Correlation, Path-Coefficient, and Economic Heterosis Studies in CMS-Based Cabbage Hybrids over Different Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Statistical Analysis
3. Results
3.1. Source of Variation
3.2. Parameters of Variability
3.3. Correlation Studies
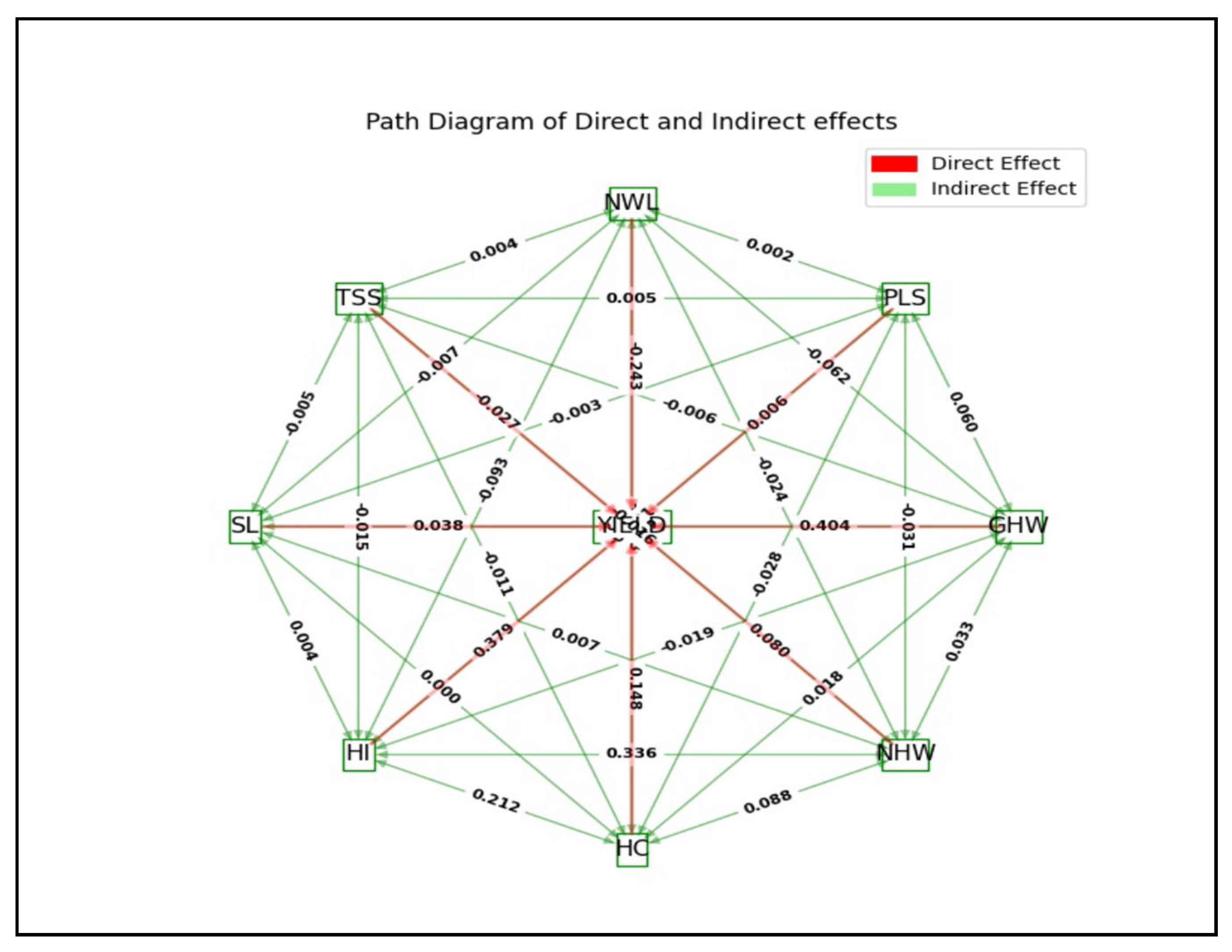
3.4. Path Coefficient Analysis
3.5. Heterosis Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yue, Z.; Zhang, G.; Wang, J.; Wang, J.; Luo, S.; Zhang, B.; Liu, Z. Comparative Study of the Quality Indices, Antioxidant Substances, and Mineral Elements in Different Forms of Cabbage. BMC Plant Biol. 2024, 24, 187. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Yang, X.; Chaobo, T.; David, E. The Brassica oleracea Genome Reveals the Asymmetrical Evolution of Polyploid Genomes. Nat. Commun. 2014, 5, 3930. [Google Scholar] [CrossRef] [PubMed]
- Samec, D.; Pavlovic, I.; Salopek-Sondi, B. White Cabbage (Brassica oleracea L. var. capitata f. alba L.): Botanical, Phytochemical, and Pharmacological Overview. Phytochem. Rev. 2017, 16, 117–135. [Google Scholar] [CrossRef]
- Liu, Z.; Alemán-Báez, J.; Visser, R.G.; Bonnema, G. Cabbage (Brassica oleracea var. capitata) Development in Time: How Differential Parenchyma Tissue Growth Affects Leafy Head Formation. Plants 2024, 13, 656. [Google Scholar] [CrossRef] [PubMed]
- Cervenski, J.; Gvozdanovic-Varga, J.; Glogovac, S. Variance Components and Correlations of Agronomic Traits Among Cabbage (Brassica oleracea L. var. capitata L.) Maturity Groups. Genetika 2012, 44, 55–68. Available online: https://doiserbia.nb.rs/Article.aspx?ID=0534-00121201055C (accessed on 1 January 2025). [CrossRef]
- Yamagishi, H.; Bhat, S.R. Cytoplasmic male sterility in Brassicaceae crops. Breed Sci. 2014, 64, 38–47. [Google Scholar] [CrossRef]
- Parkash, C.; Kumar, S.K.; Singh, R.; Kumar, A.; Kumar, S.; Dey, S.S.; Kumar, R. ‘Ogura’-Based ‘CMS’ Lines with Different Nuclear Backgrounds of Cabbage Revealed Substantial Diversity at Morphological and Molecular Levels. 3 Biotech 2018, 8, 27. [Google Scholar] [CrossRef]
- Verma, S.; Yashveer, S.; Rehman, S.; Gyawali, S.; Kumar, Y.; Chao, S.; Sarker, A.; Verma, R.P.S. Genetic and Agro-morphological diversity in global barley (Hordeum vulgare L.) collection at ICARDA. Genet. Resour. Crop Evol. 2021, 68, 1315–1330. [Google Scholar] [CrossRef]
