Effects of Light Spectra on Growth, Physiological Responses, and Antioxidant Capacity in Five Radish Varieties in an Indoor Vertical Farming System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Condition
2.2. Spectral Light Conditions
2.3. Plant Growth Measurements
2.4. Physiological Measurements
2.5. Secondary Metabolite Quantification
2.5.1. Sample Extraction
2.5.2. Determination of Total Phenolic Compounds (TPCs)
2.5.3. Determination of Flavonoid Content
2.5.4. Determination of Anthocyanin Content
2.5.5. DPPH Radical Scavenging Activity
2.6. Statistical Analysis
3. Results
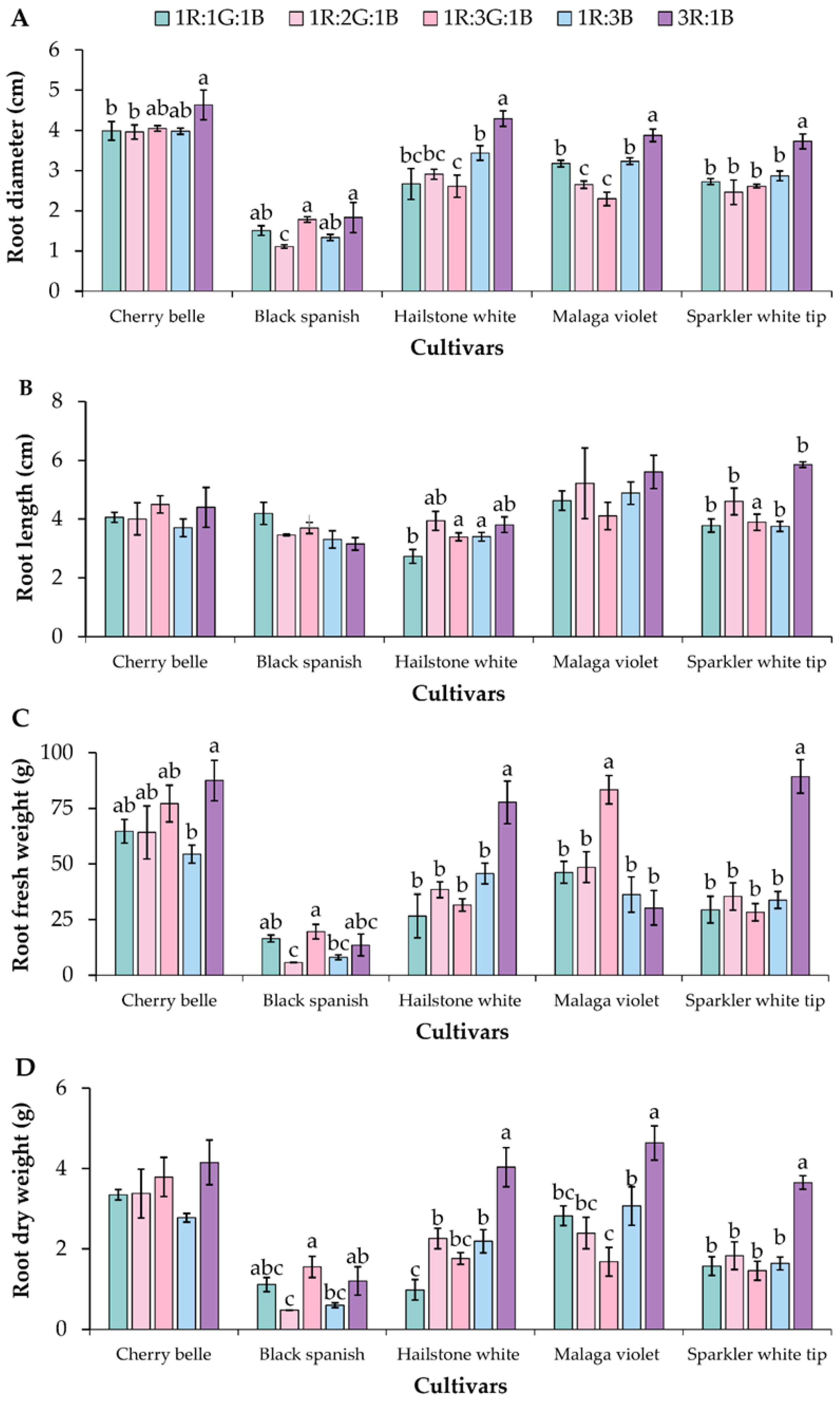
3.1. Biomass Accumulation and Plant Growth
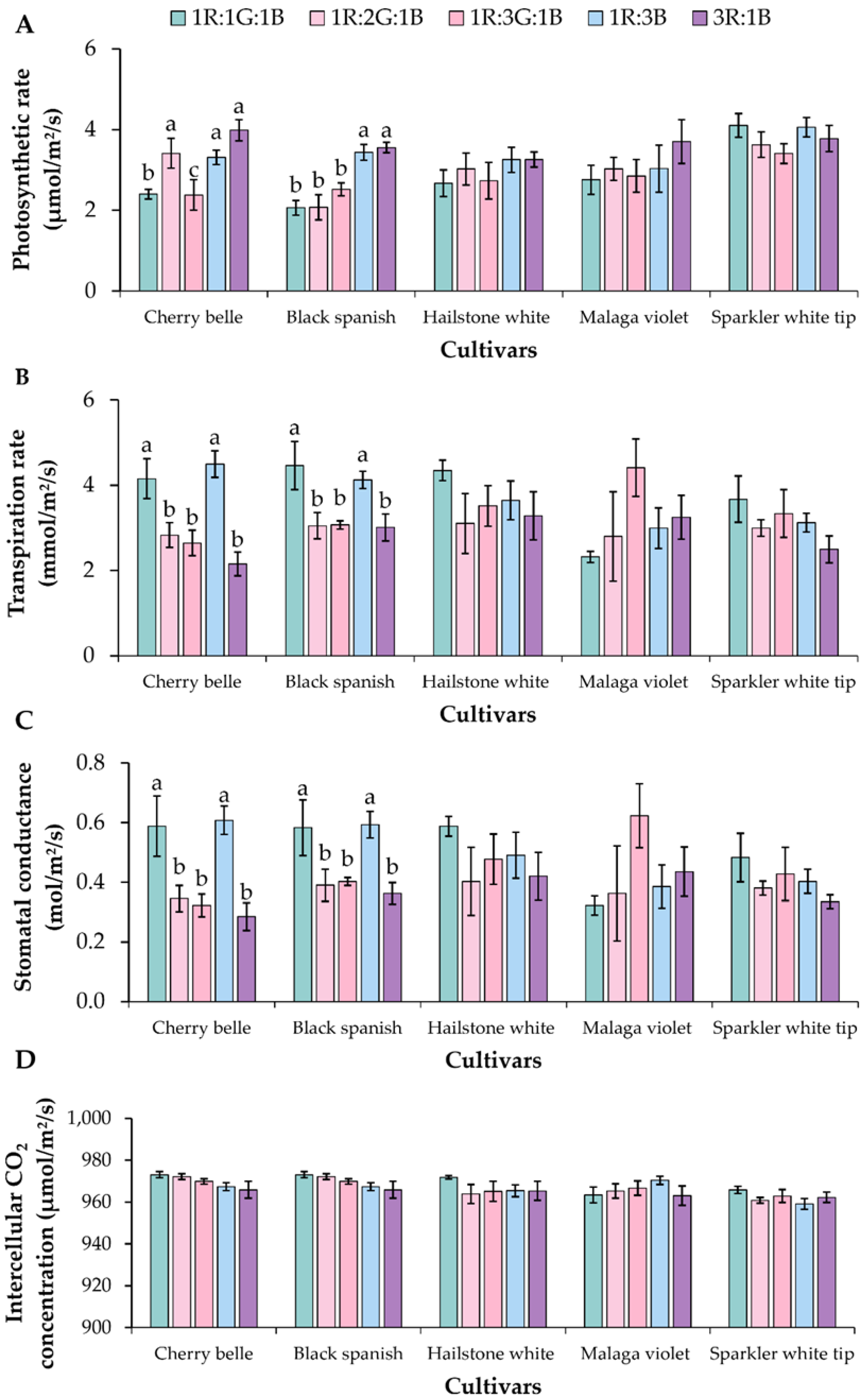
3.2. Physiological Responses
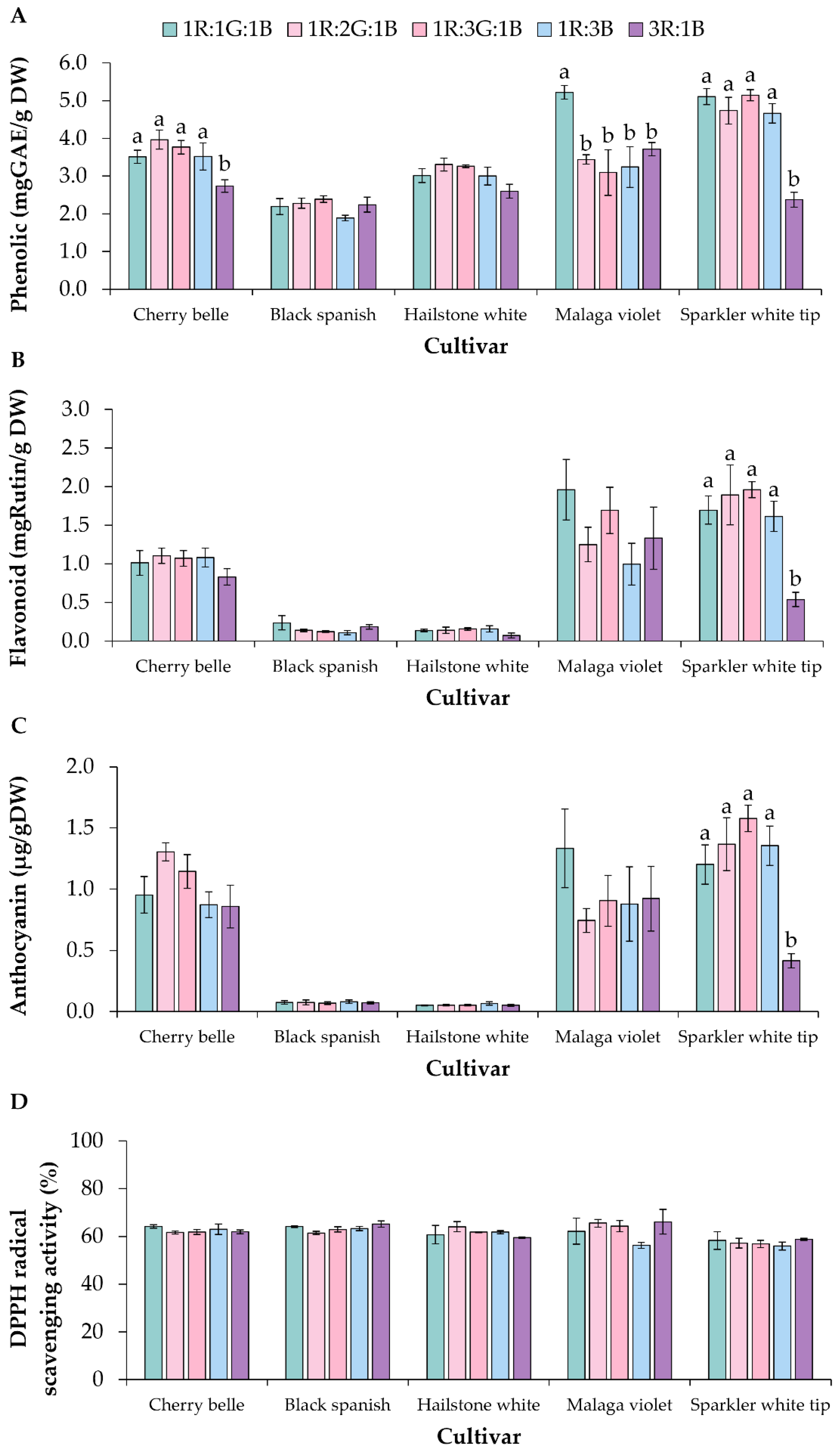
3.3. Secondary Metabolite Quantification
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishio, T. Economic and Academic Importance of Radish. In The Radish Genome; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–10. [Google Scholar]
- Kitashiba, H.; Li, F.; Hirakawa, H.; Kawanabe, T.; Zou, Z.; Hasegawa, Y.; Tonosaki, K.; Shirasawa, S.; Fukushima, A.; Yokoi, S.; et al. Draft sequences of the radish (Raphanus sativus L.) genome. DNA Res. 2014, 21, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Cao, J.; Yu, X.; Xiang, X.; Chen, H. Mapping QTLs for root morphological traits in Brassica rapa L. based on AFLP and RAPD markers. J. Appl. Genet. 2008, 49, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Kopta, T.; Pokluda, R. Yields, quality and nutritional parameters of radish (Raphanus sativus) cultivars when grown organically in the Czech Republic. Hortic. Sci. 2013, 40, 16–21. [Google Scholar] [CrossRef]
- Banihani, S.A. Radish (Raphanus sativus) and Diabetes. Nutrients 2017, 9, 1014. [Google Scholar] [CrossRef]
- Papetti, A.; Milanese, C.; Zanchi, C.; Gazzani, G. HPLC–DAD–ESI/MSn characterization of environmentally friendly polyphenolic extract from Raphanus sativus L. var. “Cherry Belle” skin and stability of its red components. Food Res. Int. 2014, 65, 238–246. [Google Scholar] [CrossRef]
