The Transcription Factor LoTDF1 Plays a Role in Early Anther Development in Lily (Lilium Oriental Hybrids)
Abstract
1. Introduction
2. Results
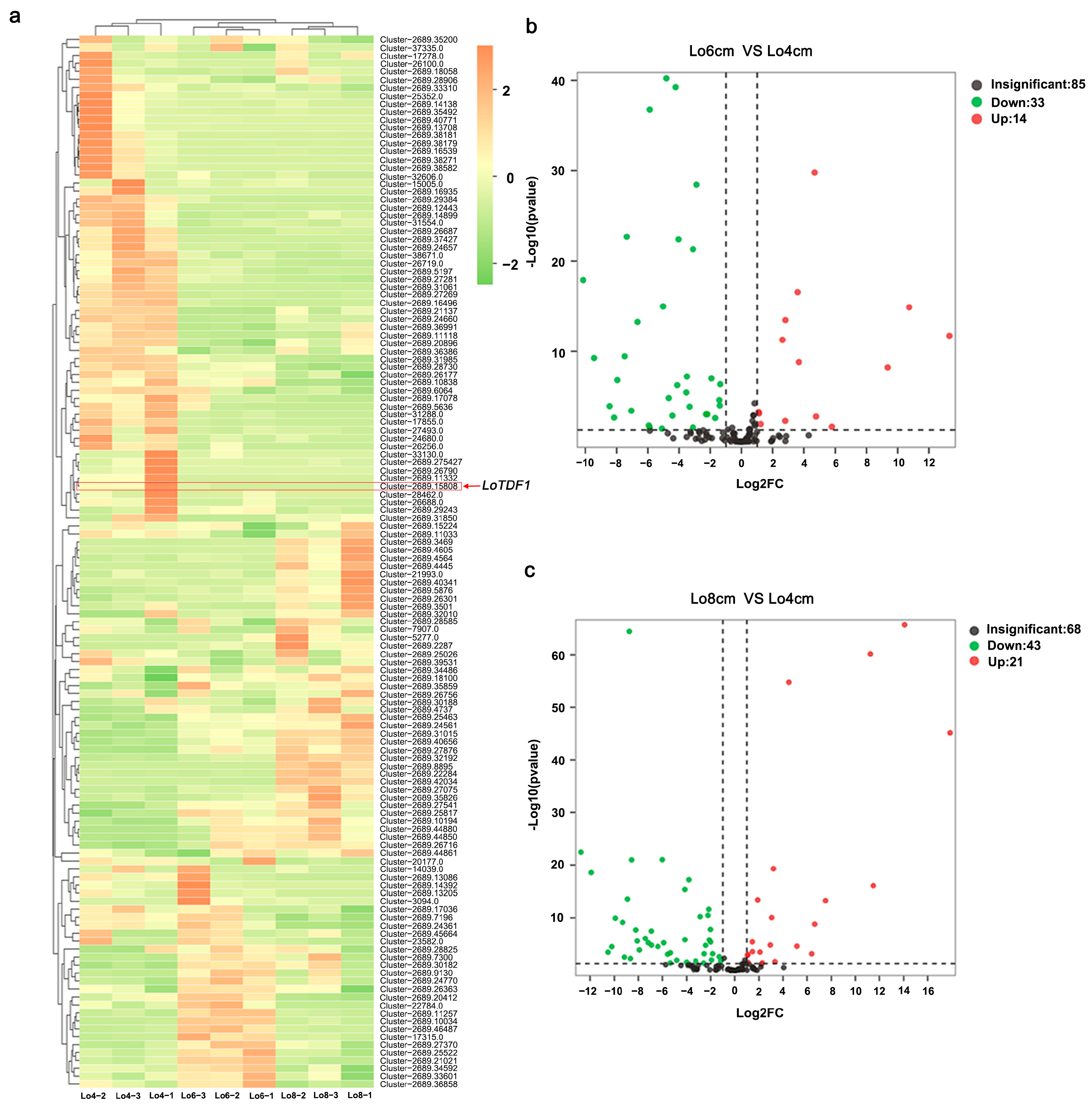
2.1. Screening the LoTDF1 Gene Based on Transcriptome Data
2.2. Molecular Characterization and Phylogenetic Analysis of LoTDF1
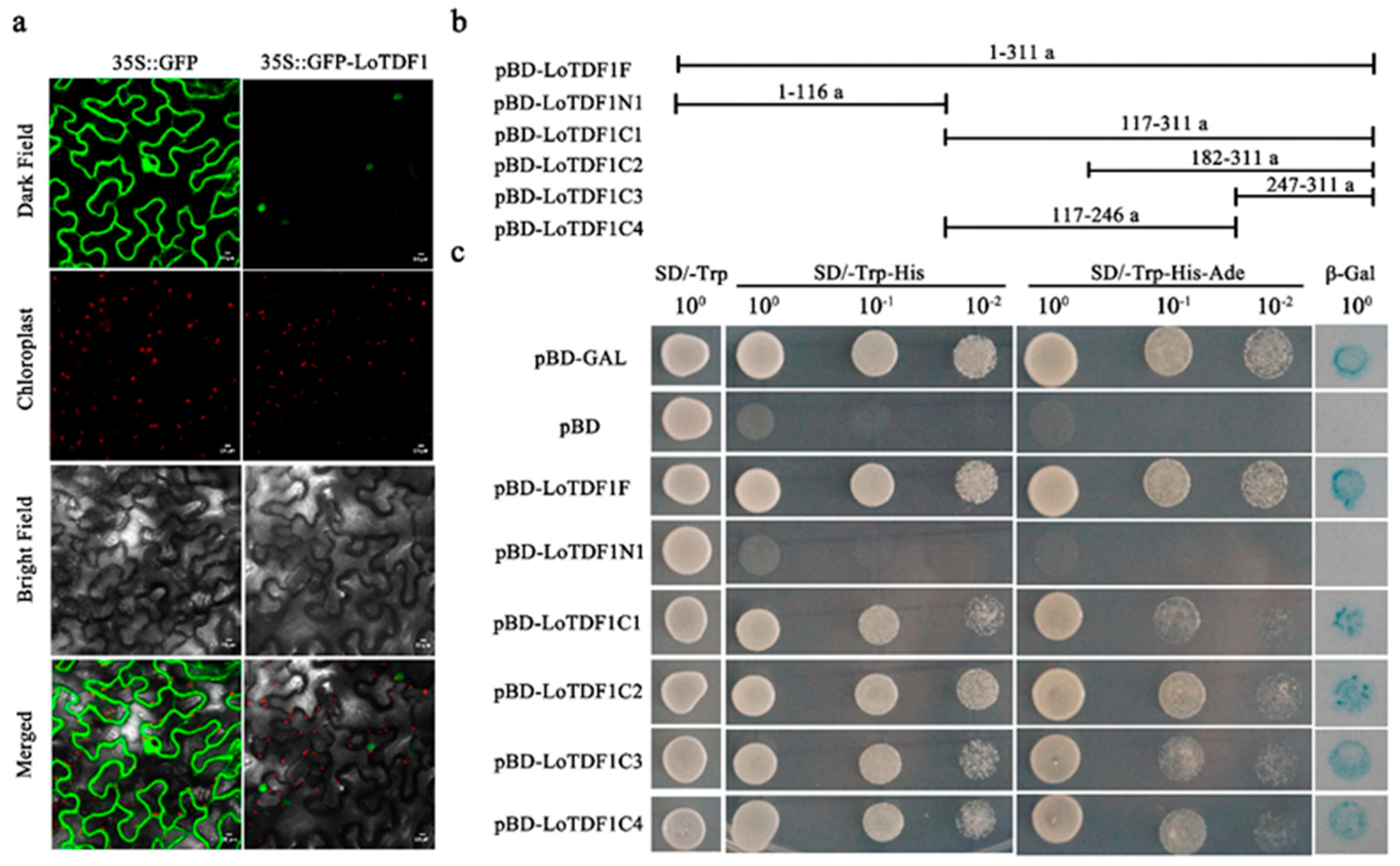
2.3. Subcellular Localization of LoTDF1
2.4. Transcriptional Activity Analysis of LoTDF1
2.5. Histological Observation of Lily Anthers and Expression Analysis of LoTDF1
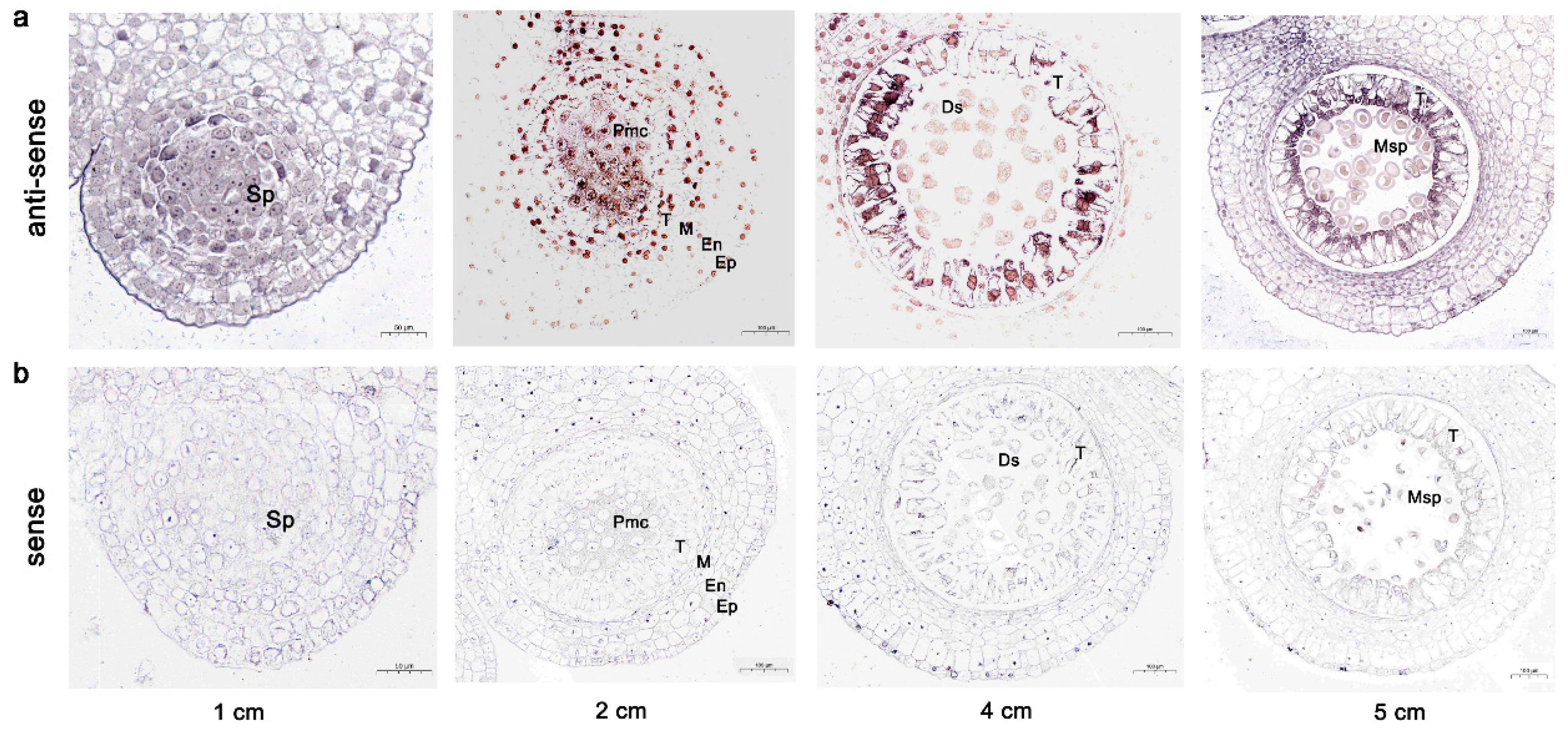
2.6. In Situ Hybridization of LoTDF1
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Transcriptome Data Analysis
4.3. Cloning and Sequence Analysis of LoTDF1
4.4. Subcellular Localization
4.5. Transcription Activation Activity Analysis
4.6. Expression Analysis of LoTDF1
4.7. Paraffin Sectioning and Histological Observation
4.8. In Situ Hybridization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldberg, R.B.; Beals, T.P.; Sanders, P.M. Anther development: Basic principles and practical applications. Plant Cell 1993, 5, 1217–1229. [Google Scholar] [PubMed]
- Sanders, P.M.; Bui, A.Q.; Weterings, K.; Mcintire, K.N.; Goldberg, R.B. Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex. Plant Reprod. 1999, 11, 297–322. [Google Scholar] [CrossRef]
- Owen, H.A.; Makaroff, C.A. Ultrastructure of microsporogenesis and microgametogenesis in Arabidopsis thaliana (L.) Heynh. ecotype Wassilewskija (Brassicaceae). Protoplasma 1995, 185, 7–21. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Singh, S.; Nandi, A. Male sterility systems in the genomics era for expediting vegetable breeding. Sci. Hortic. 2024, 338, 113774. [Google Scholar] [CrossRef]
- Siopa, C.; Castro, H.; Loureiro, J.; Castro, S. PolLimCrop, a global dataset of pollen limitation in crops. Sci. Data 2023, 10, 905. [Google Scholar] [CrossRef]
