Abstract
Citrus orchards in semi-arid regions are increasingly exposed to drought conditions due to climate change. This study compared the physiological and growth responses of ‘W. Murcott’ tangor (WM) grafted onto Citrus macrophylla (M), Swingle citrumelo (SC), C-35 citrange (C35), or bitter citrandarin (C22) rootstock subjected to two irrigation treatments: daily irrigation to replace 100% of the water lost daily by evapotranspiration (ET; control treatment) or daily irrigation to replace 75% of the water lost daily by ET (water deficit treatment). For trees in each treatment, leaf gas exchange, relative chlorophyll content, chlorophyll fluorescence, midday stem water potential, trunk cross-sectional area, and shoot length were measured 46 days after treatments were initiated. The results showed that WM on SC or C22 rootstock exhibited isohydric behavior, where decreased stomatal conductance limited transpiration in the water deficit treatment. WM on M rootstock exhibited an anisohydric response in the water deficit treatment, where there was no stomatal control of water loss by transpiration. Among the rootstocks tested for WM, the most tolerant to soil water deficit was SC, whereas trees on M rootstock were the most negatively affected by soil water deficit.
1. Introduction
The citrus industry is often a major contributor to the economies of semi-arid and arid regions. ‘W. Murcott’ tangor is a hybrid of mandarin and orange that has a fruit consumption of 40,000 tons per year in the United States [1]. The variety is characterized by seedless fruit due to self-pollination, easy peeling, good fruit quality, high vigor and productivity, and precociousness [2]. Low rainfall and high summer temperatures in production regions make this variety extremely vulnerable to climate change, which can result in long-term drought [3,4,5] leading to crop stress and negative impacts on fruit yield and fruit quality [5,6,7].
Breeding programs for high yields and fruit quality, resistance or tolerance to soil salinity, diseases, or hypoxia, among other traits, have led to a wide diversity of citrus rootstocks and cultivars. However, there are still challenges to understanding the response of many of these rootstocks and cultivars to water stress [4]. Understanding the response of the main citrus rootstocks and cultivars to drought stress will allow for the future selection of the most productive cultivars in semi-arid and arid regions where the occurrence of drought is expected to increase due to climate change. In recent years, research on the drought tolerance of citrus has primarily focused on describing the response to moderate water stress. The responses to moderate water stress are highly variable among citrus rootstocks and scion–rootstock combinations [6,7,8].
Compared with other fruit tree species, the response of citrus species and cultivars to water deficits is very diverse. The main responses reported are osmotic regulation, reduction of water potential, and stomatal closure [9]. However, these responses have been inconsistent, probably due to the influence of the rootstock [5,8]. For example, ‘Valencia’ sweet orange [Citrus sinensis (L.) Osbeck] grafted onto ‘Swingle’ citrumelo [Citrus paradisi Macf × Poncirus trifoliata (L.) Raf] rootstock does not tolerate drought; consequently, biomass is reduced under drought conditions. In contrast, ‘Valencia’ sweet orange grafted onto Rangpur lime (Citrus limonia Osbeck) rootstock exhibits strong stomatal regulation under water stress and thus maintains its biomass after exposure to drought conditions [6]. Jamshidi et al. [3] reported strong stomatal control in Washington navel orange [Citrus sinensis (L.) Osbeck] trees under water stress, which resulted in the maintenance of biomass when exposed to moderate stress but loss of biomass during severe stress when stomatal closure inhibited net CO2 assimilation. Sampaio et al. [5] showed that ‘Pera’ sweet orange grafted onto citrandarins or trifoliate orange rootstock exhibited reduced leaf gas exchange and leaf water potential but maintained yield and fruit quality under drought conditions. Thus, the responses of citrus to drought conditions seem to not only be dependent on the scion or rootstock cultivars but also on the scion–rootstock interaction. Also, the type of soil and the quality of water for irrigation can have a significant effect on the response of citrus to drought [6,10]. As climatic conditions are changing and semi-arid areas are expected to experience increased periods of drought, a better understanding of the physiological response of citrus trees to water stress is increasingly important for selecting cultivars and rootstocks for arid and semi-arid regions [5,7,8,11]. Thus, the objective of this study was to characterize and compare the physiological and growth responses to water deficit conditions among four different rootstocks compatible with and widely used in semi-arid areas for ‘W. Murcott’ (Citrus reticulata Blanco), also known as Afourer or Nadorcott.
2. Materials and Methods
2.1. Plant Material and Experimental Design
The experiment was conducted in the Paine area of Aculeo Lake, Region Metropolitana, Chile (33°51′ S 70°38′ O), from November to December 2021 (spring season) in a greenhouse. The climatic conditions inside the greenhouse were recorded with a HOBO Prov v2 datalogger (Onset Computer Corp., Bourner, MA, USA) located about 1 m above the top of the tree canopies. Relative humidity inside the greenhouse ranged from 25 to 92% with a mean of 70%, and daily air temperature ranged from 12 and 39 °C with a mean of 20 °C.
Two-year old ‘W. Murcott’ tangor scions were grafted onto one of four rootstocks: Macrophylla (Citrus macrophylla W.), Swingle citrumelo [Citrus paradisi Macf. × Poncirus trifoliata (L.) Raf], C-35 citrange [Citrus sinensis (L.) Obs. × Poncirus trifoliata (L.) Raf.], or bitter citrandarin (C-22) (Citrus sunki Hort. Ex. Tan. × Poncirus trifoliata), whose characteristics are described in Table 1 [12,13]. All trees were grown in 10 L plastic pots filled with soil collected from the local area. The soil texture was classified as a silty clay loam with a pH of 4.6, bulk density of 1.4 g/cm3, electric conductivity of 3.20 mS/m, and organic matter content of 6.88%. During the experimental period, the plants did not require fertilizer because four months before the beginning of the experiment, they were fertilized with N-P-K slow-release fertilizer (Basacote® 12M) (45 g per plant) according to standard nursery management practices.

