Aloe vera Cuticle: A Promising Organic Water-Retaining Agent for Agricultural Use
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of the Organic Agent
2.2. Measurement of Water-Holding Capacity (WHC) and Water Solubility
2.2.1. Water-Holding Capacity (WHC)
2.2.2. Water Solubility
2.3. Comparison with a Commercial Polyacrylamide Hydrogel
2.4. Experimental Design and Statistical Analysis
3. Results and Discussion
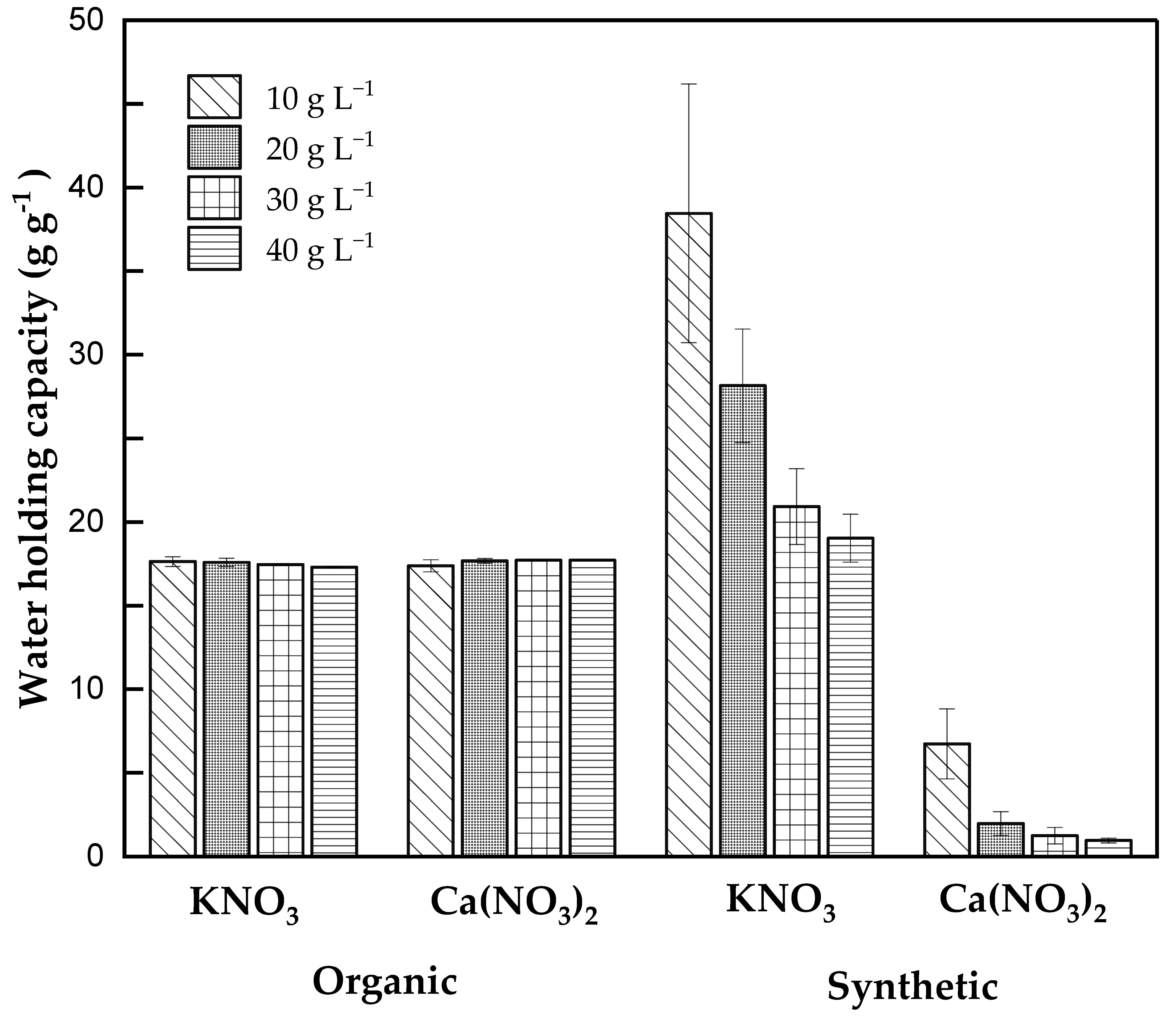
3.1. Water-Holding Capacity (WHC) Assessment
3.2. Water Solubility Evaluation
3.3. Performance Comparison against a Commercial Product
4. Conclusions
5. Patent
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pérez-Blanco, C.D.; Hrast-Essenfelder, A.; Perry, C. Irrigation Technology and Water Conservation: A Review of the Theory and Evidence. Rev. Environ. Econ. Policy 2020, 14, 216–239. [Google Scholar] [CrossRef]
- McDermid, S.; Nocco, M.; Lawston-Parker, P.; Keune, J.; Pokhrel, Y.; Jain, M.; Jägermeyr, J.; Brocca, L.; Massari, C.; Jones, A.D.; et al. Irrigation in the Earth System. Nat. Rev. Earth Environ. 2023, 4, 435–453. [Google Scholar] [CrossRef]
- Chehab, H.; Tekaya, M.; Mechri, B.; Jemai, A.; Guiaa, M.; Mahjoub, Z.; Boujnah, D.; Laamari, S.; Chihaoui, B.; Zakhama, H.; et al. Effect of the Super Absorbent Polymer Stockosorb® on Leaf Turgor Pressure, Tree Performance and Oil Quality of Olive Trees Cv. Chemlali Grown under Field Conditions in an Arid Region of Tunisia. Agric. Water Manag. 2017, 192, 221–231. [Google Scholar] [CrossRef]
- Harisha, C.B.; Rane, J.; Halagunde Gowda, G.R.; Chavan, S.B.; Chaudhary, A.; Verma, A.K.; Ravi, Y.; Asangi, H.; Halli, H.M.; Boraiah, K.M.; et al. Effect of Deficit Irrigation and Intercrop Competition on Productivity, Water Use Efficiency and Oil Quality of Chia in Semi-Arid Regions. Horticulturae 2024, 10, 101. [Google Scholar] [CrossRef]
- Tobar, S.; Gil, P.M.; Schaffer, B.; Schwember, A.R.; Cautín, R.; Mártiz, J. Physiological and Growth Responses of W. Murcott Tangor Grafted on Four Rootstocks under Water Restriction. Horticulturae 2024, 10, 352. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of Plants to Water Stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef]
- Khormizi, H.Z.; Malamiri, H.R.G.; Ferreira, C.S.S. Estimation of Evaporation and Drought Stress of Pistachio Plant Using UAV Multispectral Images and a Surface Energy Balance Approach. Horticulturae 2024, 10, 515. [Google Scholar] [CrossRef]
