Growth and Photosynthetic Responses of Lactuca sativa L. to Different Zinc Fertilizer Sources and Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Pot Experiment
2.2. Plant and Soil Analysis
2.3. Statistical Analysis
3. Results
3.1. Effect of Zn Treatments on Soil
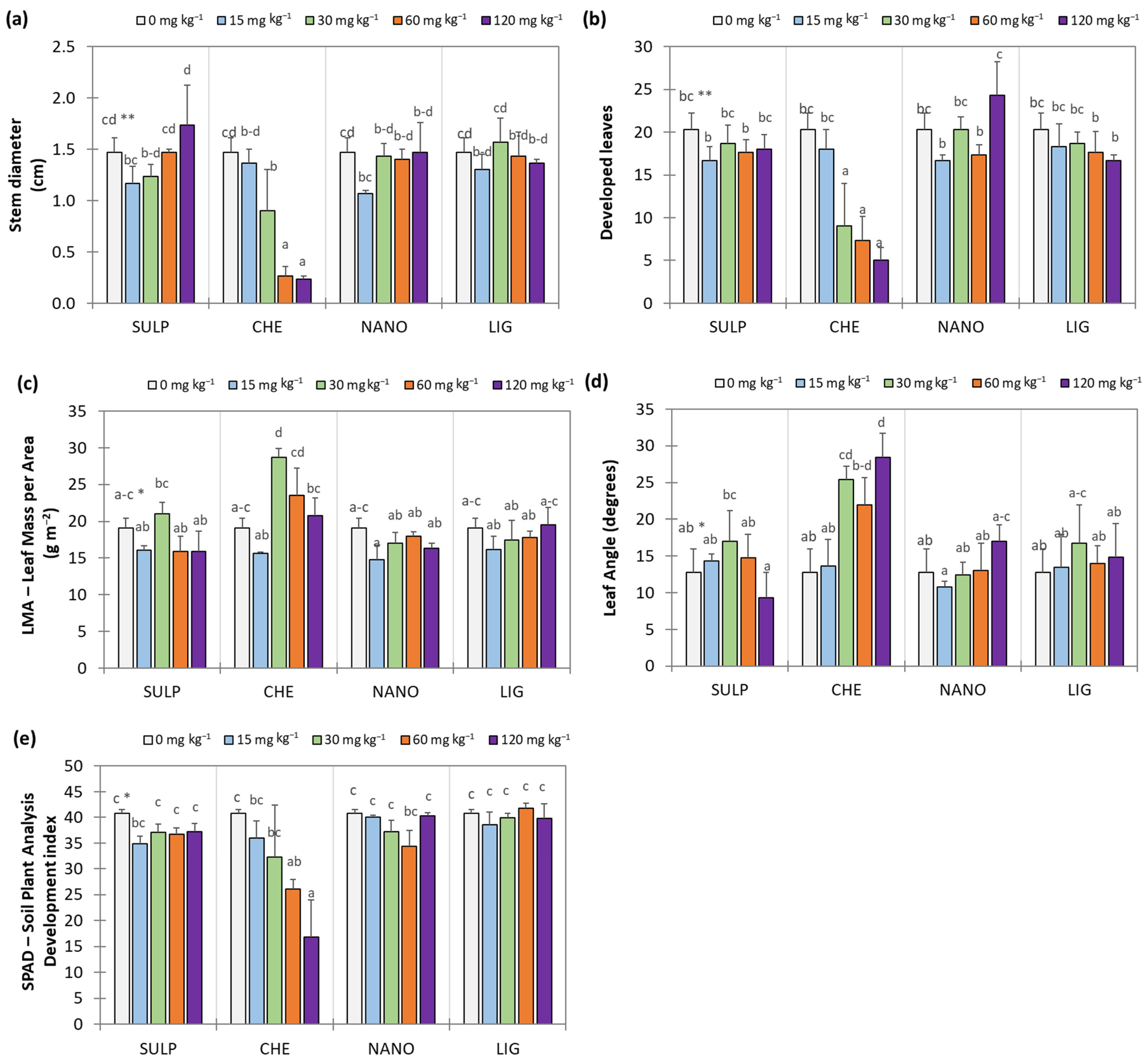
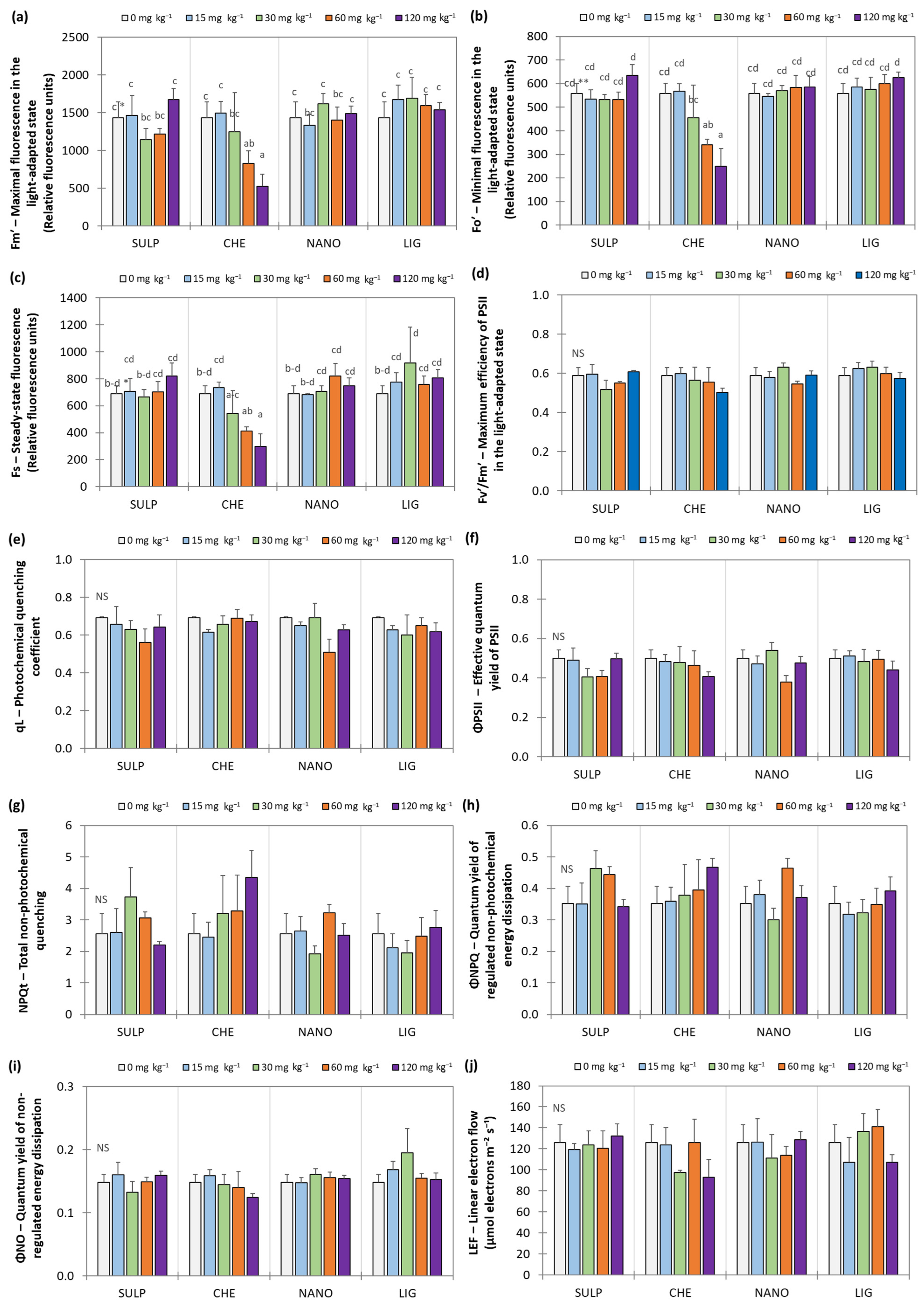
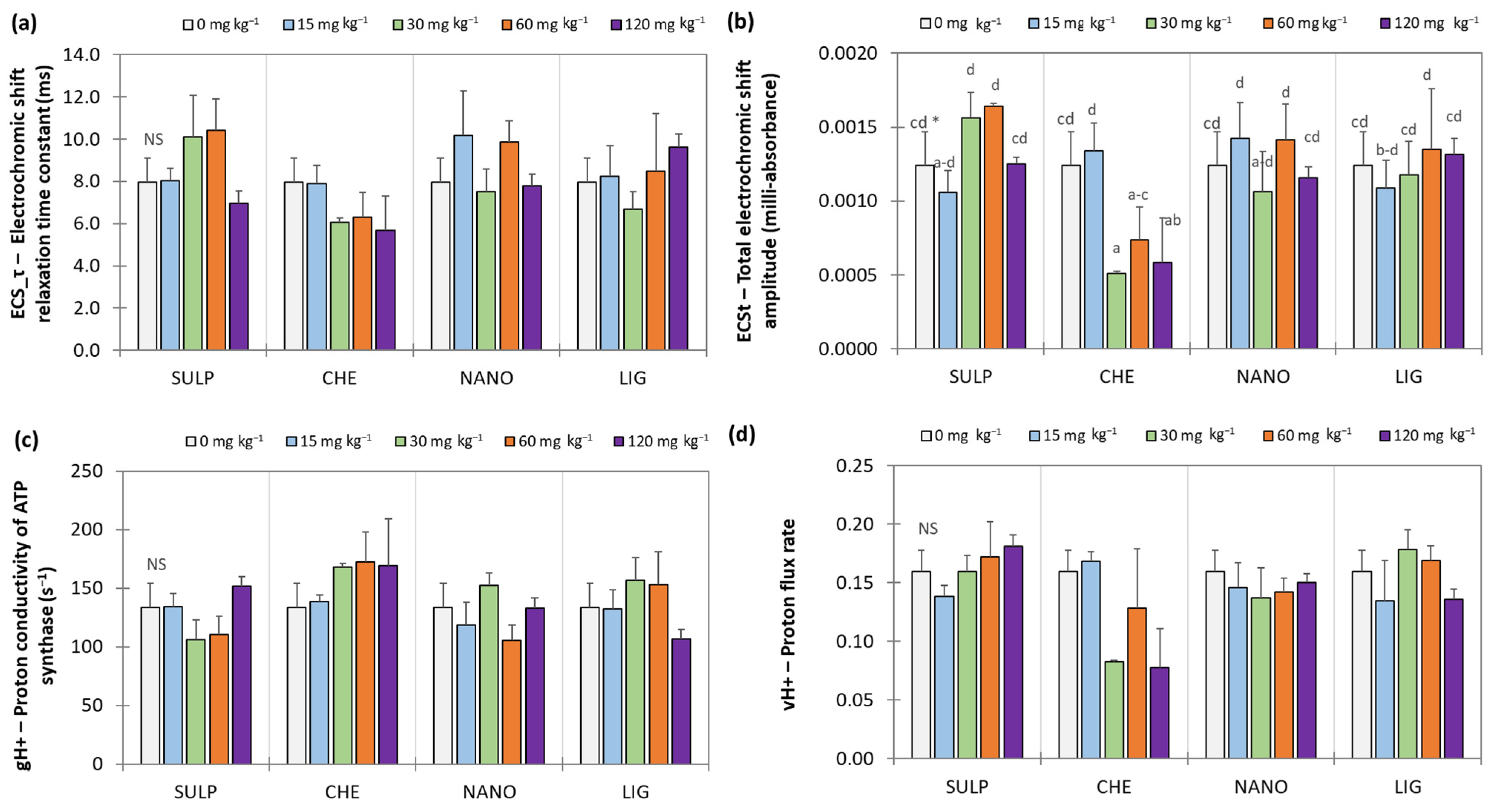
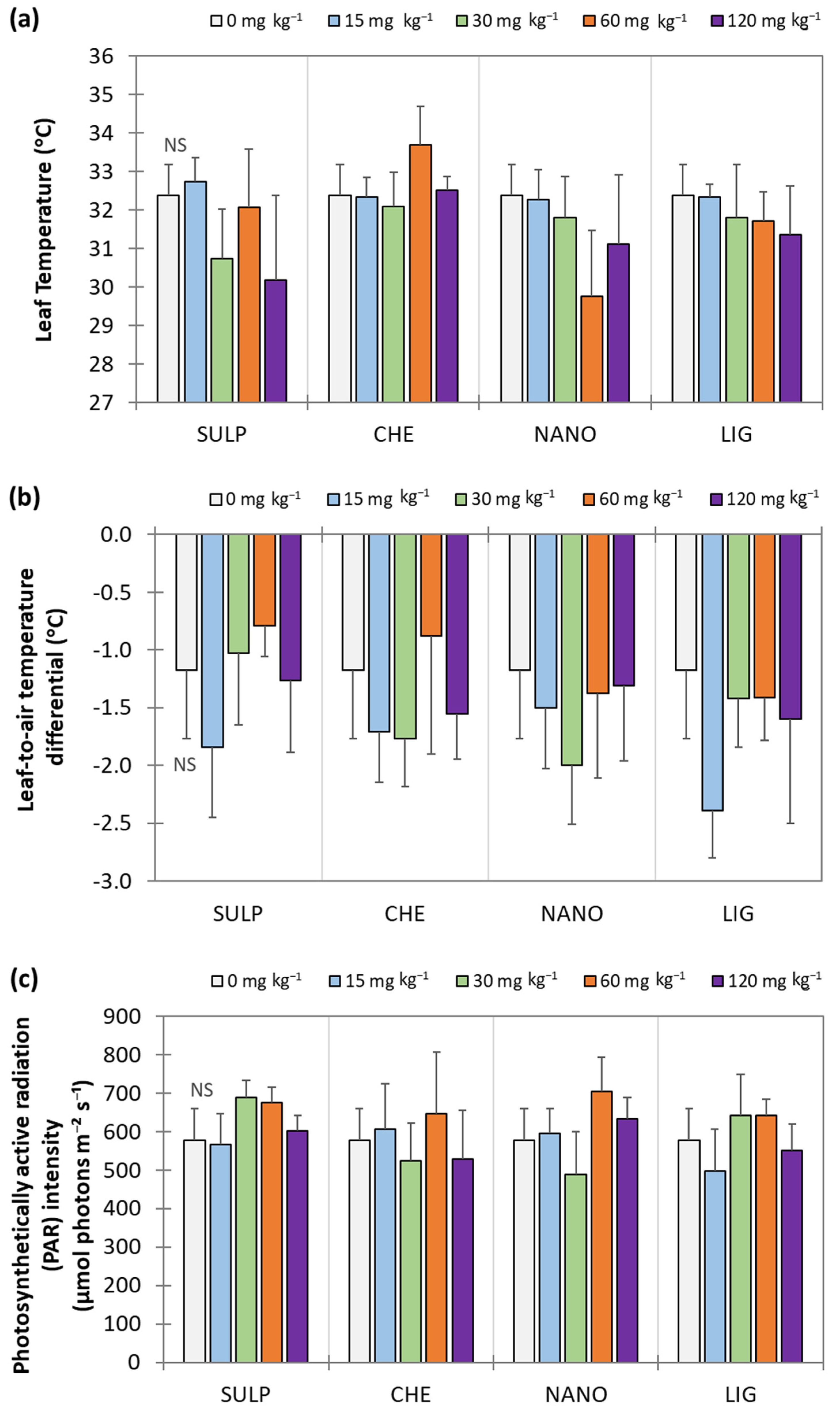
3.2. Effect of Zn Treatments on Lettuce
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Zn | Zinc |
| SULP | zinc sulfate heptahydrate |
| CHE | Zn chelated with DTPA, EDTA and HEDTA |
| DTPA | diethylenetriaminepentaacetic acid |
| EDTA | ethylenediaminetetraacetic acid |
| HEDTA | N-(2-Hydroxyethyl)ethylenediamine-N,N,N″-triacetic acid |
| LIG | Zn–lignosulphonate complex |
| NANO | ZnO nanoparticles |
| TEM | Transmission electron microscopy |
| DLS | Dynamic light scattering |
| LMA | leaf mass per area |
| PSI | Photosystem I |
| PSII | Photosystem II |
| Fm′ | maximum fluorescence in the light-adapted state |
| Fo′ | minimal fluorescence in the light-adapted state |
| Fs | steady-state fluorescence |
| Fv′/Fm′ | variable fluorescence relative to Fm′ |
| qL | photochemical quenching |
| ΦPSII | effective quantum yield of PSII |
| NPQt | total non-photochemical quenching |
| ΦNPQ | fraction of energy dissipated by regulated non-photochemical quenching (NPQ) |
| ΦNO | fraction of energy dissipated non-regulated |
| LEF | linear electron flow |
| kP700 | kinetic constant of P700 |
| tP700 | P700 relaxation time |
| vinitialP700 | initial rate of P700 oxidation |
| ECS | Proton gradient and electrochromic shift |
| ECS_τ | ECS relaxation time constant |
| ECSt, mAU | total ECS amplitude |
| gH+ | proton conductance through ATP synthase |
| vH+ | proton flux rate |
| PAR | photosynthetically active radiation |
| LMWOAs | low-molecular-weight organic acids |
