Phytohormones and Mineral Nutrient Changes in Young Plants of Grapevine Genotypes at Different Growth Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Conditions
2.2. Plant Growth and Sampling
2.3. Stomatal Conductance
2.4. Analyses of Phytohormones
2.5. Determination of Foliar Mineral Elements
2.6. Statistical Analyses and Experimental Design
3. Results and Discussion
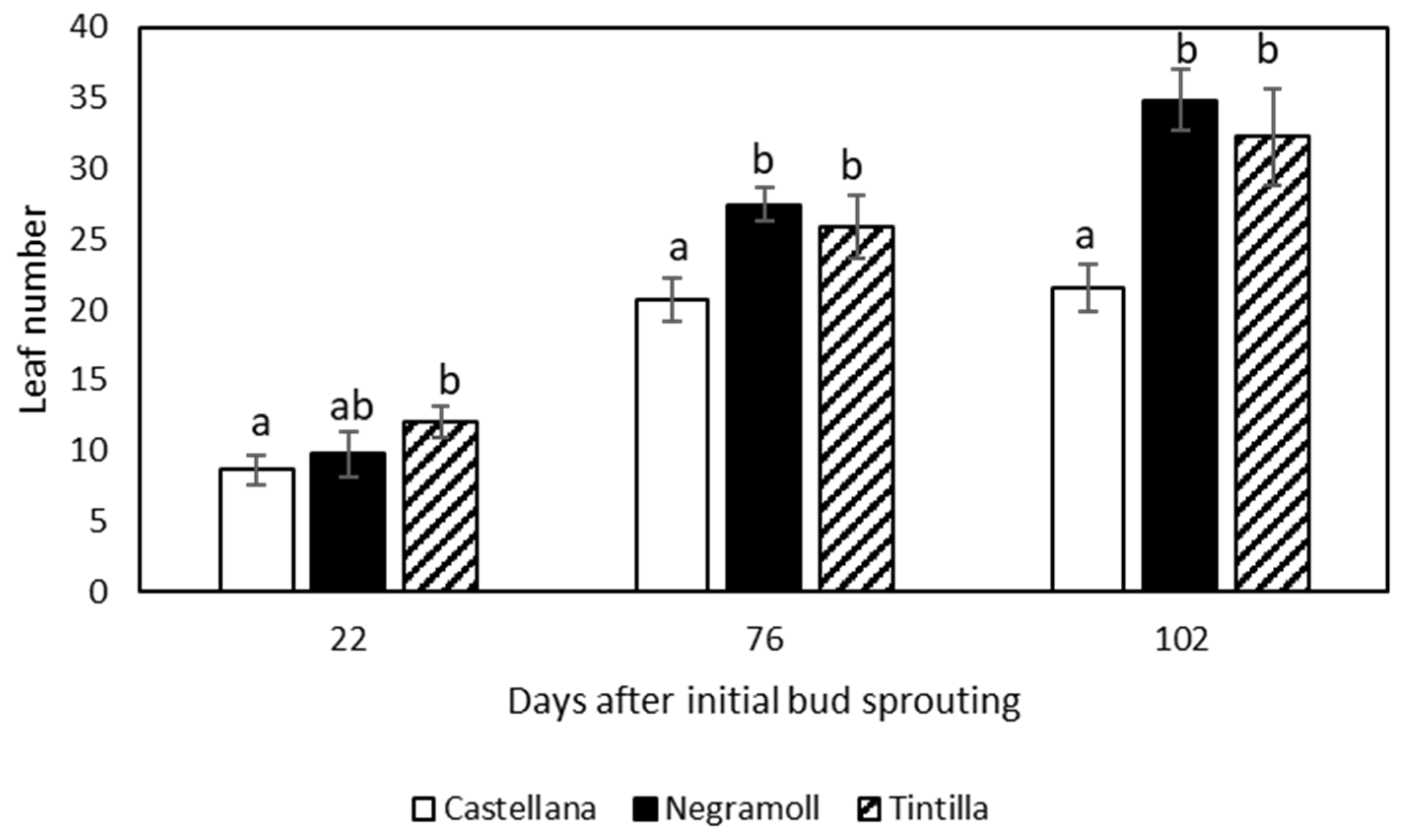
3.1. Plant Growth
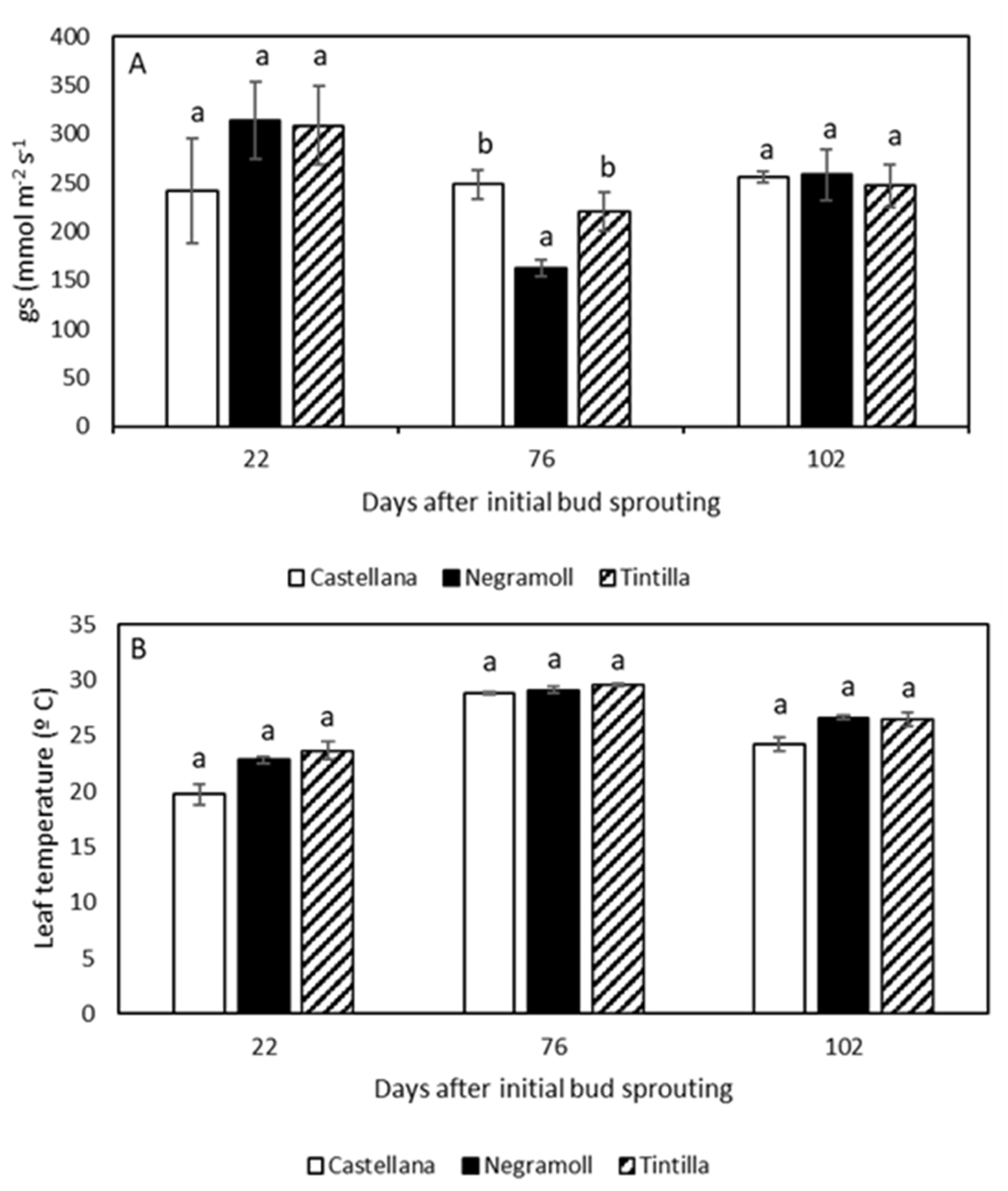
3.2. Stomatal Conductance and Leaf Temperature
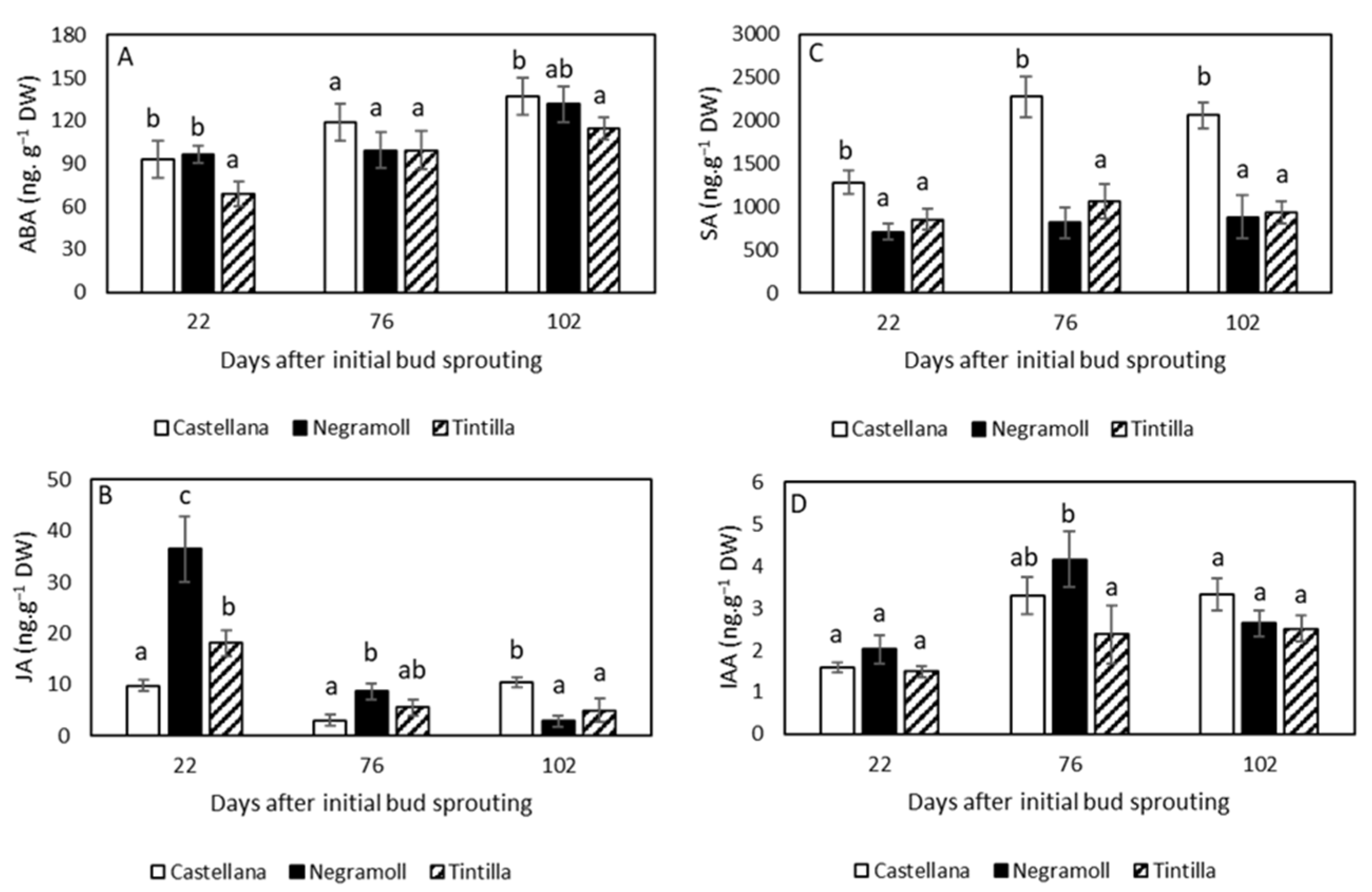
3.3. Plant Hormones Changes
3.4. Leaf Mineral Nutrients Changes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rafique, R.; Ahmad, T.; Ahmed, M.; Khan, M.A. Exploring key physiological attributes of grapevine cultivars under the influence of seasonal environmental variability. OENO One 2023, 57, 381–397. [Google Scholar] [CrossRef]
- Rafique, R.; Ahmad, T.; Ahmed, M.; Khan, M.A.; Wilkerson, C.J.; Hoogenboom, G. Seasonal variability in the effect of temperature on key phenological stages of four table grapes cultivars. Int. J. Biometeorol. 2023, 67, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Salvucci, M.E.; Crafts-Brandner, S.J. Relationship between the heat tolerance of photosynthesis and the thermal stability of rubisco activase in plants from contrasting thermal environments. Plant Physiol. 2004, 134, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.T.; Wang, J.F.; Cramer, G.; Dai, Z.W.; Duan, W.; Xu, H.G.; Wu, B.H.; Fan, P.G.; Wang, L.J.; Li, S.H. Transcriptomic analysis of grape (Vitis vinifera L.) leaves during and after recovery from heat stress. BMC Plant Biol. 2012, 12, 174. [Google Scholar] [CrossRef]
