Abstract
Brazil is home to some of the world’s greatest biodiversity, providing an immeasurable number of new opportunities and unexplored sources of native plants for the development of products, extracts, foods, and compounds of social and economic interest. Among these, plants of the genus Eugenia stand out because of the large number of species found in Brazilian territory, many of which are endemic and exclusive to Brazil. These plants have emerged as potential sources for obtaining essential oils with relevant biological activities. In this context, the present review provides an overview of essential oils derived from the main native plants of Brazilian socio-biodiversity from the genus Eugenia with food value (Eugenia stipitata, Eugenia dysenterica, Eugenia involucrata, Eugenia brasiliensis, Eugenia klotzschiana, Eugenia uniflora, and Eugenia pyriformis) and their phytochemical profile and health beneficial effects. The compiled data showed that the essential oils of these plants are composed mainly of sesquiterpenes and, in smaller quantities, monoterpenes and other compounds. These compounds contribute to different biological activities, including antimicrobial, antioxidant, anticancer, and antiparasitic effects. These findings demonstrate that the essential oils of Brazilian native plants of the genus Eugenia can be a promising raw material for active ingredients to develop innovative and sustainable food products, drugs, and cosmetics.
1. Introduction
Uncontrolled urban growth has been the subject of constant discussion worldwide because of its growing detrimental impact on social, environmental, and economic sectors. Therefore, adopting environmentally responsible development approaches aiming to conserve resources, protect ecosystems, and promote long-term well-being for both humans and the planet is of vital importance to meet the needs of the current society without compromising the ability of future generations to meet their own needs [1,2]. In this regard, stemming from the continuous efforts of the United Nations, in 2015, its members adopted “The 2030 Agenda for Sustainable Development”, which contains 17 Sustainable Development Goals (SDGs). These objectives provide a comprehensive framework to guide policies and practices at all levels, aiming to ensure that economic, social, and environmental development is achieved in an integrated and equitable manner, to attain an eco-friendly world for everyone [3,4].
The preservation of biological diversity is one of the fundamental aspects to ensure sustainable development since it is essential for ecosystem health and offers multiple benefits to humanity [1]. Brazil is recognized for harboring some of the greatest biodiversity in the world, comprising approximately 15–20% of global biological diversity. This immense biodiversity is due to the presence of many ecological biomes that provide specific conditions for the development of different species. Thus, the Brazilian territory represents an available and unique source to explore and discover food species and active principles with the potential to improve people’s health and well-being [1,5]. However, the expansion of agriculture and urbanization has promoted a growing destruction of natural areas in Brazilian biomes. Consequently, many plant species may disappear even before being cataloged and studied. Therefore, it has become urgent to investigate and rediscover the richness of Brazil’s natural heritage to protect it and build a more sustainable future [1].
Recent studies have evidenced the action of different native Brazilian plants in the prevention and treatment of non-communicable chronic diseases, including cancer, cardiovascular diseases, diabetes, obesity, and metabolic syndrome, among others [6,7]. Thus, native Brazilian plants emerge as strategic key ingredients for developing high-value-added products, such as functional foods, nutraceuticals, drugs, and cosmetics. Among the numerous plant species with exploratory potential, plants belonging to the genus Eugenia stand out as the largest genus in terms of species within the family Myrtaceae, with more than 1000 species scattered around the world, 414 of which are found in Brazilian territory, with 300 being endemic and exclusive to Brazil [8].
Native Brazilian plants and fruits belonging to the genus Eugenia are rich in biologically active compounds (e.g., phenolic compounds, carotenoids, vitamins, fibers, sugars, and volatile compounds, among others) with therapeutic effects, delaying the onset and/or development of some diseases such as diabetes, cancer, and obesity [9,10]. Among the genera belonging to the family Myrtaceae, the genus Eugenia stands out as the fourth most important genus in essential oil production, trailing only behind the genera Eucalyptus, Melaleuca, and Psidium. Essential oils from Eugenia species are highly complex natural mixtures. Studies conducted on these essential oils have already identified approximately 300 compounds, with a predominance of cyclic sesquiterpenes and lesser amounts of monoterpenes. Additionally, aromatic and aliphatic compounds can be found in a few species. Essential oils derived from these plants and their terpenoid compounds have versatile applications in different industrial sectors such as food, pharmaceutical, and cosmetics [11]. Several studies have demonstrated that essential oils from Eugenia species and their phytochemicals exert a range of biological activities, including antioxidant, anticancer, anticholinesterase, antiviral, antimicrobial, antiparasitic, and insecticidal activities [12,13].
The biological properties of essential oils from native Brazilian plants belonging to the genus Eugenia have stimulated the interest of researchers, industries, producers, and consumers. Thus, the essential oils of these plants can be sources of opportunities to produce innovative products, protecting biodiversity and the environment, fostering economic development, and improving public health and well-being, contributing significantly to sustainable development by meeting at least three United Nations SDGs including SDG 3 (good health and well-being), SDG 9 (industry, innovation, and infrastructure), and SDG 12 (responsible consumption and production).
From this perspective, we summarize the most recent scientific data available in the literature regarding the chemical composition and biological properties of essential oils from native Brazilian plants belonging to the genus Eugenia with food value, aiming to stimulate the production of innovative and eco-friendly products as a strategy for promoting health and well-being. Thus, this review can serve as reference material to support researchers and industries in the development of innovative and sustainable products derived from Brazilian biodiversity to help achieve the mentioned SDGs.
2. Search Strategy and Study Selection
In the current literature review, we performed a search strategy to find data regarding the chemical composition and biological properties of essential oils from native Brazilian plants of the genus Eugenia. The identification of plant species to be included in this review was conducted by consulting the 2021 official list of native plants of Brazilian socio-biodiversity with food value from the Ministérios da Agricultura, Pecuária e Abastecimento e do Meio Ambiente (MAPA/MMA) [14]. From this official list, seven plant species belonging to the genus Eugenia were found (for more details regarding the popular names, scientific names, natural geographic distribution, and use for food purposes of these species, refer to Table 1). After identifying the species, this review was initiated by electronic searching using the main repositories of the world’s scientific data (Scopus, Google Scholar, Science Direct, Web of Science, Scielo, and PubMed databases) to gather relevant articles published in scientific journals in the last decade (2014 to the present). We used the following terms to perform our bibliographic search: “Eugenia stipitata” OR “Eugenia dysenterica” OR “Eugenia involucrata” OR “Eugenia brasiliensis” OR “Eugenia klotzschiana” OR “Eugenia uniflora” OR “Eugenia pyriformis” AND “essential oil”. The abovementioned terms were searched for in the article title, abstract, and keywords. The search was not restricted to any specific language. The articles retrieved from the databases were selected for a full-text review. Inclusion and exclusion criteria were determined by the authors to avoid biases in the selection of articles. Inclusion parameters included articles that reported results concerning essential oil, chemical composition, and biological properties. Meanwhile, exclusion parameters included duplicates, publications before 2014, theses, editorials, communications, and conference abstracts.

Table 1.
The official list containing general information about native species of the Brazilian socio-biodiversity from the genus Eugenia with food value a.
3. Chemical Composition of Essential Oils
Essential oils are complex mixtures of low molecular weight compounds obtained from various plant parts, such as leaves, stems, seeds, fruits, and flowers [15,16]. They mostly consist of volatile and aromatic compounds, such as monoterpene and sesquiterpene hydrocarbons and their oxygenated derivatives, along with other compounds like phenylpropanoids, aldehydes, esters, alcohols, and ketones, present in low amounts [15]. These compounds are different secondary metabolites produced by plants, which play an important role in defense mechanisms against herbivores, pathogens, and environmental stressors [17]. Additionally, the volatile compounds present in essential oils possess various biological properties related to human health, including antioxidant, anti-inflammatory, antimicrobial, anticancer activities, and others [18,19,20].
The 10 most abundant compounds found in the essential oils from Brazilian native Eugenia species with food value are listed in Table 2. Additionally, the plant part used, the extraction method, the percentage yield, the total number of identified compounds, and the total identified area of essential oils are provided.

Table 2.
Major compounds found in essential oils from native Brazilian plants of the genus Eugenia.
These native Eugenia species are distributed in several Brazilian biomes; therefore, the essential oils of these plants have more than 80 compounds in their composition. The sesquiterpene hydrocarbons (e.g., germacrene A, germacrene B, germacrene D, α-humulene, β-caryophyllene, β-elemene, γ-elemene, δ-cadinene, and bicyclogermacrene) and their oxygenated derivatives (e.g., atractylone, curzerene, selina-1,3,7(11)-trien-8-one, selina-1,3,7(11)-trien-8-one epoxide, germacrone, globulol, spathulenol, viridiflorol, caryophyllene oxide, and α-cadinol) are commonly found. However, monoterpene hydrocarbons (e.g., α-pinene, limonene, γ-terpinene, and p-cymene) and their oxygenated derivatives (e.g., 1,8-cineole), and other classes of compounds, such as phenylpropanoids, are also present, albeit in smaller proportions, contributing to the complexity and uniqueness of the essential oils of these Brazilian native plants. The chemical structures of the volatile compounds most frequently found in the essential oils of native Brazilian Eugenia species are shown in Figure 1.
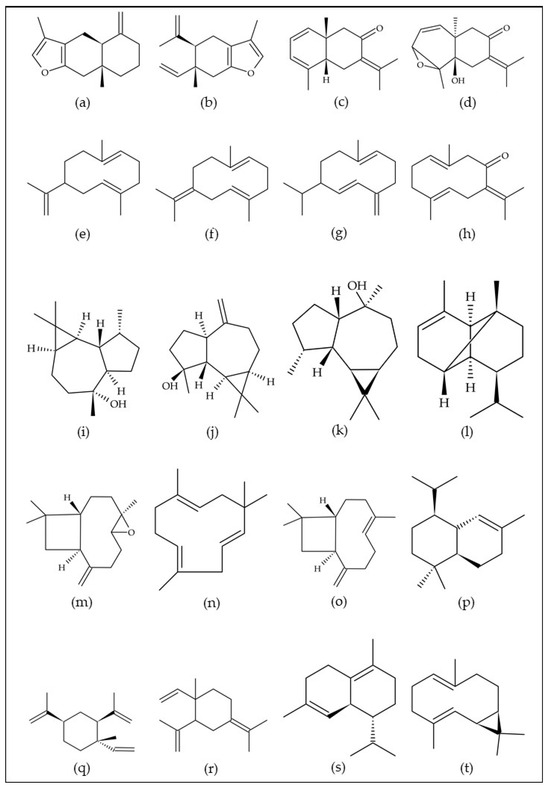
Figure 1.
Chemical structures of the 20 volatile compounds most frequently found in the essential oils of native Brazilian plants of the genus Eugenia. (a) Atractylone, (b) curzerene, (c) selina-1,3,7(11)-trien-8-one, (d) selina-1,3,7(11)-trien-8-one epoxide, (e) germacrene A, (f) germacrene B, (g) germacrene D, (h) germacrone, (i) globulol, (j) spathulenol, (k) viridiflorol, (l) α-copaene, (m) caryophyllene oxide, (n) α-humulene, (o) β-caryophyllene, (p) α-cadinol, (q) β-elemene, (r) γ-elemene, (s) δ-cadinene, and (t) bicyclogermacrene. Own authorship created by ChemSketch software version 2021.2.1.