Table 1.
Main characteristics reported of the four citrus rootstocks used according to abiotic stress.
The evapotranspiration (ET) rate was determined for plants on each rootstock using the water balance equation described by Evett et al. [14]
where R is the amount of irrigation applied, P is the precipitation, Pf is deep percolation, and ∆H is the difference between the weight of the pot at pot capacity and the weight of the pot the following day (at the same time of day). Precipitation (P) was not considered in this study because the experiment was conducted in a greenhouse.
ET = R + P − Pf + ΔH
The irrigation rate was calculated based on ET. The application rate was determined using the water balance method (Equation (1)), which consisted of irrigating the pot with a known volume of water (R). The weight of each pot was determined prior to irrigation. Each pot was placed in a plastic saucer to allow the drainage water to be collected. After irrigation, the pots were allowed to drain to soil pot capacity (−0.001 MPa). Each day, the weight of the irrigated, drained pot was determined. Also, the weight of the drainage water (Pf) in the saucer was determined. Four plants in the control treatment were used to estimate the mean ET for each rootstock.
The irrigation setup (number of drippers and flow rate), irrigation time, mean precipitation rate and volumetric water content of each pot at field capacity (pot capacity) are described in Table 2. Pot capacity was maintained for the control as this is considered the ideal water state to achieve a good balance of water and air; allowing plants to absorb water without difficulty while maintaining good oxygenation in this experiment [15]. Soil water tension was monitored with a tensiometer (SR series Irrometer Company, Inc., Riverside, CA, USA), and soil moisture content in the pots was monitored with a frequency domain reflectometry (FDR) probe (ECH2O EC-5 soil moisture sensor Meter Group, Inc., Pullman, WA, USA).

Table 2.
Irrigation design characteristics.
The experimental design was a split plot with two factors. The first factor was the irrigation treatment with two levels: (1) irrigation so that 100% of ET was replaced daily (control treatment) and (2) irrigation so that 75% of ET was replaced daily (moderate water deficit treatment). The second factor was the rootstock with four levels: (1) ‘W. Murcott’ tangor (WM) on Citrus macrophylla (WM/M), (2) WM on Swingle citrumelo (WM/SC), (3) WM on C-35 citrange (WM/C35), and (4) WM on bitter citrandarin (WM/C22). The main plot was the irrigation level, and the split plots within each irrigation level were the rootstocks with 6 single-plant replications per rootstock in each irrigation treatment. The water supply was reduced over time to simulate a situation that could be encountered in an orchard. Therefore, the plants were subjected to moderate water stress for a period of 46 days according to Santos et al. [8].
2.2. Leaf Gas Exchange, Relative Chlorophyll Content, and Chlorophyll Fluorescence
Net CO2 assimilation (A), stomatal conductance of water vapor (gs), transpiration (E), and internal CO2 concentration (Ci) were measured on a recently matured leaf in the middle of the canopy of each tree with a portable gas analyzer (CIRAS-3 PP Systems, Amesbury, MA, USA). The measurements were performed on the last day of the experiment (day 46) at 10:00 and 12:00 h.
Relative chlorophyll content (Chl) was measured on the adaxial surface of two leaves per tree with a chlorophyll meter (model, CL-01 Hansatech Instruments Ltd., Norfolk, United Kingdom). The ratio of variable to maximum chlorophyll fluorescence (Fv/Fm) was measured on the adaxial surface of two leaves per tree with a portable fluorescence meter (Pocket PEA Hansatech Instruments Ltd., Norfolk, UK). Leaves were acclimated in the dark for 30 min prior to measurements. Chl and Fv/Fm were also determined 46 days after water stress treatments began.
2.3. Stem Water Potential
Stem water potential (Ψs) was measured at solar midday with a Scholander pressure chamber (Model 600 PMS Instrument Company, Albany OR, USA) [8,16]. Prior to measurement, the leaves were covered with aluminum foil and wrapped in plastic bags for 30 min [16]. The measurements were performed on the last day of the experiment (day 46) at 10:00 and 12:00 h.
2.4. Trunk Cross-Sectional Area and Shoot Length
The trunk cross-sectional area (TCSA) above and below the graft union and shoot length was measured at the beginning and end of the experiment. The measurement at the beginning of the experiment was considered the covariance. The TCSA was calculated from the diameter of the trunk, 2 cm above and below the graft union, using the equation: Area = πr2, where r is the trunk radius. Shoot length was measured on 2 shoots in the middle of the canopy per tree.
2.5. Data Analyses
A 2-way analysis of variance (ANOVA) was used to determine if there were significant interactions between irrigation treatment and rootstock for all response variables. Before the ANOVA analysis, a homoscedasticity test was applied. For TCSA and shoot length, the variances were heterogeneous; for the other dependent variables, the data were square transformed (x = y2) to obtain homogeneous variances. Treatment differences for all response variables were determined by a one-way ANOVA and a Fisher’s least significant difference (LSD) test at the 95% confidence level. All statistical analyses were completed using InfoStat statistical software (version 2020 Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Córdoba, Argentina).