- Faysal, A.S.M.; Ali, L.; Azam, M.G.; Sarker, U.; Ercisli, S.; Golokhvast, K.S.; Marc, R.A. Genetic Variability, Character Association, and Path Coefficient Analysis in Transplant Aman Rice Genotypes. Plants 2022, 11, 2952. [Google Scholar] [CrossRef]
- Yadav, A.K.; Kumar, A.; Grover, N.; Ellur, R.K.; Bollinedi, H.; Krishnan, S.G.; Bhowmick, P.K.; Vinod, K.K.; Nagarajan, M.; Singh, A.K. Genome-Wide Association Study Reveals Marker-Trait Associations for Early Vegetative Stage Salinity Tolerance in Rice. Plants 2021, 10, 559. [Google Scholar] [CrossRef]
- Usman, M.G.; Rafii, M.Y.; Martini, M.Y.; Oladosu, Y.; Kashiani, P. Genotypic Character Relationship and Phenotypic Path Coefficient Analysis in Chili Pepper Genotypes Grown Under Tropical Condition. J. Sci. Food Agric. 2017, 97, 1164–1171. [Google Scholar] [CrossRef]
- Khan, M.M.H.; Rafii, M.Y.; Ramlee, S.I.; Jusoh, M.; Al Mamun, M. Genetic Analysis and Selection of Bambara Groundnut (Vigna subterranea L. Verdc.) Landraces for High Yield Revealed by Qualitative and Quantitative Traits. Sci. Rep. 2021, 11, 7597. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.H.; Rafii, M.Y.; Ramlee, S.I. Path-Coefficient and Correlation Analysis in Bambara Groundnut (Vigna subterranea L. Verdc.) Accessions Over Environments. Sci. Rep. 2022, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Yan, L.; Chen, Y.; Ken, Y.; Huai, D.; Wang, X.; Jiang, H.; Lei, Y.; Liao, B. Correlation and Variability Analysis of Yield and Quality Related Traits in Different Peanut Varieties Across Various Ecological Zones of China. Oil Crop Sci. 2023, 8, 236–242. [Google Scholar] [CrossRef]
- Correa, E.; Malla, S.; Crosby, K.M.; Avila, C.A. Evaluation of Genotypes and Association of Traits in Watermelon Across Two Southern Texas Locations. Horticulturae 2020, 6, 67. [Google Scholar] [CrossRef]
- Chakraborty, N.R.; Lakshman, S.S.; Debnath, S.; Rahimi, M. Yield Stability and Economic Heterosis Analysis in Newly Bred Sunflower Hybrids throughout Diverse Agro-Ecological Zones. BMC Plant Biol. 2022, 22, 579. [Google Scholar] [CrossRef]
- Singh, S.; Kalia, P.; Mangal, M.; Prakash, V.; Parihar, B.R. Development of Ogura CMS Lines in Indian Cauliflower and Their Characterization Using Agro-Morphological Traits and mtDNA Markers. Sci. Hort. 2022, 291, 110589. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, Y.; Lou, P.; Liu, G. Current Trends in Cabbage Breeding. J. New Seeds 2005, 6, 75–107. [Google Scholar] [CrossRef]