- Rani, A.; Arfat, Y.; Aziz, R.S.; Ali, L.; Ahmed, H.; Asim, S.; Rashid, M.; Hocart, C.H. Enzymatically assisted extraction of antioxidant and anti-mutagenic compounds from radish (Raphanus sativus). Environ. Technol. Innov. 2021, 23, 101620. [Google Scholar] [CrossRef]
- Rakhmawati, R. Potency of Lobak Leaves (Raphanus sativus L. var. hortensis Back) as Anticancer and Antimicrobial Candidates. Biodiversitas J. Biol. Divers. 2009, 10, 158–162. [Google Scholar] [CrossRef]
- Curtis, I. The noble radish: Past, present and future. Trends Plant Sci. 2003, 8, 305–307. [Google Scholar] [CrossRef]
- Kumar, S. The importance of antioxidant and their role in pharmaceutical science—A review. Asian J. Res. Chem. Pharm. Sci. 2014, 1, 27–44. [Google Scholar]
- Hu, Y.; Zheng, Z.; Zhai, D.; Liang, P.; Wang, Z.; Jiang, C.; Guo, Y.; Chen, H.; Shen, C.; Wu, Y.; et al. A Mini-Review: Exploring the Application Prospects of the Three Major Rules in the Field of Antioxidants. J. Mol. Struct. 2024, 1304, 137746. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Sezgin-Bayindir, Z.; Paiva-Martins, F.; Bravo-Díaz, C. Biochemistry of Antioxidants: Mechanisms and Pharmaceutical Applications. Biomedicines 2022, 10, 3051. [Google Scholar] [CrossRef] [PubMed]
- Kurutas, E. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Antioxidants: Basic Principles, Emerging Concepts, and Problems. Biomed. J. 2014, 37, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Goyeneche, R.; Roura, S.; Ponce, A.G.; Vega-Galvez, A.; Quispe, I.; Uribe, E.; Di Scala, K. Chemical characterization and antioxidant capacity of red radish (Raphanus sativus L.) leaves and roots. J. Funct. Foods 2015, 16, 256–264. [Google Scholar] [CrossRef]
- Kozai, T. Propagation, grafting and transplant production in closed systems with artificial lighting for commercialization in Japan. Propag. Ornam. Plants 2007, 7, 145–149. [Google Scholar]