- Canton, M.; Borghezan, M.; Silva, T.C.; Welter, J.F.; Villar, L.; Rosa, D.J.; Silva, A.L.; Pescador, R. Chlorophyll evaluation on leaves of ‘Sauvignon Blanc’ during vegetative growth in São Joaquim, Santa Catarina, Brazil. Acta Hortic. 2017, 1188, 15–20. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Coito, J.L.; Colaço, S.; Sangiogo, M.; Amâncio, S. Heat stress in grapevine: The pros and cons of acclimation. Plant Cell Env. 2015, 38, 777–789. [Google Scholar] [CrossRef]
- Higgins, S.S.; Larsen, F.E.; Bendel, R.B.; Radamaker, G.K.; Bassman, J.H.; Bidlake, W.R.; Wir, A.A. Comparative gas exchange characteristics of potted, glasshouse-grown almond, apple, fig, grape, olive, peach and Asian pear. Sci. Hortic. 1992, 52, 313–329. [Google Scholar] [CrossRef]
- Harris, J. Abscisic acid: Hidden architect of root system structure. Plants 2015, 4, 548–572. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddym, K.R.; Li, J. Abscisic Acid and Abiotic Stress Tolerance in Crop Plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef]
- Khandani, Y.; Sarikhani, H.; Gholami, M.; Rad, A.C.; Yousefi, S.; Sodini, M.; Sivilotti, P. Exogenous Auxin Improves the Growth of Grapevine (Vitis vinifera L.) under Drought Stress by Mediating Physiological, Biochemical and Hormonal Modifications. J. Soil Sci. Plant Nutr. 2024, 24, 3422–3440. [Google Scholar] [CrossRef]
- Lovisolo, C.; Hartung, W.; Schubert, A. Whole-plant hydraulic conductance and root-to-shoot flow of abscisic acid are independently affected by water stress in grapevines. Funct. Plant Biol. 2002, 29, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Perrone, I.; Gambino, G.; Chitarra, W.; Vitali, M.; Pagliarani, C.; Riccomagno, N.; Balestrini, R.; Kaldenhoff, R.; Uehlein, N.; Gribaudo, I.; et al. The grapevine root-specific aquaporin VvPIP2;4N controls root hydraulic conductance and leaf gas exchange under well-watered conditions but not under water stress. Plant Physiol. 