CmSN Regulates Fruit Skin Netting Formation in Melon
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Transient Transformation of Melon Fruits
2.3. Virus-Induced Gene Silencing (VIGS) in Melon
2.4. Tomato Transformation and Phenotype Observation
2.5. Total RNA Extraction and RT-qPCR Analysis
2.6. Statistical Analysis
3. Results
3.1. Measurement of CmSN Gene Expression in Different Rind Types
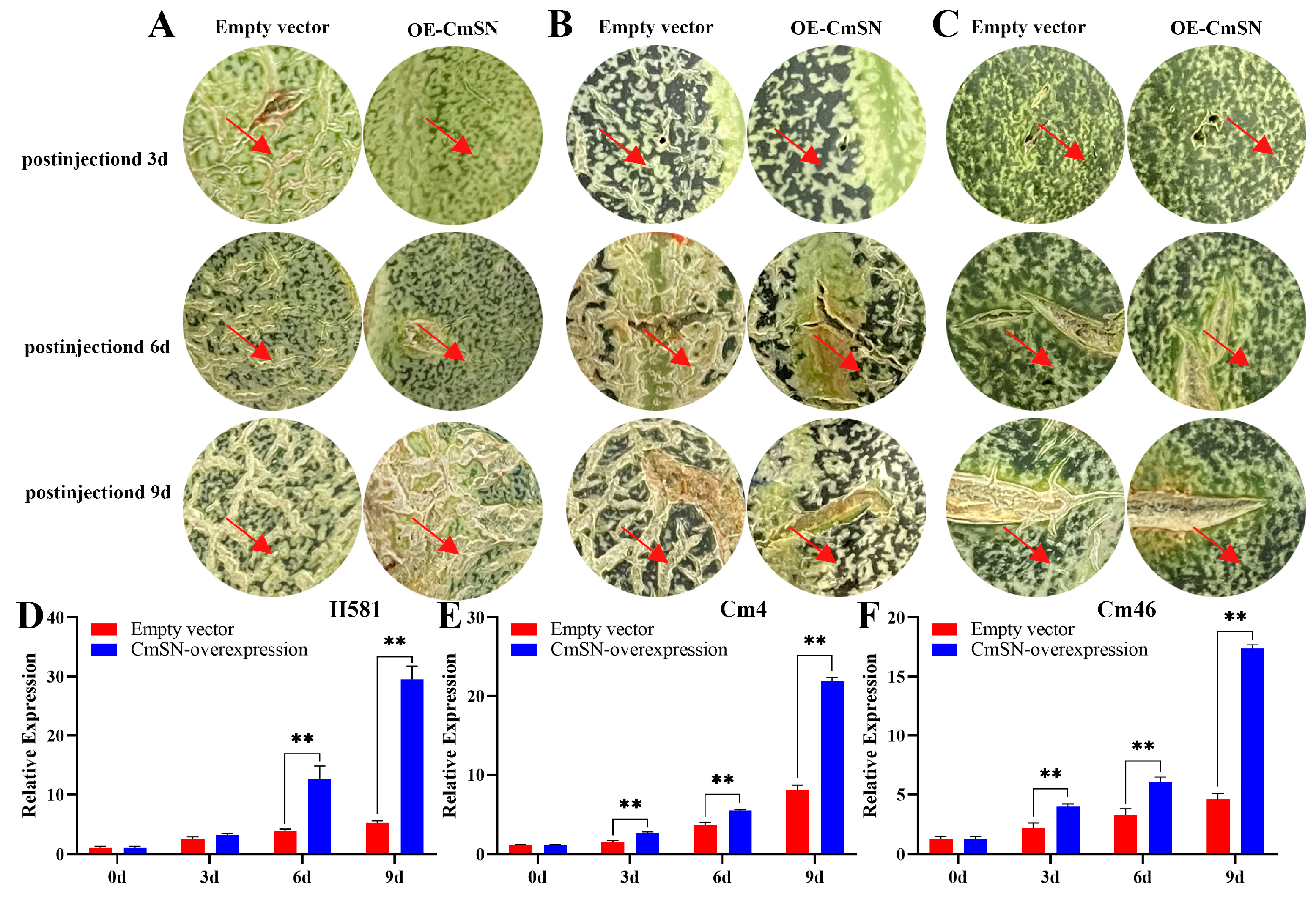
3.2. Overexpression of CmSN Inhibits Netting Formation in Melon Fruit Skin
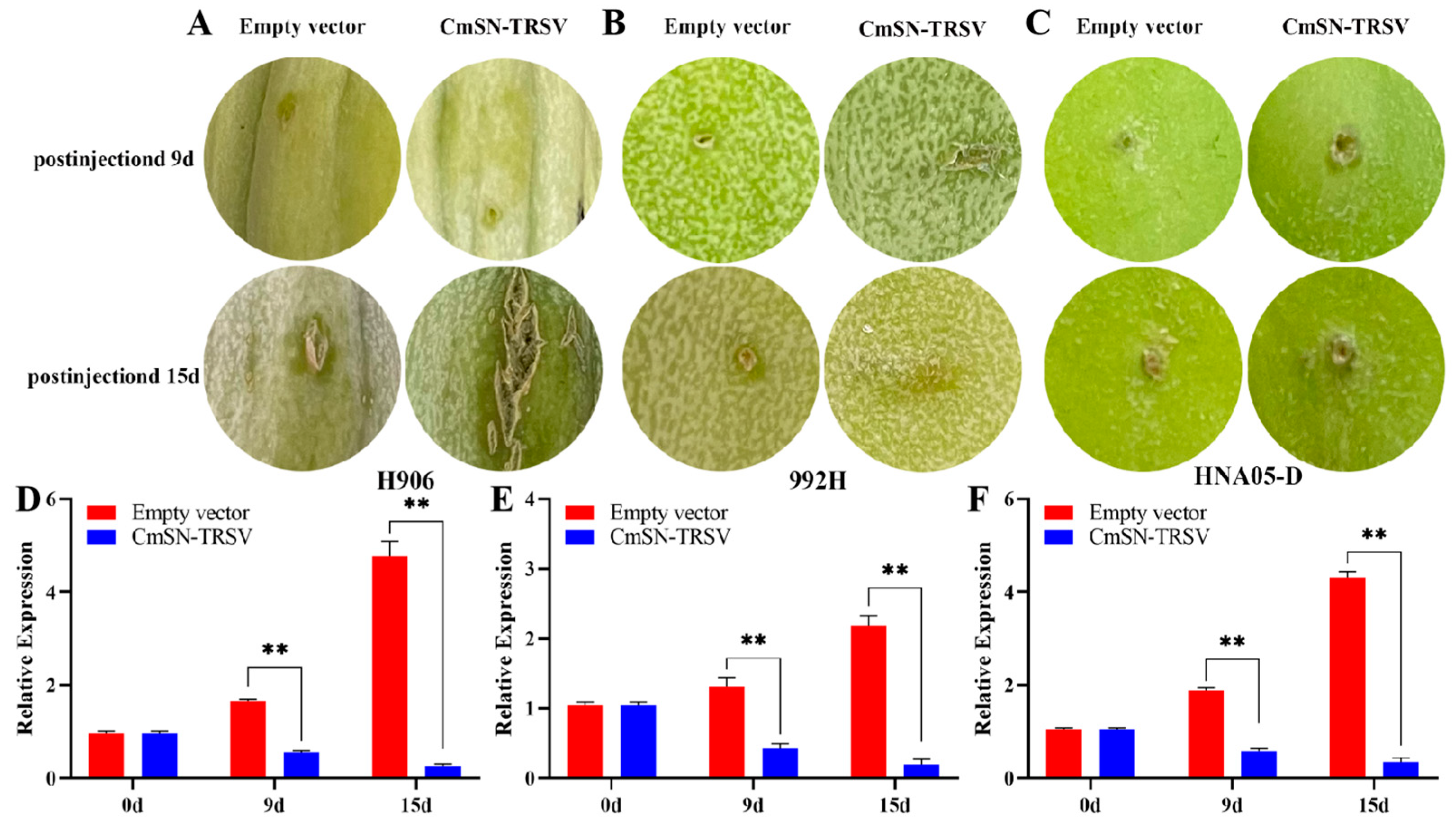
3.3. Silencing CmSN Enhances Netting Formation in Melon Fruit Skin
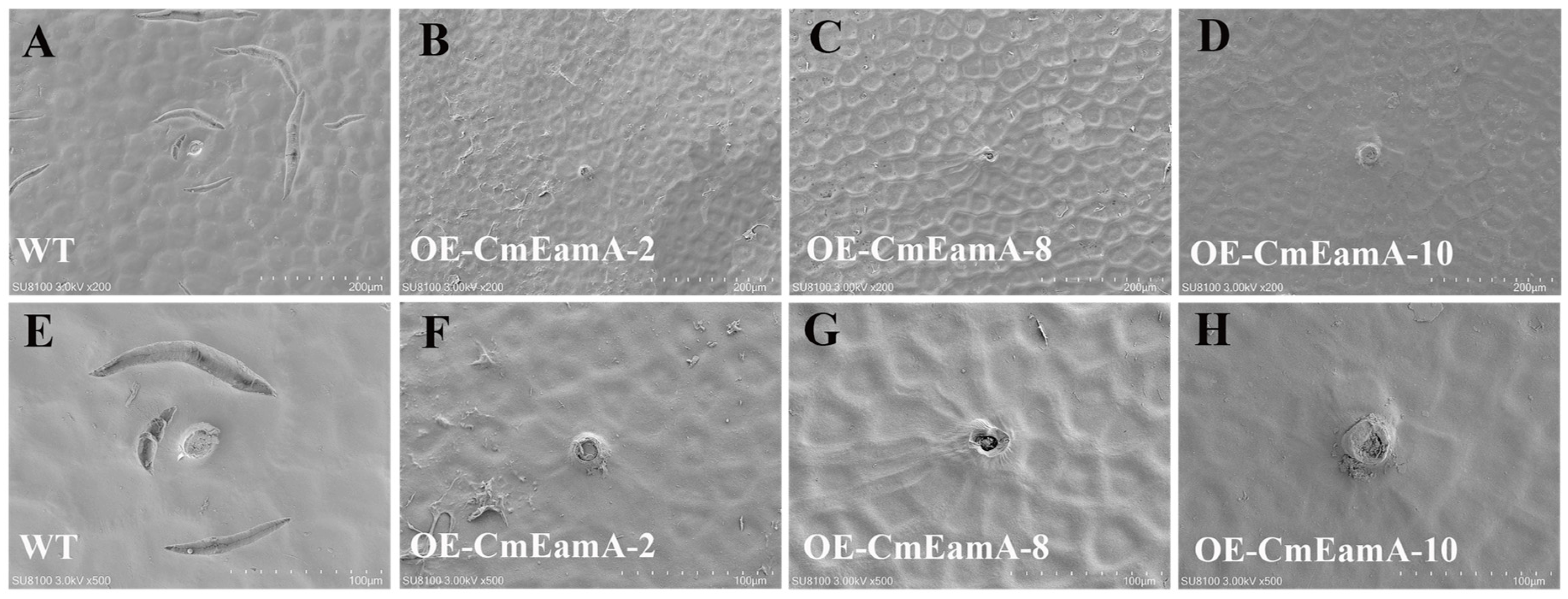
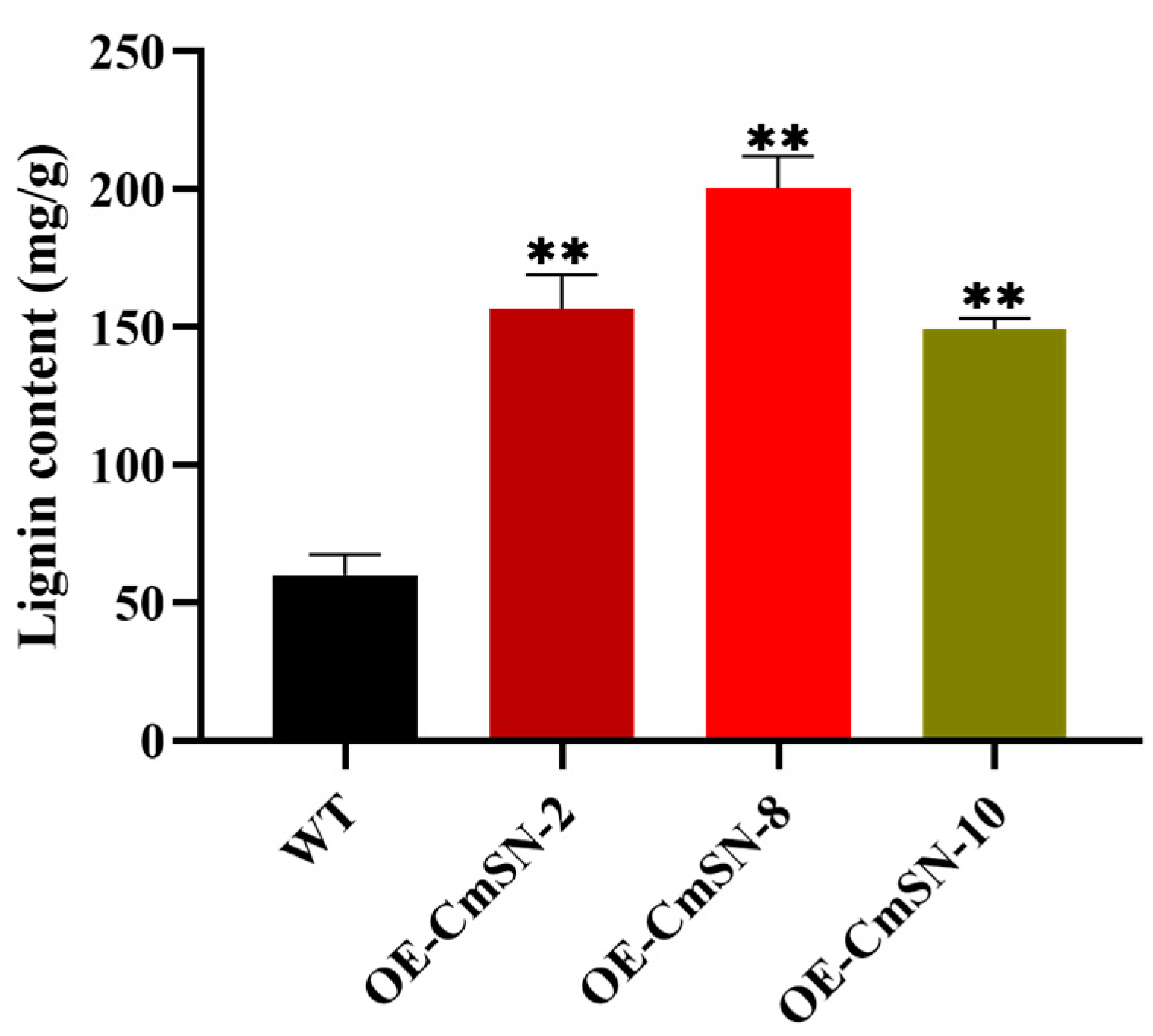
3.4. Heterologous Transformation of CmSN in Tomato Leads to Skin Roughness
3.5. NIL_CmSN Exhibits Netted Skin Phenotype During Fruit Development Process
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pitrat, M. Melon. In Vegetables I. Handbook of Plant Breeding; Prohens, J., Nuez, F., Eds.; Springer: New York, NY, USA, 2008; Volume 1. [Google Scholar] [CrossRef]
- Pitrat, M. Phenotypic diversity in wild and cultivated melons (Cucumis melo). Plant Biotechnol. 2013, 30, 273–278. [Google Scholar] [CrossRef]
- Keren-Keiserman, A.; Tanami, Z.; Shoseyov, O.; Ginzberg, I. Differing rind characteristics of developing fruits of smooth and netted melons (Cucumis melo). J. Hortic. Sci. Biotechnol. 2004, 79, 107–113. [Google Scholar] [CrossRef]
- Nishizawa, T.; Okafuji, K.; Murayama, H. Storability and development of physiological disorder of netted melon ‘life’ fruit as influenced by storage conditions. Acta Hortic. 2009, 2009, 147–154. [Google Scholar] [CrossRef]