3. Results
There was a significant statistical interaction between irrigation and rootstock for E (p < 0.0001), gs (p < 0.0001), Chl (p = 0.0462), TCSA (p < 0.0001), and shoot length (p = 0.0278). Therefore, for all physiological and growth variables, differences between irrigation treatments were analyzed separately for each rootstock, and differences among rootstocks were analyzed separately within each irrigation treatment.
3.1. Physiological Variables
There was a significant difference in A values among rootstocks for each irrigation treatment. In the control treatment, WM/M had higher A than WM/C35 and WM/C22 (p = 0.0353), whereas WM/SC showed A values that were statistically similar among rootstocks. However, in the water deficit treatment, WM/M had higher A values than WM/SC, WM/C35, and WM/C22 (p < 0.0001), whereas WMSC, WM/C35, and WM/C22 were statistically similar to each other in the water deficit treatment. There was a significant difference in A values between irrigation treatments only for some rootstocks. There was no significant irrigation treatment effect on A for WM/M (p = 0.7064) and WM/C22 (p = 0.0865), whereas for WM/SC (p = 0.0433) and WM/C35 (p = 0.0485), A was 26.5% and 33.1% lower, respectively, in the moderate water deficit treatment compared with the control irrigation treatment (Figure 1a).
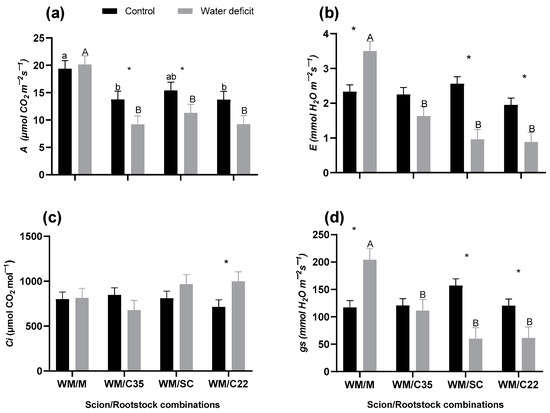
Figure 1.
(a) Net CO2 assimilation (A), (b) transpiration (E), (c) internal CO2 concentration (Ci), and (d) stomatal conductance (gs) of ‘W. Murcott’ tangor (WM) grafted onto Citrus macrophlylla (M), C-35 citrange (C35), ‘Swingle’ citrumelo (SC), or bitter citrandarin (C22) in control and water deficit treatments. Lowercase letters indicate significant differences among rootstocks in the control treatment, capital letters indicate significant differences among rootstocks in the water deficit treatment, and asterisks indicate significant differences between irrigation treatments for each rootstock according to Fisher’s LSD test (p ≤ 0.05). The bars represent the means, and the error bars represent the standard errors (n = 6).
There was no significant difference in E among rootstocks in the control treatment (p = 0.2204). However, in the water deficit treatment, there was a significant difference in E among rootstocks (p < 0.0001), with WM/M having higher E values than trees on the other rootstocks. Also, WM/SC and WM/C22 had lower E values than the WM/M in the water deficit treatment. There were significant differences in E between irrigation treatments for WM/M (p = 0.0052), WM/SC (p < 0.0001), and WM/C22 (p = 0.0138), but no difference for WM/C35 (p = 0.090). For WM/M, E was 50.2% lower in the water deficit treatment than in the control treatment. For WM/SC and WM/C22, E was 62.5% and 54.8% lower, respectively, in the water deficit treatment than in the control treatment (Figure 1b).
There was no difference in Ci among rootstocks in either irrigation treatment (p = 0.6837). There was also no difference in Ci between irrigation treatments for WM/M, WM/C35, or WM/SC (p = 0.1394). However, there was a significant difference in Ci between irrigation treatments for C22 (p = 0.0343), with Ci being 40.0% lower in the water deficit treatment than in the control treatment (Figure 1c).
There was no difference in gs among rootstocks in the control irrigation treatment (p = 0.0899). However, there was a difference in gs among rootstocks in the water deficit treatment (p < 0.0001). In the water deficit treatment, WM/M had a higher gs than the other rootstocks. There were significant differences in gs between irrigation treatments for WM/M (p = 0.0039), WM/SC (p < 0.0001), and WM/C22 (p = 0.0474), with WM/M having 74.3% higher gs in the water deficit treatment than in the control treatment. Also, WM/SC and WM/C22 had 61.9% and 49.1% lower gs, respectively, in the water deficit treatment compared with the control treatment. For C35, there was no significant difference in gs between irrigation treatments (p = 0.6935) (Figure 1d).
In the control irrigation treatment, there was no significant difference in Chl among rootstocks (p = 0.7863). However, there was a significant difference in Chl among rootstocks in the water deficit treatment (p = 0.0008), where WM/M and WM/C35 had higher Chl than WM/SC or WM/C22. There was a significant difference between irrigation treatments for WM/SC (p = 0.0375) and WM/C22 (p= 0.030), with these rootstocks having a 28.5% and 50.0% lower Chl, respectively, in the water deficit treatment compared with the control treatment. However, there was no significant difference in Chl between irrigation treatment for WM/M (p = 0.1816) or WM/C35 (p = 0.7682) (Figure 2a).