2012, 160, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Perrone, I.; Pagliarani, C.; Lovisolo, C.; Chitarra, W.; Roman, F.; Schubert, A. Recovery from water stress affects grape leaf petiole transcriptome. Planta 2012, 235, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- Ferrandino, A.; Lovisolo, C. Abiotic stress effects on grapevine (Vitis vinifera L.): Focus on abscisic acid-mediated consequences on secondary metabolism and berry quality. Environ. Exp. Bot. 2010, 103, 38–147. [Google Scholar] [CrossRef]
- Janda, T.; Szalai, G.; Pál, M. Salicylic acid signalling in plants. Int. J. Mol. Sci. 2020, 21, 2655. [Google Scholar] [CrossRef]
- Li, Q.; Guan, C.; Zhao, Y.; Duan, X.; Yang, Z.; Zhu, J. Salicylic acid alleviates Zn-induced inhibition of growth via enhancing antioxidant system and glutathione metabolism in alfalfa. Ecotoxicol. Environ. Saf. 2023, 265, 115500. [Google Scholar] [CrossRef]
- Wen, P.F.; Chen, J.Y.; Wan, S.B.; Kong, W.F.; Zhang, P.; Wang, W.; Zhan, J.C.; Pan, Q.H.; Huang, W.D. Salicylic acid activates phenylalanine ammonia-lyasein grape berry in response to high temperature stress. Plant Grow. Regul. 2008, 55, 1–10. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, S.; Ye, N.; Jiang, M.; Cao, J.; Zhang, J. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef]
- Shen, X.; Guo, X.; Guo, X.; Zhao, D.; Zhao, W.; Chen, J.; Li, T. PacMYBA, a sweet cherry R2R3-MYB transcription factor, is a positive regulator of salt stress tolerance and pathogen resistance. Plant Physiol. Biochem. 2017, 112, 302–311. [Google Scholar] [CrossRef]
- Yoon, Y.; Seo, D.H.; Shin, H.; Kim, H.J.; Kim, C.M.; Jang, G. The Role of stress-responsive transcription factors in modulating abiotic stress tolerance in plants. Agronomy 2020, 10, 788. [Google Scholar] [CrossRef]
