Evaluation of Bioactive Functions and Quantitative Analysis of Phenolic Compounds of Glehnia littoralis from Different Regions
Abstract
1. Introduction
2. Materials and Methods
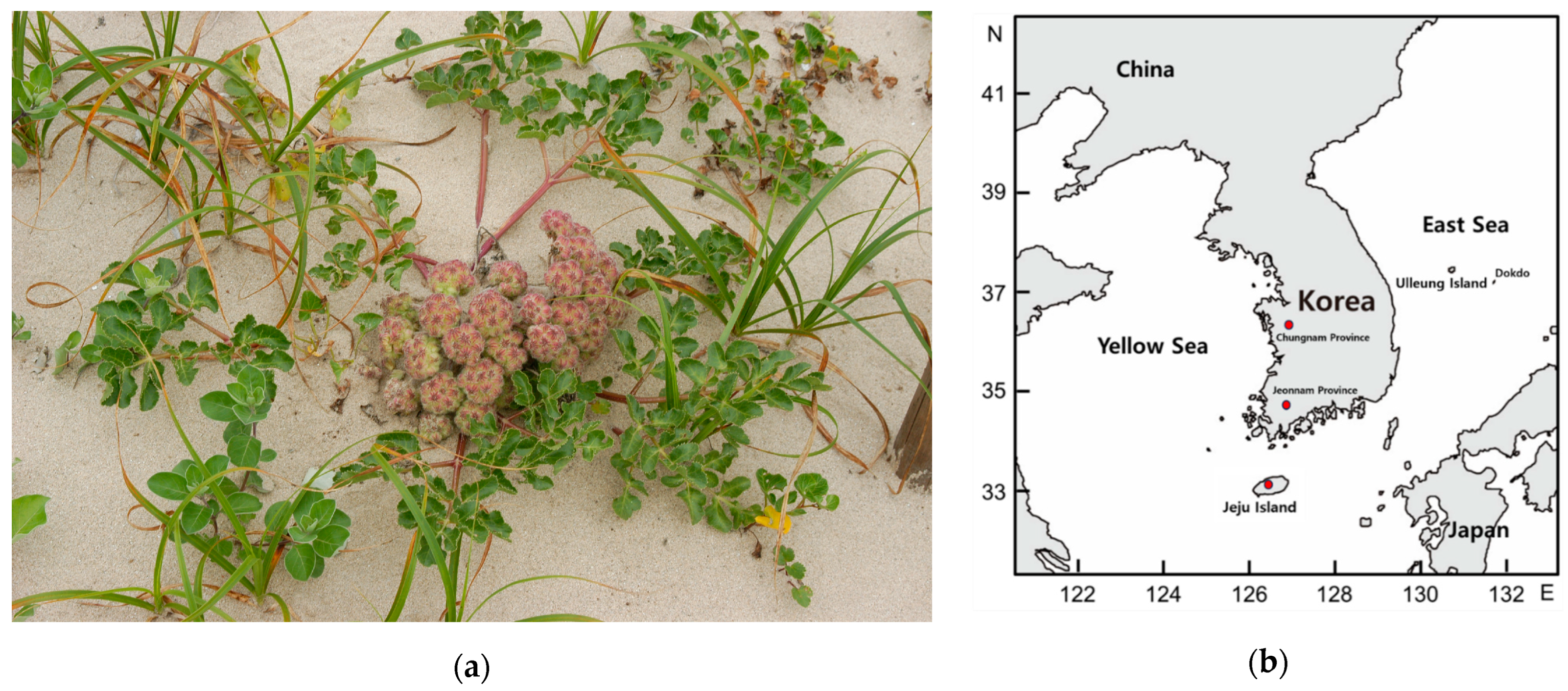
2.1. Plant Materials
2.2. Equipment and Reagents
2.3. Chemical Compounds
2.4. Sample Preparation
2.5. 2,2-Diphenyl-1-picrylhydraxyl (DPPH)-Radical-Scavenging Assay
2.6. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+)-Radical-Scavenging Assay
2.7. HPLC Conditions
2.8. Calibration Curves
2.9. Statistical Analysis
3. Results
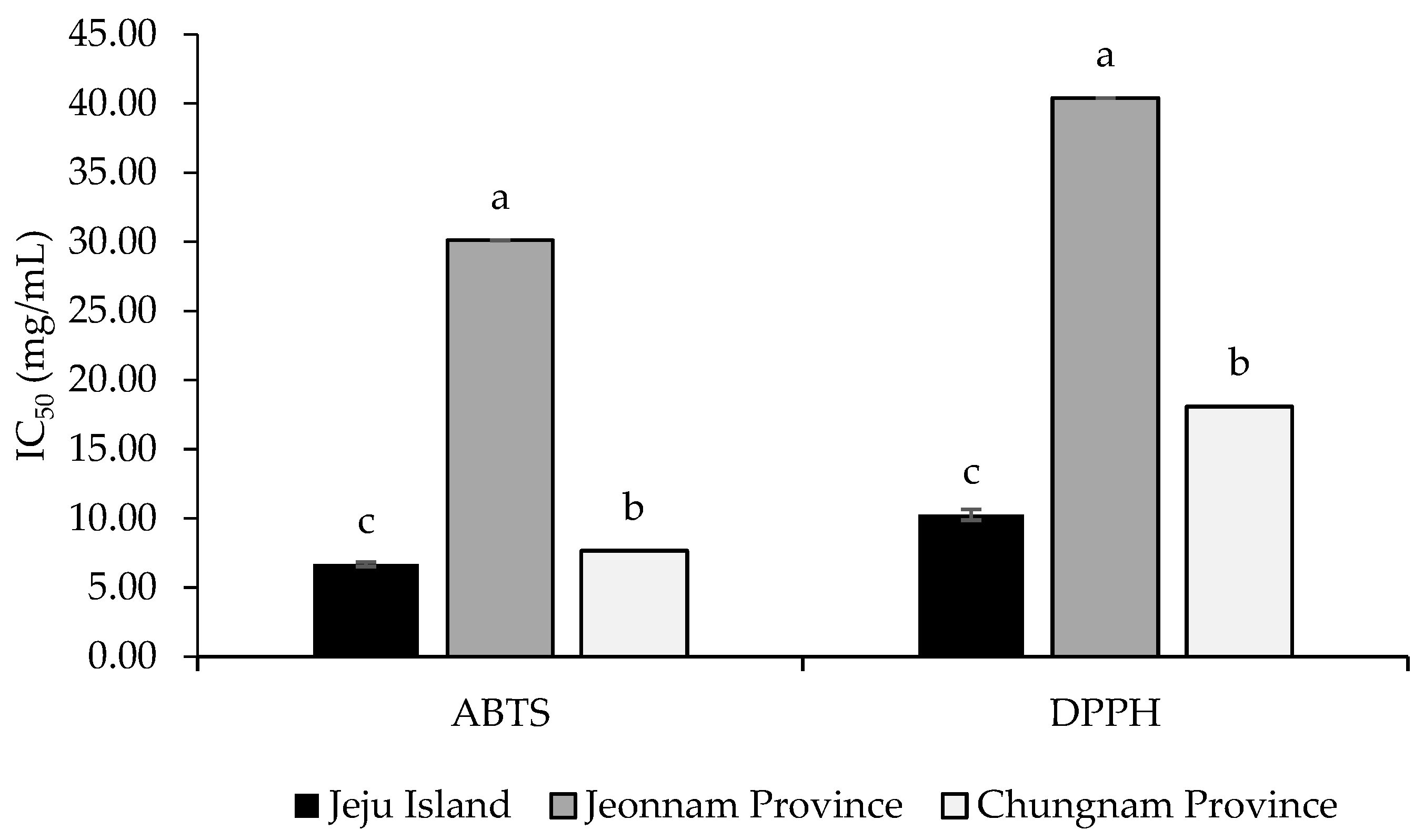
3.1. Radical-Scavenging Activities
3.2. Characterization of Phenolic Compound Content by HPLC
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.C.; Lee, H.O.; Kim, K.; Kim, S.; Yang, T.J. The complete chloroplast genome sequence of the medicinal plant Glehnia littoralis F. Schmidt ex. Miq. (Apiaceae). Mitochondrial DNA A DNA Mapp. Seq. Anal. 2015, 27, 3674–3675. [Google Scholar] [PubMed]
- Li, B.; Wang, A.; Zhang, P.; Li, W. Genetic diversity and population structure of endangered Glehnia littoralis (Apiaceae) in China based on AFLP analysis. Biotechnol. Biotechnol. Equip. 2019, 33, 331–337. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, C.; Jin, Q.; Yeon, E.T.; Lee, D.H.; Kim, S.Y.; Han, S.B.; Hong, J.T.; Lee, M.K.; Hwang, B.Y. Pyranocoumarins from Glehnia littoralis inhibit the LPS-induced NO production in macrophage RAW 264.7 cells. Bioorg. Med. Chem. Lett. 2014, 24, 2717–2719. [Google Scholar] [CrossRef]
- Ishikawa, A.; Kitamura, Y.; Ozeki, Y.; Watanabe, M. Different responses of shoot and root cultures of Glehnia littoralis to yeast extract. J. Nat. Med. 2006, 61, 30–37. [Google Scholar] [CrossRef]
- Matsuura, H.; Saxena, G.; Farmer, S.W.; Hancock, R.E.W.; Towers, G.H.N. Antibacterial and antifungal polyine compounds from Glehnia littoralis ssp. leiocarpa. Nature 1996, 62, 256–259. [Google Scholar]