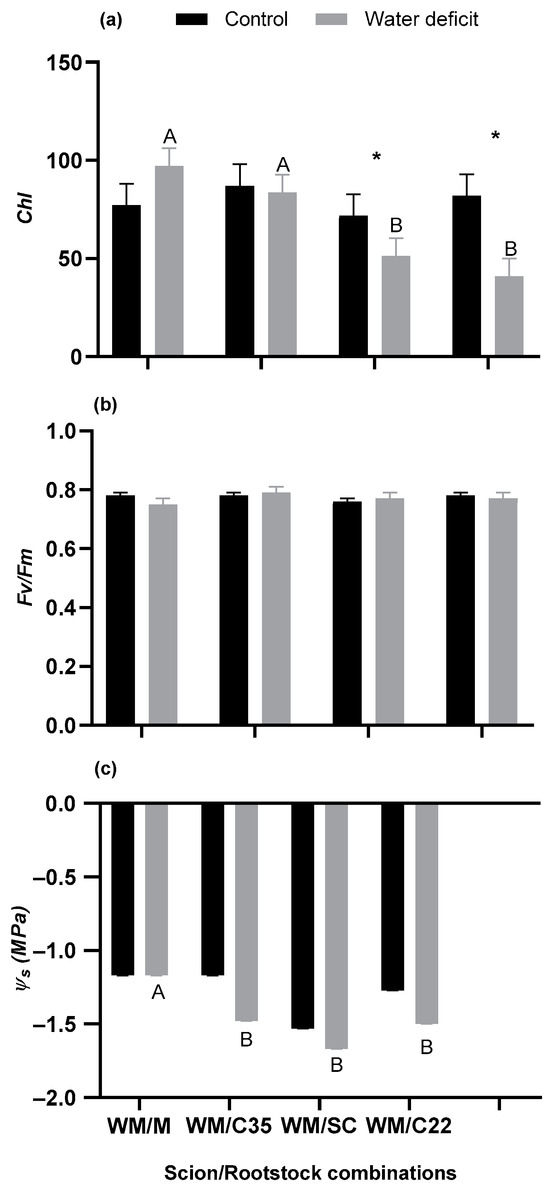
Figure 2.
(a) Relative chlorophyll content (Chl), (b) the ratio of variable to maximum chlorophyll fluorescence (Fv/Fm), and (c) stem water potential (Ψs) of ‘W. Murcott’ tangor (WM) grafted onto Citrus macrophlylla (M), C-35 citrange (C35), ‘Swingle’ citrumelo (SC), or bitter citrandarin (C22) rootstock in the control and water deficit treatment. Capital letters indicate significant differences among rootstocks in the water deficit treatment, and asterisks indicate significant differences between irrigation treatments for each rootstock according to Fisher’s LSD test (p ≤ 0.05). The bars represent the means, and the error bars represent the standard errors (n = 6).
There were no differences in Fv/Fm between irrigation treatments for any rootstock (p values for WM/M = 0.3752, WM/C35 = 0.5014, WM/SC = 0.3560 and WM/C22 = 0.3192) or among rootstocks within each irrigation treatment (P values for the water deficit treatment = 0.4656 and the control treatment = 0.1471) (Figure 2b).
There was no difference in Ψs among rootstocks in the control treatment (p = 0.1653). However, in the water deficit treatment, there was a significant difference in Ψs among rootstocks (p = 0.0291). In the water deficit treatment, the WM/M rootstock had higher Ψs than the other rootstocks. There was no difference in Ψs between irrigation treatments for any rootstock (P values for WM/M > 0.999, WM/C35 = 0.1054, WM/SC = 0.2943 and WM/C22 = 0.2222) (Figure 2c).
3.2. Vegetative Growth
There was no difference in the TCSA of the scion between irrigation treatments for WM/C35 (p = 0.2929), WM/SC (p = 0.1724), or WM/C22 (p = 0.5374). However, there was a significant difference in scion TCSA between irrigation treatments for WM/M (p = 0.0025), with the TCSA of the scion being 18.3% higher in the water deficit treatment than in the control treatment (Figure 3a). There was no difference in scion TCSA among rootstocks in the control irrigation treatment (p = 0.7827) or the water deficit treatment (p = 0.1798).
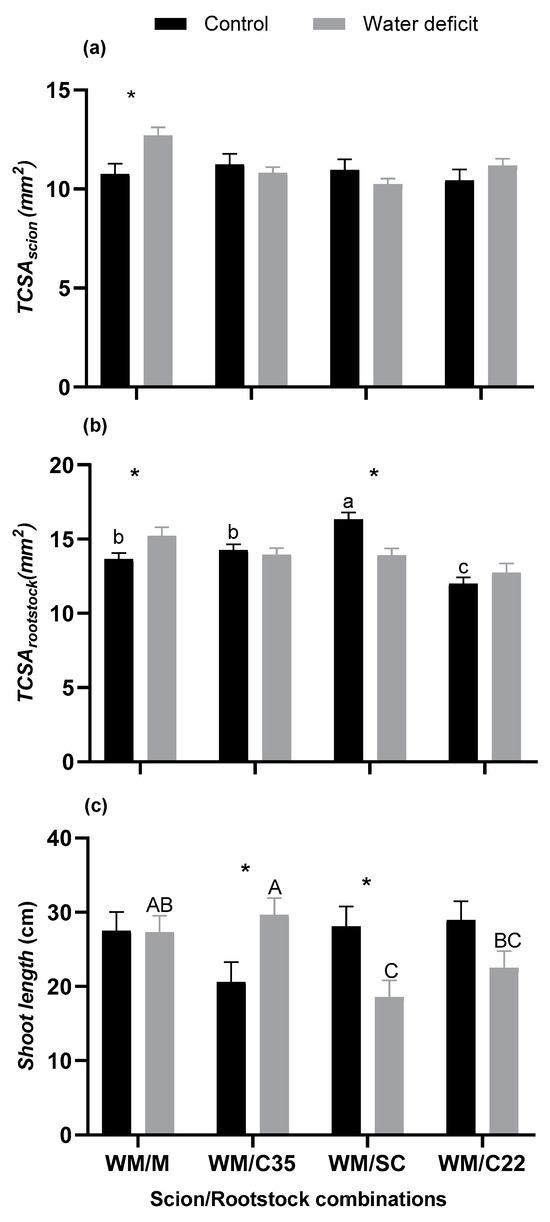
Figure 3.