| FAAS | flame atomic absorption spectrophotometry |
References
- Ramos, C.; Pomares, F. Abonado de Los Cultivos Hortícolas. In Guía Práctica de la Fertilización Racional de los Cultivos en España. Parte II; Ministerio de Medio Ambiente y Medio Rural y Marino, Ed.; Secretaría General Técnica: Madrid, Spain, 2010; pp. 181–192. ISBN 978-84-491-0997-3. [Google Scholar]
- Marschner, H. Marschner’s Mineral Nutrition of Plants, 4th ed.; Rengel, Z., Cakmak, I., White, P., Eds.; Elsevier: London, UK, 2023; ISBN 978-0-12-819773-8. [Google Scholar]
- Fu, C.; Li, M.; Zhang, Y.; Zhang, Y.; Yan, Y.; Wang, Y. Morphology, Photosynthesis, and Internal Structure Alterations in Field Apple Leaves under Hidden and Acute Zinc Deficiency. Sci. Hortic. 2015, 193, 47–54. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Physiological Limits to Zinc Biofortification of Edible Crops. Front. Plant Sci. 2011, 2, 80. [Google Scholar] [CrossRef] [PubMed]
- Chaney, R.L. Zinc Phytotoxicity. In Zinc in Soils and Plants. Developments in Plant and Soil Sciences; Robson, A., Ed.; Springer: Dordrecht, The Netherlands, 1993; Volume 55, pp. 135–150. ISBN 978-94-011-0878-2. [Google Scholar]
- Balafrej, H.; Bogusz, D.; Triqui, Z.-E.A.; Guedira, A.; Bendaou, N.; Smouni, A.; Fahr, M. Zinc Hyperaccumulation in Plants: A Review. Plants 2020, 9, 562. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Garg, N. Zinc Toxicity in Plants: A Review. Planta 2021, 253, 129. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, H.; Carmona, V.M.V.; Inocêncio, M.F.; Furtini Neto, A.E.; Cecílio Filho, A.B.; Mauad, M. Soil Type and Zinc Doses in Agronomic Biofortification of Lettuce Genotypes. Agronomy 2020, 10, 124. [Google Scholar] [CrossRef]
- Almendros, P.; Gonzalez, D.; Fernandez, M.D.; Garcia-Gomez, C.; Obrador, A. Both Zn Biofortification and Nutrient Distribution Pattern in Cherry Tomato Plants Are Influenced by the Application of ZnO Nanofertilizer. Heliyon 2022, 8, e09130. [Google Scholar] [CrossRef]