The chemical profile of the essential oils obtained from the dried leaves of E. stipitata consists mainly of sesquiterpene hydrocarbons and their oxygenated derivatives such as germacrene D (11.8%), (Z)-α-bisabolene (8.4%), and cadin-4-en-10-ol (6.1%) [23]. Additionally, other authors have found different compositions of the essential oil from E. stipitata leaves [21,22,24]. For example, Costa et al. [24] obtained compounds such as β-eudesmol (15.28%), γ-eudesmol (10.85%), and elemol (10.21%). Meanwhile, Rivera et al. [21] found γ-muurolene (22.34%), β-caryophyllene (12.11%), and α-copaene (5.20%) as their major compounds. Similarly, sesquiterpene hydrocarbons and their oxygenated derivatives are the most abundant compounds for essential oils obtained from E. involucrata leaves. Toledo et al. [30] noticed that approximately 50% of the total identified area of essential oil from dried leaves of E. involucrata was composed of elixene (26.53%), β-caryophyllene (13.16%), and α-copaene (8.41%). Another study with dried leaves of E. involucrata found that around 50% of the identified compounds were represented by germacrene B (22.17%), bicyclogermacrene (19.76%), and β-elemene (10.86%) [29]. On the other hand, besides sesquiterpenes and their derivatives, monoterpenes can be found abundantly in the essential oils from E. dysenterica leaves. For example, Galheigo et al. [25] found the monoterpene (Z)-β-ocimene (19.14%) to be the most abundant compound present in the essential oil from fresh leaves of E. dysenterica, followed by sesquiterpenes (β-caryophyllene (15.36%) and caryophyllene oxide (8.23%)). Another study reported the presence of monoterpene limonene (16%) as the most abundant compound in the essential oil from dried leaves of E. dysenterica [28]. These differences in the composition of essential oils can also be observed for the other plants studied, such as E. brasiliensis, E. klotzschiana, E. uniflora, and E. pyriformis (Table 2). Several factors highly influence the diverse composition of essential oils, including the type of corresponding family/genus, plant part, time and period of harvesting, physiological age and stage of development of the plants, geographical and climatic conditions, post-harvest drying, plant storage conditions, and extraction methods, among others [58,59].
As demonstrated in Table 2, it can be observed that the species and the part of the plant used contributed to obtaining different profiles of essential oils. dos Santos et al. [33] identified that β-caryophyllene (7.70%), spathulenol (6.42%), and δ-cadinene (5.55%) were the most abundant compounds in the essential oil obtained from dried leaves of E. brasiliensis. In comparison, the essential oil from E. uniflora leaves was predominantly composed of germacrone (37.82%), curzerene (16.60%), and germacrene B (13.54%). Carneiro et al. [34] identified several common compounds in essential oils obtained from the fresh leaves and flowers of E. klotzschiana (e.g., β-elemene, spathulenol α-cadinol, globulol, γ-selinene, α-humulene, γ-elemene, and α-copaene), while others were found exclusively in the leaves (e.g., caryophyllene oxide and τ-muurolol) or flowers (e.g., germacrene D, α-caryophyllene, α-farnesol, γ-gurjunene, eugenol, and ledene oxide). Besides the profile difference noted among the essential oils obtained from different parts of E. klotzschiana, it was observed that the major compounds also changed. The essential oil from the fresh flowers was mainly composed of germacrene D (29.90%), γ-elemene (12.06%), and α-caryophyllene (10.14%), whereas the essential oil from the fresh leaves was predominantly composed of β-elemene (10.65%), spathulenol (8.76%), and caryophyllene oxide (7.44%). Additionally, the method used to obtain an essential oil affects the diversity and quantity of these compounds. It is noted in Table 2 that hydrodistillation is the most commonly employed method to obtain essential oils from native Brazilian Eugenia species, while few studies use the steam distillation method. Additionally, a variation in extraction time (1 to 6 h) can be observed. These different methods may result in significant variations in the composition and yield of the essential oils. In this review, only one study was found that evaluated the effect of hydrodistillation time on the yield of essential oils from fresh leaves of E. pyriformis [54].
In addition to the factors described above, different drying methods, the age of the leaves used, and the specimens also affect the composition of essential oils. For example, Carneiro et al. [34] found variations in the composition of essential oils from E. klotzschiana leaves before and after different drying methods (shade or kiln drying), where α-caryophyllene was not found in fresh leaves but became the major compound after drying. Similarly, an increase in the content of other compounds was observed after the drying processes, including γ-elemene (1.5 to 5.5-fold), where kiln drying led to a more significant increase. On the other hand, there was a reduction in the content of β-elemene by almost 90% after the drying process. Assis et al. [51] observed an 11.83% increase in sesquiterpenes of essential oils from E. uniflora leaves when dried at 45 °C compared with room temperature (22 °C). Santos et al. [41] verified that the age of the E. uniflora leaves resulted in different compositions of essential oils. The authors identified that germacrone was present only in young leaves, representing about 38% of the total composition of the essential oil, while curzerene was predominant in mature leaves (around 22%). Additionally, furanodiene was identified in large quantities only in mature leaves, accounting for 19%. Figueiredo et al. [46] studied the essential oils obtained from the leaves of five E. uniflora specimens. They observed that curzerene was not detected in specimens 1, 2, or 3, while in specimen 4, this compound accounted for around 50% of the identified compounds. On the other hand, compounds selina-1,3,7(11)-trien-2-one (18.1–32.6%) and selina-1,3,7(11)-trien-2-one epoxide (16.0–30.4%) were the majority for specimens 1, 2, and 3, while they were not found in specimens 4 or 5. Cipriano et al. [52] examined the composition of essential oils obtained from leaves of 36 different E. uniflora genotypes. Through the cluster analysis method, they classified these essential oils into six groups according to their different chemical compositions. Selina-1,3,7(11)-trien-8-one, selina-1,3,7(11)-trien-8-one epoxide, curzerene, germacrone, 7-epi-α-selinene, furanodiene, and 7,14-anhydro-amorpha-4,9-diene were the key components that differentiated the groups. Ramalho et al. [53] investigated the variability in the composition of essential oils in E. uniflora fruits from three fruit biotypes (red, red-orange, and purple) and at different stages of ripeness. The constituents were subjected to multivariate analysis, and the fruit samples were differentiated by selina-1,3,7(11)-trien-8-one (36.06–41.22%), and selina-1,3,7(11)-trien-8-one epoxide (19.36–34.28%) for the red-orange biotype, curzerene (14.41–15.75%) and atractylone (6.90–10.10%) for the red biotype, and germacrone (51.56–56.80%) and spathulenol (5.46–8.36%) for the purple biotype. Furthermore, as other studies report, climatic influence and seasonality affect the content and composition of essential oils from native Brazilian Eugenia species. For example, Luz et al. [49] found significant variations in the total area identified of essential oils from E. uniflora leaves in rainy and dry periods (99.98% and 95.06%, respectively). Although major compounds remained the same during these periods (curzerene, germacrone, and β-elemenone), some other compounds were found only in the rainy season (e.g., γ-elemene and δ-elemene) or in the dry season (e.g., humulene). Siebert et al. [31] observed that spathulenol was the major compound found in essential oil from E. brasiliensis leaves from the spring (16.02%) and summer (18.17%), and τ-cadinol (12.83%) in essential oil from the autumn, both sesquiterpene compounds. On the other hand, they found α-pinene (15.94%), a monoterpene, to be the major compound in essential oil from the winter. Monoterpenes are smaller molecules, which allows them to be retained in the plant in more humid and colder climates, possibly explaining their higher content in winter essential oils [31].
Therefore, essential oils from native Brazilian Eugenia species demonstrate a profound complexity in their composition due to the series of factors previously discussed. These include the botanical species, the specific part of the plant used, the stage of maturation, the season, the climatic conditions, and the drying and extraction methods. Consequently, a more comprehensive understanding of the determinants that shape the profile and composition of these essential oils is needed. This knowledge guarantees the quality, efficiency, and sustainability of production processes and reveals new ways of using and applying these valuable natural reservoirs.
4. Biological Activities of Essential Oils
As seen in the section above, essential oils from Brazilian native plants of the genus Eugenia exhibit a complex mixture of phytochemicals in varying concentrations, including terpenes, terpenoids, and aromatic and aliphatic components. The presence of these compounds may contribute to the wide range of health benefits associated with essential oils. Essential oils from Brazilian native plants have been used for centuries in folk medicine for treating various pathologies and promoting health and well-being. Consequently, many recent studies have been conducted to validate the effects reported by folk medicine and identify other biological activities and therapeutic effects of these essential oils. In the sections below, we present and discuss the main biological activities reported for essential oils obtained from native Brazilian Eugenia species, highlighting the identification of biological targets and potential mechanisms of action.
4.1. Antimicrobial Activity
Food spoilage microorganisms, such as bacteria, yeasts, and fungi, contribute to annual food losses reaching up to 40%. Additionally, foodborne pathogenic microorganisms are a constant public health concern because of the various disorders they can cause [60]. Several in vitro studies have demonstrated that essential oils obtained from native Brazilian Eugenia species exhibit antimicrobial activity against food spoilage and pathogenic microorganisms (see Table 3).

Table 3.
A summary of studies showing the in vitro antimicrobial activities of essential oils obtained from native Brazilian plants of the genus Eugenia.
The antimicrobial activity of essential oils is attributed to several cellular mechanisms and is related to their composition [50]. The main characteristic demonstrating the antimicrobial potential of essential oils lies in the hydrophobic character of their compounds [63]. These compounds play a crucial role in disrupting the lipid membranes of microbial cells, making them permeable and causing the leakage of intracellular constituents, such as ions, glucose, and ATP, resulting in microbial death [63,64]. These oils also have the ability to interfere with enzymatic systems involved in energy production and the synthesis of structural compounds [30,63].
The antimicrobial activity of essential oils obtained from native Brazilian Eugenia species has been evaluated in vitro for a myriad of foodborne and oral microorganisms, mainly bacteria, yeast, and fungi (Table 3). Among these, essential oil from E. uniflora stands out as the most commonly investigated, which could be related to its higher bioavailability in different Brazilian regions and pleasant aroma and properties [10]. Additionally, most studies prioritize evaluating the activity of essential oils from plant leaves (Table 3), while some authors have also evaluated the essential oils from other plant parts, such as twigs [57] and flowers [34]. This is associated with the high content of aromatic compounds present in leaves, their ease of harvesting, consistency in their chemical composition, and their historical use [64].
Regarding the in vitro methods commonly applied for antimicrobial screening of essential oils from native Brazilian Eugenia species, the determination of the minimum inhibitory concentration (MIC) using broth dilution methods has been the most applied, while some studies further determined the minimal bactericidal concentration (MBC) and/or the minimum fungicidal concentration (MFC) of the essential oils (Table 3). The MIC represents the lowest concentration of an essential oil required to inhibit the growth of microorganisms under certain conditions, while the MBC and MFC refer to the lowest concentration of an essential oil that results in the inactivation of the majority (usually ≥99.9%) of bacterial and fungal populations, respectively [65].
In the following sections, the main results derived from research on the antimicrobial effects of essential oils from native Brazilian Eugenia species are described.
4.1.1. Antimicrobial Activity against Bacteria
As seen in Table 3, essential oils from native Brazilian Eugenia species have been evaluated for their antimicrobial effectiveness against both collection bacterial strains and clinical and food isolates. The in vitro activity of essential oils from native Brazilian Eugenia species has been frequently tested against foodborne bacterial pathogens, mainly Gram-positive bacteria including Staphylococcus aureus, Bacillus subtilis, and Listeria monocytogenes and Gram-negative bacteria including Escherichia coli, Salmonella spp., and Pseudomonas aeruginosa (Table 3). These bacteria are frequently studied because of their prevalence in food contamination and their significant impact on food safety and public health [63]. On the other hand, the oral bacteria most commonly exposed to essential oils from native Brazilian Eugenia species in in vitro tests are Streptococcus salivarius, Streptococcus mutans, Streptococcus mitis, Streptococcus sanguinis, and Streptococcus sobrinus. These bacteria play an important role in dental caries, periodontal diseases, and other oral health conditions, and are frequently studied in oral microbiology [28,34,48].
As extensively documented in the literature on plant essential oils and extracts, Gram-positive bacteria have shown lower resistance when exposed to essential oils from native Brazilian Eugenia species compared with Gram-negative bacteria [22,30,39,56]. For example, in the study by Toledo et al. [30], essential oil from E. involucrata leaves showed activity against the Gram-positive bacteria S. aureus, S. epidermidis, and B. subtilis (MIC = 875 µg/mL). In contrast, no antimicrobial effect of the essential oil against Gram-negative bacteria (e.g., E. coli, S. Enteritidis, and S. Typhimurium) was recorded. Moreover, the MIC of the essential oil from E. uniflora leaves against S. aureus was reported to be at least four times lower than the value recorded for E. coli (Table 3) [39].