- An, X.; Ma, B.; Duan, M.; Dong, Z.; Liu, R.; Yuan, D.; Hou, Q.; Wu, S.; Zhang, D.; Liu, D.; et al. Molecular regulation of ZmMs7 required for maize male fertility and development of a dominant male-sterility system in multiple species. Proc. Natl. Acad. Sci. USA 2020, 117, 23499–23509. [Google Scholar] [CrossRef]
- Tong, Z.; Li, Q.; Yang, Y.; Dai, F.; Gao, J.; Hong, B. Isolation and expression analysis of LoPIP2, a lily (Lilium Oriental Hybrids) aquaporin gene involved in desiccation-induced anther dehiscence. Sci. Hortic. 2013, 164, 316–322. [Google Scholar] [CrossRef]
- Sui, J.; He, J.; Wu, J.; Gong, B.; Cao, X.; Seng, S.; Wu, Z.; Wu, C.; Liu, C.; Yi, M. Characterization and Functional Analysis of Transcription Factor LoMYB80 Related to Anther Development in Lily (Lilium Oriental Hybrids). J. Plant Growth Regul. 2015, 34, 545–557. [Google Scholar] [CrossRef]
- Feng, J.; Wu, Z.; Wang, X.; Zhang, Y.; Teng, N. Analysis of Pollen Allergens in Lily by Transcriptome and Proteome Data. Int. J. Mol. Sci. 2019, 20, 5892. [Google Scholar] [CrossRef]
- Sui, J.; Cao, X.; Yi, M.; Wu, J.; He, J. Isolation and characterization of LoAMS gene in anther development of lily (Lilium oriental hybrids). N. Zealand J. Crop Hortic. Sci. 2020, 48, 257–269. [Google Scholar] [CrossRef]
- Zhang, S.; Fang, Z.; Zhu, J.; Gao, J.; Yang, Z. OsMYB103 is required for rice anther development by regulating tapetum development and exine formation. Plant Dev. Biol. 2010, 55, 3288–3297. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, H.; Gao, J.; Jiang, H.; Wang, C.; Guan, Y.; Yang, Z. Defective in Tapetal development and function 1 is essential for anther development and tapetal function for microspore maturation in Arabidopsis. Plant J. 2008, 55, 266–277. [Google Scholar] [CrossRef]
- Pei, Y.; Xue, Q.; Zhang, Z.; Shu, P.; Deng, H.; Bouzayen, M.; Hong, Y.; Liu, M. β-1,3-GLUCANASE10 regulates tomato development and disease resistance by modulating callose deposition. Plant Physiol. 2023, 192, 2785–2802. [Google Scholar] [CrossRef]
- Lu, Z.; Zhu, L.; Liang, G.; Li, X.; Li, Q.; Li, Y.; He, S.; Wu, J.; Liu, X.; Zhang, J. MORE FLORET1 controls anther development by negatively regulating key tapetal genes in both diploid and tetraploid rice. Plant Physiol. 2024, 195, 1981–1994. [Google Scholar] [CrossRef] [PubMed]