- Ponce-García, O.C.; Noperi-Mosqueda, L.C.; Soto-Parra, J.M.; Yáñez-Muñoz, R.M.; Pérez-Leal, R.; Navarro-León, E.; Sánchez, E. Assaying the Efficiency of Sulfate, Chelate and Zinc Nanoparticle Fertilizers in Green Bean Grown in Alkaline Soil. J. Plant Nutr. 2023, 46, 653–664. [Google Scholar] [CrossRef]
- Ortiz, R.; Gascó, G.; Méndez, A.; Sanchez-Martín, L.; Obrador, A.; Almendros, P. Comparative Study of Traditional and Environmentally Friendly Zinc Sources Applied in Alkaline Fluvisol Soil: Lettuce Biofortification and Soil Zinc Status. Agronomy 2023, 13, 3014. [Google Scholar] [CrossRef]
- Ortiz, R.; Gascó, G.; Méndez, A.; Obrador, A.; González, D.; Almendros, P. Zinc Biofortification of Lettuce Using Environmentally Friendly Zinc Sources in an Acidic Soil. Sci. Hortic. 2024, 338, 113620. [Google Scholar] [CrossRef]
- Garza-Alonso, C.A.; Juárez-Maldonado, A.; González-Morales, S.; Cabrera-De la Fuente, M.; Cadenas-Pliego, G.; Morales-Díaz, A.B.; Trejo-Téllez, L.I.; Tortella, G.; Benavides-Mendoza, A. ZnO Nanoparticles as Potential Fertilizer and Biostimulant for Lettuce. Heliyon 2023, 9, e12787. [Google Scholar] [CrossRef]
- Alloway, B. Bioavailability of Elements in Soil. In Essentials of Medical Geology; Selinus, O., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2013; pp. 351–373. ISBN 9789400743755. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2011; ISBN 9780429192036. [Google Scholar]
- De-Francisco, M.; Romeiro, A.; Durães, L.; Álvarez-Torrellas, S.; Ibañez, M.; Almendros, P. Environmental Behaviour of Synthesized and Commercial Agricultural Zinc Products: Leaching, Migration, and Availability in Soils. J. Soil Sci. Plant Nutr. 2024, 24, 5293–5308. [Google Scholar] [CrossRef]