Differences in the effects on Gram-negative and Gram-positive bacteria following exposure to the essential oils are related to the presence of an outer phospholipid membrane enveloping the Gram-negative bacteria cell wall [66]. This membrane incorporates hydrophilic lipopolysaccharides, which act as a barrier against macromolecules and hydrophobic compounds, enhancing tolerance to certain antimicrobial agents, such as compounds of essential oils [48,66]. Although Gram-positive bacteria possess a cell wall thicker than Gram-negative bacteria, it is primarily composed of lipophilic ends of lipoteichoic acids, which facilitates the direct interaction of essential oils with the bacterial cell [30].
Interestingly, studies revealed variability in susceptibilities among strains of the same microbial species (e.g., S. aureus) and/or the same microbial genus (e.g., Staphylococcus spp.) when exposed to native Brazilian Eugenia species. For example, a methicillin-resistant S. aureus strain was more susceptible to the essential oil from E. pyriformis leaves than a non-resistant S. aureus strain, with MIC values equal to 125 and 250 µg/mL, respectively [56]. Additionally, S. Typhimurium was more sensitive to the essential oil from E. uniflora leaves than S. Gallinarum, with MIC values equal to 0.78 and 3.13 mg/mL, respectively [38]. Bacillus licheniformis was also more susceptible to the essential oil from E. uniflora leaves compared with B. subtillis, with MIC values equal to 0.625 and 5 mg/mL, respectively [44].
Variability in susceptibilities among strains of the same microbial species or genus when exposed to native Brazilian Eugenia species can be attributed to several factors. Firstly, microbial strains exhibit genetic variability, leading to differences in membrane permeability, cell wall composition, and the expression of resistance genes, which consequently affect their response to essential oils. Moreover, resistant strains may possess specific resistance mechanisms, including efflux pumps or enzymatic degradation pathways, while susceptible strains may lack or have reduced expression of these mechanisms. Differences in cellular physiology, growth characteristics, and metabolic activity among strains can also impact their susceptibility to essential oils [63].
As discussed in Section 3, different factors can affect the chemical composition of essential oils, which, in turn, can reflect on their antimicrobial activities. For example, in the study by Siebert et al. [31], the essential oil from E. brasiliensis leaves extracted in the autumn and winter was more active against S. saprophyticus and P. aeruginosa than essential oils obtained in the spring and summer (Table 3). Furthermore, in the study by Sviech et al. [45], no antimicrobial effect was observed for essential oil from E. uniflora leaves against the E. coli strain ATCC 25922 (no inhibition zones by the diffusion method when exposed to 40 µL of a 25% (v/v) essential oil solution), while the same bacterial species was susceptible to the essential oil from E. uniflora leaves in the study by Antonelo et al. [38] (MIC = 0.78 mg/mL) (Table 3). In the first study, the compounds germacrome, spathulenol, and curzerene were present in the essential oil at minor concentrations (13.57, 7.49, and 5.31%, respectively), whereas in the latter, essential oil showed major cyclic oxygenated compounds, including curzerene (10.15%), as well as phenolic compounds, like acetosyringone (12.08%), which could partially explain the differences in antimicrobial effects [38,45].
Because of their confirmed antibacterial potential, essential oils obtained from native Brazilian Eugenia species can be used in combination with other microbial inactivation methods, such as antibiotics and non-thermal technologies (e.g., high-pressure processing and pulsed electric fields for food decontamination). In the study by Pereira et al. [39], essential oil from E. uniflora leaves was combined with antibiotics (i.e., amikacin and gentamicin) to inactivate S. aureus and E. coli. Overall, the authors observed a reduction in the antibiotic concentrations required to achieve desired levels of microbial inactivation, evidencing a synergistic effect. However, complex interactions among different substances may be elucidated to optimize the efficacy of these essential oils as antibacterial agents.
4.1.2. Antimicrobial Activity against Fungi and Yeast
The susceptibilities of different fungi and yeast to essential oils obtained from native Brazilian Eugenia species have been assessed in different studies, mostly against Candida albicans (Table 3) [27,30,38,40,44,56,62]. Other species evaluated in previous investigations include Candida parapsilosis [44,56,62], Candida krusei [40,62], Candida tropicalis [40], Aspergillus brasiliensis [27], Malassezia furfur [57], and Fusarium oxysporum [33]. Although these species have been associated with human infections, they are not considered foodborne pathogens.
C. albicans has shown different susceptibilities to essential oils obtained from native Brazilian Eugenia species. The MIC values of the essential oils from leaves of E. dysenterica, E. involucrata, and E. uniflora against C. albicans strain ATCC 10231 were higher than 10 mg/mL [27], equal to 1.75 mg/mL [30], and equal to 0.39 mg/mL [38], respectively. These results are associated with the diversity of chemical compounds found in essential oils of different plant species. Furthermore, C. parapsilosis was shown to be more sensitive to essential oil from E. uniflora leaves than C. albicans [44], whereas the latter was more sensitive than C. tropicalis and C. krusei when subjected to the same plant essential oil [40,62]. Besides differences in the chemical compositions of essential oils, the biological variability between yeast and fungal species, as discussed for bacterial strains and genera, also plays a relevant role in their sensitivities.
The anti-yeast and antifungal potential of essential oils obtained from native Brazilian Eugenia species is attributed to the presence of some phytochemicals including monoterpenes and sesquiterpenes [34,36,56]. Because of its lipophilic nature, the essential oil can permeate the yeast and fungal cell walls, disrupting the activity of enzymes crucial for its synthesis. Furthermore, essential oils can also create a pH gradient across the cytoplasmic membrane, avoiding energy production and inducing changes in yeast and fungal membrane structure [66].
Besides the determination of the MIC and MFC, researchers have applied the agar diffusion method to evaluate the effects of essential oils from leaves of E. brasiliensis and E. uniflora on the growth of F. oxysporum (Table 3). A significant level of inhibition was observed for both essential oils 72 h after their application (66.5% inhibition). Germacrone, which accounted for approximately one-third of the composition of the essential oil from E. uniflora leaves (34.59%), is considered a potent antimicrobial compound, while the presence of oxygenated terpenoids, phenolic alcohols, and aldehydes present in the essential oil from E. brasiliensis leaves are relevant for its fungicidal activity [33].
Overall, the results of antimicrobial screening tests of essential oils obtained from native Brazilian Eugenia species reported in previous investigations showed different levels of microbial inactivation, which can be attributed to their highly diverse chemical composition. These results provide information for developing biopreservation solutions for foods and alternative drugs to treat infections caused by the evaluated microorganisms. Future studies should focus on the optimization of strategies for the application of these essential oils in foods. Additionally, the antimicrobial efficacy of their application in combination with currently applied antibiotics or methods for microbial inactivation must also be considered and evaluated.
4.2. Antioxidant Activity
Oxidative stress or cellular damage triggered by free radicals or reactive oxygen/nitrogen species (ROS/RNS) appears to be the fundamental mechanism behind the development of various non-communicable diseases (NCDs), including inflammatory, cardiovascular, neurodegenerative, and other chronic diseases. Compounds naturally present in foods or medicinal plants can scavenge free radicals and ROS/RNS, acting as antioxidants and consequently reducing oxidative damage [67]. Several in vitro studies have demonstrated that essential oils obtained from native Brazilian Eugenia species exhibit antioxidant activity in different assays (see Table 4).

Table 4.
A summary of studies showing the in vitro antioxidant activity of essential oils obtained from native Brazilian plants of the genus Eugenia.
Among native Brazilian Eugenia species, essential oils from E. uniflora were the most investigated for their antioxidant activity. Furthermore, most studies evaluated the antioxidant activity of the plant leaves (Table 4), while only one study assessed the essential oil of other plant parts, namely, the flowers [34]. Regarding the commonly used in vitro methods for screening the antioxidant activity of Eugenia species essential oils, the DPPH assay has been the most applied. At the same time, some studies have also conducted ABTS, FRAP, β-carotene/linoleic acid, iron-reducing power, and phosphomolybdenum-reducing assays. Additionally, most studies chose to determine the antioxidant activity of essential oils through the IC50, which refers to the concentration of essential oil that results in the inhibition and/or sequestration of 50% of a radical species compared with the control [45].
Several researchers have used IC50 values obtained from the DPPH assay to classify plant essential oils according to their antioxidant activity. Essential oils with IC50 values ≤50 µg/mL, 51–100 µg/mL, 101–250 µg/mL, 251–500 µg/mL, and >500 µg/mL are considered very strongly active, strongly active, moderately active, weakly active, and inactive, respectively [68]. According to this classification, only the essential oils from E. stipitata leaves (IC50 value of 12.868 µg/mL) [21] and E. klotzschiana flowers and leaves (IC50 values of 5.70 and 6.48–29.77 µg/mL, respectively) can be considered potent antioxidants (very strongly active) [34], while the essential oil from E. uniflora leaves is moderately active (IC50 value of 100.96 µg/mL) [45]. The remaining essential oils were classified as inactive (IC50 values >500 µg/mL).
Plant essential oils consist of a complex mixture of compounds that can exhibit different antioxidant mechanisms of action. Therefore, for a better understanding of the antioxidant potential of an essential oil, it is necessary to subject it to various antioxidant assays that evaluate different mechanisms of action, including assays based on hydrogen atom transfer (e.g., β-carotene/linoleic acid) and single electron transfer (e.g., FRAP, phosphomolybdenum-reducing, and iron-reducing power), as well as mixed-mode assays (e.g., DPPH and ABTS) [69]. Considering the different mechanisms of action evaluated by the various antioxidant assays, all essential oils from native Brazilian Eugenia species exhibited some antioxidant activity. For example, the essential oil from E. stipitata leaves was most active in the β-carotene bleaching assay, indicating that its compounds act particularly through hydrogen atom transfer [21]. On the other hand, essential oils from E. brasiliensis and E. uniflora leaves seemed to be more effective as single electron donors (they showed high antioxidant activity values in the FRAP, iron-reducing power, and phosphomolybdenum-reducing assays) and almost ineffective as hydrogen atom donors (they showed low antioxidant activity values in the β-carotene/linoleic acid assay) [31,38,45,46]. The essential oil from E. stipitata leaves showed similar antioxidant activity values in the DPPH and ABTS assays, as both assays are mixed-mode tests [21,22]. The different profiles and contents of compounds present in the essential oils are responsible for the variation in antioxidant activity values and observed mechanisms of action among the essential oils of native Brazilian Eugenia species. Carneiro et al. [34] evaluated the effect of plant part and drying method on the antioxidant activity of E. klotzschiana essential oils and observed that both factors substantially impact the antioxidant activity values observed in the DPPH and ABTS assays. The authors reported that the essential oil obtained from the flowers (IC50 value of 5.70 µg/mL for DPPH and 104.61 µM Trolox equivalents/g for ABTS) exhibits higher antioxidant activity than the essential oil from the leaves (IC50 value of 29.77 µg/mL for DPPH and 57.81 µM Trolox equivalents/g for ABTS). Additionally, they found that the essential oil obtained from shade-dried leaves (IC50 value of 6.48 µg/mL for DPPH and 143.85 µM Trolox equivalents/g for ABTS) was more active than that obtained from leaves dried by forced air circulation at 40 °C (IC50 value of 7.61 µg/mL for DPPH and 106.27 µM Trolox equivalents/g for ABTS). Both the plant part and the drying method affected the profile and content of active principles in E. klotzschiana essential oils, explaining the different antioxidant activity values found (for more details on the chemical composition, see Table 2).