- Kim, H.; Seomun, S.; Yoon, Y.; Jang, G. Jasmonic Acid in Plant Abiotic Stress Tolerance and Interaction with Abscisic Acid. Agronomy 2021, 11, 1886. [Google Scholar] [CrossRef]
- De Ollas, C.; Hernando, B.; Arbona, V.; Gómez-Cadenas, A. Jasmonic acid transient accumulation is needed for abscisic acid increase in citrus roots under drought stress conditions. Physiol. Plant. 2013, 147, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.; Jing, Y.; Shi, P.; Liu, J.; Chen, J.; Yan, J.; Chu, J.; Chen, K.M.; Sun, J. JAZ proteins modulate seed germination through interaction with ABI 5 in bread wheat and Arabidopsis. New Phytol. 2019, 223, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- De Ollas, C.; Dodd, I.C. Physiological impacts of ABA–JA interactions under water limitation. Plant Mol. Biol. 2016, 91, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Deytieux-Belleau, C.; Gagne, S.; L’Hyvernay, A.; Donèche, B.; Geny, L. Possible roles of both absicisic acid and indol-acetic acid in controlling grape berry ripening process. J. Int. Sci. Vigne. Vin. 2007, 41, 141–148. [Google Scholar] [CrossRef]
- Böttcher, C.; Burbidge, C.A.; Boss, P.K.; Davies, C. Interactions between ethylene and auxin are crucial to the control of grape (Vitis vinifera L.) berry ripening. BMC Plant Biol. 2013, 13, 222. [Google Scholar] [CrossRef]
- Dinis, L.T.; Bernardo, S.; Conde, A.; Pimentel, D.; Ferreira, H.; Félix, L.; Gerós, H.; Correia, C.M.; Moutinho-Pereira, J. Kaolin exogenous application boosts antioxidant capacity and phenolic content in berries and leaves of grapevine under summer stress. J. Plant Physiol. 2016, 191, 45–53. [Google Scholar] [CrossRef]