- Sillanpää, M. Environmental Fate of EDTA and DTPA. Rev. Environ. Contam. Toxicol. 1997, 152, 85–111. [Google Scholar] [CrossRef] [PubMed]
- Wurzer, G.K.; Hettegger, H.; Bischof, R.H.; Fackler, K.; Potthast, A.; Rosenau, T. Agricultural Utilization of Lignosulfonates. Holzforschung 2022, 76, 155–168. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Fedorenko, A.; Chernikova, N.; Hassan, T.; Mandzhieva, S.; Sushkova, S.; Lysenko, V.; Soldatov, M.A.; Burachevskaya, M. Effects of Zinc Oxide Nanoparticles on Physiological and Anatomical Indices in Spring Barley Tissues. Nanomaterials 2021, 11, 1722. [Google Scholar] [CrossRef] [PubMed]
- Azarin, K.; Usatov, A.; Minkina, T.; Duplii, N.; Kasyanova, A.; Fedorenko, A.; Khachumov, V.; Mandzhieva, S.; Rajput, V.D. Effects of Bulk and Nano-ZnO Particles on Functioning of Photosynthetic Apparatus in Barley (Hordeum vulgare L.). Environ. Res. 2023, 216, 114748. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Chen, S.; Li, Q.; Wang, W.; Hou, C.; Gao, X.; Wang, L.; Wang, S. Zinc Oxide Nanoparticles Affect Biomass Accumulation and Photosynthesis in Arabidopsis. Front. Plant Sci. 2015, 6, 1243. [Google Scholar] [CrossRef]
- Pedruzzi, D.P.; Araujo, L.O.; Falco, W.F.; Machado, G.; Casagrande, G.A.; Colbeck, I.; Lawson, T.; Oliveira, S.L.; Caires, A.R.L. ZnO Nanoparticles Impact on the Photosynthetic Activity of Vicia Faba: Effect of Particle Size and Concentration. NanoImpact 2020, 19, 100246. [Google Scholar] [CrossRef]
- Mazaheri-Tirani, M.; Dayani, S. Growth, Flowering and Physiological Response of Trachyspermum ammi L. to Zinc Oxide Micro- and Nanoparticles. Russ. J. Plant Physiol. 2022, 69, 14. [Google Scholar] [CrossRef]
- Montoya, M.; Vallejo, A.; Recio, J.; Guardia, G.; Alvarez, J.M. Zinc–Nitrogen Interaction Effect on Wheat Biofortification and Nutrient Use Efficiency. J. Plant Nutr. Soil Sci. 2020, 183, 169–179. [Google Scholar] [CrossRef]