- Saha, A.; Sekharan, S.; Manna, U. Superabsorbent Hydrogel (SAH) as a Soil Amendment for Drought Management: A Review. Soil Tillage Res. 2020, 204, 104736. [Google Scholar] [CrossRef]
- Aalam, F.; Rezaei Nejad, A.; Mousavi-Fard, S.; Raji, M.; Nikoloudakis, N.; Goumenaki, E.; Fanourakis, D. Water Deficit Severity during the Preceding Year Determines Plant Tolerance to Subsequent Year Drought Stress Challenges: A Case Study in Damask Rose. Horticulturae 2024, 10, 462. [Google Scholar] [CrossRef]
- Pedroza-Sandoval, A.; Minjares-Fuentes, J.R.; Trejo-Calzada, R.; Gramillo-Avila, I. Physiological and Productivity Responses in Two Chili Pepper Morphotypes (Capsicum annuum L.) under Different Soil Moisture Contents. Horticulturae 2024, 10, 92. [Google Scholar] [CrossRef]
- Jaramillo-Quiceno, N.; Carmona, D.M.; Torres-Taborda, M.; Hincapié-Llanos, G.A.; Álvarez-López, C. Multilayer Silk Sericin-Based Coating for Controlled Release of Water and Nutrients in Soil: Development, Characterization, and Performance Evaluation in Agricultural Production Model. Horticulturae 2024, 10, 273. [Google Scholar] [CrossRef]
- Fayek, M.A.; Abdel-Mohsen, M.A.; Laz, S.I.; EL-Sayed, S.M. Impact of Super Absorbent Polymer and a Bentonite as Soil Amendments under Irrigation Regimes in Olive Orchard. Plant Arch. 2020, 20, 723–730. [Google Scholar]
- Guancha-Chalapud, M.A.M.A.; Serna-Cock, L.; Tirado, D.F.D.F. Hydrogels Are Reinforced with Colombian Fique Nanofibers to Improve Techno-Functional Properties for Agricultural Purposes. Agriculture 2022, 12, 117. [Google Scholar] [CrossRef]
- Cheng, Y.-C.; Wang, C.-P.; Liu, K.-Y.; Pan, S.-Y. Towards Sustainable Management of Polyacrylamide in Soil-Water Environment: Occurrence, Degradation, and Risk. Sci. Total Environ. 2024, 926, 171587. [Google Scholar] [CrossRef]
- Xiong, B.; Loss, R.D.; Shields, D.; Pawlik, T.; Hochreiter, R.; Zydney, A.L.; Kumar, M. Polyacrylamide Degradation and Its Implications in Environmental Systems. Npj Clean Water 2018, 1, 17. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.T.; Rubira, A.F.; Muniz, E.C. Superabsorbent Hydrogels Based on Polysaccharides for Application in Agriculture as Soil Conditioner and Nutrient Carrier: A Review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef]