- Wu, J.; Gao, W.; Song, Z.; Xiong, Q.; Xu, Y.; Han, Y.; Yuan, J.; Zhang, R.; Cheng, Y.; Fang, J.; et al. Anticancer activity of polysaccharide from Glehnia littoralis on human lung cancer cell line A549. Int. J. Biol. Macromol. 2018, 106, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.Y.; Hong, J.H.; Gu, Y.R.; Kim, I.D.; Dhungana, S.K.; Moon, K.D. Hot water extract of Glehnia littoralis leaf showed skin-whitening and anti-wrinkle properties. S. Afr. J. Bot. 2019, 127, 104–109. [Google Scholar] [CrossRef]
- Masuda, T.; Takasugi, M.; Anetal, M. Psoralen and other linear furanocoumarins as phytoalexins in Glehnia littoralis. Phytochemistry 1998, 47, 13–16. [Google Scholar] [CrossRef]
- Park, Y.J.; Choi, Y.B.; Oh, S.B.; Moon, J.; Truong, T.Q.; Huynh, P.K.; Kim, S.M. Development and application of a high-performance liquid chromatography diode-array detection (HPLC-DAD) method for the simultaneous quantification of phenolic compounds in the aerial part of Glehnia littoralis. Appl. Biol. Chem. 2024, 67, 34. [Google Scholar] [CrossRef]
- Eum, H.L.; Choi, M.H.; Park, M.H.; Lee, J.S.; Chang, M.S. Influence of MAP on the postharvest quality of Glehnia littoralis Fr. Schmidt ex Miq. Processes 2021, 9, 2052. [Google Scholar] [CrossRef]
- Xu, Y.; Gu, X.; Yuan, Z. Lignan and neolignan glycosides from the roots of Glehnia littoralis. Planta Med. 2010, 76, 1706–1709. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, N.; Fang, Q.; Cheng, X.; Chen, J.; Liu, P.; Li, L.; Wang, C.; Liu, W. Glehnia littoralis Fr. Schmidtex Miq.: A systematic review on ethnopharmacology, chemical composition, pharmacology and quality control. J. Ethnopharmacol. 2023, 317, 116831. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.T.M.; Tran, G.H.; Nguyen, T.K.; Lim, J.H.; Lee, S. Antioxidant activity of different cultivars of Chrysanthemum morifolium and quantitative analysis of phenolic compounds by HPLC/UV. Appl. Biol. Chem. 2024, 67, 17. [Google Scholar] [CrossRef]