(a) Trunk cross-sectional area of the scion (TCSA scion), (b) trunk cross-sectional area of the rootstock (TCSA rootstock), and (c) shoot length of ‘W. Murcott’ tangor (WM) grafted onto Citrus macrophlylla (M), C-35 citrange (C35), ‘Swingle’ citrumelo (SC), or bitter citrandarin (C22) in control and water deficit treatments. Lowercase letters indicate significant differences among rootstocks in the control treatment, capital letters indicate significant differences among rootstocks in the water deficit treatment, and asterisks indicate significant differences between irrigation treatment for each rootstock according to Fisher’s LSD test (p ≤ 0.05). The bars represent the means, and the error bars represent the standard errors (n = 6).
There was a significant difference in rootstock TCSA among rootstocks in the control irrigation treatment (p < 0.0001), with WM/SC exhibiting a higher rootstock TCSA than the other rootstocks. Also, the rootstock TCSA was similar for WM/C35 and WM/M, whereas WM/C22 had a lower rootstock TCSA than WM/C35 or WM/M. However, there was no difference in rootstock TCSA among rootstocks in the water deficit treatment (p = 0.0903). There was a significant difference in rootstock TCSA between irrigation treatments for WM/M (p = 0.0004) and WM/SC (p = 0.0430), where the rootstock TCSA of WM/M was 21.9% higher in the water deficit treatment than in the control treatment. Also, for WM/SC, the rootstock TCSA was 11.5% lower in the water deficit treatment compared with the control treatment (Figure 3b). There was no difference between irrigation treatments for WM/C35 (p = 0.5161) or WM/C22 (p = 0.4698).
There was no difference in shoot length among rootstocks in the control irrigation treatment (p = 0.1222), but there was a significant difference in shoot length among rootstocks in the water deficit treatment (p = 0.0064). In the water deficit treatment, WM/C35 and WM/M had longer shoots than WM/C22 or WM/SC. In addition, there was a significant difference in shoot length between irrigation treatments for WM/C35 (p = 0.0483) and WM/SC (p = 0.0006), but there was no difference for WM/M (p = 0.9137) or WM/C22 (p = 0.1432). For WM/C35, the shoot length was 43.7% greater in the water deficit treatment than in the control treatment, whereas for WM/SC, the shoot length was 38.9% lower in the water deficit treatment compared with the control treatment (Figure 3c).
4. Discussion
Several authors have reported that physiological and morphological responses of several citrus species to water stress negatively impact yield and fruit quality [3,11,17,18,19,20]. In our study, we observed a significant effect of the rootstock on the leaf gas exchange of ‘W. Murcott’ tangor plants under water restriction. Water deficit conditions promoted an isohydric-type response in trees grafted on SC or C22 rootstocks [21,22]. With these rootstocks, ‘W. Murcott’ showed a significant reduction in gs and E under water stress, which could help to maintain their leaf water potential. This response is mainly controlled by the stomata, which close partially or completely in response to water stress [9], limiting water loss but also reducing the entry of CO2 into the leaf [23]. Thus, in isohydric plants, the stomatal response to the water deficit condition is a strategy that allows for drought avoidance, favoring survival at the expense of reduced photo-assimilation [9]. In contrast, trees on C35 rootstock appeared to be tolerant of water stress, exhibiting no significant reduction in leaf gas exchange in the water deficit treatment compared with the control treatment. Therefore, the main mechanism for drought tolerance in plants on this rootstock was likely due to non-stomatal factors [23]. Among those factors, citrus has been reported to show osmotic adjustment, which involves metabolic regulation of sugars, amino acids, and osmoprotective proteins within the cell, as well as hydraulic adaptation, thereby avoiding the potential of xylem cavitation and maintaining constant xylem sap flow [9,23].
‘W. Murcott’ tangor on C22 or C35 rootstocks exhibited better drought avoidance behavior than trees grafted on the other rootstocks tested, suggesting that these two rootstocks would better avoid yield and quality loss compared with the other rootstocks tested under water-restricted conditions. Both C22 and C35 are hybrids of Poncirus trifoliata, which has been described as tolerant to several abiotic stresses, including water stress [24]. This may explain the greater drought tolerance observed for C22 and C35 compared with the other rootstocks tested in this study.
In the present study, ‘W. Murcott’ tangor on M rootstock showed a classic anisohydric type response to water stress due to a lack of stomatal regulation in response to drought [22,25,26]. For trees on this rootstock subjected to water deficit irrigation, leaf gas exchange values remained equal to, or even greater than, those of plants in the control irrigation treatment where plants received adequate water. Our results agree with those of Robles et al. [11], who reported that the rootstocks with the most vigor, such as Citrus macrophylla, are the most susceptible to drought stress, which can negatively affect yield and fruit quality [9,13,24].