- Doolette, C.L.; Read, T.L.; Li, C.; Scheckel, K.G.; Donner, E.; Kopittke, P.M.; Schjoerring, J.K.; Lombi, E. Foliar Application of Zinc Sulphate and Zinc EDTA to Wheat Leaves: Differences in Mobility, Distribution, and Speciation. J. Exp. Bot. 2018, 69, 4469–4481. [Google Scholar] [CrossRef]
- Lucini, L.; Bernardo, L. Comparison of Proteome Response to Saline and Zinc Stress in Lettuce. Front. Plant Sci. 2015, 6, 240. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Bernardo, L.; Kane, D.; Trevisan, M.; Lucini, L. Zinc Excess Triggered Polyamines Accumulation in Lettuce Root Metabolome, as Compared to Osmotic Stress under High Salinity. Front. Plant Sci. 2016, 7, 842. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.R.; Harbinson, J.; Kramer, D.M. Determining the Limitations and Regulation of Photosynthetic Energy Transduction in Leaves. Plant. Cell Environ. 2007, 30, 1107–1125. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, E.T.F.; Lamont, B.B. Leaf Specific Mass Confounds Leaf Density and Thickness. Oecologia 1991, 88, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Falster, D.S.; Westoby, M. Leaf Size and Angle Vary Widely across Species: What Consequences for Light Interception? New Phytol. 2003, 158, 509–525. [Google Scholar] [CrossRef]
- Zeng, Z.-L.; Sun, H.; Wang, X.-Q.; Zhang, S.-B.; Huang, W. Regulation of Leaf Angle Protects Photosystem I under Fluctuating Light in Tobacco Young Leaves. Cells 2022, 11, 252. [Google Scholar] [CrossRef]
- Flexas, J.; Escalona, J.; Evain, S.; Gulas, J.; Moya, I.; Osmond, C.; Medrano, H. Steady-State Chlorophyll Fluorescence (Fs) as an Indicator of Leaf Photosynthesis and Stomatal Conductance under Drought Conditions. In Proceedings of the 12th International Congress on Photosynthesis; Brisbane Convention & Exhibition Centre: Queensland, Australia, 2001; Volume 3, pp. 231–240. Available online: https://www.publish.csiro.au (accessed on 10 September 2025).