- Meissner, F. Die Korkbildung der Fruèchte von Aesculusund Cucumis-Arten. Osterreichsche Bot. Z. 1952, 99, 606–623. [Google Scholar] [CrossRef]
- Cutter, E.G. Plant Anatomy: Experiment and Interpretation; Addison-Wesley: Menlo Park, CA, USA, 1969. [Google Scholar]
- Webster, B.D.; Craig, M.E. Net morphogenesis and characteristics of the surface of muskmelon fruit. J. Am. Soc. Hortic. Sci. 1976, 101, 412–415. [Google Scholar] [CrossRef]
- Martin, L.B.; Rose, J.K. There’s more than one way to skin a fruit: Formation and functions of fruit cuticles. J. Exp. Bot. 2014, 65, 4639–4651. [Google Scholar] [CrossRef]
- Liang, X.; Li, Q.; Cao, L.; Du, X.; Qiang, J.; Hou, J.; Li, X.; Zhu, H.; Yang, S.; Liu, D.; et al. Natural allelic variation in the EamA-like transporter, CmSN, is associated with fruit skin netting in melon. Theor. Appl. Genet. 2023, 136, 192. [Google Scholar] [CrossRef]
- Ranocha, P.; Denance, N.; Vanholme, R.; Freydier, A.; Martinez, Y.; Hoffmann, L.; Kohler, L.; Pouzet, C.; Renou, J.P.; Sundberg, B.; et al. Walls are thin 1 (WAT1), an Arabidopsis homolog of Medicago truncatula NODULIN21, is a tonoplast-localized protein required for secondary wall formation in fibers. Plant J. 2010, 63, 469–483. [Google Scholar] [CrossRef]