- Choi, J.; Lee, H.D.; Cho, H.; Lee, C.-D.; Tran, G.H.; Kim, H.; Moon, S.K.; Lee, S. Antioxidative phenolic compounds from the aerial parts of Cyperus exaltatus var. iwasakii and their HPLC analysis. Appl. Biol. Chem. 2023, 66, 61. [Google Scholar]
- Ahmed, S.; Khan, H.; Aschner, M.; Mirzae, H.; Akkol, E.K.; Capasso, R. Anticancer potential of furanocoumarins: Mechanistic and therapeutic aspects. Int. J. Mol. Sci. 2020, 21, 5622. [Google Scholar] [CrossRef] [PubMed]
- Bruni, R.; Barreca, D.; Proti, M.; Brighenti, V.; Righetti, L.; Anceschi, L.; Mercolini, L.; Bervenuti, S.; Gattuso, G.; Pellati, F. Botanical sources, chemistry, analysis, and biological activity of furanocoumarins of pharmaceutical interest. Molecules 2019, 24, 2163. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.; Bhardwaj, N.; Kumar, S.; Jain, S.K. Phytochemical and pharmacological aspects of psoralen-a bioactive furanocoumarin from Psoralea corylifolia Linn. Chem. Biodivers. 2023, 20, e202300867. [Google Scholar] [CrossRef] [PubMed]
- Sumorek-Wiadro, J.; Zajgc, A.; Maciejczyk, A.; Jakubowicz-Gil, J. Furanocoumarins in anticancer therapy-for and against. Fitoterapia 2020, 142, 104492gyt6. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.N.; Han, L.; Zhang, Z.; Wang, Y.L.; Zhang, X.P.; Wu, Y.J.; Xiu, G.L. Synthesis and antioxidant properties of psoralen derivatives. Chem. Biodivers. 2023, 20, e202300620. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. The role of phenolic hydroxy groups in the free radical scavenging activity of betalains. J. Nat. Prod. 2009, 72, 1142–1146. [Google Scholar] [CrossRef]
- Wang, J.; Cao, X.; Jiang, H.; Qi, Y.; Chin, K.L.; Yue, Y. Antioxidant activity of leaf extracts from different Hibiscus sabdariffa accessions and simulataneous determination five major antioxidant compounds by LC-Q-TOF-MS. Molecules 2014, 19, 21226–21238. [Google Scholar] [CrossRef] [PubMed]
- So, J.; Lee, H.D.; Kim, J.H.; Lee, S.; Lim, J.H. Antioxidant, antimicrobial, and skin-whitening effects and quantitative analysis of phenolic compounds in Korean wild Chrysanthemum flowers via HPLC/UV. Hortic. Environ. Biotechnol. 2024, 65, 215–227. [Google Scholar] [CrossRef]