- Ritchie, G. A Chlorophyll Fluorescence: What Is It and What Do the Numbers Mean? In National Proceedings: Forest and Conservation Nursery Associations; Riley, L., Dumroese, R., Landis, T., Eds.; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2006; pp. 34–43. Available online: https://research.fs.usda.gov/treesearch/26654 (accessed on 10 September 2025).
- Makhtoum, S.; Sabouri, H.; Gholizadeh, A.; Ahangar, L.; Katouzi, M.; Mastinu, A. Genomics and Physiology of Chlorophyll Fluorescence Parameters in Hordeum vulgare L. under Drought and Salt Stresses. Plants 2023, 12, 3515. [Google Scholar] [CrossRef]
- MAPA Métodos Oficiales de Análisis. Tomo III; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 1994.
- IUSS. Working Group WRB World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; ISBN 9798986245119. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA Soil Test for Zinc, Iron, Manganese, and Copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- García-Gómez, C.; García, S.; Obrador, A.; Almendros, P.; González, D.; Fernández, M.D. Effect of Ageing of Bare and Coated Nanoparticles of Zinc Oxide Applied to Soil on the Zn Behaviour and Toxicity to Fish Cells Due to Transfer from Soil to Water Bodies. Sci. Total Environ. 2020, 706, 135713. [Google Scholar] [CrossRef]
- Feng, M.H.; Shan, X.; Zhang, S.; Wen, B. A Comparison of the Rhizosphere-Based Method with DTPA, EDTA, CaCl2, and NaNO3 Extraction Methods for Prediction of Bioavailability of Metals in Soil to Barley. Environ. Pollut. 2005, 137, 231–240. [Google Scholar] [CrossRef]
- Almendros, P.; Obrador, A.; Alvarez, J.M.; Gonzalez, D. Zn-DTPA-HEDTA-EDTA Application: A Strategy to Improve the Yield and Plant Quality of a Barley Crop While Reducing the N Application Rate. J. Soil Sci. Plant Nutr. 2019, 19, 920–934. [Google Scholar] [CrossRef]
- Smith, R.M.; Martell, A.E.; Motekaitis, R.J. NIST Critically Selected Stability Constants of Metal Complexes Database. NIST Standard Reference Database 46; National Institute of Standard and Technology: Gaithersburg, MD, USA, 2004; Volume 2. [Google Scholar] [CrossRef]
- Huang, J.W.; Chen, J.; Berti, W.R.; Cunningham, S.D. Phytoremediadon of Lead-Contaminated Soils: Role of Synthetic Chelates in Lead Phytoextraction. Environ. Sci. Technol. 1997, 31, 800–805. [Google Scholar] [CrossRef]
- Tewari, G.; Pareek, N.; Pachauri, S.; Pandey, S. Impact of Chelation/Complexation Phenomenon on Soil Environment. Int. J. Agric. Sci. 2018, 9107, 7314–7316. Available online: https://bioinfopublication.org/pages/article.php?id=BIA0004599 (accessed on 10 September 2025).