- Cohen, H.; Dong, Y.; Szymanski, J.; Lashbrooke, J.; Meir, S.; Almekias-Siegl, E.; Zeisler-Diehl, V.V.; Schreiber, L.; Aharoni, A. A Multilevel Study of Melon Fruit Reticulation Provides Insight into Skin Ligno-Suberization Hallmarks. Plant Physiol. 2019, 179, 1486–1501. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, R.; Gu, T.; Gan, L. Analysis of RNA-Seq-based expression profles during adventitious shoot regeneration in Arabidopsis thaliana. J. Nanjing Agric. Univ. 2018, 41, 308–320. [Google Scholar]
- Dou, J.; Wang, Y.; Yang, H.; Niu, H.; Liu, D.; Yang, S.; Zhu, H.; Sun, S.; Yang, L. Development of branchless watermelon near isogenic lines by marker assisted selection. Hortic. Plant J. 2022, 8, 627–636. [Google Scholar] [CrossRef]
- Li, L.; Li, Q.; Chen, B.; Wang, J.; Ding, F.; Wang, P.; Zhang, X.; Hou, J.; Luo, R.; Li, X.; et al. Identification of candidate genes that regulate the trade-off between seedling cold tolerance and fruit quality in melon (Cucumis melo L.). Hortic. Res. Engl. 2023, 10, d93. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Lu, J.; Han, M.; Lu, L.; Tian, J.; Zheng, H.; Liu, S.; Zhong, F.; Hou, M. Identification and Functional Analysis of 1-Deoxy-D-xylulose-5-phosphate Synthase Gene in Tomatoes (Solanum lycopersicum). Horticulturae 2024, 10, 304. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, S.; Jia, Z.; Zhang, J.; Li, Y.; Ma, X.; Fan, B.; Wang, P.; Gao, Y.; Ye, Z.; et al. Exploring the influence of a single-nucleotide mutation in EIN4 on tomato fruit firmness diversity through fruit pericarp microstructure. Plant Biotechnol. J. 2024, 22, 2379–2394. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, K.; Zhu, H.; Zhang, X.; Yan, W.; Xu, N.; Liu, D.; Hu, J.; Wu, Y.; Weng, Y.; et al. Melon short internode (CmSi) encodes an ERECTA-like receptor kinase regulating stem elongation through auxin signaling. Hortic. Res. Engl. 2020, 7, 2632–2645. [Google Scholar] [CrossRef]