- Guancha-Chalapud, M.A.; Serna-Cock, L.; Tirado, D.F. Aloe vera Rind Valorization to Improve the Swelling Capacity of Commercial Acrylic Hydrogels. Fibers 2022, 10, 73. [Google Scholar] [CrossRef]
- Singh, P.; Hundal, J.S.; Patra, A.K.; Wadhwa, M.; Sharma, A. Sustainable Utilization of Aloe vera Waste in the Diet of Lactating Cows for Improvement of Milk Production Performance and Reduction of Carbon Footprint. J. Clean. Prod. 2021, 288, 125118. [Google Scholar] [CrossRef]
- Kalderis, D.; Stavroulakis, G.; Tsubota, T.; Çalhan, S.D. Valorization of Aloe vera Waste for the Production of Ca and P-Rich Hydrochars. Sustain. Chem. Environ. 2024, 5, 100057. [Google Scholar] [CrossRef]
- Ayala-Aponte, A.; Cárdenas-Nieto, J.D.; Tirado, D.F. Aloe vera Gel Drying by Refractance Window®: Drying Kinetics and High-Quality Retention. Foods 2021, 10, 1445. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.A.; Ismail, A.; Hamid, A.A.; Azlan, A.; Al-sheraji, S.H. Characterisation of Fibre-Rich Powder and Antioxidant Capacity of Mangifera Pajang K. Fruit Peels. Food Chem. 2011, 126, 283–288. [Google Scholar] [CrossRef]
- Robertson, J.A.; de Monredon, F.D.; Dysseler, P.; Guillon, F.; Amado, R.; Thibault, J.-F. Hydration Properties of Dietary Fibre and Resistant Starch: A European Collaborative Study. LWT Food Sci. Technol. 2000, 33, 72–79. [Google Scholar] [CrossRef]
- Serna-Cock, L.; Torres-León, C.; Ayala-Aponte, A. Evaluation of Food Powders Obtained from Peels of Mango (Mangifera indica) as Sources of Functional Ingredients. Inf. Tecnol. 2015, 26, 41–50. [Google Scholar] [CrossRef]
- Hosseini, A.; Moradinezhad, F.; Khayyat, M.; Aminifard, M.H. Bioactive Compounds and Quality Attributes of Fresh Seedless Barberry (Berberis vulgaris L.) at Cold Storage as Influenced by Multiple Spraying of Calcium Nitrate and Potassium Nitrate. J. Agric. Sci. 2022, 17, 258–269. [Google Scholar] [CrossRef]
- Mohd Nizam, N.H.; Mohammad Rawi, N.F.; Mhd Ramle, S.F.; Abd Aziz, A.; Abdullah, C.K.; Rashedi, A.; Mohamad Kassim, M.H. Physical, Thermal, Mechanical, Antimicrobial and Physicochemical Properties of Starch Based Film Containing Aloe vera: A Review. J. Mater. Res. Technol. 2021, 15, 1572–1589. [Google Scholar] [CrossRef]
- Ahmed, J.; Al-Attar, H.; Arfat, Y.A. Effect of Particle Size on Compositional, Functional, Pasting and Rheological Properties of Commercial Water Chestnut Flour. Food Hydrocoll. 2016, 52, 888–895. [Google Scholar] [CrossRef]