- Dinis, L.T.; Malheiro, A.C.; Luzio, A.; Fraga, H.; Ferreira, H.; Gonçalves, I.; Pinto, G.; Correia, C.M.; Moutinho-Pereira, J. Improvement of grapevine physiology and yield under summer stress by kaolin-foliar application: Water relations, photosynthesis and oxidative damage. Photosynthetica 2017, 56, 641–651. [Google Scholar] [CrossRef]
- Dinis, L.T.; Bernardo, S.; Luzio, A.; Pinto, G.; Meijón, M.; Pintó-Marijuan, M.; Cotado, A.; Correia, C.; Moutinho-Pereira, J. Kaolin modulates ABA and IAA dynamics and physiology of grapevine under Mediterranean summer stress. J. Plant Physiol. 2018, 220, 181–192. [Google Scholar] [CrossRef]
- Amorós, J.A.; Pérez-de-los Reyes, C.; García Navarro, F.J.; Bravo, S.; Chacón, J.L.; Martínez, J.; Jiménez Ballesta, R. Bioaccumulation of mineral elements in grapevine varieties cultivated in “La Mancha”. J. Plant Nutr. Soil Sci. 2013, 176, 843–850. [Google Scholar] [CrossRef]
- Toumi, M.; Nedjimi, B.; Halitim, A.; Garcia, M. Effects of K-Mg ratio on growth and cation nutrition of Vitis vinifera L. cv. “Dattier de Beiruth” grafted on SO4 rootstock. J. Plant Nutr. 2016, 39, 904–911. [Google Scholar] [CrossRef]
- Tagliavini, M.; Scandellari, F. Methodologies and concepts in the study of nutrient uptake requirements and partitioning in fruit trees. Proceedings of the Seventh International Symposium on Mineral Nutrition of Fruit Crops. Acta Horticul. 2013, 984, 47–56. [Google Scholar] [CrossRef]
- Salisbury, F.B.; Ross, C.W. Plant Physiology, 4th ed.; Salisbury, F.B., Ross, C.W., Eds.; Wadsworth Publishing Company: Belmont, CA, USA, 1992; 682p, ISBN 0-534-15162-0. [Google Scholar]
- Proffitt, T.; Campbell-Clause, J. Managing Grapevine Nutrition and Vineyard Soil Health. 2012. Available online: https://winewa.asn.au/wp-content/uploads/2020/04/Grapevine_Nutrition_LR_2.pdf (accessed on 18 June 2024).