- Oren, E.; Tzuri, G.; Dafna, A.; Meir, A.; Kumar, R.; Katzir, N.; Elkind, Y.; Freilich, S.; Schaffer, A.A.; Tadmor, Y.; et al. High-density NGS-based map construction and genetic dissection of fruit shape and rind netting in Cucumis melo. Theor. Appl. Genet. 2020, 133, 1927–1945. [Google Scholar] [CrossRef]
- Aharoni, A.; Dixit, S.; Jetter, R.; Thoenes, E.; van Arkel, G.; Pereira, A. The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis. Plant Cell 2004, 16, 2463–2480. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Tan, J.; Weng, Y. Functional copy number variation of CsSHINE1 is associated with fruit skin netting intensity in cucumber, Cucumis sativus. Theor. Appl. Genet. 2022, 135, 2101–2119. [Google Scholar] [CrossRef]
- Cao, Y.; Tang, X.; Giovannoni, J.; Xiao, F.; Liu, Y. Functional characterization of a tomato COBRA-like gene functioning in fruit development and ripening. BMC Plant Biol. 2012, 12, 211. [Google Scholar] [CrossRef]
- Blaker, K.M.; Olmstead, J.W. Cell wall composition of the skin and flesh tissue of crisp and standard texture southern highbush blueberry genotypes. J. Berry Res. 2015, 5, 9–15. [Google Scholar] [CrossRef]