- Verma, N. Transcriptional regulation of anther development in Arabidopsis. Gene 2019, 689, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Tang, P.; Wang, R.; Ren, Y.; Wu, B.; Yan, N.; Zhang, G.; Niu, N.; Song, Y. The characteristic analysis of TaTDF1 reveals its function related to male sterility in wheat (Triticum aestivum L.). BMC Plant Biol. 2024, 24, 746. [Google Scholar] [CrossRef]
- Ferguson, A.C.; Pearce, S.; Band, L.R.; Yang, C.; Ferjentsikova, I.; King, J.; Yuan, Z.; Zhang, D.; Wilson, Z.A. Biphasic regulation of the transcription factor ABORTED MICROSPORES (AMS) is essential for tapetum and pollen development in Arabidopsis. New Phytol. 2017, 213, 778–790. [Google Scholar] [CrossRef]
- Zhu, J.; Lou, Y.; Xu, X.; Yang, Z.N. A Genetic Pathway for Tapetum Development and Function in Arabidopsis. J. Integr. Plant Biol. 2011, 53, 892–900. [Google Scholar] [CrossRef]
- Li, D.D.; Xue, J.S.; Zhu, J.; Yang, Z.N. Gene Regulatory Network for Tapetum Development in Arabidopsis thaliana. Front. Plant Sci. 2017, 8, 1559. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, Y.; Timofejeva, L.; Chen, C.; Grossniklaus, U.; Ma, H. Regulation of Arabidopsis tapetum development and function by DYSFUNCTIONAL TAPETUM1 (DYT1) encoding a putative bHLH transcription factor. Development 2006, 133, 3085–3095. [Google Scholar] [CrossRef]
- Gu, J.N.; Zhu, J.; Yu, Y.; Teng, X.D.; Lou, Y.; Xu, X.F.; Liu, J.L.; Yang, Z.N. DYT1 directly regulates the expression of TDF1 for tapetum development and pollen wall formation in Arabidopsis. Plant J. 2014, 80, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.F.; Zhu, J.; Lou, Y.; Guo, Z.L.; Xiong, S.X.; Wang, K.; Yang, Z.N. The functional analysis of OsTDF1 reveals a conserved genetic pathway for tapetal development between rice and Arabidopsis. Sci. Bull. 2015, 60, 1073–1082. [Google Scholar] [CrossRef]