- Sánchez-Mendoza, N.A.; Ruiz-Ruiz, J.C.; Dávila-Ortiz, G.; Jiménez-Martínez, C. Techno-Functional and Biological Properties of Flour, Isolated and Majority Protein Fractions from Seeds of Inga Paterno. CyTA—J. Food 2017, 15, 400–408. [Google Scholar] [CrossRef]
- Laftah, W.A.; Hashim, S.; Ibrahim, A.N. Polymer Hydrogels: A Review. Polym. Plast. Technol. Eng. 2011, 50, 1475–1486. [Google Scholar] [CrossRef]
- Hossen, M.M.; Hossain, M.L.; Mitra, K.; Hossain, B.; Bithi, U.H.; Uddin, M.N. Phytochemicals and In-Vitro Antioxidant Activity Analysis of Aloe vera by-Products (Skin) in Different Solvent Extract. J. Agric. Food Res. 2022, 10, 100460. [Google Scholar] [CrossRef]
- Cheng, S.; Panthapulakkal, S.; Ramezani, N.; Asiri, A.M.; Sain, M. Aloe vera Rind Nanofibers: Effect of Isolation Process on the Tensile Properties of Nanofibre Films. BioResources 2014, 9, 7653–7665. [Google Scholar] [CrossRef][Green Version]
- Maan, A.A.; Nazir, A.; Khan, M.K.I.; Ahmad, T.; Zia, R.; Murid, M.; Abrar, M. The Therapeutic Properties and Applications of Aloe vera: A Review. J. Herb. Med. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Hęś, M.; Dziedzic, K.; Górecka, D.; Jędrusek-Golińska, A.; Gujska, E. Aloe vera (L.) Webb.: Natural Sources of Antioxidants—A Review. Plant Foods Hum. Nutr. 2019, 74, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Illés, E.; Tombácz, E. The Effect of Humic Acid Adsorption on PH-Dependent Surface Charging and Aggregation of Magnetite Nanoparticles. J. Colloid Interface Sci. 2006, 295, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Biela, M.; Kleinová, A.; Klein, E. Phenolic Acids and Their Carboxylate Anions: Thermodynamics of Primary Antioxidant Action. Phytochemistry 2022, 200, 113254. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-Z.; Deng, G.; Guo, R.; Fu, Z.-M.; Chen, D.-F. Effects of Different Ester Chains on the Antioxidant Activity of Caffeic Acid. Bioorg. Chem. 2020, 105, 104341. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Reports 2019, 24, e00370. [Google Scholar] [CrossRef]
- Benzidia, B.; Barbouchi, M.; Hammouch, H.; Belahbib, N.; Zouarhi, M.; Erramli, H.; Ait Daoud, N.; Badrane, N.; Hajjaji, N. Chemical Composition and Antioxidant Activity of Tannins Extract from Green Rind of Aloe vera (L.) Burm. F. J. King Saud Univ. Sci. 2019, 31, 1175–1181. [Google Scholar] [CrossRef]
- United Nations. The 17 Goals: Sustainable Development Goals; United Nations: New York, NY, USA, 2024.