- Kılıç, M.; Käpylä, V.; Gollan, P.J.; Aro, E.-M.; Rintamäki, E. PSI Photoinhibition and Changing CO2 Levels Initiate Retrograde Signals to Modify Nuclear Gene Expression. Antioxidants 2023, 12, 1902. [Google Scholar] [CrossRef] [PubMed]
- Miyake, C. Molecular Mechanism of Oxidation of P700 and Suppression of ROS Production in Photosystem I in Response to Electron-Sink Limitations in C3 Plants. Antioxidants 2020, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G. Non-Photochemical Quenching (NPQ) in Photoprotection: Insights into NPQ Levels Required to Avoid Photoinactivation and Photoinhibition. New Phytol. 2025, 246, 1967–1974. [Google Scholar] [CrossRef]
- de Francisco, M.; Ortiz, R.; Obrador, A.; Gonzalez, D.; Gascó, G.; Almendros, P. The Effect of Complexed, Nanosized, and Conventional Zinc Sources Applied at Varying Rates to an Acidic Mediterranean Soil on Two Successive Lettuce Crops. Agronomy 2025, 15, 896. [Google Scholar] [CrossRef]
- Hall, J.L. Cellular Mechanisms for Heavy Metal Detoxification and Tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef]
- Ejaz, U.; Khan, S.M.; Khalid, N.; Ahmad, Z.; Jehangir, S.; Fatima Rizvi, Z.; Lho, L.H.; Han, H.; Raposo, A. Detoxifying the Heavy Metals: A Multipronged Study of Tolerance Strategies against Heavy Metals Toxicity in Plants. Front. Plant Sci. 2023, 14, 1154571. [Google Scholar] [CrossRef]
- Zhao, F.-J.; Tang, Z.; Song, J.-J.; Huang, X.-Y.; Wang, P. Toxic Metals and Metalloids: Uptake, Transport, Detoxification, Phytoremediation, and Crop Improvement for Safer Food. Mol. Plant 2022, 15, 27–44. [Google Scholar] [CrossRef]
- Saraswat, S.; Rai, J.P.N. Complexation and Detoxification of Zn and Cd in Metal Accumulating Plants. Rev. Environ. Sci. Bio/Technology 2011, 10, 327–339. [Google Scholar] [CrossRef]


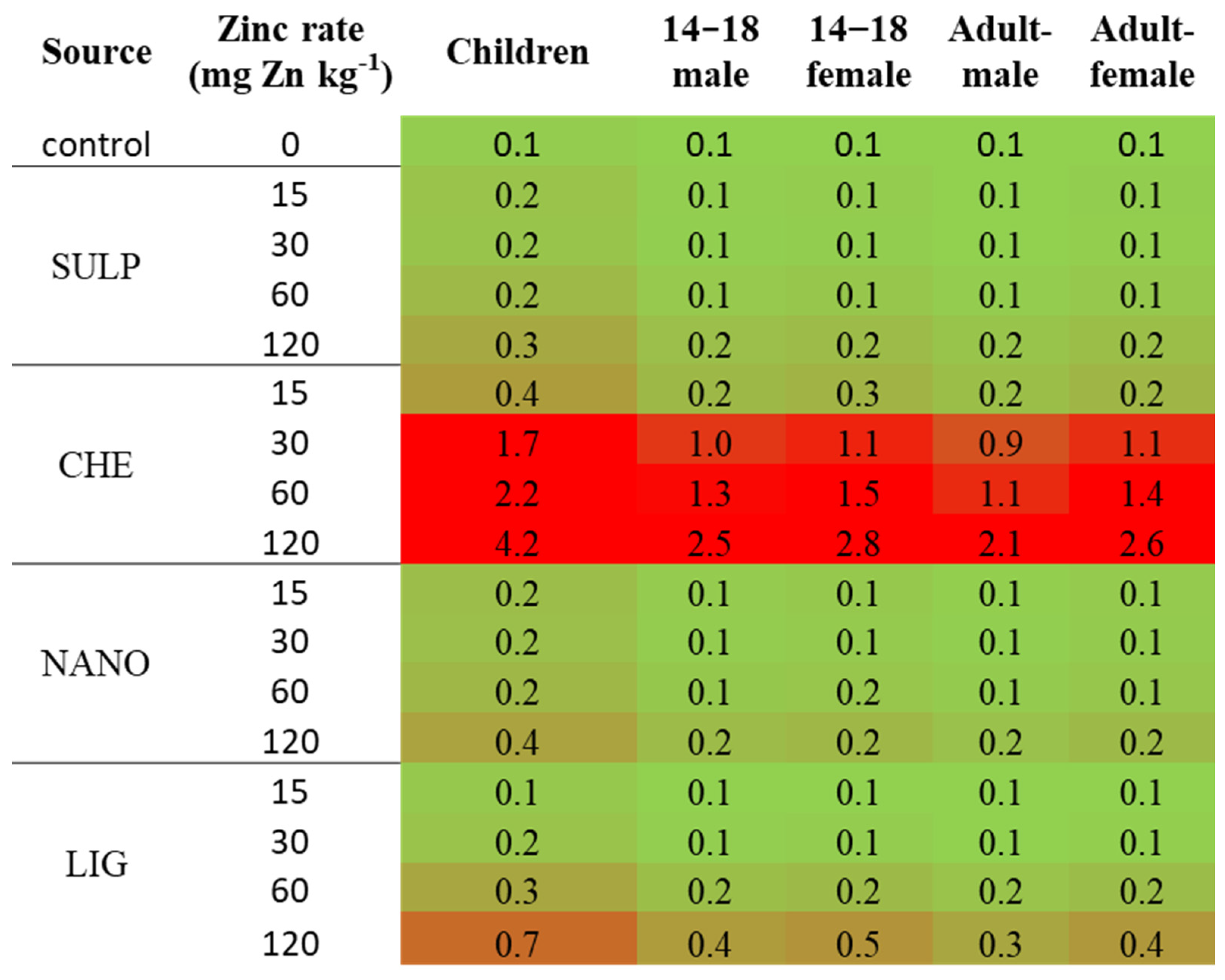
| Source 1 | Rate | Available Zn Concentration in Soil 2 | |
|---|---|---|---|
| mg Zn·Kg−1 Soil | mg Zn·Kg−1 Soil | ||
| control | 0 | 0.40 ± 0.01 | a |
| SULP | 15 | 0.29 ± 0.00 | a |
| 30 | 0.53 ± 0.03 | a | |
| 60 | 0.86 ± 0.17 | ab | |
| 120 | 5.15 ± 2.22 | c | |
| CHE | 15 | 0.56 ± 0.09 | a |
| 30 | 1.88 ± 0.77 | ab | |
| 60 | 2.96 ± 0.66 | b | |
| 120 | 12.55 ± 1.32 | d | |
| NANO | 15 | 0.47 ± 0.05 | a |
| 30 | 0.55 ± 0.07 | a | |
| 60 | 0.73 ± 0.04 | a | |
| 120 | 3.06 ± 0.84 | b | |
| LIG | 15 | 0.40 ± 0.02 | a |
| 30 | 0.58 ± 0.06 | a | |
| 60 | 1.18 ± 0.27 | ab | |
| 120 | 2.30 ± 0.41 | ab | |
| p-value | 0.0000 | ||
| Source effect | CHE | 4.49 ± 1.47 | B |
| LIG | 1.11 ± 0.25 | A | |
| NANO | 1.20 ± 0.37 | A | |