- Kozai, T.; Kubota, C.; Chun, C.; Afreen, F.; Ohyama, K. Necessity and Concept of the Closed Transplant Production System. In Transplant Production in the 21st Century: Proceedings of the International Symposium on Transplant Production in Closed System for Solving the Global Issues on Environmental Conservation, Food, Resources and Energy; Springer: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Morimoto, T.; Torii, T.; Hashimoto, Y. Optimal control of physiological processes of plants in a green plant factory. Control Eng. Pract. 1995, 3, 505–511. [Google Scholar] [CrossRef]
- Nicole, C.C.s.; Charalambous, F.; Martinakos, S.; Voort, S.; Li, Z.; Verhoog, M.; Krijn, M. Lettuce growth and quality optimization in a plant factory. Acta Hortic. 2016, 1134, 231–238. [Google Scholar] [CrossRef]
- Kang, J.; Sugumaran, K.; Atulba, S.L.; Jeong, B.R.; Hwang, S. Light intensity and photoperiod influence the growth and development of hydroponically grown leaf lettuce in a closed-type plant factory system. Horticulture 2013, 54, 501–509. [Google Scholar] [CrossRef]
- Shimizu, H. Light Environment Optimization for Lettuce Growth in Plant Factory. IFAC Proc. Vol. 2011, 18, 605–609. [Google Scholar] [CrossRef]
- Kozai, T. Resource use efficiency of closed plant production system with artificial light: Concept, estimation and application to plant factory. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2013, 89, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Bantis, F.; Ouzounis, T.; Radoglou, K. Artificial LED lighting enhances growth characteristics and total phenolic content of Ocimum basilicum, but variably affects transplant success. Sci. Hortic. 2016, 198, 277–283. [Google Scholar] [CrossRef]
- Shao, L.; Fu, Y.; Liu, H.; Liu, H. Changes of the antioxidant capacity in Gynura bicolor DC under different light sources. Sci. Hortic. 2015, 184, 40–45. [Google Scholar] [CrossRef]