| Particle Size (µm) | Temperature (°C) | pH | WHC (g g−1) | Particle Size (µm) | Temperature (°C) | pH | WHC (g g−1) |
|---|---|---|---|---|---|---|---|
| 180 | 10 | 4.5 | 8.7 ± 0.4 | 250 | 10 | 4.5 | 15.6 ± 0.4 |
| 6.0 | 7.9 ± 0.6 | 6.0 | 15.7 ± 0.6 | ||||
| 7.0 | 7.3 ± 0.7 | 7.0 | 15.1 ± 0.4 | ||||
| 20 | 4.5 | 7.0 ± 0.7 | 20 | 4.5 | 17.9 ± 0.9 | ||
| 6.0 | 6.8 ± 0.8 | 6.0 | 18.0 ± 0.3 | ||||
| 7.0 | 6.4 ± 0.9 | 7.0 | 17.5 ± 0.5 | ||||
| 30 | 4.5 | 6.8 ± 1.5 | 30 | 4.5 | 16.6 ± 0.4 | ||
| 6.0 | 5.6 ± 2.4 | 6.0 | 17.1 ± 0.3 | ||||
| 7.0 | 7.5 ± 2.1 | 7.0 | 16.6 ± 0.8 | ||||
| 40 | 4.5 | 7.5 ± 0.8 | 40 | 4.5 | 15.9 ± 0.4 | ||
| 6.0 | 6.3 ± 2.6 | 6.0 | 16.1 ± 0.6 | ||||
| 7.0 | 7.6 ± 2.2 | 7.0 | 17.0 ± 0.4 |
| Particle Size (µm) | Temperature (°C) | pH | Solubility (%) | Particle Size (µm) | Temperature (°C) | pH | Solubility (%) |
|---|---|---|---|---|---|---|---|
| 180 | 10 | 4.5 | 65.2 ± 3.4 | 250 | 10 | 4.5 | 72.8 ± 4.5 |
| 6.0 | 51.2 ±1.8 | 6.0 | 65.3 ± 5.0 | ||||
| 7.0 | 49.9 ± 4.4 | 7.0 | 54.9 ± 3.5 | ||||
| 20 | 4.5 | 75.3 ± 1.6 | 20 | 4.5 | 65.1 ± 2.9 | ||
| 6.0 | 49.7 ± 4.9 | 6.0 | 45.1 ± 1.3 | ||||
| 7.0 | 56.7 ± 3.9 | 7.0 | 53.1 ± 2.6 | ||||
| 30 | 4.5 | 63.8 ± 12.8 | 30 | 4.5 | 71.1 ± 4.7 | ||
| 6.0 | 51.9 ± 4.3 | 6.0 | 42.7 ± 1.3 | ||||
| 7.0 | 50.1 ± 3.3 | 7.0 | 45.7 ± 5.1 | ||||
| 40 | 4.5 | 59.4 ± 3.1 | 40 | 4.5 | 45.4 ± 2.2 | ||
| 6.0 | 50.4 ± 4.8 | 6.0 | 46.4 ± 3.7 | ||||
| 7.0 | 52.4 ± 1.8 | 7.0 | 47.5 ± 2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luligo-Montealegre, W.E.; Prado-Alzate, S.; Ayala-Aponte, A.; Tirado, D.F.; Serna-Cock, L. Aloe vera Cuticle: A Promising Organic Water-Retaining Agent for Agricultural Use. Horticulturae 2024, 10, 797. https://doi.org/10.3390/horticulturae10080797
Luligo-Montealegre WE, Prado-Alzate S, Ayala-Aponte A, Tirado DF, Serna-Cock L. Aloe vera Cuticle: A Promising Organic Water-Retaining Agent for Agricultural Use. Horticulturae. 2024; 10(8):797. https://doi.org/10.3390/horticulturae10080797
Chicago/Turabian StyleLuligo-Montealegre, Wilmer E., Santiago Prado-Alzate, Alfredo Ayala-Aponte, Diego F. Tirado, and Liliana Serna-Cock. 2024. "Aloe vera Cuticle: A Promising Organic Water-Retaining Agent for Agricultural Use" Horticulturae 10, no. 8: 797. https://doi.org/10.3390/horticulturae10080797
APA StyleLuligo-Montealegre, W. E., Prado-Alzate, S., Ayala-Aponte, A., Tirado, D. F., & Serna-Cock, L. (2024). Aloe vera Cuticle: A Promising Organic Water-Retaining Agent for Agricultural Use. Horticulturae, 10(8), 797. https://doi.org/10.3390/horticulturae10080797