- Nedi, G.; Tulu, L.; Alamerew, S. Standard Heterosis of the Selected Maize (Zea mays L.) Inbred Line Hybrids for Grain Yield and Yield Components at Jimma, South West Oromia Region, Ethiopia. J. Biol. Agric. Healthc. 2017, 7, 51–58. [Google Scholar]
- Mogesse, W.; Zelleke, H.; Nigussie, M. Standard Heterosis for Grain Yield and Yield-Related Traits in Maize (Zea mays L.) Inbred Lines in Haramaya District, Eastern Ethiopia. East Afr. J. Sci. 2020, 14, 51–64. [Google Scholar]
- Selvakumar, R. A Textbook on Glaustas Olericulture; New Vishal Publications: New Delhi, India, 2014; 618p. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; Wiley & Sons Inc.: New York, NY, USA, 1984; p. 680. [Google Scholar]
- Liu, Z.; Jiang, J.; Ren, A.; Xu, X.; Zhang, H.; Zhao, T.; Jiang, X.; Sun, Y.; Li, J.; Yang, H. Heterosis and combining ability analysis of fruit yield, early maturity, and quality in tomato. Agronomy 2021, 11, 807. [Google Scholar] [CrossRef]
- Wright, S. The theory of path coefficients a reply to Niles’s criticism. Genetics 1923, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Dewey, D.R.; Lu, K.H. A Correlation and Path Coefficient Analysis of Components of Crested Wheat Grass Seed Population. Agron. J. 1959, 51, 515–518. [Google Scholar] [CrossRef]
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics, 4th ed.; Longman: London, UK, 1996. [Google Scholar]
- Allard, R.W. Principles of Plant Breeding; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Sharma, K.C. Genetic Variability, Character Association and Path Analysis in Exotic Lines of Cabbage under Mid-Hill, Sub-Humid Conditions of Himachal Pradesh. J. Hill Agric. 2010, 1, 146–150. [Google Scholar]
- Sharma, P.; Singh, S.P.; Dogra, B.S.; Rani, R.; Verma, A. Assessment of genetic variability, correlation and path analysis studies in cabbage (Brassica oleracea) under low hill conditions of Himachal Pradesh. Indian J. Agric. Sci. 2023, 93, 1375–1379. [Google Scholar] [CrossRef]
- Ozer, M.O.; Kar, H.; Bekar, N.K.; Dogru, S.M.; Beşirli, G.; Sönmez, İ. Correlation, genetic variability, heritability and genetic advance for some morphological traits in red cabbage lines (Brassica oleracea L. var. capitata subvar. rubra). J. Agric. Fac. Gaziosmanpaşa Univ. 2023, 40, 58–65. [Google Scholar]
- Chittora, A.; Singh, D.K. Genetic variability studies in early cauliflower (Brassica oleracea var. botrytis L.). Elec. J. Plant. Breed. 2015, 6, 842–847. [Google Scholar]