- Lahaye, M.; Bouin, C.; Barbacci, A.; Le Gall, S.; Foucat, L. Water and cell wall contributions to apple mechanical properties. Food Chem. 2018, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P.; Phillips, A.L. Gibberellin metabolism: New insights revealed by the genes. Trends Plant Sci. 2000, 5, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Romero, P.; Rose, J.K.C. A relationship between tomato fruit softening, cuticle properties and water availability. Food Chem. 2019, 295, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Saladie, M.; Matas, A.J.; Isaacson, T.; Jenks, M.A.; Goodwin, S.M.; Niklas, K.J.; Xiaolin, R.; Labavitch, J.M.; Shackel, K.A.; Fernie, A.R.; et al. A reevaluation of the key factors that influence tomato fruit softening and integrity. Plant Physiol. 2007, 144, 1012–1028. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, Y.; Ma, Q.; Li, F.; Tao, R.; Li, T.; Zhu, M.; Ding, J.; Li, C.; Guo, W.; et al. Comparative transcriptomic and metabolomic analysis revealed molecular mechanism of two wheat near-isogenic lines response to nitrogen application. Plant Physiol. Biochem. 2023, 195, 47–57. [Google Scholar] [CrossRef]
- Zhang, T.; Hong, Y.; Zhang, X.; Yuan, X.; Chen, S. Relationship between key environmental factors and the architecture of fruit shape and size in near-isogenic lines of cucumber (Cucumis sativus L.). Int. J. Mol. Sci. 2022, 23, 14033. [Google Scholar] [CrossRef]
- Xu, N.; Fang, X.; Xie, K.; Cheng, S.; Wang, Y.; Yang, S.; Zhu, H.; Sun, S.; Weng, Y.; Yang, L. Transcriptional and phytohormone regulatory network involved in LITTLELEAF-mediated organ size development in cucumber (Cucumis sativus). Sci. Hortic. 2023, 321, 112294. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Y.; Zhang, Z.; Chen, X.; Huang, J.; Chen, Z.; Zheng, J.; Jiang, L.; Huang, Y.; Wang, H.; et al. Transcriptomic, proteomic, and phosphoproteomic analyses reveal dynamic signaling networks influencing long-grain rice development. Crop J. 2022, 10, 716–728. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, B.; Qiao, L.; Chen, F.; Zhao, J.; Cheng, Z.; Weng, Y. Phenotypic characterization and fine mapping of a major-effect fruit shape QTL FS5.2 in cucumber, Cucumis Sativus L., with near-isogenic line-derived segregating populations. Int. J. Mol. Sci. 2022, 23, 13384. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Wang, P.; Luo, C.; Li, X.; Mao, W.; Hou, J.; Fan, J.; Guo, Y.; Cheng, Z.; Li, Q.; et al. CmSN Regulates Fruit Skin Netting Formation in Melon. Horticulturae 2024, 10, 1115. https://doi.org/10.3390/horticulturae10101115
Liang X, Wang P, Luo C, Li X, Mao W, Hou J, Fan J, Guo Y, Cheng Z, Li Q, et al. CmSN Regulates Fruit Skin Netting Formation in Melon. Horticulturae. 2024; 10(10):1115. https://doi.org/10.3390/horticulturae10101115
Chicago/Turabian StyleLiang, Xiaoxue, Panqiao Wang, Chen Luo, Xiang Li, Wenwen Mao, Juan Hou, Junlong Fan, Yan Guo, Zhiqiang Cheng, Qiong Li, and et al. 2024. "CmSN Regulates Fruit Skin Netting Formation in Melon" Horticulturae 10, no. 10: 1115. https://doi.org/10.3390/horticulturae10101115
APA StyleLiang, X., Wang, P., Luo, C., Li, X., Mao, W., Hou, J., Fan, J., Guo, Y., Cheng, Z., Li, Q., & Hu, J. (2024). CmSN Regulates Fruit Skin Netting Formation in Melon. Horticulturae, 10(10), 1115. https://doi.org/10.3390/horticulturae10101115