- Lou, Y.; Zhou, H.S.; Han, Y.; Zeng, Q.Y.; Zhu, J.; Yang, Z.N. Positive regulation of AMS by TDF1 and the formation of a TDF1–AMS complex are required for anther development in Arabidopsis thaliana. New Phytol. 2018, 271, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, A.M.; Krber, S.; Unte, U.S.; Huijser, P.; Dekker, K.; Saedler, H. The Arabidopsis aborted microspores (ams) gene encodes a MYC class transcription factor. Plant J. 2003, 33, 413–423. [Google Scholar] [CrossRef]
- Xu, J.; Yang, C.; Yuan, Z.; Zhang, D.; Gondwe, M.Y.; Ding, Z.; Liang, W.; Zhang, D.; Wilson, Z.A. The ABORTED MICROSPORES Regulatory Network Is Required for Postmeiotic Male Reproductive Development in Arabidopsis thaliana. Plant Cell 2010, 22, 91–107. [Google Scholar] [CrossRef]
- Xu, J.; Ding, Z.; Vizcay-Barrena, G.; Shi, J.; Liang, W.; Yuan, Z.; Werck-Reichhart, D.; Schreiber, L.; Wilson, Z.A.; Zhang, D. ABORTED MICROSPORES Acts as a Master Regulator of Pollen Wall Formation in Arabidopsis. Plant Cell 2014, 26, 1544–1556. [Google Scholar] [CrossRef]
- Phan, H.A.; Iacuone, S.; Li, S.F.; Parish, R.W. The MYB80 transcription factor is required for pollen development and the regulation of tapetal programmed cell death in Arabidopsis thaliana. Plant Cell 2011, 23, 2209–2224. [Google Scholar] [CrossRef]
- Xiong, S.X.; Lu, J.Y.; Lou, Y.; Teng, X.D.; Gu, J.N.; Zhang, C.; Shi, Q.S.; Yang, Z.N.; Zhu, J. The transcription factors MS188 and AMS form a complex to activate the expression of CYP703A2 for sporopollenin biosynthesis in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2016, 88, 936–946. [Google Scholar] [CrossRef]
- Takuya, I.; Noriko, N.; Yoshu, Y.; Masaru, O.T.; Hong, M.; Kazuo, S. Arabidopsis MALE STERILITY1 encodes a PHD-type transcription factor and regulates pollen and tapetum development. Plant Cell 2007, 19, 3549–3562. [Google Scholar]