- Shiga, T.; Shoji, K.; Shimada, H.; Hashida, S.-n.; Goto, F.; Yoshihara, T. Effect of light quality on rosmarinic acid content and antioxidant activity of sweet basil, Ocimum basilicum L. Plant Biotechnol. 2009, 26, 255–259. [Google Scholar] [CrossRef]
- Chutimanukul, P.; Wanichananan, P.; Janta, S.; Toojinda, T.; Darwell, C.T.; Mosaleeyanon, K. The influence of different light spectra on physiological responses, antioxidant capacity and chemical compositions in two holy basil cultivars. Sci. Rep. 2022, 12, 588. [Google Scholar] [CrossRef]
- Chutimanukul, P.; Jindamol, H.; Thongtip, A.; Korinsak, S.; Romyanon, K.; Toojinda, T.; Darwell, C.T.; Wanichananan, P.; Panya, A.; Kaewsri, W.; et al. Physiological responses and variation in secondary metabolite content among Thai holy basil cultivars (Ocimum tenuiflorum L.) grown under controlled environmental conditions in a plant factory. Front. Plant Sci. 2022, 13, 1008917. [Google Scholar] [CrossRef]
- Yorio, N.C.; Goins, G.D.; Kagie, H.R.; Wheeler, R.M.; Sager, J.C. Improving spinach, radish, and lettuce growth under red light-emitting diodes (LEDs) with blue light supplementation. HortScience 2001, 36, 380–383. [Google Scholar] [CrossRef]
- Zha, L.; Liu, W. Effects of light quality, light intensity, and photoperiod on growth and yield of cherry radish grown under red plus blue LEDs. Hortic. Environ. Biotechnol. 2018, 59, 511–518. [Google Scholar] [CrossRef]
- Gam, D.T.; Khoi, P.H.; Ngoc, P.B.; Linh, L.K.; Hung, N.K.; Anh, P.T.L.; Thu, N.T.; Hien, N.T.T.; Khanh, T.D.; Ha, C.H. LED Lights Promote Growth and Flavonoid Accumulation of Anoectochilus roxburghii and Are Linked to the Enhanced Expression of Several Related Genes. Plants 2020, 9, 1344. [Google Scholar] [CrossRef]
- Pritchard, S.G.; Prior, S.A.; Rogers, H.H.; Davis, M.A.; Runion, G.B.; Popham, T.W. Effects of elevated atmospheric CO2 on root dynamics and productivity of sorghum grown under conventional and conservation agricultural management practices. Agric. Ecosyst. Environ. 2006, 113, 175–183. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, B.; Su, J.; Liao, J.; Lin, C.; Oka, Y. Cryptochromes Orchestrate Transcription Regulation of Diverse Blue Light Responses in Plants. Photochem. Photobiol. 2017, 93, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Kang, C.; Kaiser, E.; Kuang, Y.; Yang, Q.; Li, T. Red/blue light ratios induce morphology and physiology alterations differently in cucumber and tomato. Sci. Hortic. 2021, 281, 109995. [Google Scholar] [CrossRef]
- Samuoliene, G.; Sirtautas, R.; Brazaitytė, A.; Miliauskienė, J.; Sakalauskienė, S.; Duchovskis, P. The impact of red and blue light-emitting diode illumination on radish physiological indices. Cent. Eur. J. Biol. 2011, 6, 821–828. [Google Scholar] [CrossRef]
- Liu, J.; van Iersel, M.W. Photosynthetic Physiology of Blue, Green, and Red Light: Light Intensity Effects and Underlying Mechanisms. Front. Plant Sci. 2021, 12, 619987. [Google Scholar] [CrossRef]
- Stirbet, A.; Lazár, D.; Guo, Y.; Govindjee, G. Photosynthesis: Basics, history and modelling. Ann. Bot. 2019, 126, 511–537. [Google Scholar] [CrossRef]
- Engineer, C.B.; Hashimoto-Sugimoto, M.; Negi, J.; Israelsson-Nordström, M.; Azoulay-Shemer, T.; Rappel, W.-J.; Iba, K.; Schroeder, J.I. CO2 Sensing and CO2 Regulation of Stomatal Conductance: Advances and Open Questions. Trends Plant Sci. 2016, 21, 16–30. [Google Scholar] [CrossRef]
- Doi, M.; Kitagawa, Y.; Shimazaki, K. Stomatal Blue Light Response Is Present in Early Vascular Plants. Plant Physiol. 2015, 169, 1205–1213. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzino, V.M.T.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochem. Adv. Res. 2006, 661, 23–67. [Google Scholar]
- Weeplian, T.; Yen, T.-B.; Ho, Y.-S. Growth, Development, and Chemical Constituents of Edible Ice Plant (Mesembryanthemum crystallinum L.) Produced under Combinations of Light-emitting Diode Lights. HortScience 2018, 53, 865–874. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Z.; Yuan, X.; Chen, X.; Lu, C. A review on the effects of light-emitting diode (LED) light on the nutrients of sprouts and microgreens. Trends Food Sci. Technol. 2020, 99, 203–216. [Google Scholar]