Seasonality is another important factor that can affect an essential oil’s chemical composition and its antioxidant activity. Some studies have demonstrated the effect of seasons on the composition and antioxidant activity of essential oils from E. brasiliensis and E. uniflora leaves [31,47,50]. When evaluating the impact of seasonality on essential oil from E. brasiliensis leaves, Siebert et al. [31] found that the highest inhibition of lipid peroxidation was observed for the essential oil from the spring (14.05% inhibition). At the same time, the best iron-reducing power was recorded for the essential oil from the autumn (94.32 mg ascorbic acid equivalents/g). Essential oil from the spring was mainly composed of spathulenol (16.02%), τ-cadinol (15.30%), 1-epi-cubenol (7.02%), and α-cadinol (6.04%), while τ-cadinol (12.83%), β-caryophyllene (8.65%), spathulenol (8.10%), and globulol (7.87%) were the predominant compounds in the essential oil from the autumn. The different profiles and contents of active compounds observed among essential oils obtained in different seasons may explain their different antioxidant activity values and mechanisms of action. On the other hand, Cipriano et al. [50] observed that the essential oil from E. uniflora leaves obtained in the summer showed the highest antioxidant activity value in the DPPH assay (IC50 value of 0.62 mg/mL), followed by the oils from the spring, winter, and autumn (IC50 values of 0.98, 2.01, and 2.73 mg/mL, respectively). The authors attributed the highest antioxidant activity values of the essential oils from the summer and spring to the higher concentration of germacrone present in them (45.00 and 48.05%, respectively) compared with essential oils from other seasons (41.15–42.68%), as germacrone has been reported as a potent free radical scavenger. Similar results were reported by da Costa et al. [47] in essential oils from E. uniflora leaves, where the most active essential oils were those obtained from leaves collected in December and January (435.0 and 436.3 mg Trolox equivalents/g, respectively), which corresponds to the Brazilian summer. The authors verified through hierarchical clustering analysis (HCA) and principal components analysis (PCA) that these essential oils exhibited great similarity in their chemical compositions.
Some studies have also shown that the specimen and genotype can also affect the antioxidant activity of essential oils from Eugenia species [46,52]. Figueiredo et al. [46] evaluated the antioxidant activity of the essential oil from the leaves obtained from five different E. uniflora specimens. They found that the highest antioxidant activities were reported for specimens 3 and 4 (228.3 and 217.0 mg Trolox equivalents/mL, respectively), which belonged to chemotypes II and III, respectively. Chemotype II displayed significant content of selina-1,3,7(11)-trien-8-one (18.1%) and selina-1,3,7(11)-trien-8-one epoxide (16.0%), followed by a highlighted amount of caryophyllene oxide (18.1%). Meanwhile, chemotype III was dominated by curzerene (50.6%), followed by germacrene B (5.2%) and germacrone (4.5%). Cipriano et al. [52] studied the antioxidant activity of essential oils obtained from the leaves of 36 E. uniflora genotypes. The authors noted that genotype significantly impacts the antioxidant activity of the essential oils, with values ranging between 176.66 and 867.57 μmol Trolox equivalents/L. Genotypes A05, A11, and A29 exhibited the most active essential oils (867.57, 861.51, and 843.33 μmol Trolox equivalents/L). The essential oils from genotypes A05 and A11 were dominated by germacrone (46.52 and 65.03%, respectively), followed by curzerene (7.95 and 9.21%, respectively). On the other hand, the essential oil from genotype A29 was particularly composed of curzerene (18.45%), followed by similar amounts of germacrene B (9.40%), germacrone (9.15%), and furanodiene (8.36%). These findings demonstrate that different chemical compositions can result in similar antioxidant activities. Thus, the antioxidant activity of essential oils should not be considered solely concerning their major constituents, as the synergistic action of other minor components may contribute to an increase in antioxidant activity.
These outcomes demonstrate that the essential oils from native Brazilian Eugenia species can be promising sources of natural antioxidants for managing diseases related to oxidative stress, such as non-communicable chronic diseases. Additionally, they can also be used in the food industry as substitutes for synthetic antioxidants, extending the shelf life of food products. The pharmaceutical and cosmetic industries can also utilize their antioxidant characteristics to develop innovative products.
4.3. Anticancer Activity
Cancer encompasses a range of conditions marked by uncontrolled and excessive cellular proliferation, leading to the formation of abnormal tissue masses. This disease poses a substantial public health challenge worldwide, impacting approximately 11 million individuals annually and contributing to one in every six deaths globally [70]. Recent studies have revealed the promising anticancer potential of essential oils from areal parts of native Brazilian Eugenia species against different types of cancer (see Table 5).

Table 5.
A summary of studies showing the in vitro anticancer activity of essential oils obtained from native Brazilian plants of the genus Eugenia.
As can be seen in Table 5, the anticancer activity of essential oil from E. uniflora leaves has been evidenced by different studies. Antonelo et al. [38] noticed that essential oil from E. uniflora leaves exhibited low cytotoxic activity against human adrenocortical carcinoma (H295R cells, IC50 value of 322.94 µg/mL), being even more cytotoxic against non-cancerous kidney epithelial cells (VERO cells, IC50 value of 101.37 µg/mL). On the other hand, Sobeh et al. [44] demonstrated that this essential oil was able to significantly inhibit the proliferation of human breast cancer cells (MCF7 cells, IC50 value of 76.4 µg/mL). A very similar result was found by da Silva et al. [48] when evaluating the effect of essential oil from E. uniflora leaves on the same cancer cell line (MCF7 cells, IC50 value of 76.5 µg/mL). Additionally, the authors demonstrated that this essential oil was also effective against glioblastoma (M059J cells) and cervical adenocarcinoma (HeLa cells) human cancer cells, with IC50 values of 84.5 and 106.2 µg/mL, respectively. However, it was highly toxic to non-cancerous human lung fibroblasts (GM07492A cells, IC50 value of 39.8 µg/mL), which would limit its use because of low selectivity. In another study, Figueiredo et al. [46] investigated the effect of essential oil obtained from the leaves of different E. uniflora specimens against three human cancer cell lines (colon (HCT116 cells), gastric (AGP01 cells), and melanoma (SKMEL-19 cells)) and non-malignant human lung fibroblast cells (MRC5 cells). Their results demonstrated that the essential oils obtained from E. uniflora specimens 2 and 4, which contained significant amounts of selina-1,3,7(11)-trien-8-one (43.1%), selina-1,3,7(11)-trien-8-one epoxide (21.7%), and curzerene (50.6%), displayed the highest antiproliferative activities against all tested cancer cell lines (IC50 values ranging from 8.73 to 16.26 µg/mL). However, once again, these essential oils showed a high cytotoxic effect against normal cells (MRC5 cells, IC50 values of 10.27 and 14.95 µg/mL, respectively). The geographical origin and growth conditions (e.g., fertilization, maturity, soil conditions, climatic conditions, and seasonal factors) of the plant had a significant impact on the chemical composition of its essential oils, directly reflecting the different results of anticancer activity presented by the evaluated E. uniflora specimens [44,46]. Figueiredo et al. [46] also evaluated the cytotoxic effect of curzerene, the main compound found in the essential oil from E. uniflora leaves of specimen 4 (50.6%), and found that this compound was highly active against all cancer cell lines (IC50 values ranging from 5.17 to 9.18 µmol/L), particularly against human melanoma cells (SKMEL-19 cells), with an IC50 value and a selectivity index of 5.17 µmol/L and 2.21, respectively. Furthermore, further analysis revealed that curzerene exerted its anticancer effect on melanoma cells by inducing cellular apoptosis and inhibiting cell migration [46].
In addition to the essential oil of E. uniflora leaves, essential oils from the aerial parts of E. stipitata, E. involucrata, and E. pyriformis also displayed antiproliferative activity (see Table 5). Jerônimo et al. [23] investigated the cytotoxic effect of the essential oil from E. stipitata leaves against four human cancer cell lines (breast (MCF7 cells), melanoma (SKMEL-19 cells), gastric (AGP01 cells), and colon (HCT116 cells)) and non-malignant human lung fibroblast cells (MRC5 cells). The essential oil was active against melanoma and breast human cancer cells (IC50 values of 17.2 and 19.1 µg/mL, respectively), but it also exerted a toxic effect on normal human fibroblasts (IC50 value of 13.8 µg/mL), demonstrating effectiveness but low selectivity against cancer cells. Similar results were reported by D’Almeida et al. [29] for the essential oil from E. involucrata leaves, in which the authors also found a highly cytotoxic effect against murine melanoma cells (B16F10 cells) and human breast cancer cells (MCF7 cells), as well as non-cancerous murine fibroblasts (McCoy cells) (IC50 values of 14.12, 24.06, and 16.59 µg/mL, respectively). Morphological analyses demonstrated that this essential oil promoted cell death through the induction of apoptosis, as morphological changes consistent with apoptosis rather than necrosis were observed in the cells, including blebs formation, chromatin condensation, and cell rounding. Similarly, Durazzini et al. [57] studied the antiproliferative effect of the essential oil from the aerial parts of E. pyriformis (leaves and twigs) against three human cancer cell lines (breast (MCF7), glioblastoma (M059J), and cervical adenocarcinoma (HeLa)) and human lung fibroblasts (GM07492A cells). The essential oil was most active against human breast cancer cells (MCF7 cells, IC50 value of 73.09 µg/mL), showing effects similar to the positive control doxorubicin (IC50 value of 62.10 µg/mL) and high selectivity (selectivity index of 1.91).
In short, the promising cytotoxic and antiproliferative effects against different types of cancer cells exhibited by essential oils from Brazilian native Eugenia species can be explained by the presence of various bioactive phytochemicals in their compositions, primarily terpene compounds (for more details regarding the composition of these essential oils, please refer to Table 2). The literature has reported that some terpenes identified in these essential oils (e.g., β-caryophyllene, β-elemene, curzerene, germacrene D, and bicyclogermacrene) have significant anticancer activity [71,72,73,74]. These compounds generally act synergistically, with their primary target in the cell being the cytoplasmic membrane. Studies have reported that these substances can disrupt cell membranes and impact crucial cellular functions, leading to cell death by apoptosis or necrosis [57]. However, the few molecular studies conducted with essential oils from Brazilian native Eugenia species have indicated that they predominantly act through cellular apoptosis [29,46].
Despite showing promising results against human cancer cells, most essential oils from Brazilian native Eugenia species exhibited a low selectivity index, demonstrating that they can also exert toxic effects on normal cells and tissues. Therefore, further isolation and/or technological approaches should be carried out to obtain active agents against tumor cells without causing harm to normal tissues. Additionally, in vivo studies should be conducted to ensure these essential oils’ efficacies and safe uses for therapeutic purposes.
4.4. Antiparasitic Activity
Trypanosomiasis and leishmaniasis are a group of vector-borne infectious diseases belonging to Neglected Tropical Diseases (NTDs) according to the World Health Organization (WHO). These diseases are caused by infection with kinetoplastid protozoan parasites, namely, Trypanosoma spp. and Leishmania spp., affecting humans and animals in tropical and subtropical areas. It is estimated that more than 1 billion people worldwide are affected annually by NTDs, generating health concerns and a cumulative economic burden worth billions of United States dollars each year [75,76]. As can be seen in Table 6, essential oils from some native Brazilian Eugenia species have shown significant activity against these human pathogenic protozoa.

Table 6.
A summary of studies showing the in vitro antiparasitic activity of essential oils obtained from native Brazilian plants of the genus Eugenia.
dos Santos et al. [22] evaluated the antiprotozoal effect of the essential oil from E. stipitata leaves against Trypanosoma cruzi epimastigotes and Leishmania braziliensis and Leishmania infantum promastigotes. The results demonstrated that the essential oil exhibited potent leishmanicidal activity (LC50 values ranging from 37.65 to 54.71 µg/mL) but lacked trypanocidal activity (LC50 value greater than 1000 µg/mL). The essential oil was more active against L. braziliensis promastigotes (LC50 value of 37.65 µg/mL) and showed low cytotoxicity against fibroblast cells (LC50 value of 511.5 µg/mL). Thus, this essential oil was highly selective for L. braziliensis and L. infantum promastigotes, with selectivity indices of 13.58 and 9.35, respectively.
Unlike the essential oil from E. stipitata leaves, the essential oils from E. dysenterica and E. uniflora leaves and E. klotzschiana flowers exhibited high trypanocidal activity (see Table 6). The essential oil from E. dysenterica leaves was highly active against T. cruzi trypomastigotes (LC50 value of 9.5 µg/mL), with an effect practically equal to that exerted by the reference drug benznidazole (LC50 value of 9.8 µg/mL) [28]. Similar results were observed by Carneiro et al. [35] for the essential oil from E. klotzschiana flowers against T. cruzi trypomastigotes (LC50 value of 20.2 µg/mL). Although less active than the reference drug benznidazole, this essential oil exhibited low cytotoxicity against fibroblast cells (LC50 value of 220.3 µg/mL), demonstrating its highly selective action against the protozoa (selectivity index of 10.91). Sobeh et al. [44] also found a high susceptibility of T. brucei brucei to the essential oil from E. uniflora leaves with a selectivity index of 6.82.
