHasegawa, Chiaki, Kaori Yamada, Natsuki Hoyano, Mao Sano, Kiei Soyama, and Hiroaki Iwai. 2025. "Salt Stress-Induced Ascorbic Acid Accumulation and Its Trade-Off with Mannan Content in Tomato" Horticulturae 11, no. 4: 400. https://doi.org/10.3390/horticulturae11040400
APA StyleHasegawa, C., Yamada, K., Hoyano, N., Sano, M., Soyama, K., & Iwai, H. (2025). Salt Stress-Induced Ascorbic Acid Accumulation and Its Trade-Off with Mannan Content in Tomato. Horticulturae, 11(4), 400. https://doi.org/10.3390/horticulturae11040400