- Durgbanshi, A.; Arbona, V.; Pozo, O.; Miersch, O.; Sancho, J.V.; Gómez-Cadenas, A. Simultaneous determination of multiple phytohormones in plant extracts by liquid chromatography-electrospray tandem mass spectrometry. J. Agric. Food Chem. 2005, 53, 8437–8442. [Google Scholar] [CrossRef]
- Sáez-Plaza, P.; Michałowski, T.; Navas, M.J.; Asuero, A.G.; Wybraniec, S. An overview of the Kjeldahl method of nitrogen determination. Part I. Early history, chemistry of the procedure, and titrimetric finish. Crit. Rev. Anal. Chem. 2013, 43, 178–223. [Google Scholar] [CrossRef]
- Sáez-Plaza, P.; Michałowski, T.; Navas, M.J.; Asuero, A.G.; Wybraniec, S. An overview of the Kjeldahl method of nitrogen determination. Part II. Sample preparation, working scale, instrumental finish, and quality control. Crit. Rev. Anal. Chem. 2013, 43, 224–272. [Google Scholar] [CrossRef]
- Barton, C.J. Photometric analysis of phosphate rock. Anal. Chem. 1948, 20, 1068–1073. [Google Scholar] [CrossRef]
- Gaines, T.P.; Mitchell, A. Boron determination in plant tissue by the azomethine H method. Commun. Soil Sci. Plant Anal. 1979, 10, 1099–1108. [Google Scholar] [CrossRef]
- Rodríguez-Torres, I. Variedades de Vid Cultivadas en Canarias: Descriptores Morfológicos, Caracterización Morfológica, Molecular, Agronómica y Enológica, 2nd ed.; Instituto Canario de Investigaciones Agrarias: Canary Islands, Spain, 2017; p. 194. [Google Scholar]
- Greer, D.H. Responses of biomass accumulation, photosynthesis and the net carbon budget to high canopy temperatures of Vitis vinifera L. cv. Semillon vines grown in field conditions. Environ. Exp. Bot. 2017, 138, 10–20. [Google Scholar] [CrossRef]
- Zsófi, Z.; Váradi, G.; Bálo, B.; Marschall, M.; Nagy, Z. Heat acclimation of grapevine leaf photosynthesis: Mezzo- and macro-climatic aspects. Funct. Plant Biol. 2009, 36, 310–322. [Google Scholar] [CrossRef]
- Greer, D.H.; Weedon, M.M. Modelling photosynthetic responses to temperature of grapevine (Vitis vinifera cv. Semillon) leaves on vines grown in a hot climate. Plant Cell Environ. 2012, 35, 1050–1064. [Google Scholar] [CrossRef] [PubMed]
- Greer, D.H.; Weston, C. Heat stress affects flowering, berry growth, sugar accumulation and photosynthesis of Vitis vinifera cv. Semillon grapevines grown in a controlled environment. Funct. Plant Biol. 2010, 37, 206–214. [Google Scholar] [CrossRef]
- Greer, D.H. Stomatal and non-stomatal limitations at different leaf temperatures to the photosynthetic process during the post-harvest period for Vitis vinifera cv. Chardonnay vines. N. Z. J. Crop Hortic. Sci. 2020, 48, 1–21. [Google Scholar] [CrossRef]