In addition to the essential oil from E. stipitata leaves, the essential oils from the aerial parts of E. uniflora and E. pyriformis also exhibited promising leishmanicidal effects (see Table 6). The essential oils from E. uniflora leaves [48], E. pyriformis leaves [77], and the aerial parts of E. pyriformis (leaves and twigs) [57] were highly active against L. amazonensis promastigotes, with LC50 values of 0.99, 19.73, and 2.16 µg/mL, respectively. da Silva et al. [48] found that the essential oil from E. uniflora leaves (LC50 value of 0.99 µg/mL) exhibited a leishmanicidal effect very close to that of the standard drug amphotericin B (LC50 value of 0.60 µg/mL). Likewise, Kauffmann et al. [77] observed that the essential oil from E. pyriformis leaves and the standard drug pentamidine isethionate showed almost equal effectiveness against L. amazonensis (LC50 values of 19.73 and 23.22 µg/mL, respectively). Meanwhile, Durazzini et al. [57] demonstrated that the essential oil from the aerial parts of E. pyriformis (leaves and twigs) had only moderate cytotoxicity against human lung fibroblasts (LC50 value of 141.27 µg/mL), being highly selective against L. amazonensis (selectivity index of 65.4).
The promising antiparasitic activity against Trypanosoma spp. and Leishmania spp. exhibited by the essential oils of native Brazilian Eugenia species may be due to the major constituents identified in each essential oil, particularly terpenes (for more details regarding the composition of these essential oils, please refer to Table 2). Different terpenes identified in these essential oils possess recognized trypanocidal and leishmanicidal activities reported in the literature (e.g., α-pinene, β-eudesmol, γ-eudesmol, caryophyllene oxide, limonene, citral, β-caryophyllene, eugenol, and nerolidol) [78,79,80,81,82,83,84,85,86,87,88]. These compounds can act synergistically, enhancing the antiparasitic effect of each essential oil. Because of their hydrophobic nature, the terpenes present in the essential oils can diffuse through a parasite’s cell membrane, thus affecting its metabolic pathways and intracellular organelles [35]. These substances can interact with polysaccharides, lipids, and phospholipid layers in parasite membranes, inducing cellular lysis and macromolecule extravasation. Once in the cytoplasm, these compounds can interrupt specific metabolic pathways of proteins and lipids or stimulate the depolymerization of the mitochondrial membrane by decreasing membrane potential, affecting ionic Ca2+ cycling and other ionic channels, and reducing the pH gradient that disturbs the proton pump and ATP pool. This depolymerization affects the fluidity of the inner and outer mitochondrial membranes, which become abnormally permeable, resulting in the release of radicals, calcium ions, cytochrome C, and proteins that, in turn, lead to the parasite’s death by necrosis and apoptosis [22,48].
These studies demonstrate that the essential oils from native Brazilian Eugenia species can be promising and innovative sources of active agents for developing drugs to treat NTDs, particularly trypanosomiasis and leishmaniasis.
4.5. Other Biological Activities
In addition to the biological activities already mentioned and addressed above, essential oils from some native Brazilian Eugenia species exhibit anti-inflammatory, antinociceptive, antipyretic, antidiarrheal, and wound healing effects, as listed in Table 7.

Table 7.
A summary of studies showing the other biological activities of essential oils obtained from leaves of native Brazilian plants of the genus Eugenia.
Endogenous or exogenous antigens can interact with the cellular immune system, leading to the generation of ROS and RNS, which, in turn, trigger signaling cascades that may result in the hyperactivation of inflammatory responses. These responses can induce oxidative stress and tissue damage phenomena, which are the main factors contributing to the development, progression, and worsening of most known diseases [89]. Studies conducted in in vitro, cellular, and animal models have demonstrated that essential oils from E. stipitata, E. dysenterica, and E. uniflora leaves exhibit promising anti-inflammatory activities (see Table 7).
Costa et al. [24] evaluated the anti-inflammatory effect of the essential oil from E. stipitata leaves in in vitro (inhibition of protein denaturation) and animal models (mice orally treated with the essential oil). Their study demonstrated that the essential oil from E. stipitata leaves was able to inhibit BSA denaturation, exhibiting greater inhibitory effects (74.38–77.20% inhibition) compared with the standard drug sodium diclofenac (45.02–67.00% inhibition) when evaluated at the same concentrations (0.25 to 1 mg/mL). The anti-inflammatory effects of this essential oil were confirmed in animal models (carrageenan-induced acute inflammation), where its oral administration (40–250 mg/kg bw) reduced paw edema volume (88.66–96.94% inhibition) and the migration of leukocytes (76.9–86.5% inhibition) and neutrophils (74.5–77.9% inhibition) into the peritoneal cavity of mice. It is important to note that these results were similar or even superior to those found for the standard drug indomethacin at 20 mg/kg bw. Furthermore, no death or alteration in behavior signs, water and food consumption, and biochemical, hematological, or histopathological parameters were observed in mice treated with 2000 mg/kg bw during the 14 experiment days, demonstrating that this essential oil is non-toxic. The authors attributed the anti-inflammatory effects particularly to β-caryophyllene, one of the major compounds in this essential oil (11.36% of the oil composition), which is capable of reducing the synthesis/expression of inflammatory mediators such as prostaglandin E2, IL-1β, and TNF-α.
da Silva et al. [26] found that the essential oil from E. dysenterica leaves and α-humulene (one of the main components of the essential oil, accounting for approximately 19.3% of its composition) also exhibited anti-inflammatory activity by inhibiting nitric oxide production in LPS-stimulated RAW 264.7 murine macrophages. Moreover, both the essential oil (146.3 and 292.6 µg/mL) and α-humulene (155.58 and 311.15 µg/mL) were significantly more active than the positive control dexamethasone (62.5 and 125 µg/mL) and showed low cytotoxicity against mouse fibroblast cells (L929) and macrophages cells (RAW 264.7) (LC50 ≤ 292 µg/mL). This essential oil is predominantly composed of α-humulene (19.3%) and β-caryophyllene (24.36%). The authors suggested that these compounds may be involved in the anti-inflammatory responses of the essential oil since the literature shows that they inhibit various pro-inflammatory mediators, including interleukins (IL-2, IL-10, and IL-1β), TNF-α, histamine, and prostaglandin E2.
Anti-inflammatory effects have also been reported for the essential oil from E. uniflora leaves. de Jesus et al. [43] found that oral treatment of mice with essential oil from E. uniflora leaves reduced xylene-induced ear edema at all tested doses (50–200 mg/kg bw; 50.26–55.17% inhibition) and carrageenan-induced leukocyte recruitment in the peritoneal cavity at a concentration of 200 mg/kg bw (47.25% inhibition). It is noteworthy that treatment with 200 mg/kg bw showed leukocyte migration inhibition results significantly equal to the control drug dexamethasone at 1 mg/kg bw. Additionally, oral administration of up to 2000 mg/kg bw did not induce behavioral changes, mortality, or impairments in hepatic and renal functions of the mice over 14 experiment days, demonstrating the absence of acute oral toxicity. The authors attributed the anti-inflammatory effects of this essential oil to its ability to inhibit the release of inflammatory mediators, reducing vascular permeability and vasodilation, and, consequently, leukocyte and neutrophil migration. Curzerene (33.4%), caryophyllene oxide (7.6%), β-elemene (6.5%), and β-caryophyllene (4.1%) were the major components identified in this essential oil. These compounds may be associated, at least in part, with the anti-inflammatory effects exerted by the essential oil from E. uniflora leaves.
It is well documented in the literature that plants exhibiting anti-inflammatory activity also exert analgesic and antipyretic effects. In fact, the essential oils from E. stipitata and E. uniflora leaves, which were potent anti-inflammatory agents, also demonstrated antinociceptive and/or antipyretic activity in animal models (see Table 7). Different doses of essential oil from E. stipitata leaves (40–250 mg/kg bw) were able to reduce rectal temperature from the first hour after the oral administration in mice induced by Saccharomyces cerevisiae. The animals’ temperatures remained below febrile values from 1 h and were maintained until the end of the experiment (4 h). Additionally, this essential oil exhibited analgesic effects by reducing the number of acetic acid-induced abdominal writhings in mice (54.1–56.6% inhibition) [24]. Similar results were reported by de Jesus et al. [43] in mice treated with essential oil from E. uniflora leaves. They noted that oral administration of this essential oil reduced the number of acetic acid-induced abdominal writhings (50–200 mg/kg bw; 33.33–61.66% inhibition), formalin-induced neurogenic (200 mg/kg bw; 63.43% inhibition), and inflammatory pain (50–200 mg/kg bw; 30.54–80.87% inhibition) but did not increase latency to response in the hot-plate test. Furthermore, no changes in motricity or sedation were observed in animals treated with essential oil from E. uniflora leaves (300 mg/kg bw), demonstrating that the analgesic results were not influenced by motor or sedative effects. These results demonstrate that these essential oils have peripheral antinociceptive action but not central. The antipyretic and analgesic effects observed in these essential oils may be associated with their major components (e.g., β-caryophyllene) that can reduce the expression of inflammatory mediators, as discussed above, and act as an endocannabinoids type 2 agonist [24,43].
In addition to anti-inflammatory activity, antidiarrheic and wound healing effects have also been reported in essential oils from E. dysenterica leaves (see Table 7). Galheigo et al. [25] noticed that oral administration of essential oil from E. dysenterica leaves (300 mg/kg bw) reduced castor oil-induced diarrhea by decreasing the total number of feces, the number of diarrheic feces, and the accumulation of intestinal fluids without altering the distance traveled by charcoal meal in the intestine. These results suggest that the antidiarrheic effect of this essential oil and its volatile components (particularly (Z)-β-ocimene (19.14%) and β-caryophyllene (15.36%)) seems to be linked to its ability to inhibit intestinal secretion and/or increase intestinal absorption without involving the inhibition of gastrointestinal motility. In another study, da Silva et al. [26] demonstrated that essential oil from E. dysenterica leaves (542.2 µg/mL) stimulated the migration of skin cells (fibroblasts) in mechanically induced wounds, promoting complete wound closure in just 12 h. This essential oil proved to be safe for dermatological applications as it did not cause irritation or injury to the skin in the chorioallantoic membrane assay at concentrations ≤ 292 µg/mL. Additionally, the essential oil exhibited anti-inflammatory activity by inhibiting nitric oxide production in LPS-induced macrophages and angiogenic effects by increasing the number of blood vessels in the chorioallantoic membrane assay. Thus, essential oil from E. dysenterica leaves may promote wound healing by modulating inflammation, cell migration, and blood vessel sprouting. Instrumental analyses have shown that this essential oil contains high concentrations of bioactive sesquiterpene compounds, such as β-caryophyllene (24.36%) and α-humulene (19.3%), suggesting that these compounds may act synergistically and be responsible for their wound healing activity.
These studies together demonstrate that the essential oils of Brazilian native Eugenia species can be promising sources of active principles for the development of drugs and cosmetics aimed at wound healing, pain relief, fever, diarrhea, and inflammatory processes.
5. Limitations, Challenges, and Future Research Directions
Although the findings compiled here have demonstrated that essential oils obtained from native Brazilian Eugenia species can inhibit or delay food spoilage microbial growth and oxidative processes, there are still significant limitations to the current findings, particularly related to their effectiveness in food systems. Moreover, more advanced analytical techniques are needed to better explore the antioxidant and antimicrobial mechanisms of these essential oils, as the specific in vitro assays that produce positive results are conducted under simple and highly controlled laboratory conditions that do not mimic the environment found in food systems, making it difficult to scale up these procedures to a commercial level. Therefore, it is essential to develop and standardize formulations with these essential oils to reduce chemical variability and consequently improve their efficacy as natural preservative agents. Additionally, the effectiveness of these essential oils needs to be evaluated in complex food systems (e.g., food packaging, edible films, food products, among others) to confirm the findings observed in controlled systems. Toxicological studies must also be conducted to ensure that the effective concentrations of these essential oils in food systems do not compromise the health and well-being of consumers.