- Dey, S.S.; Bhatia, R.; Sharma, S.; Sharma, K.; Kumar, R.; Sharma, V.K.; Parkash, C. Genetic divergence and interaction among CUPRAC, FRAP and total phenolics content in cauliflower (Brassica oleracea var. botrytis L.). Indian. J. Gen. Plant Breed. 2015, 75, 79–85. [Google Scholar] [CrossRef]
- Chittora, A.; Sıngh, D.K.; Kaushal, A. Genetic variability, heritability and genetic advance in early maturing cauliflower. Ann. Plant Soil Res. 2016, 18, 251–256. [Google Scholar]
- Wudneh, A.Y. Genetıc variability, heritability and genetic advance for vegetable yield and its component traits in ethıopıan kale (Brassica carinata A.). Inter. J. Plant Breed. Crop Sci. 2020, 7, 946–952. [Google Scholar]
- Soni, S.; Kumar, S.; Maji, S. Correlation and Path Coefficient Analysis Studies for the Yield and Its Traits in Cabbage (Brassica oleracea L. var. capitata L.). Ann. Hortic. 2013, 6, 331–336. [Google Scholar]
- Thakur, H.V. Combining Ability, Gene Action and Heterosis Studies Involving SI and CMS Lines and Testers in Cabbage. Green Farming 2016, 7, 580–585. [Google Scholar]
- Singh, B.; Mishra, A.K.; Sanwal, S.K.; Singh, P.K.; Rai, M. Genetic Variability and Character Association Analysis in Cabbage Hybrids. Indian J. Hortic. 2013, 70, 296–299. [Google Scholar]
- Milatovic, D.D.; Nikolic, D.D. Variability, heritability and correlations of some factors affecting productivity in peach. Hortic. Sci. 2010, 37, 79–87. [Google Scholar] [CrossRef]
- Kutty, C.N.; Sreelatha, U.; Prameela, K.P. Variance components and correlation of economic traits in tropical cabbage (Brassica oleracea L. var. capitata L.). Indian J. Agric. Sci. 2017, 87, 240–244. [Google Scholar] [CrossRef]
- Ahmet, B.; Yanmaz, R.; Apaydin, A.; Kar, H. Morphological Characterization of White Head Cabbage (Brassica oleracea var. capitata subvar. alba) Genotypes in Turkey. N. Z. J. Crop Hortic. Sci. 2010, 33, 75–107. [Google Scholar]
- Katoch, V.; Shiwani, K.; Sharma, A.; Kumari, V. Correlation and Path Coefficient Analysis in Sprouting Broccoli (Brassica oleracea L. var. italica L.). Indian J. Agric. Sci. 2014, 84, 49–57. [Google Scholar] [CrossRef]
- Kibar, B.; Karaagac, O.; Kar, H. Heterosis for Yield-Contributing Head Traits in Cabbage (Brassica oleracea L. var. capitata L.). Sci. Investig. Agrar. 2015, 42, 205–216. [Google Scholar] [CrossRef]
- Biswal, P.P.; Verma, A.; Sharma, D. To Assess Association Correlation in Mid-Season Cauliflower (Brassica oleracea L. var. botrytis L.). Pharma Innov. J. 2022, 11, 1827–1829. [Google Scholar]