- Sharma, S.; Ali, A.; Ali, J.; Sahni, J.K.; Baboota, S. Rutin: Therapeutic potential and recent advances in drug delivery. Expert Opin. Investig. 2013, 22, 1063–1079. [Google Scholar] [CrossRef] [PubMed]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxid. Med. Cell. Lonogev. 2018, 2018, 6241017. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Choi, S.S.; Park, H.R.; Lee, K.A. A comparative study of rutin and rutin glycoside: Antioxidant activity, anti-inflammatory effect, effect on platelet aggregation and blood coagulation. Antioxidants 2021, 10, 1696. [Google Scholar] [CrossRef] [PubMed]
- Girsang, E.; Lister, I.N.E.; Ginting, C.N.; Sholihah, I.A.; Raif, M.A.; Kunrdi, S.; Million, H.; Widowati, W. Antioxidant and antiaging activity of rutin and caffeic acid. Pharmaciana 2020, 10, 147–156. [Google Scholar] [CrossRef]
- Atanassova, M.; Bagdassarian, V. Rutin content in plant products. J. Univ. Chem. Technol. Metall. 2009, 44, 201–203. [Google Scholar]
- Tang, E.; Hu, T.; Jiang, Z.; Shen, X.; Lin, H.; Xian, H.; Wu, X. Isoquercitrin alleviates lipopolysaccharide-induced intestinal mucosal barrier damage in mice by regulating TLR4/MyD88/NF-kB signaling pathway and intestinal flora. Food Funct. 2024, 15, 295–309. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Q.; Wang, T.; Liu, J.; Chen, D. Comparison of the antioxidant effects of quercitrin and isoquercitrin: Understanding the role of the 6″-OH group. Molecules 2016, 21, 1246. [Google Scholar] [CrossRef]
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Krěn, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Lee, H.; Ko, H.J.; Woo, E.R.; Lee, D.G. Fungicidal effect of isoquercitrin via inducing membrane disturbance. Biochim. Biophys. Acta Biomembr. 2015, 1848, 695–701. [Google Scholar] [CrossRef]