- Taulavuori, K.; Pyysalo, A.; Taulavuori, E.; Julkunen-Tiitto, R. Responses of phenolic acid and flavonoid synthesis to blue and blue-violet light depends on plant species. Environ. Exp. Bot. 2018, 150, 183–187. [Google Scholar] [CrossRef]
- Batista, D.S.; Felipe, S.H.S.; Silva, T.D.; Castro, K.M.; Mamedes-Rodrigues, T.C.; Miranda, N.A.; Ríos-Ríos, A.M.; Faria, D.V.; Fortini, E.A.; Chagas, K.; et al. Light quality in plant tissue culture: Does it matter? In Vitro Cell. Dev. Biol. Plant 2018, 54, 195–215. [Google Scholar]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.-N.; Yoshihara, T. Blue Light-emitting Diode Light Irradiation of Seedlings Improves Seedling Quality and Growth after Transplanting in Red Leaf Lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Jang, E.B.; Ho, T.-T.; Park, S.-Y. Effect of light quality and tissue origin on phenolic compound accumulation and antioxidant activity in Camellia japonica calli. In Vitro Cell. Dev. Biol. Plant 2020, 56, 567–577. [Google Scholar] [CrossRef]
- Kondo, S.; Tomiyama, H.; Rodyoung, A.; Okawa, K.; Ohara, H.; Sugaya, S.; Terahara, N.; Hirai, N. Abscisic acid metabolism and anthocyanin synthesis in grape skin are affected by light emitting diode (LED) irradiation at night. J. Plant Physiol. 2014, 171, 823–829. [Google Scholar] [CrossRef]
- Stutte, G.W. Light-emitting Diodes for Manipulating the Phytochrome Apparatus. HortScience 2009, 44, 231–234. [Google Scholar] [CrossRef]
- Wong, C.E.; Teo, Z.W.N.; Shen, L.; Yu, H. Seeing the lights for leafy greens in indoor vertical farming. Trends Food Sci. Technol. 2020, 106, 48–63. [Google Scholar] [CrossRef]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: A review. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, S.S.; Akbar, F.; Kanwal, F. Correlation of different spectral lights with biomass accumulation and production of antioxidant secondary metabolites in callus cultures of medicinally important Prunella vulgaris L. J. Photochem. Photobiol. B Biol. 2016, 159, 1–7. [Google Scholar] [CrossRef]