These essential oils can be sources of active principles for the development of cosmetics, personal hygiene products, and medications to treat/manage various pathological conditions, such as foodborne intoxications and infections caused by pathogenic microorganisms, oxidative stress, cancer, inflammation, pain, fever, diarrhea, wounds, and diseases associated with human parasites, including Chagas disease and leishmaniasis. However, most of these studies have been conducted in vitro under conditions that do not always replicate the complex biological environments found in vivo. Furthermore, the chemical complexity of these essential oils complicates the standardization of treatments and the precise prediction of therapeutic outcomes. Translating the results reported in in vitro and animal studies into clinical applications faces several obstacles, including toxicity, volatility, and instability. Thus, it is essential to conduct clinical studies to validate health claims and determine the toxic dose of these essential oils to ensure that the developed products are safe and effective for consumers. Furthermore, cutting-edge technologies for encapsulating these essential oils (e.g., nanoencapsulation, liposomes, and emulsions) can be applied to increase stability and reduce the volatility of the active principles and control and direct their release in a target tissue, thereby improving their efficacy and safety. The synergy between these essential oils and other conventional drugs can also be another research area to explore, as their combination may reduce the effective doses required for managing pathologies and minimize the side effects of conventional medications.
6. Conclusions
Brazilian native plants have attracted growing interest from consumers and researchers around the world because of their unique flavor and aroma characteristics as well as claims of beneficial health effects. Among these, plants belonging to the genus Eugenia stand out because of the large number of species spread across Brazilian territory. The data collected here demonstrate that some native Brazilian Eugenia species have emerged as potential sources for obtaining essential oils with significant biological properties, such as E. stipitata, E. dysenterica, E. involucrata, E. brasiliensis, E. klotzschiana, E. uniflora, and E. pyriformis. Instrumental analyses have shown that the essential oils obtained from these plants are composed of extremely complex mixtures of phytochemicals, potentially containing more than 80 compounds. Sesquiterpene hydrocarbons and their oxygenated derivatives are the main components of these essential oils, with monoterpenes and other classes of compounds (e.g., phenylpropanoids) being found only in small quantities. The synergism among these different classes of compounds may explain the biological activities of these essential oils observed in in vitro assays and animal trials, as essential oils with different compositions presented similar effects. Evidence has shown that several factors can affect the chemical composition of these essential oils and consequently their biological activities, including plant part, sample preparation, extraction conditions, soil composition and climatic conditions where the plant grows, season, age and vegetative cycle stage, and genotypes or specimens, among others.
The discoveries made so far demonstrate that the native Brazilian Eugenia species can be a valuable source of essential oils with active principles for developing innovative and sustainable products for food, medicinal, and cosmetic applications. Thus, these essential oils can be used as a strategic action to meet some SDGs, particularly SDG 3 (good health and well-being), SDG 9 (industry, innovation, and infrastructure), and SDG 12 (responsible consumption and production). However, some gaps, particularly those related to the effectiveness of these essential oils as preservative agents in food systems and the confirmation of their safety and beneficial effects through clinical/toxicological studies, still need to be further explored in future studies. Furthermore, it is important to emphasize that sustainable practices must be adopted throughout the production chain to ensure the preservation of Brazilian biodiversity.
Author Contributions
Conceptualization, H.S.A., A.P. and F.T.B.; methodology, H.S.A.; investigation, H.S.A., A.P. and F.T.B.; resources, H.S.A. and G.M.P.; data curation, H.S.A. and F.T.B.; writing—original draft preparation, H.S.A., A.P. and F.T.B.; writing—review and editing, all authors; supervision, H.S.A.; project administration, H.S.A.; funding acquisition, H.S.A. and G.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES, Finance Code 001), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant number 406820/2018-0), and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grant number 2020/08761-4). Henrique Silvano Arruda thanks the CAPES (grant number 88887.469390/2019-00) for his postdoctoral assistantship. Felipe Tecchio Borsoi thanks the CAPES (Finance Code 001) for his doctorate assistantship.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of this manuscript; or in the decision to publish the results.
References
- Peixoto Araujo, N.M.; Berni, P.; Zandoná, L.R.; de Toledo, N.M.V.; da Silva, P.P.M.; de Toledo, A.A.; Maróstica Junior, M.R. Potential of Brazilian Berries in Developing Innovative, Healthy, and Sustainable Food Products. Sustain. Food Technol. 2024, 2, 506–530. [Google Scholar] [CrossRef]
- Arruda, H.S.; Silva, E.K.; Peixoto Araujo, N.M.; Pereira, G.A.; Pastore, G.M.; Marostica Junior, M.R. Anthocyanins Recovered from Agri-Food By-Products Using Innovative Processes: Trends, Challenges, and Perspectives for Their Application in Food Systems. Molecules 2021, 26, 2632. [Google Scholar] [CrossRef] [PubMed]
- United Nations. The 17 Goals—Sustainable Development. Available online: https://sdgs.un.org/goals# (accessed on 19 March 2024).
- Henderson, K.; Loreau, M. A Model of Sustainable Development Goals: Challenges and Opportunities in Promoting Human Well-Being and Environmental Sustainability. Ecol. Model. 2023, 475, 110164. [Google Scholar] [CrossRef]
- Arruda, H.S.; Angolini, C.F.F.; Eberlin, M.N.; Pastore, G.M.; Marostica Junior, M.R. UHPLC-ESI-QTOF-MS/MS Profiling of Phytochemicals from Araticum Fruit (Annona crassiflora Mart.) and Its Antioxidant Activity. Foods 2023, 12, 3456. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, A.P.A.; Conte-Junior, C.A. Health Benefits of Phytochemicals from Brazilian Native Foods and Plants: Antioxidant, Antimicrobial, Anti-Cancer, and Risk Factors of Metabolic/Endocrine Disorders Control. Trends Food Sci. Technol. 2021, 111, 534–548. [Google Scholar] [CrossRef]
- Farias, T.R.B.; Sanches, N.B.; Petrus, R.R. The Amazing Native Brazilian Fruits. Crit. Rev. Food Sci. Nutr. 2023, 1–18. [Google Scholar] [CrossRef] [PubMed]
- REFLORA. Eugenia in Flora e Funga Do Brasil. Available online: https://floradobrasil.jbrj.gov.br/consulta/?grupo=6&familia=171&genero=Eugenia&especie=&autor=&nomeVernaculo=&nomeCompleto=&formaVida=null&substrato=null&ocorreBrasil=QUALQUER&ocorrencia=OCORRE&endemismo=TODOS&origem=TODOS®iao=QUALQUER&ilhaOceanica=32767&estado=QUALQUER&domFitogeografi-cos=QUALQUER&vegetacao=TODOS&mostrarAte=SUBESP_VAR&opcoesBusca=TODOS_OS_NOMES&loginUsuario=Visitante&senhaUsuario=&contexto=consulta-publica&pagina=1# (accessed on 20 March 2024).
- Peixoto Araujo, N.M.; Arruda, H.S.; de Paulo Farias, D.; Molina, G.; Pereira, G.A.; Pastore, G.M. Plants from the Genus Eugenia as Promising Therapeutic Agents for the Management of Diabetes Mellitus: A Review. Food Res. Int. 2021, 142, 110182. [Google Scholar] [CrossRef]
- de Araújo, F.F.; Neri-Numa, I.A.; de Paulo Farias, D.; da Cunha, G.R.M.C.; Pastore, G.M. Wild Brazilian Species of Eugenia Genera (Myrtaceae) as an Innovation Hotspot for Food and Pharmacological Purposes. Food Res. Int. 2019, 121, 57–72. [Google Scholar] [CrossRef] [PubMed]
- de Souza, A.; de Oliveira, C.; de Oliveira, V.; Betim, F.; Miguel, O.; Miguel, M. Traditional Uses, Phytochemistry, and Antimicrobial Activities of Eugenia Species—A Review. Planta Med. 2018, 84, 1232–1248. [Google Scholar] [CrossRef]
- da Costa, J.S.; da Cruz, E.D.N.S.; Setzer, W.N.; da Silva, J.K.D.R.; Maia, J.G.S.; Figueiredo, P.L.B. Essentials Oils from Brazilian Eugenia and Syzygium Species and Their Biological Activities. Biomolecules 2020, 10, 1155. [Google Scholar] [CrossRef]
- da Silva, L.A.; da Silva, R.S.; de Oliveira, M.R.; Guimarães, A.C.; Takeara, R. Chemical Composition and Biological Activities of Essential Oils from Myrtaceae Species Growing in Amazon: An Updated Review. J. Essent. Oil Res. 2023, 35, 103–116. [Google Scholar] [CrossRef]
- MAPA/MMA. Espécies Nativas da Sociobiodiversidade Brasileira de Valor Alimentício—Portaria Interministerial dos Ministérios da Agricultura, Pecuária e Abastecimento e do Meio Ambiente (MAPA/MMA) No 10 de 21 de Julho de 2021. Available online: https://in.gov.br/en/web/dou/-/portaria-interministerial-mapa/mma-n-10-de-21-de-julho-de-2021-333502918 (accessed on 18 March 2024).