- Lu, J.Y.; Xiong, S.X.; Yin, W.; Teng, X.D.; Lou, Y.; Zhu, Y.; Zhang, C.; Gu, J.N.; Wilson, Z.A.; Yang, Z.N. MS1, a direct target of MS188, regulates the expression of key sporophytic pollen coat protein genes in Arabidopsis. J. Exp. Bot. 2020, 71, 4877–4889. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Z.; Feng, J.; Yuan, G.; He, L.; Zhang, D.; Teng, N. A Novel R2R3-MYB Gene LoMYB33 from Lily Is Specifically Expressed in Anthers and Plays a Role in Pollen Development. Front. Plant Sci. 2021, 12, 730007. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Jia, W.; Xin, Y.; Zhang, Y. Transcriptomics-Based Identification of Genes Related to Tapetum Degradation and Microspore Development in Lily. Genes 2022, 13, 366. [Google Scholar] [CrossRef]
- Ko, S.S.; Li, M.J.; Ho, Y.C.; Yu, C.P.; Yang, T.T.; Lin, Y.J.; Hsing, H.C.; Chen, T.K.; Jhong, C.M.; Li, W.H.; et al. Rice transcription factor GAMYB modulates bHLH142 and is homeostatically regulated by TDR during anther tapetal and pollen development. J. Exp. Bot. 2021, 72, 4888–4903. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.; Yoon, H.; Suh, H.S.; Chung, Y.Y. Male-sterility of thermosensitive genic male-sterile rice is associated with premature programmed cell death of the tapetum. Planta 2003, 217, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Yin, W.; Gómez, J.F.; Tidy, A.; Xing, G.; Zong, J.; Shi, S.; Wilson, Z.A. Barley TAPETAL DEVELOPMENT and FUNCTION1 (HvTDF1) gene reveals conserved and unique roles in controlling anther tapetum development in dicot and monocot plants. New Phytol. 2023, 240, 173–190. [Google Scholar] [CrossRef]
- Moriyama, T.; Shea, D.J.; Yokoi, N.; Imakiire, S.; Saito, T.; Ohshima, H.; Saito, H.; Okamoto, S.; Fukai, E.; Okazaki, K. Identification of a Male Sterile Candidate Gene in Lilium x formolongi and Transfer of the Gene to Easter Lily (L. longiflorum) via Hybridization. Front. Plant Sci. 2022, 13, 914671. [Google Scholar] [CrossRef]
- Valentina, C.; Daniela, C.; Nadia, N.; Roberta, G.; Patrizia, B.; Paolo, C.; Maura, C. An auxin maximum in the middle layer controls stamen development and pollen maturation in Arabidopsis. New Phytol. 2017, 213, 1194–1207. [Google Scholar]