- Impa, H.R.; Kumar, R.; Dogra, R.K. Estimation of Mean Performance and Heterosis in Cytoplasmic Male Sterility (CMS)-Based Hybrids in Cabbage. Crop Breed. Appl. Biotechnol. 2023, 23, 43802313. [Google Scholar] [CrossRef]

| Sr. No. | Hybrids | Morphological Characters |
|---|---|---|
| 1. | UHF-CAB-HYB-1 | Spherical, Green |
| 2. | UHF-CAB-HYB-2 | Spherical, Green |
| 3. | UHF-CAB-HYB-3 | Spherical, Green |
| 4. | UHF-CAB-HYB-4 | Spherical, Light green |
| 5. | UHF-CAB-HYB-5 | Spherical, Light green |
| 6. | UHF-CAB-HYB-6 | Spherical, Green |
| 7. | UHF-CAB-HYB-7 | Spherical, Green |
| 8. | UHF-CAB-HYB-8 | Spherical, Green |
| 9. | Pusa Hybrid-81 (Check-1) | Spherical, Green |
| 10. | Pusa Cabbage-1 (Check-2) | Spherical, Green |
| Location | Latitude | Longitude | Altitude (MSL) | Av Temp (°C) | Av Hum (%) | Rainfall (mm) |
|---|---|---|---|---|---|---|
| 2021 | ||||||
| Location I | 30°5″ N | 77°80″ E | 1270 | 24.90–7.06 | 55.56 | 25.4 |
| Location II | 30°30′20″ N | 77°20′30″ E | 468 | 26.35–14.66 | 72.87 | 7.06 |
| Location III | 32.8° N | 74.7° E | 1090 | 23.91–3.70 | 62.71 | 25.85 |
| 2022 | ||||||
| Location I | 30°5″ N | 77°80″ E | 1270 | 23.02–7.57 | 58.63 | 2.96 |
| Location II | 30°30′20″ N | 77°20′30″ E | 468 | 24.50–13.80 | 48.65 | 55.35 |
| Location III | 32.8° N | 74.7° E | 1090 | 22.72–5.61 | 63.42 | 39 |
| Response Variable | SED | GCV | PCV | Heritability | Gen-Adv % Means |
|---|---|---|---|---|---|
| YIELD | 1.03 | 9.29 | 10.16 | 83.63 | 17.51 |
| DTFMM | 3.77 | 8.67 | 9.00 | 92.75 | 17.20 |
| NWL | 1.03 | 5.96 | 7.66 | 60.66 | 9.57 |
| PLS | 3.09 | 2.07 | 4.00 | 26.86 | 2.21 |
| GHW | 76.73 | 4.57 | 5.45 | 70.45 | 7.91 |
| NHW | 51.71 | 11.25 | 12.12 | 86.12 | 21.50 |
| HC | 4.42 | 16.12 | 17.66 | 83.32 | 30.31 |
| HI | 4.46 | 9.07 | 10.80 | 70.48 | 15.68 |
| PD | 1.03 | 3.32 | 5.95 | 31.21 | 3.83 |
| ED | 1.04 | 3.65 | 5.90 | 38.22 | 4.64 |
| SL | 0.34 | 3.50 | 6.18 | 31.98 | 4.07 |
| CL | 0.30 | 9.98 | 10.92 | 83.55 | 18.79 |
| TSS | 0.41 | 4.50 | 5.12 | 77.02 | 8.13 |
| AA | 0.63 | 12.98 | 13.08 | 98.41 | 26.53 |
| Yield | DTFMM | NWL | PLS | GHW | NHW | HC | HI | PD | ED | SL | CL | TSS | AA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield | 1.00 | |||||||||||||
| DTFMM | −0.04 | 1.00 | ||||||||||||
| NWL | 0.06 | −0.03 | 1.00 | |||||||||||
| PLS | 0.02 | −0.28 | 0.04 | 1.00 | ||||||||||
| GHW | −0.36 | 0.09 | 0.07 | 0.27 | 1.00 | |||||||||
| NHW | 0.57 ** | 0.22 | −0.24 | −0.15 | −0.11 | 1.00 | ||||||||