- Maurya, A.; Prasad, J.; Das, S.; Dwivedy, A.K. Essential Oils and Their Application in Food Safety. Front. Sustain. Food Syst. 2021, 5, 653420. [Google Scholar] [CrossRef]
- Silvestre, W.P.; Livinalli, N.F.; Baldasso, C.; Tessaro, I.C. Pervaporation in the Separation of Essential Oil Components: A Review. Trends Food Sci. Technol. 2019, 93, 42–52. [Google Scholar] [CrossRef]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; El Basuini, M.F.; Zaineldin, A.I.; Yilmaz, S.; Hasan, M.T.; Ahmadifar, E.; El Asely, A.M.; Abdel-Latif, H.M.R.; Alagawany, M.; Abu-Elala, N.M.; et al. Antiparasitic and Antibacterial Functionality of Essential Oils: An Alternative Approach for Sustainable Aquaculture. Pathogens 2021, 10, 185. [Google Scholar] [CrossRef]
- Sharma, M.; Grewal, K.; Jandrotia, R.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Essential Oils as Anticancer Agents: Potential Role in Malignancies, Drug Delivery Mechanisms, and Immune System Enhancement. Biomed. Pharmacother. 2022, 146, 112514. [Google Scholar] [CrossRef]
- Ramsey, J.T.; Shropshire, B.C.; Nagy, T.R.; Chambers, K.D.; Li, Y.; Korach, K.S. Essential Oils and Health. Yale J. Biol. Med. 2020, 93, 291–305. [Google Scholar]
- Rivera, N.; Uría, L.; Ronquillo, C.; Galarza, C.; Martínez, I. Antioxidant Potential of Five Essential Oils from Kutukú Biological Station. Pharmacologyonline 2018, 3, 161–169. [Google Scholar]
- dos Santos, C.R.B.; Sampaio, M.G.V.; Vandesmet, L.C.S.; dos Santos, B.S.; de Menezes, S.A.; Portela, B.Y.M.; Gomes, D.W.R.; Correia, M.T.S.; Gomez, M.C.V.; de Alencar Menezes, I.R.; et al. Chemical Composition and Biological Activities of the Essential Oil from Eugenia stipitata McVaugh Leaves. Nat. Prod. Res. 2023, 37, 3844–3850. [Google Scholar] [CrossRef]
- Jerônimo, L.B.; da Costa, J.S.; Pinto, L.C.; Montenegro, R.C.; Setzer, W.N.; Mourão, R.H.V.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Antioxidant and Cytotoxic Activities of Myrtaceae Essential Oils Rich in Terpenoids from Brazil. Nat. Prod. Commun. 2021, 16, 1–13. [Google Scholar] [CrossRef]
- Costa, W.K.; de Oliveira, J.R.S.; de Oliveira, A.M.; da Silva Santos, I.B.; da Cunha, R.X.; de Freitas, A.F.S.; da Silva, J.W.L.M.; Silva, V.B.G.; de Aguiar, J.C.R.d.O.F.; da Silva, A.G.; et al. Essential Oil from Eugenia stipitata McVaugh Leaves Has Antinociceptive, Anti-Inflammatory and Antipyretic Activities without Showing Toxicity in Mice. Ind. Crops Prod. 2020, 144, 112059. [Google Scholar] [CrossRef]
- Galheigo, M.R.U.; Prado, L.C.D.S.; Mundin, A.M.M.; Gomes, D.O.; Chang, R.; Lima, A.M.C.; Canabrava, H.A.N.; Bispo-da-Silva, L.B. Antidiarrhoeic Effect of Eugenia dysenterica DC (Myrtaceae) Leaf Essential Oil. Nat. Prod. Res. 2016, 30, 1182–1185. [Google Scholar] [CrossRef]
- da Silva, S.M.; Costa, C.R.; Gelfuso, G.M.; Guerra, E.S.; Nóbrega, Y.D.M.; Gomes, S.; Pic-Taylor, A.; Fonseca-Bazzo, Y.; Silveira, D.; Magalhães, P. Wound Healing Effect of Essential Oil Extracted from Eugenia dysenterica DC (Myrtaceae) Leaves. Molecules 2018, 24, 2. [Google Scholar] [CrossRef]
- Oliveira, D.C.S.; Kaneko, T.M.; Young, M.C.M.; Murakami, C.; Cordeiro, I.; Moreno, P.R.H. Chemical Composition, Antimicrobial and Antioxidant Activities of Eugenia dysenterica DC Essential Oil. Emerg. Sci. J. 2018, 2, 410. [Google Scholar] [CrossRef]
- Santos, L.S.; Alves, C.C.F.; Estevam, E.B.B.; Martins, C.H.G.; Silva, T.D.S.; Esperandim, V.R.; Miranda, M.L.D. Chemical Composition, in vitro Trypanocidal and Antibacterial Activities of the Essential Oil from the Dried Leaves of Eugenia dysenterica DC from Brazil. J. Essent. Oil Bear. Plants 2019, 22, 347–355. [Google Scholar] [CrossRef]
- D’Almeida, W.; Monteiro, L.M.; Raman, V.; Rehman, J.U.; Paludo, K.S.; de Noronha Sales Maia, B.H.L.; Casapula, I.; Khan, I.A.; Farago, P.V.; Budel, J.M. Microscopy of Eugenia involucrata, Chemical Composition and Biological Activities of the Volatile Oil. Rev. Bras. Farmacogn. 2021, 31, 239–243. [Google Scholar] [CrossRef]
- Toledo, A.G.; de Souza, J.G.D.L.; da SIilva, J.P.B.; Favreto, W.A.J.; da Costa, W.F.; Pinto, F.G.D.S. Chemical Composition, Antimicrobial and Antioxidant Activity of the Essential Oil of Leaves of Eugenia involucrata DC. Biosci. J. 2020, 36, 568–577. [Google Scholar] [CrossRef]
- Siebert, D.A.; Tenfen, A.; Yamanaka, C.N.; De Cordova, C.M.M.; Scharf, D.R.; Simionatto, E.L.; Alberton, M.D. Evaluation of Seasonal Chemical Composition, Antibacterial, Antioxidant and Anticholinesterase Activity of Essential Oil from Eugenia brasiliensis Lam. Nat. Prod. Res. 2015, 29, 289–292. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.O.M.; Castro, J.W.G.; Dantas Junior, O.D.M.; de Araújo, A.C.J.; Leandro, M.K.D.N.S.; Costa, R.J.O.; Pinto, L.L.; Leandro, L.M.G.; da Silva, L.E.; do Amaral, W.; et al. Photoinduced Antibacterial Activity of the Essential Oils from Eugenia brasiliensis Lam and Piper mosenii C. DC. by Blue Led Light. Antibiotics 2019, 8, 242. [Google Scholar] [CrossRef]
- dos Santos, F.R.; Rezende, S.R.D.F.; dos Santos, L.V.; da Silva, E.R.M.N.; Caiado, M.S.; de Souza, M.A.A.; Pontes, E.G.; de Carvalho, M.G.; Braz Filho, R.; Castro, R.N. Larvicidal and Fungicidal Activity of the Leaf Essential Oil of Five Myrtaceae Species. Chem. Biodivers. 2023, 20, e202300823. [Google Scholar] [CrossRef]
- Carneiro, N.S.; Alves, C.C.F.; Alves, J.M.; Egea, M.B.; Martins, C.H.G.; Silva, T.S.; Bretanha, L.C.; Balleste, M.P.; Micke, G.A.; Silveira, E.V.; et al. Chemical Composition, Antioxidant and Antibacterial Activities of Essential Oils from Leaves and Flowers of Eugenia klotzschiana Berg (Myrtaceae). Acad. Bras. Cienc. 2017, 89, 1907–1915. [Google Scholar] [CrossRef]
- Carneiro, N.S.; Alves, J.M.; Alves, C.C.F.; Esperandim, V.R.; Miranda, M.L.D. Essential Oil of Flowers from Eugenia klotzschiana (Myrtaceae): Chemical Composition and In Vitro Trypanocidal and Cytotoxic Activities. Rev. Virtual Química 2017, 9, 1381–1392. [Google Scholar] [CrossRef]
- Franciscato, L.M.S.D.S.; da Silva, M.R.; Andrich, F.; Sakai, O.A.; Doyama, J.T.; Fernandes Júnior, A.; Moritz, C.M.F. Chemical Characterization and Antimicrobial Activity of Essential Oils of Mint (Mentha spicata L.) and Surinam Cherry (Eugenia uniflora L.). Orbital Electron. J. Chem. 2018, 10, 475–481. [Google Scholar] [CrossRef]
- Barbosa, L.N.; Probst, I.D.S.; Andrade, B.F.M.T.; Alves, F.C.B.; Albano, M.; da Cunha, M.; de, L.R.d.S.; Doyama, J.T.; Rall, V.L.M.; Júnior, A.F. In Vitro Antibacterial and Chemical Properties of Essential Oils Including Native Plants from Brazil against Pathogenic and Resistant Bacteria. J. Oleo Sci. 2015, 64, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Antonelo, F.A.; Rodrigues, M.S.; Cruz, L.C.; Pagnoncelli, M.G.; da Cunha, M.A.A.; Bonatto, S.J.R.; Busso, C.; Wagner Júnior, A.; Montanher, P.F. Bioactive Compounds Derived from Brazilian Myrtaceae Species: Chemical Composition and Antioxidant, Antimicrobial and Cytotoxic Activities. Biocatal. Agric. Biotechnol. 2023, 48, 102629. [Google Scholar] [CrossRef]
- Pereira, N.L.F.; Aquino, P.E.A.; Júnior, J.G.A.S.; Cristo, J.S.; Vieira Filho, M.A.; Moura, F.F.; Ferreira, N.M.N.; Silva, M.K.N.; Nascimento, E.M.; Correia, F.M.A.; et al. In Vitro Evaluation of the Antibacterial Potential and Modification of Antibiotic Activity of the Eugenia uniflora L. Essential Oil in Association with Led Lights. Microb. Pathog. 2017, 110, 512–518. [Google Scholar] [CrossRef]
- dos Santos, J.F.S.; Rocha, J.E.; Bezerra, C.F.; Silva, M.K.D.N.; de Matos, Y.M.L.S.; de Freitas, T.S.; dos Santos, A.T.L.; da Cruz, R.P.; Machado, A.J.T.; Rodrigues, T.H.S.; et al. Chemical Composition, Antifungal Activity and Potential Anti-Virulence Evaluation of the Eugenia uniflora Essential Oil against Candida spp. Food Chem. 2018, 261, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.R.; Braz-Filho, R.; Castro, R.N. Influence of Age of the Leaves of Eugenia uniflora L. on Chemical Composition of Essential Oil. Quim. Nova 2015, 38, 762–768. [Google Scholar] [CrossRef]
- Ascari, J.; Pereira, M.F.M.; Schaffka, V.M.; Nunes, D.S.; Magalhães, C.G.; Santos, J.S.; Granato, D.; do Carmo, M.A.V.; Azevedo, L.; Archilha, M.V.L.R.; et al. Selina-1,3,7(11)-Trien-8-one and Oxidoselina-1,3,7(11)-Trien-8-one from Eugenia uniflora Leaf Essential Oil and Their Cytotoxic Effects on Human Cell Lines. Molecules 2021, 26, 740. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, E.N.S.; Tavares, M.S.; Barros, P.A.C.; Miller, D.C.; da Silva, P.I.C.; Freitas, J.J.S.; de Lima, A.B.; Setzer, W.N.; da Silva, J.K.R.; Figueiredo, P.L.B. Chemical Composition, Antinociceptive and Anti-Inflammatory Activities of the Curzerene Type Essential Oil of Eugenia uniflora from Brazil. J. Ethnopharmacol. 2023, 317, 116859. [Google Scholar] [CrossRef]
- Sobeh, M.; Braun, M.S.; Krstin, S.; Youssef, F.S.; Ashour, M.L.; Wink, M. Chemical Profiling of the Essential Oils of Syzygium aqueum, Syzygium samarangense and Eugenia uniflora and Their Discrimination Using Chemometric Analysis. Chem. Biodivers. 2016, 13, 1537–1550. [Google Scholar] [CrossRef] [PubMed]
- Sviech, F.; Pianoski, K.E.; Kruger, R.L.; Kotovicz, V.; Bainy, E.M.B.M.; Sakata, G.S.B.; Bombardelli, M.C.M. Biological Activity of Essential Oil of Pitanga (Eugenia uniflora L.) Leaves. Bol. Cent. Pesqui. Process. Aliment. 2019, 36, 28–38. [Google Scholar] [CrossRef]
- Figueiredo, P.L.B.; Pinto, L.C.; da Costa, J.S.; da Silva, A.R.C.; Mourão, R.H.V.; Montenegro, R.C.; da Silva, J.K.R.; Maia, J.G.S. Composition, Antioxidant Capacity and Cytotoxic Activity of Eugenia uniflora L. Chemotype-Oils from the Amazon. J. Ethnopharmacol. 2019, 232, 30–38. [Google Scholar] [CrossRef]
- da Costa, J.S.; Barroso, A.S.; Mourão, R.H.V.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Seasonal and Antioxidant Evaluation of Essential Oil from Eugenia uniflora L., Curzerene-Rich, Thermally Produced in Situ. Biomolecules 2020, 10, 328. [Google Scholar] [CrossRef] [PubMed]
- da Silva, V.P.; Alves, C.C.F.; Miranda, M.L.D.; Bretanha, L.C.; Balleste, M.P.; Micke, G.A.; Silveira, E.V.; Martins, C.H.G.; Ambrosio, M.A.L.V.; de Souza Silva, T.; et al. Chemical Composition and in vitro Leishmanicidal, Antibacterial and Cytotoxic Activities of Essential Oils of the Myrtaceae Family Occurring in the Cerrado Biome. Ind. Crops Prod. 2018, 123, 638–645. [Google Scholar] [CrossRef]