- Pantin, F.; Simonneau, T.; Muller, B. Coming of leaf age: Control of growth by hydraulics and metabolics during leaf ontogeny. New Phytol. 2012, 196, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Amiri, J.; Eshghi, S.; Tafazoli, E.; Kholdebarin, B.; Abbaspour, N. Ameliorative effects of salicylic acid on mineral concentrations in roots and leaves of two grapevine (Vitis vinifera L.) cultivars under salt stress. Vitis 2014, 53, 181–188. [Google Scholar] [CrossRef]
- Ali, I.; Wang, X.; Abbas, W.M.; Hassan, M.U.; Shafique, M.; Tareen, M.J.; Fiaz, S.; Ahmed, W.; Qayyum, A. Quality Responses of Table Grapes “Flame Seedless” as Effected by Foliarly Applied Micronutrients. Horticulturae 2021, 7, 462. [Google Scholar] [CrossRef]
- Mohamed, A.A. Impact of foliar application of nanomicronutrient fertilizers on some quantitative and qualitative traits of “Thompson seedless” grapevine. Middle East J. Appl. Sci. 2020, 10, 435–441. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, M.; Liu, Z.; Chenb, H.; Li, Y.C.; Sun, Y.; Ma, Q.; Zhao, C. Effects of foliar application of the mixture of copper and chelated iron on the yield, quality, photosynthesis, and microelement concentration of table grape (Vitis vinifera L.). Sci. Hortic. 2019, 254, 106–115. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in Plants: From Acquisition to Subcellular Allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef]
| Days After Initial Bud Sprouting | |||
|---|---|---|---|
| Macronutrients | 22 | 76 | 102 |
| “Cas” N (%DW) | 3.02 ± 0.01 a | 2.66 ± 0.14 a | 2.53 ± 0.17 a |
| “Neg” N (%DW) | 3.17 ± 0.05 a | 2.80 ± 0.15 a | 2.51 ± 0.07 a |
| “Tin” N (%DW) | 3.11 ± 0.19 a | 2.66 ± 0.08 a | 2.36 ± 0.08 a |
| “Cas” P (%DW) | 0.20 ± 0.01 a | 0.38 ± 0.09 a | 0.51 ± 0.07 a |
| “Neg” P (%DW) | 0.24 ± 0.02 ab | 0.27 ± 0.04 a | 0.38 ± 0.04 a |
| “Tin” P (%DW) | 0.29 ± 0.02 b | 0.31 ± 0.06 a | 0.43 ± 0.04 a |
| “Cas” K+ (%DW) | 0.85 ± 0.02 a | 0.87 ± 0.06 a | 0.75 ± 0.05 a |
| “Neg” K+ (%DW) | 0.84 ± 0.03 a | 0.91 ± 0.05 a | 0.94 ± 0.05 a |
| “Tin” K+ (%DW) | 0.78 ± 0.05 a | 0.72 ± 0.06 a | 0.83 ± 0.07 a |
| “Cas” Ca2+ (%DW) | 0.90 ± 0.08 a | 1.57 ± 0.13 a | 1.84 ± 0.09 b |
| “Neg” Ca2+ (%DW) | 0.96 ± 0.07 a | 1.19 ± 0.14 a | 1.37 ± 0.02 a |
| “Tin” Ca2+ (%DW) | 1.20 ± 0.04 b | 1.51 ± 0.18 a | 1.81 ± 0.11 b |
| “Cas” Mg2+ (%DW) | 0.12 ± 0.01 a | 0.15 ± 0.01 a | 0.16 ± 0.02 a |
| “Neg” Mg2+ (%DW) | 0.14 ± 0.01 a | 0.15 ± 0.01 a | 0.15 ± 0.01 a |