| HC | 0.48 ** | 0.41 | 0.11 | 0.15 | −0.14 | 0.59 ** | 1.00 | |||||||
| HI | 0.36 | 0.41 | −0.21 | −0.26 | −0.63 ** | 0.84 ** | 0.53 ** | 1.00 | ||||||
| PD | −0.34 | −0.23 | −0.11 | −0.21 | 0.35 | 0.11 | −0.31 | −0.11 | 1.00 | |||||
| ED | −0.58 ** | 0.13 | −0.34 | −0.22 | 0.07 | 0.19 | −0.23 | 0.11 | 0.47 ** | 1.00 | ||||
| SL | 0.09 | −0.12 | 0.15 | 0.28 | 0.07 | 0.16 | 0.40 | 0.10 | 0.02 | −0.15 | 1.00 | |||
| CL | −0.31 | 0.08 | −0.10 | −0.31 | −0.46 | 0.17 | −0.15 | 0.39 | −0.07 | 0.24 | 0.27 | 1.00 | ||
| TSS | −0.07 | 0.39 | 0.11 | −0.22 | 0.24 | 0.18 | 0.16 | 0.02 | 0.15 | 0.43 | 0.09 | −0.04 | 1.00 | |
| AA | 0.11 | −0.29 | 0.24 | 0.08 | 0.24 | 0.04 | −0.14 | −0.11 | 0.24 | −0.07 | 0.06 | −0.06 | −0.01 | 1.00 |
| Variable | Yield | DTFMM | NWL | PLS | GHW | NHW | HC | HI | PD | ED | SL | CL | TSS | AA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield | 1.00 | −0.34 | −0.70 ** | −0.57 ** | 0.72 ** | 0.82 ** | 0.56 ** | 0.62 ** | 0.03 | 0.17 | 0.37 ** | −0.18 | 0.54 ** | 0.27 |
| DTFMM | −0.30 | 1.00 | 0.76 ** | 1.19 | −0.08 | −0.48 ** | −0.49 ** | −0.54 ** | 0.58 ** | −0.08 | −0.38 | 0.15 | −0.05 | −0.60 ** |
| NWL | −0.49 ** | 0.57 ** | 1.00 | 0.85 ** | −0.27 | −0.35 | −0.63 ** | −0.26 | 0.44 | 0.48 ** | −0.58 ** | 0.14 | −0.25 | −0.34 |
| PLS | −0.27 | 0.53 ** | 0.36 | 1.00 | 0.06 | −0.71 ** | −0.52 ** | −0.89 ** | 0.88 ** | −0.40 | −0.96 ** | 0.22 | −0.19 | −0.67 ** |
| GHW | 0.47 ** | −0.05 | −0.15 | 0.15 | 1.00 | 0.56 ** | 0.20 | 0.19 | 0.46 | 0.12 | 0.41 | −0.26 | 0.20 | −0.05 |
| NHW | 0.73 ** | −0.37 | −0.31 | −0.38 | 0.41 | 1.00 | 0.59 ** | 0.92 ** | −0.30 | 0.17 | 0.26 | −0.08 | 0.70 ** | 0.61 ** |
| HC | 0.55 ** | −0.38 | −0.42 | −0.19 | 0.12 | 0.59 ** | 1.00 | 0.58 ** | −0.99 ** | −0.75 ** | −0.26 | −0.38 | 0.47 ** | 0.68 ** |
| HI | 0.56 ** | −0.37 | −0.25 | −0.50 ** | −0.05 | 0.89 ** | 0.56 ** | 1.00 | −0.55 ** | 0.20 | 0.14 | 0.04 | 0.76 ** | 0.76 ** |
| PD | −0.10 | 0.26 | 0.10 | 0.11 | 0.38 | −0.12 | −0.61 ** | −0.31 | 1.00 | 0.61 ** | 0.39 | 0.42 | −0.29 | −0.84 ** |
| ED | −0.09 | −0.02 | 0.06 | −0.27 | 0.09 | 0.15 | −0.50 ** | 0.15 | 0.52 ** | 1.00 | 0.95 ** | 0.35 | −0.17 | −0.27 |
| SL | 0.22 | −0.23 | −0.18 | −0.08 | 0.22 | 0.19 | 0.02 | 0.11 | 0.13 | 0.23 | 1.00 | 0.43 | 0.29 | −0.10 |
| CL | −0.20 | 0.14 | 0.07 | −0.01 | −0.30 | −0.04 | −0.34 | 0.12 | 0.19 | 0.28 | 0.31 | 1.00 | 0.42 | 0.02 |
| TSS | 0.42 | 0.01 | −0.14 | −0.18 | 0.21 | 0.61 ** | 0.41 | 0.56 ** | −0.08 | 07.0 | 0.18 | 0.33 | 1.00 | 0.65 ** |