- Luz, T.R.S.A.; Leite, J.A.C.; de Mesquita, L.S.S.; Bezerra, S.A.; Gomes Ribeiro, E.C.; Silveira, D.P.B.; de Mesquita, J.W.C.; do Amaral, F.M.M.; Coutinho, D.F. Seasonal Variation in the Chemical Composition and Larvicidal Activity against Aedes aegypti L. of Essential Oils from Brazilian Amazon. Exp. Parasitol. 2022, 243, 108405. [Google Scholar] [CrossRef] [PubMed]
- Cipriano, R.R.; Duarte, M.C.T.; Maciel Tomazzoli, M.; de Sales Maia, B.H.N.; Deschamps, C. Yield, Composition and Biological Activities of Eugenia uniflora L. Essential Oil According to Seasonality. J. Essent. Oil Res. 2023, 35, 406–413. [Google Scholar] [CrossRef]
- De Assis, A.L.A.; Cipriano, R.R.; Cuquel, F.L.; Deschamps, C. Effect of Drying Method and Storage Conditions on the Essential Oil Yield and Composition in Eugenia uniflora L. Leaves. Rev. Colomb. Cienc. Hortícolas 2020, 14, 275–282. [Google Scholar] [CrossRef]
- Cipriano, R.R.; Maia, B.H.L.N.S.; Deschamps, C. Chemical Variability of Essential Oils of Eugenia uniflora L. Genotypes and Their Antioxidant Activity. Acad. Bras. Cienc. 2021, 93, e20181299. [Google Scholar] [CrossRef]
- Ramalho, R.R.F.; Barbosa, J.M.G.; Ferri, P.H.; Santos, S.d.C. Variability of Polyphenols and Volatiles during Fruit Development of Three Pitanga (Eugenia uniflora L.) Biotypes. Food Res. Int. 2019, 119, 850–858. [Google Scholar] [CrossRef]
- Durazzini, A.M.S.; Machado, C.H.M.; Fernandes, C.C.; Miranda, M.L.D. Chemical Composition and Effect of Hydrodistillation Times on the Yield of Essential Oil from Eugenia pyriformis Leaves. Orbital Electron. J. Chem. 2019, 11, 334–338. [Google Scholar] [CrossRef]
- Armstrong, L.; Merino, F.J.Z.; Miguel, M.D.; Lordello, A.L.L.; Migue, O.G. Chemical Profile of Essential Oil, Extracts and Fractions of Eugenia pyriformis Cambess. and Its Antioxidant, Cytotoxic and Allelopathic Activities. Braz. Arch. Biol. Technol. 2023, 66, e23220352. [Google Scholar] [CrossRef]
- de Souza, A.M.; de Oliveira, V.B.; de Oliveira, C.F.; Betim, F.C.M.; Pacheco, S.D.G.; Cogo, L.L.; Miguel, O.G.; Miguel, M.D. Chemical Composition and In Vitro Antimicrobial Activity of the Essential Oil Obtained from Eugenia pyriformis Cambess. (Myrtaceae). Braz. Arch. Biol. Technol. 2021, 64, e21200663. [Google Scholar] [CrossRef]
- Durazzini, A.M.S.; Machado, C.H.M.; Fernandes, C.C.; Willrich, G.B.; Crotti, A.E.M.; Candido, A.C.B.B.; Magalhães, L.G.; Squarisi, I.S.; Ribeiro, A.B.; Tavares, D.C.; et al. Eugenia pyriformis Cambess: A Species of the Myrtaceae Family with Bioactive Essential Oil. Nat. Prod. Res. 2019, 35, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nahar, L.; El-Seedi, H.R.; Khalifa, S.A.M.; Mohammadhosseini, M.; Sarker, S.D. Ruta Essential Oils: Composition and Bioactivities. Molecules 2021, 26, 4766. [Google Scholar] [CrossRef] [PubMed]
- Mohammadhosseini, M.; Sarker, S.D.; Akbarzadeh, A. Chemical Composition of the Essential Oils and Extracts of Achillea Species and Their Biological Activities: A Review. J. Ethnopharmacol. 2017, 199, 257–315. [Google Scholar] [CrossRef] [PubMed]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts against Food Pathogens and Spoilage Microorganisms. Front. Microbiol. 2018, 9, 389103. [Google Scholar]
- Costa, W.K.; de Oliveira, A.M.; Santos, I.B.D.S.; Silva, V.B.G.; da Silva, E.K.C.; Alves, J.V.D.O.; da Silva, A.P.S.A.; de Menezes Lima, V.L.; dos Santos Correia, M.T.; da Silva, M.V. Antibacterial Mechanism of Eugenia stipitata McVaugh Essential Oil and Synergistic Effect against Staphylococcus aureus. S. Afr. J. Bot. 2022, 147, 724–730. [Google Scholar] [CrossRef]
- de Souza, J.M.; Rodrigues, M.V.P.; Cirqueira, R.T.; Alves, M.J.Q.D.F.; Lordelo, E.P.; de Oliveira, C.F.; Pietro, R.C.L.R. Evaluation of Antimicrobial, Hypotensive and Diuretic Effect of Eugenia uniflora Extracts. Mundo Saude 2018, 42, 269–282. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. The Inhibitory Effect of Plant Essential Oils on Foodborne Pathogenic Bacteria in Food. Crit. Rev. Food Sci. Nutr. 2019, 59, 3281–3292. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.; Ademiluyi, A.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential Oils: Sources of Antimicrobials and Food Preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Khan, H. Advances in Antioxidant Potential of Natural Alkaloids. Curr. Bioact. Compd. 2017, 13, 101–108. [Google Scholar] [CrossRef]
- Ilmi, Z.N.; Wulandari, P.A.C.; Husen, S.A.; Winarni, D.; Alamsjah, M.A.; Awang, K.; Vastano, M.; Pellis, A.; Macquarrie, D.; Pudjiastuti, P. Characterization of Alginate from Sargassum duplicatum and the Antioxidant Effect of Alginate–Okra Fruit Extracts Combination for Wound Healing on Diabetic Mice. Appl. Sci. 2020, 10, 6082. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Arruda, H.S.; Borsoi, F.T.; Andrade, A.C.; Pastore, G.M.; Marostica Junior, M.R. Scientific Advances in the Last Decade on the Recovery, Characterization, and Functionality of Bioactive Compounds from the Araticum Fruit (Annona crassiflora Mart.). Plants 2023, 12, 1536. [Google Scholar] [CrossRef]
- Da Silva, E.B.P.; Matsuo, A.L.; Figueiredo, C.R.; Chaves, M.H.; Sartorelli, P.; Lago, J.H.G. Chemical Constituents and Cytotoxic Evaluation of Essential Oils from Leaves of Porcelia macrocarpa (Annonaceae). Nat. Prod. Commun. 2013, 8, 1934578X1300800. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Guo, J.; Wang, Q.; Zhu, S.; Gao, S.; Yang, C.; Wei, M.; Pan, X.; Zhu, W.; et al. Cytotoxic and Antitumor Effects of Curzerene from Curcuma longa. Planta Med. 2017, 83, 23–29. [Google Scholar] [CrossRef]
- Dai, Z.J.; Tang, W.; Lu, W.F.; Gao, J.; Kang, H.F.; Ma, X.B.; Min, W.L.; Wang, X.J.; Wu, W.Y. Antiproliferative and Apoptotic Effects of β-Elemene on Human Hepatoma HepG2 Cells. Cancer Cell Int. 2013, 13, 27. [Google Scholar] [CrossRef]
- Dahham, S.; Tabana, Y.; Iqbal, M.; Ahamed, M.; Ezzat, M.; Majid, A.; Majid, A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Altamura, F.; Rajesh, R.; Catta-Preta, C.M.C.; Moretti, N.S.; Cestari, I. The Current Drug Discovery Landscape for Trypanosomiasis and Leishmaniasis: Challenges and Strategies to Identify Drug Targets. Drug Dev. Res. 2022, 83, 225–252. [Google Scholar] [CrossRef] [PubMed]
- Kourbeli, V.; Chontzopoulou, E.; Moschovou, K.; Pavlos, D.; Mavromoustakos, T.; Papanastasiou, I.P. An Overview on Target-Based Drug Design against Kinetoplastid Protozoan Infections: Human African Trypanosomiasis, Chagas Disease and Leishmaniases. Molecules 2021, 26, 4629. [Google Scholar] [CrossRef] [PubMed]
- Kauffmann, C.; Ethur, E.M.; Buhl, B.; Scheibel, T.; Machado, G.M.C.; Cavalheiro, M.M.C. Potential Antileishmanial Activity of Essential Oils of Native Species from Southern Brazil. Environ. Nat. Resour. Res. 2016, 6, 18. [Google Scholar] [CrossRef][Green Version]
- Baldissera, M.D.; Grando, T.H.; Souza, C.F.; Cossetin, L.F.; Sagrillo, M.R.; Nascimento, K.; da Silva, A.P.T.; Dalla Lana, D.F.; Da Silva, A.S.; Stefani, L.M.; et al. Nerolidol Nanospheres Increases Its Trypanocidal Efficacy against Trypanosoma evansi: New Approach against Diminazene Aceturate Resistance and Toxicity. Exp. Parasitol. 2016, 166, 144–149. [Google Scholar] [CrossRef]
- Moemenbellah-Fard, M.D.; Abdollahi, A.; Ghanbariasad, A.; Osanloo, M. Antibacterial and Leishmanicidal Activities of Syzygium aromaticum Essential Oil versus Its Major Ingredient, Eugenol. Flavour. Fragr. J. 2020, 35, 534–540. [Google Scholar] [CrossRef]
- Arruda, D.C.; Miguel, D.C.; Yokoyama-Yasunaka, J.K.U.; Katzin, A.M.; Uliana, S.R.B. Inhibitory Activity of Limonene against Leishmania Parasites in vitro and in vivo. Biomed. Pharmacother. 2009, 63, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Moreno, É.M.; Leal, S.M.; Stashenko, E.E.; García, L.T. Induction of Programmed Cell Death in Trypanosoma cruzi by Lippia alba Essential Oils and Their Major and Synergistic Terpenes (Citral, Limonene and Caryophyllene Oxide). BMC Complement. Altern. Med. 2018, 18, 225. [Google Scholar] [CrossRef]
- Otoguro, K.; Iwatsuki, M.; Ishiyama, A.; Namatame, M.; Nishihara-Tukashima, A.; Kiyohara, H.; Hashimoto, T.; Asakawa, Y.; Mura, S.; Yamada, H. In Vitro Antitrypanosomal Activity of Plant Terpenes against Trypanosoma brucei. Phytochemistry 2011, 72, 2024–2030. [Google Scholar] [CrossRef]
- Bailen, M.; Martínez-Díaz, R.A.; Hoffmann, J.J.; Gonzalez-Coloma, A. Molecular Diversity from Arid-Land Plants: Valorization of Terpenes and Biotransformation Products. Chem. Biodivers. 2020, 17, e1900663. [Google Scholar] [CrossRef]
- Soares, D.C.; Portella, N.A.; Ramos, M.F.D.S.; Siani, A.C.; Saraiva, E.M. Trans-β-Caryophyllene: An Effective Antileishmanial Compound Found in Commercial Copaiba Oil (Copaifera spp.). Evid.-Based Complement. Altern. Med. 2013, 2013, 761323. [Google Scholar] [CrossRef] [PubMed]
- Adão, I.S.; Garcia, A.R.; Sette, K.M.; Adade, C.M.; de Andrade Silva, J.R.; Amaral, A.C.F.; Pinheiro, A.S.; Rodrigues, I.A. Enantioselectivity of Pinene against Leishmania amazonensis. Med. Chem. Res. 2024, 33, 127–135. [Google Scholar] [CrossRef]
- Amaral, R.G.; Baldissera, M.D.; Grando, T.H.; Couto, J.C.M.; Posser, C.P.; Ramos, A.P.; Sagrillo, M.R.; Vaucher, R.A.; Da Silva, A.S.; Becker, A.P.; et al. Combination of the Essential Oil Constituents α-Pinene and β-Caryophyllene as a Potentiator of Trypanocidal Action on Trypanosoma evansi. J. Appl. Biomed. 2016, 14, 265–272. [Google Scholar] [CrossRef]
- Polanco-Hernández, G.; Escalante-Erosa, F.; García-Sosa, K.; Rosado, M.E.; Guzmán-Marín, E.; Acosta-Viana, K.Y.; Giménez-Turba, A.; Salamanca, E.; Peña-Rodríguez, L.M. Synergistic Effect of Lupenone and Caryophyllene Oxide against Trypanosoma cruzi. Evid.-Based Complement. Altern. Med. 2013, 2013, 435398. [Google Scholar] [CrossRef] [PubMed]
- Polanco-Hernández, G.M.; Giménez-Turba, A.; Salamanca, E.; Getti, G.; Rai, R.; Acosta-Viana, K.Y.; Arana-Argáez, V.E.; Torres-Romero, J.C.; Fernández-Martín, K.G.; Segura-Campos, M.R.; et al. Leishmanicidal Activity and Immunomodulatory Effect of a Mixture of Lupenone and β-Caryophyllene Oxide. Rev. Bras. Farmacogn. 2021, 31, 199–206. [Google Scholar] [CrossRef]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Cianciosi, D.; Quiles, J.L.; Mezzetti, B.; Xiao, J.; Giampieri, F.; Battino, M. The Efficacy of Berries against Lipopolysaccharide-Induced Inflammation: A Review. Trends Food Sci. Technol. 2021, 117, 74–91. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).