| “Tin” Mg2+ (%DW) | 0.17 ± 0.01 b | 0.18 ± 0.01 a | 0.20 ± 0.00 b |
| Days After Initial Bud Sprouting | |||
|---|---|---|---|
| Micronutrients | 22 | 76 | 102 |
| “Cas” Na+ (%DW) | 0.04 ± 0.01 a | 0.03 ± 0.00 a | 0.03 ± 0.01 a |
| “Neg” Na+ (%DW) | 0.06 ± 0.01 a | 0.03 ± 0.00 a | 0.04 ± 0.01 a |
| “Tin” Na+ (%DW) | 0.04 ± 0.00 a | 0.02 ± 0.00 a | 0.04 ± 0.00 a |
| “Cas” Fe2+ (ppm) | 44.47 ± 0.78 a | 53.41 ± 2.64 a | 0.03 ± 0.01 a |
| “Neg” Fe2+ (ppm) | 47.89 ± 3.13 a | 63.43 ± 2.48 bc | 0.04 ± 0.01 a |
| “Tin” Fe2+ (ppm) | 48.97 ± 3.37 a | 57.31 ± 1.21 ab | 0.04 ± 0.00 a |
| “Cas” Mn2+ (ppm) | 36.33 ± 4.63 a | 88.70 ± 1.67 b | 87.41 ± 10.13 bc |
| “Neg” Mn2+ (ppm) | 40.02 ± 2.66 a | 39.85 ± 4.78 a | 48.43 ± 11.41 a |
| “Tin” Mn2+ (ppm) | 33.16 ± 1.98 a | 39.01 ± 3.00 a | 64.41 ± 9.98 ab |
| “Cas” Cu2+ (ppm) | 1.86 ± 0.24 ab | 1.14 ± 0.31 a | 4.11 ± 1.94 ab |
| “Neg” Cu2+ (ppm) | 2.48 ± 0.19 b | 1.25 ± 0.27 a | 8.47 ± 1.11 b |
| “Tin” Cu2+ (ppm) | 1.27 ± 0.30 a | 1.59 ± 0.34 a | 3.53 ± 0.18 a |
| “Cas” Zn2+ (ppm) | 6.08 ± 0.46 b | 8.43 ± 1.37 a | 7.71 ± 0.82 a |
| “Neg” Zn2+ (ppm) | 7.14 ± 0.40 b | 7.38 ± 0.79 a | 10.20 ± 1.83 a |
| “Tin” Zn2+ (ppm) | 4.78 ± 0.29 a | 8.06 ± 1.69 a | 7.25 ± 0.36 a |
| “Cas” B (ppm) | 17.70 ± 1.07 b | 26.56 ± 6.34 a | 27.40 ± 3.93 a |
| “Neg” B (ppm) | 13.60 ± 0.77 a | 18.30 ± 1.19 a | 21.99 ± 3.96 a |
| “Tin” B (ppm) | 16.08 ± 1.21 ab | 19.58 ± 3.52 a | 28.65 ± 8.77 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbano-Gálvez, A.; López-Climent, M.F.; Gómez-Cadenas, A.; Mahouachi, J. Phytohormones and Mineral Nutrient Changes in Young Plants of Grapevine Genotypes at Different Growth Stages. Horticulturae 2024, 10, 1114. https://doi.org/10.3390/horticulturae10101114
Urbano-Gálvez A, López-Climent MF, Gómez-Cadenas A, Mahouachi J. Phytohormones and Mineral Nutrient Changes in Young Plants of Grapevine Genotypes at Different Growth Stages. Horticulturae. 2024; 10(10):1114. https://doi.org/10.3390/horticulturae10101114
Chicago/Turabian StyleUrbano-Gálvez, Antonio, María F. López-Climent, Aurelio Gómez-Cadenas, and Jalel Mahouachi. 2024. "Phytohormones and Mineral Nutrient Changes in Young Plants of Grapevine Genotypes at Different Growth Stages" Horticulturae 10, no. 10: 1114. https://doi.org/10.3390/horticulturae10101114
APA StyleUrbano-Gálvez, A., López-Climent, M. F., Gómez-Cadenas, A., & Mahouachi, J. (2024). Phytohormones and Mineral Nutrient Changes in Young Plants of Grapevine Genotypes at Different Growth Stages. Horticulturae, 10(10), 1114. https://doi.org/10.3390/horticulturae10101114