| SULP | 1.79 ± 0.84 | A | |
| p-value | 0.0000 | ||
| Zn rate effect | 15 | 0.43 ± 0.04 | A |
| 30 | 0.88 ± 0.24 | B | |
| 60 | 1.48 ± 0.34 | C | |
| 120 | 5.77 ± 1.36 | D | |
| p-value | 0.0000 | ||
| Parameter | Correlation with Zn Conc | |
|---|---|---|
| Agronomic and crop quality | ||
| Yield | −0.682 *** | |
| Zn concentration (young leaves) | 0.694 *** | |
| Available Zn conc. in soil | 0.847 *** | |
| Morphological and structural leaf traits | ||
| Stem diameter | −0.677 *** | |
| Total leaves | −0.667 *** | |
| LMA | 0.393 * | |
| Leaf angle | 0.576 *** | |
| SPAD | −0.712 *** | |
| Chlorophyll fluorescence & PSII | ||
| Fm′ | −0.599 *** | |
| Fo′ | −0.717 *** | |
| Fs | −0.610 *** | |
| Fv′/Fm′ | −0.338 * | |
| qL | NS | |
| ΦPSII | NS | |
| NPQt | 0.378 * | |
| ΦNPQ | NS | |
| ΦNO | −0.334 * | |
| LEF | −0.283 * | |
| Photosystem I (PSI) parameters | ||
| kP700 | NS | |
| tP700 | −0.417 * | |
| vinitial P700 | −0.386 * | |
| PSI Active Centers | −0.548 *** | |
| PSI Open Centers | NS | |
| PSI Oxidized Centers | NS | |
| Proton gradient & ECS parameters | ||
| ECS_τ | −0.366 * | |
| ECSt | −0.489 ** | |
| gH+ | 0.280 * | |
| vH+ | −0.490 ** | |
| Environmental & light-related parameters | ||
| Leaf temperature | NS | |
| Leaf-to-air temperature differential | NS | |
| PAR | NS | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de-Francisco, M.; Hernández-Montes, E.; DeSanto, S.; Montoya, M.; Obrador, A.; Almendros, P. Growth and Photosynthetic Responses of Lactuca sativa L. to Different Zinc Fertilizer Sources and Applications. Horticulturae 2025, 11, 1221. https://doi.org/10.3390/horticulturae11101221
de-Francisco M, Hernández-Montes E, DeSanto S, Montoya M, Obrador A, Almendros P. Growth and Photosynthetic Responses of Lactuca sativa L. to Different Zinc Fertilizer Sources and Applications. Horticulturae. 2025; 11(10):1221. https://doi.org/10.3390/horticulturae11101221
Chicago/Turabian Stylede-Francisco, Marina, Esther Hernández-Montes, Sarah DeSanto, Monica Montoya, Ana Obrador, and Patricia Almendros. 2025. "Growth and Photosynthetic Responses of Lactuca sativa L. to Different Zinc Fertilizer Sources and Applications" Horticulturae 11, no. 10: 1221. https://doi.org/10.3390/horticulturae11101221
APA Stylede-Francisco, M., Hernández-Montes, E., DeSanto, S., Montoya, M., Obrador, A., & Almendros, P. (2025). Growth and Photosynthetic Responses of Lactuca sativa L. to Different Zinc Fertilizer Sources and Applications. Horticulturae, 11(10), 1221. https://doi.org/10.3390/horticulturae11101221