The only trees to exhibit a significant reduction in TCSA and shoot length under water stress were those on the SC rootstock. The reduced biomass of trees on the SC rootstock in the water stress treatment was presumably due to stomatal closure limiting A. In contrast, the growth of trees on C35 or C22 rootstocks subjected to deficit irrigation had similar or slightly reduced growth compared with trees on these rootstocks in the control irrigation treatment. This was presumably due to A being less negatively impacted by water stress in trees on C35 or C22 rootstock compared with trees on the other rootstocks, thus allowing them to maintain cell growth [18,27,28,29]. ‘W. Murcott’ tangor on M rootstock had significantly greater stem growth in the water deficit treatment compared with the control irrigation treatment. Bhusal et al. [24] indicated that increased trunk growth is clear evidence that the plant cannot generate a response to water stress because it cannot perceive a water reduction in the soil and therefore continues to grow [6]. It also may be due to a strategy to redistribute and/or reduce the size of the canopy during drought [11,25]. Another compensatory response to water shortage in trees on M rootstock may have been the increase in the leaf Chl, which helped to maintain photosynthesis [30]. However, Chl and Fv/Fm are unreliable indicators of water stress in citrus trees because the response of these variables to water stress varies among rootstocks and scion cultivars [6,10].
Stem water potential is affected by rootstock and irrigation levels independently. In the present study, M was the only rootstock with a higher stem water potential in the water deficit treatment. However, there were no differences in stem water potential in the control irrigation treatment among rootstocks or between irrigation treatments. This could indicate that the plants in this experiment were able to respond to water stress, avoiding significant stem water potential reduction. Previous studies have demonstrated that citrus and other fruit-bearing trees are prone to responding to water stress using different strategies, such as osmotic adjustment [9,29]. Maintaining the water potential derives from the plant’s ability to perceive a decrease in soil water content and osmotically adjust as a result, thereby improving hydraulic conductivity to maintain the soil–water–plant–atmosphere continuity [31,32,33,34,35]. The reduction in water potential indicates the inability of plants to respond to water stress. Moreover, a change in stem water potential due to water stress is related to the expression of aquaporins [36,37], which are small proteins within cell membranes that are channels for water transport [38]. Paudel et al. [10] observed that in ‘Rio Red’ grapefruit (Citrus paradise Macfadyen) grafted onto volkameriana (Citrus volkameriana Volkamer), there was an upregulation in the mRNA expression of the aquaporin genes CvPIP1:2, CvPIP2:1, and CvPIP2:2 in the root with decreasing irrigation. Aquaporins facilitate the entry of water into the plant and maintain the integrity of the water column through the xylem even when soil water content is low [36]. The expression of aquaporins allows hydraulic regulation in roots and leaves, conferring greater tolerance to drought [39]. Thus, future studies on the relative drought tolerance of ‘W. Murcott’ tangor grafted on different rootstocks would benefit from examining the role of aquaporins in the stress response to provide greater insight into the mechanisms of the plant’s response to water stress.
5. Conclusions
The response of ‘W. Murcott’ tangor to soil water deficit is regulated by the rootstock. Grafting trees onto Swingle citrumelo rootstock resulted in the greatest soil water deficit tolerance of ‘W. Murcott’ tangor, with stomata closing in response to low soil moisture, resulting in reduced A and a significant decrease in growth, which can be described as strict isohydric-type behavior. Bitter citrandarin and C-35 citrange responded similarly to water stress, but the response was not strictly isohydric. The Citrus macrophylla rootstock was the most susceptible to water stress, with high water loss due to an increase in stomatal conductance and transpiration, which can be described as strict anisohydric-type behavior. In the present study, water-stressed trees were only subjected to moderate water deficit, with about a 25% reduction in soil water content for 46 days. This research suggests that P. trifoliata hybrid rootstocks could be better at conferring tolerance to moderate drought than the other rootstocks tested in this study due to their flexible response to water stress. Therefore, C-35 and C-22 rootstocks could be more suitable for areas prone to drought conditions. Future studies should focus on identifying the response of different rootstocks for ‘W. Murcott’ tangor subjected to different levels of water availability for different durations and studying phenotypic traits associated with the genetic expression that modulates the physiological response of these rootstocks to water stress. Ultimately, the effects of these rootstocks on fruit yield and quality under water stress in the field also need to be evaluated to select the best rootstock for drought-prone semi-arid regions.
Author Contributions
Conceptualization, S.T., P.M.G. and J.M.; methodology, S.T., P.M.G., J.M., A.R.S. and R.C.; formal analysis and writing—original draft preparation, S.T.; writing—review and editing, S.T., P.M.G., J.M., A.R.S., R.C. and B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ph.D. scholarship N° 21200679; Agencia Nacional de Investigación y Desarrollo; Ministerio de Ciencia, Tecnología, Conocimiento e Innovación; Gobierno de Chile together with Innova-Chile-CORFO Sumate an Innovar project N°20SN151588, Escuela de Agronomía; Pontificia Universidad Católica de Valparaíso, Chile; and Viveros Deliplant.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We thank Viveros Deliplant for providing access to all plant materials and locations for the experiments. We also thank the Departamento de Investigación y Postgrado de la Facultada de Agronomía y Sistemas Naturales de la Pontificia Universidad Católica de Chile.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analysis or interpretation of the data, in the writing of the manuscript, or in the decision to publish the results.
References
- FAO. “Resultados.” Estados Unidos de América Importaciones—Cantidad Tangerinas, Mandarinas, Clementinas. 2021. Available online: https://www.fao.org/faostat/en/#compare (accessed on 5 December 2023).