| AA | 0.25 | −0.58 ** | −0.24 | −0.34 | −0.02 | 0.57 ** | 0.61 ** | 0.62 ** | −0.44 | −0.18 | −0.05 | 0.01 | 0.56 ** | 1.00 |
| Hybrids | E1 | E2 | E3 | E4 | E5 | E6 | E7 | E8 | E9 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SC1 | SC2 | SC1 | SC2 | SC1 | SC2 | SC1 | SC2 | SC1 | SC2 | SC1 | SC2 | SC1 | SC2 | SC1 | SC2 | SC1 | SC2 | |
| UHF-CAB-HYB-1 | 45.82 * | 22.84 * | 28.85 * | 30.61 * | 14.50 * | 25.41 * | 29.69 * | 23.64 * | 18.57 * | 21.47 * | 26.62 * | 12.30 * | 29.39 * | 52.20 * | 21.63 * | 26.00 * | 51.15 * | 33.62 * |
| UHF-CAB-HYB-2 | 27.57 * | 7.47 * | 4.69 | 6.12 | 1.75 | 11.44 * | 5.47 | 0.55 | −5.79 | −3.49 | 8.63 * | −3.66 | −2.48 | 14.71 * | −1.46 | 2.07 | 17.25 * | 3.65 |
| UHF-CAB-HYB-3 | 23.72 * | 4.23 | −3.54 | −2.22 | 0.47 | 10.04 * | −3.87 | −8.36 * | −5.14 | −2.82 | −1.86 | −12.96 * | −13.93 * | 1.25 | −15.13 * | −12.09 * | 0.88 | −10.82 * |
| UHF-CAB-HYB-4 | 26.82 * | 6.84 * | −0.21 | 1.15 | 5.69 | 15.76 * | −12.81 * | −16.88 * | −17.62 * | −15.61 * | −3.52 | −14.44 * | −17.64 * | −3.12 | −9.50 * | −6.25 | −0.21 | −11.78 * |
| UHF-CAB-HYB-5 | 12.24 * | −5.44 | 5.19 | 6.62 | −8.52 * | 0.19 | −1.97 | −6.55 | −8.94 * | −6.71 * | −3.36 | −14.29 * | −19.88 * | −5.75 | −20.34 * | −17.48 * | −4.51 | −15.58 * |
| UHF-CAB-HYB-6 | 21.43 * | 2.30 | 17.07 * | 18.67 * | −21.49 * | −14.01 * | 2.25 | −2.52 | −2.71 | −0.33 | 8.46 * | −3.80 | −4.13 | 12.77 * | −5.93 | −2.56 | 20.46 * | 6.49 |
| UHF-CAB-HYB-7 | 23.94 * | 4.41 | −2.65 | −1.32 | −5.05 | 3.99 | −9.76 * | −13.98 * | −20.81 * | −18.87 * | −2.06 | −13.13 * | −16.01 * | −1.20 | −12.82 * | −9.69 * | 4.72 | −7.43 |
| UHF-CAB-HYB-8 | −5.38 | −20.29 * | 11.10 * | 12.62 * | −19.18 * | −11.48 * | 9.10 * | 4.01 | −2.81 | −0.44 | 12.59 * | −0.14 | −8.46 * | 7.68 * | −11.62 * | −8.45 * | 9.54 | −3.17 |
| Sr. No. | Hybrids | E1 | E2 | E3 | E4 | E5 | E6 | E7 | E8 | E9 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SC1 | SC2 | SC1 | SC2 | SC1 | SC2 | SC1 | SC2 | SC1 | SC2 | SC1 | SC2 | SC1 | SC2 | SC1 | SC2 | SC1 | SC2 | ||
| 1 | UHF-CAB-HYB-1 | 40.25 * | 18.97 * | 15.17 * | 31.43 * | 27.00 * | 41.94 * | 29.58 * | 14.83 * | 16.64 * | 19.41 * | 10.43 * | 1.62 | 6.50 | 41.65 * | 9.94 * | 23.79 * | 25.64 * | 29.75 * |
| 2 | UHF-CAB-HYB-2 | 15.85 * | −1.73 | −2.77 | 10.96 | 5.41 | 17.82 * | 6.28 | −5.82 | −2.75 | −0.45 | 6.82 | −1.70 | −20.14 * | 6.21 | −13.64 * | −2.76 | 8.22 | 11.76 |