- Wu, Z.; Liang, J.; Wang, C.; Ding, L.; Zhao, X.; Cao, X.; Xu, S.; Teng, N.; Yi, M. Alternative Splicing Provides a Mechanism to Regulate LlHSFA3 Function in Response to Heat Stress in Lily. Plant Physiol. 2019, 181, 1651–1667. [Google Scholar] [CrossRef]
- Ding, L.; Wu, Z.; Teng, R.; Xu, S.; Cao, X.; Yuan, G.; Zhang, D.; Teng, N. LlWRKY39 is involved in thermotolerance by activating LlMBF1c and interacting with LlCaM3 in lily (Lilium longiflorum). Hortic. Res. 2021, 8, 36. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Wang, H.; Feng, X.; Zhang, Y.; Wei, D.; Zhang, Y.; Jin, Q.; Cai, Y. PbUGT72AJ2-Mediated Glycosylation Plays an Important Role in Lignin Formation and Stone Cell Development in Pears (Pyrus bretschneideri). Int. J. Mol. Sci. 2022, 23, 7893. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wu, Z.; Zheng, J.; Koskela, E.A.; Fan, L.; Fan, G.; Gao, D.; Dong, Z.; Hou, S.; Feng, Z.; et al. The GATA factor HANABA TARANU promotes runner formation by regulating axillary bud initiation and outgrowth in cultivated strawberry. Plant J. 2022, 110, 1237–1254. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, J.; Tang, Y.; Cao, X.; Yang, J. The Transcription Factor LoTDF1 Plays a Role in Early Anther Development in Lily (Lilium Oriental Hybrids). Horticulturae 2025, 11, 398. https://doi.org/10.3390/horticulturae11040398
Sui J, Tang Y, Cao X, Yang J. The Transcription Factor LoTDF1 Plays a Role in Early Anther Development in Lily (Lilium Oriental Hybrids). Horticulturae. 2025; 11(4):398. https://doi.org/10.3390/horticulturae11040398
Chicago/Turabian StyleSui, Juanjuan, Yan Tang, Xing Cao, and Jingxia Yang. 2025. "The Transcription Factor LoTDF1 Plays a Role in Early Anther Development in Lily (Lilium Oriental Hybrids)" Horticulturae 11, no. 4: 398. https://doi.org/10.3390/horticulturae11040398
APA StyleSui, J., Tang, Y., Cao, X., & Yang, J. (2025). The Transcription Factor LoTDF1 Plays a Role in Early Anther Development in Lily (Lilium Oriental Hybrids). Horticulturae, 11(4), 398. https://doi.org/10.3390/horticulturae11040398