- Barry, G.H.; Caruso, M.; Gmitter, F.G. Commercial Scion Varieties. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Jr., Eds.; Woodhead Publishing: Duxford, UK, 2020; pp. 83–104. [Google Scholar]
- Jamshidi, S.; Zand, S.; Dev, P. Physiological Responses of Orange Trees Subject to Regulated Deficit Irrigation and Partial Root Drying. Irrig. Sci. 2021, 39, 441–455. [Google Scholar] [CrossRef]
- Puig-Sirera, A.; Provenzano, G.; González-Altozano, P.; Intrigliolo, D.S.; Rallo, G. Irrigation Water Saving Strategies in Citrus Orchards: Analysis of the Combined Effects of Timing and Severity of Soil Water Deficit. Agric. Water Manag. 2021, 248, 106773. [Google Scholar] [CrossRef]
- Sampaio, A.; Oliveira Silva, R.; Franca Brito, R.B.; Soares, W.; da Silva, A.; Duarte, L.; Coelho, M. Sweet Orange Acclimatisation to Water Stress: A Rootstock Dependency. Sci. Hortic. 2021, 276, 109727. [Google Scholar] [CrossRef]
- Miranda, T.; Da Silva, S.F.; Silveira, N.; Pereira, L.; Machado, E.C.; Ribeiro, R.V. Root Osmotic Adjustment and Stomatal Control of Leaf Gas Exchange Are Dependent on Citrus Rootstocks Under Water Deficit. J. Plant Growth Regul. 2020, 40, 11–19. [Google Scholar] [CrossRef]
- Puglisi, I.; Nicolosi, E.; Vanella, D.; Lo Piero, A.; Stagno, F.; Saitta, D.; Roccuzzo, G.; Consoli, S.; Baglieri, A. Physiological and Biochemical Responses of Orange Trees to Different Deficit Irrigation Regimes. Plants 2019, 8, 423. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.S.; Neves, D.; Santana-Vieira, D.D.S.; Almeida, L.; Costa, M.; Soares, W.; Pirovani, C.; Coelho, M.; Ferreira, C.; Gesteira, A. Citrus Scion and Rootstock Combinations Show Changes in DNA Methylation Profiles and ABA Insensitivity under Recurrent Drought Conditions. Sci. Hortic. 2020, 267, 109313. [Google Scholar] [CrossRef]
- Dong, T.; Xi, L.; Bo, X.; Qiu, X.; Huang, S.; Xu, W.; Wang, J.; Wang, B.; Yao, Y.; Duan Ch Tang, X.; et al. Drought Resistance in Harumi Tangor Seedlings Grafted onto Different Rootstocks. Funct. Plant Biol. 2021, 48, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Paudel, I.; Cohen, S.; Shlizerman, L.; Jaiswal, A.K.; Shaviv, A.; Sadka, A. Reductions in Root Hydraulic Conductivity in Response to Clay Soil and Treated Waste Water Are Related to PIPs Down-Regulation in Citrus. Sci. Rep. 2017, 7, 15429. [Google Scholar] [CrossRef] [PubMed]
- Robles, J.M.; Botía, P.; Pérez-Pérez, J.G. Sour Orange Rootstock Increases Water Productivity in Deficit Irrigated ‘Verna’ Lemon Trees Compared with Citrus Macrophylla. Agric. Water Manag. 2017, 186, 98–107. [Google Scholar] [CrossRef]
- Jiménez, R.; Zamora, V. Principales Cultivares y Patrones Utilizados En La Citricultura. In Taller Reg. Sobre Viveros De Cítricos; Instituto de Investigaciones en Fruticultura Tropical: La Habana, Cuba, 2010; Volume 44, ISBN 978-959-296-020-6. [Google Scholar]
- Bowman, K.D.; Joubert, J. Citrus Rootstocks. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Jr., Eds.; Woodhead Publishing: Duxford, UK, 2020; pp. 105–127. [Google Scholar]
- Evett, S.R.; Schwartz, R.; Casanova, J.; Heng, L. Soil Water Sensing for Water Balance, ET and WUE. Agric. Water Manag. 2012, 104, 1–9. [Google Scholar] [CrossRef]
- Passioura, J.B. Viewpoint: The perils of pot experiments. Funct.Plant Biol. 2006, 33, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, U. Facilitating Protocols While Maintaining Accuracy in Grapevine Pressure Chamber Measurements-Comments on Levin 2019. Agric. Water Manag. 2020, 227, 2019–2021. [Google Scholar] [CrossRef]
- Jiménez, S.; Fattahi, M.; Bedis, K.; Nasrolahpour-Moghadam, S.; Irigoyen, J.; Gogorcena, Y. Interactional Effects of Climate Change Factors on the Water Status, Photosynthetic Rate, and Metabolic Regulation in Peach. Front. Plant Sci. 2020, 11, 43. [Google Scholar] [CrossRef]
- Tu, A.; Xie, S.; Mo, M.; Song, Y.; Li, Y. Water Budget Components Estimation for a Mature Citrus Orchard of Southern China Based on HYDRUS-1D Model. Agric. Water Manag. 2021, 243, 106426. [Google Scholar] [CrossRef]
- Singh, G.; Singh, H.; Gupta, M.; Singh Sidhu, G. Standardization of Stage Wise Water Requirement in Drip Irrigated Kinnow Mandarin Orchards under Sub-Tropical Conditions. J. Agrometeorol. 2020, 22, 305–312. [Google Scholar] [CrossRef]