- Mustafa, R.A.; Hamid, A.A.; Mohamed, S.; Bakar, F.A. Total phenolic compounds, flavonoids, and radical scavenging activity of 21 selected tropical plants. J. Food Sci. 2010, 75, C28–C35. [Google Scholar] [CrossRef] [PubMed]
- Tremi, J.; Šmejkal, K. Flavonoids as potent scavengers of hydroxyl radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The biological activity mechanism of chlorogenic acid and its applications in food industry: A review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Niu, L.; Chen, Y.; Qiu, T.; Zhiu, M.; Wang, M.; Mo, H.; Xiao, S. Recent advance in the biological activity of chlorogenic acid and its application in food industry. Int. J. Food. Sci. Technol. 2023, 58, 4931–4947. [Google Scholar] [CrossRef]
- Ma, K.; Li, F.; Zhe, T.; Sun, X.; Zhang, X.; Wan, P.; Na, H.; Zhao, J.; Wang, L. Biopolymer films incorporated with chlorogenic acid nanoparticles for active food packaging application. Food Chem. 2024, 435, 137552. [Google Scholar] [CrossRef] [PubMed]
- Kabtni, S.; Sdouga, D.; Bettaid Rebey, I.; Save, M.; Trifi-Farah, N.; Fauconnier, M.L.; Marghali, S. Influence of climate variation on phenolic composition and antioxidant capacity of Medicago minima populations. Sci. Rep. 2020, 10, 8293. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, D.; Seo, C.; Ryu, J.; Chae, Y.; Baek, G.; Bae, C. Vulnerability assessment on the location of industrial complex considering climate change-focusing on physical and economic features of province industrial complex. Korean Soc. Environ. Impact Assess. 2013, 22, 627–637. [Google Scholar] [CrossRef][Green Version]
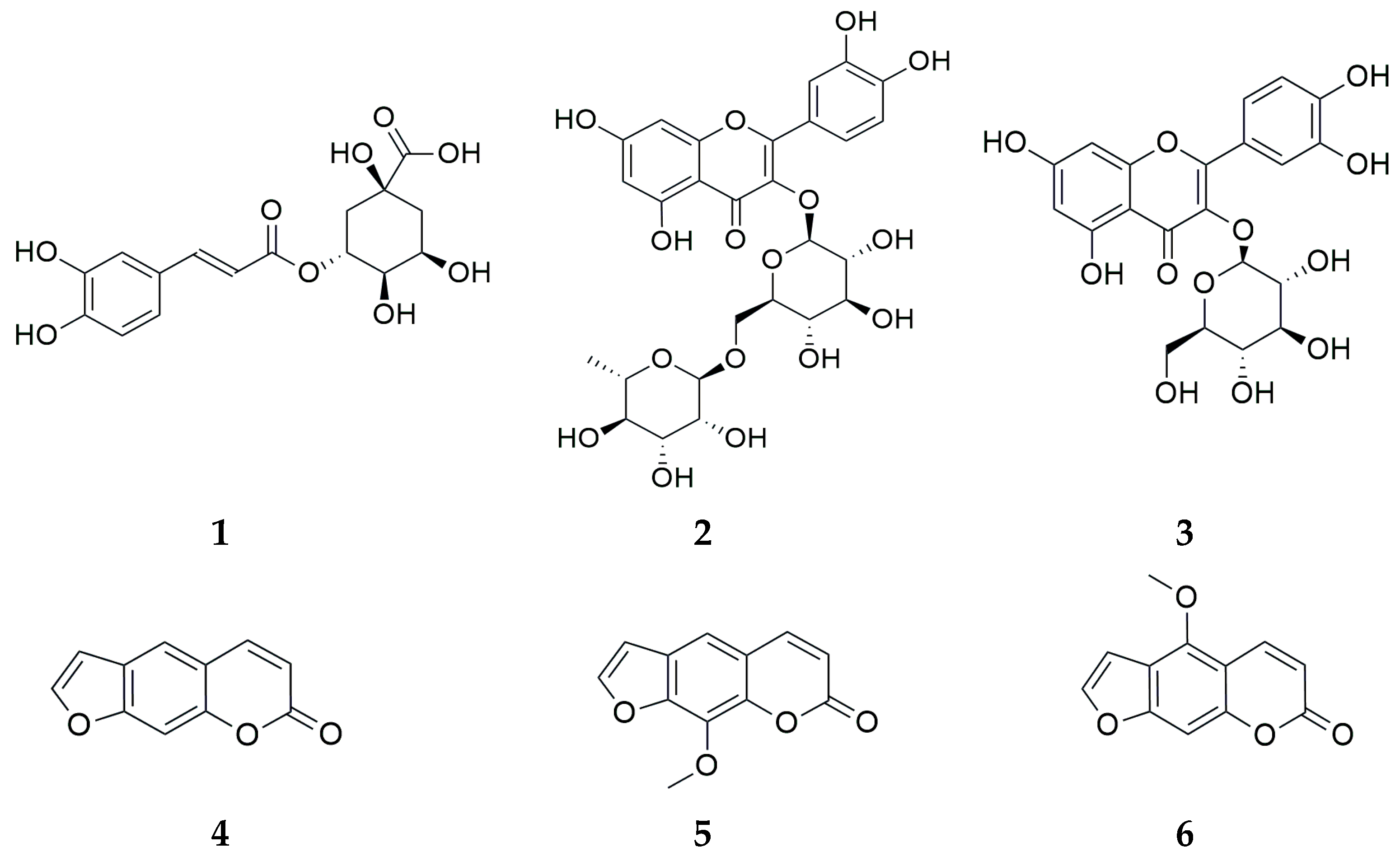

| Compound | ABTS+-Scavenging Activity (Relative VCEAC Values a) | DPPH-Scavenging Activity (Relative VCEAC Values) |
|---|---|---|
| Chlorogenic acid (1) | 1.12 | 0.96 |
| Rutin (2) | 2.27 | 1.31 |
| Isoquercitrin (3) | 0.57 | 0.54 |
| Psoralen (4) | - | - |
| 8-Methoxy psoralen (5) | - | - |
| Bergapten (6) | - | - |
| Vitamin C b | 1.00 | 1.00 |
| Compound | tr a | Calibration Equation b | R-Value c |