| 3 | UHF-CAB-HYB-3 | 26.26 * | 7.10 | −14.67 * | −2.62 | 4.89 | 17.23 * | −8.68 | −19.08 * | −14.77 * | −12.76 * | −17.30 * | −23.89 * | −20.20 * | 6.13 | −20.56 * | −10.55 * | −20.12 * | −17.51 * |
| 4 | UHF-CAB-HYB-4 | 24.48 * | 5.59 | −2.21 | 11.60 * | 18.94 * | 32.94 * | −5.70 | −16.43 * | −7.05 | −4.85 | −0.17 | −8.13 | −25.58 * | −1.01 | −14.34 * | −3.55 | −2.19 | 1.01 |
| 5 | UHF-CAB-HYB-5 | 10.08 | −6.63 | 9.60 | 25.08 * | −3.75 | 7.57 | −7.53 | −18.06 * | −19.20 * | −17.29 * | −16.11 * | −22.81 * | −13.11 * | 15.56 * | −33.91 * | −25.58 | −20.12 * | −17.51 * |
| 6 | UHF-CAB-HYB-6 | 1.32 | −14.05 * | 8.88 | 24.25 * | −6.48 | 4.52 | −5.34 | −16.12 * | −9.20 * | −7.05 | −8.21 | −15.53 * | −3.49 | 28.37 * | −17.66 * | −7.28 | −2.02 | 1.18 |
| 7 | UHF-CAB-HYB-7 | 10.23 | −6.50 | −16.41 * | −4.60 | 21.79 * | 36.12 * | −10.57 * | −20.75 * | −18.21 * | −16.27 * | −5.83 | −13.34 * | −15.69 * | 12.14 | −16.95 * | −6.49 | −7.08 | −4.05 |
| 8 | UHF-CAB-HYB-8 | −13.64 * | −26.74 * | 0.50 | 14.69 * | −20.54 * | −11.18 * | 16.19 * | 2.96 | −2.55 | −0.25 | 4.33 | −3.99 | 5.91 | 40.86 * | −20.40 * | −10.38 | −1.51 | 1.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parmar, S.S.; Kumar, R.; Vikram, A.; Dogra, R.K.; Gupta, M.; Singh, A.; Ghazaryan, K.; Singh, R.K.; Sousa, J.R. Correlation, Path-Coefficient, and Economic Heterosis Studies in CMS-Based Cabbage Hybrids over Different Environments. Horticulturae 2025, 11, 606. https://doi.org/10.3390/horticulturae11060606
Parmar SS, Kumar R, Vikram A, Dogra RK, Gupta M, Singh A, Ghazaryan K, Singh RK, Sousa JR. Correlation, Path-Coefficient, and Economic Heterosis Studies in CMS-Based Cabbage Hybrids over Different Environments. Horticulturae. 2025; 11(6):606. https://doi.org/10.3390/horticulturae11060606
Chicago/Turabian StyleParmar, Shipra Singh, Ramesh Kumar, Amit Vikram, Rajesh Kumar Dogra, Meenu Gupta, Abhishek Singh, Karen Ghazaryan, Rupesh Kumar Singh, and João Ricardo Sousa. 2025. "Correlation, Path-Coefficient, and Economic Heterosis Studies in CMS-Based Cabbage Hybrids over Different Environments" Horticulturae 11, no. 6: 606. https://doi.org/10.3390/horticulturae11060606
APA StyleParmar, S. S., Kumar, R., Vikram, A., Dogra, R. K., Gupta, M., Singh, A., Ghazaryan, K., Singh, R. K., & Sousa, J. R. (2025). Correlation, Path-Coefficient, and Economic Heterosis Studies in CMS-Based Cabbage Hybrids over Different Environments. Horticulturae, 11(6), 606. https://doi.org/10.3390/horticulturae11060606