- Nagaz, K.; El Mokh, F.; Ben Hassen, N.; Masmoudi, M.M.; Ben Mechlia, N.; Baba Sy, M.O.; Belkheiri, O.; Ghiglieri, G. Impact of Deficit Irrigation on Yield and Fruit Quality of Orange Trees (Citrus sinensis, L. Osbeck, CV. Meski maltaise) in Southern Tunisia. Irrig. Drain. 2020, 69, 186–193. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, F.; Gou, X.; Fonti, P.; Xia, J.; Cao, Z.; Liu, J.; Wang, Y.; Zhang, J. Seasonal Variations in Leaf-Level Photosynthesis and Water Use Efficiency of Three Isohydric to Anisohydric Conifers on the Tibetan Plateau. Agric. For. Meteorol. 2021, 308–309, 108581. [Google Scholar] [CrossRef]
- Hochberg, U.; Rockwell, F.E.; Michele Holbrook, N.; Cochard, H. Iso/Anisohydry: A Plant–Environment Interaction Rather Than a Simple Hydraulic Trait. Trends Plant Sci. 2018, 23, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Salmon, Y.; Lintunen, A.; Dayet, A.; Chan, T.; Dewar, R.; Vesala, T.; Hölttä, T. Leaf Carbon and Water Status Control Stomatal and Nonstomatal Limitations of Photosynthesis in Trees. New Phytol. 2020, 226, 690–703. [Google Scholar] [CrossRef]
- Caruso, M.; Continella, A.; Modica, G.; Pannitteri, C.; Russo, R.; Salonia, F.; Arlotta, C.; Gentile, A.; Russo, G. Rootstocks Influence Yield Precocity, Productivity, and Pre-Harvest Fruit Drop of Mandared Pigmented Mandarin. Agronomy 2020, 10, 1305. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Reum Han, A.; Han, A.; Seok Kim, H. Responses to Drought Stress in Prunus Sargentii and Larix Kaempferi Seedlings Using Morphological and Physiological Parameters. For. Ecol. Manag. 2019, 465, 118099. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Garcia-Forner, N. Water Potential Regulation, Stomatal Behaviour and Hydraulic Transport under Drought: Deconstructing the Iso/Anisohydric Concept. Plant Cell Environ. 2017, 40, 962–976. [Google Scholar] [CrossRef] [PubMed]
- Livellara, N.; Saavedra, F.; Salgado, E. Plant Based Indicators for Irrigation Scheduling in Young Cherry Trees. Agric. Water Manag. 2011, 98, 684–690. [Google Scholar] [CrossRef]
- Meza, F.J.; Montes, C.; Bravo-Martínez, F.; Serrano-Ortiz, P.; Kowalski, A.S. Soil Water Content Effects on Net Ecosystem CO2 Exchange and Actual Evapotranspiration in a Mediterranean Semiarid Savanna of Central Chile. Sci. Rep. 2018, 8, 8570. [Google Scholar] [CrossRef] [PubMed]
- Serra, I.; Strever, A.; Myburgh, P.A.; Deloire, A. Review: The Interaction between Rootstocks and Cultivars (Vitis vinifera L.) to Enhance Drought Tolerance in Grapevine. Aust. J. Grape Wine Res. 2014, 20, 1–14. [Google Scholar] [CrossRef]
- Nankishore, A.; Farrell, A.D. The Response of Contrasting Tomato Genotypes to Combined Heat and Drought Stress. J. Plant Physiol. 2016, 202, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Landi, S.; Nurcato, R.; De Lillo, A.; Lentini, M.; Grillo, S.; Esposito, S. Glucose-6-Phosphate Dehydrogenase Plays a Central Role in the Response of Tomato (Solanum lycopersicum) Plants to Short and Long-Term Drought. Plant Physiol. Biochem. 2016, 105, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C. Turgor Maintenance by Osmotic Adjustment, an Adaptive Mechanism for Coping with Plant Water Deficits. Plant Cell Environ. 2017, 40, 1–3. [Google Scholar] [CrossRef]
- Kosar, F.; Aisha Akram, N.; Sadiq, M.; Al-Qurainy, F.; Ashraf, M. Trehalose: A Key Organic Osmolyte Effectively Involved in Plant Abiotic Stress Tolerance. J. Plant Growth Regul. 2019, 38, 606–618. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, Q.; Li, Y.; Wang, Q.; Xu, F.; Dang, X.; Xu, W.; Zhang, J.; Miao, R. Abscisic Acid Is Required for Root Elongation Associated With Ca2+ Influx in Response to Water Stress. Front. Plant Sci. 2020, 11, 127–137. [Google Scholar] [CrossRef]
- Fàbregas, N.; Fernie, A.R. The Metabolic Response to Drought. J. Exp. Bot. 2019, 70, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Carminati, A.; Javaux, M. Soil Rather Than Xylem Vulnerability Controls Stomatal Response to Drought. Trends Plant Sci. 2020, 25, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zuo, Q.; Shi, J.; Wang, J.; Xue, X.; Ben-Gal, A. Introducing Water Stress Hysteresis to the Feddes Empirical Macroscopic Root Water Uptake Model. Agric. Water Manag. 2020, 240, 106293. [Google Scholar] [CrossRef]
- Takata, K.; Matsuzaki, T.; Tajika, Y. Aquaporins: Water Channel Proteins of the Cell Membrane. Prog. Histochem. Cytochem. 2004, 39, 1–83. [Google Scholar] [CrossRef]
- Wei, Q.; Ma, Q.; Ma, Z.; Zhou, G.; Feng, F.; Le, S.; Lei Ch Gu, Q. Genome-Wide Identification and Characterization of Sweet Orange (Citrus sinensis) Aquaporin Genes and Their Expression in Two Citrus Cultivars Differing in Drought Tolerance. Tree Genet. Genomes 2019, 15, 17. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).