|---|---|---|---|
| Chlorogenic acid (1) | 11.7 | Y = 10,686x + 9024.1 | 0.9999 |
| Rutin (2) | 17.5 | Y = 19,141x − 766.57 | 1 |
| Isoquercitrin (3) | 18.2 | Y = 26,810x + 40,996 | 0.9999 |
| Psoralen (4) | 30.6 | Y = 35,255x + 5221.5 | 0.9999 |
| 8-Methoxy psoralen (5) | 32.2 | Y = 49,634x + 24,987 | 1 |
| Bergapten (6) | 36.3 | Y = 27,694x + 13,860 | 1 |
| Compound | Jeju Island (µmoL/g) | Jeonnam Province (µmoL/g) | Chungnam Province (µmoL/g) |
|---|---|---|---|
| Chlorogenic acid (1) | 4.34 ± 0.14 b | 0.88 ± 0.00 c | 10.31 ± 0.08 a |
| Rutin (2) | 24.13 ± 0.03 a | tr | 15.95 ± 0.02 b |
| Isoquercitrin (3) | 2.69 ± 0.04 a | tr | 1.55 ± 0.02 b |
| Psoralen (4) | ND | 2.15 ± 0.05 | tr |
| 8-Methoxy psoralen (5) | ND | 5.41 ± 0.05 b | 6.85 ± 0.09 a |
| Bergapten (6) | tr | 13.42 ± 0.05 b | 44.44 ± 0.65 a |
| Total | 31.16 | 21.86 | 79.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, N.; Lee, S.; Choi, K.; Ku, J.; Lee, S. Evaluation of Bioactive Functions and Quantitative Analysis of Phenolic Compounds of Glehnia littoralis from Different Regions. Horticulturae 2024, 10, 764. https://doi.org/10.3390/horticulturae10070764
Yoon N, Lee S, Choi K, Ku J, Lee S. Evaluation of Bioactive Functions and Quantitative Analysis of Phenolic Compounds of Glehnia littoralis from Different Regions. Horticulturae. 2024; 10(7):764. https://doi.org/10.3390/horticulturae10070764
Chicago/Turabian StyleYoon, Nari, Sullim Lee, Kyung Choi, Jajung Ku, and Sanghyun Lee. 2024. "Evaluation of Bioactive Functions and Quantitative Analysis of Phenolic Compounds of Glehnia littoralis from Different Regions" Horticulturae 10, no. 7: 764. https://doi.org/10.3390/horticulturae10070764
APA StyleYoon, N., Lee, S., Choi, K., Ku, J., & Lee, S. (2024). Evaluation of Bioactive Functions and Quantitative Analysis of Phenolic Compounds of Glehnia littoralis from Different Regions. Horticulturae, 10(7), 764. https://doi.org/10.3390/horticulturae10070764