- Tang, Y.; Mao, R.; Guo, S. Effects of LED spectra on growth, gas exchange, antioxidant activity and nutritional quality of vegetable species. Life Sci. Space Res. 2020, 26, 77–84. [Google Scholar] [CrossRef]
- Heo, J.; Lee, Y.; Kim, D.; Chang, Y.; Chun, C. Effects of Supplementary LED Lighting on Growth and Biochemical Parametets in Dieffenbachia amoena ‘Camella’ and Ficus elastica ‘Melany’. J. Korean Hort. Sci. Tech. 2010, 28, 51–58. [Google Scholar]
- Martineau, V.; Lefsrud, M.; Naznin, T.; Kopsell, D. Comparison of Light-emitting Diode and High-pressure Sodium Light Treatments for Hydroponics Growth of Boston Lettuce. HortScience 2012, 47, 477–482. [Google Scholar] [CrossRef]
- Barros, L.; Ferreira, M.-J.; Queirós, B.; Ferreira, I.; Baptista, P. Total phenols, ascorbic acid, B-Carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 2007, 103, 413–419. [Google Scholar] [CrossRef]
- Murakami, M.; Yamaguchi, T.; Takamura, H.; Matoba, T. Effects of Ascorbic Acid and α-Tocopherol on Antioxidant Activity of Polyphenolic Compounds. J. Food Sci. 2006, 68, 1622–1625. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chutimanukul, P.; Piew-ondee, P.; Dangsamer, T.; Thongtip, A.; Janta, S.; Wanichananan, P.; Thepsilvisut, O.; Ehara, H.; Chutimanukul, P. Effects of Light Spectra on Growth, Physiological Responses, and Antioxidant Capacity in Five Radish Varieties in an Indoor Vertical Farming System. Horticulturae 2024, 10, 1059. https://doi.org/10.3390/horticulturae10101059
Chutimanukul P, Piew-ondee P, Dangsamer T, Thongtip A, Janta S, Wanichananan P, Thepsilvisut O, Ehara H, Chutimanukul P. Effects of Light Spectra on Growth, Physiological Responses, and Antioxidant Capacity in Five Radish Varieties in an Indoor Vertical Farming System. Horticulturae. 2024; 10(10):1059. https://doi.org/10.3390/horticulturae10101059
Chicago/Turabian StyleChutimanukul, Panita, Pakin Piew-ondee, Thanyaluk Dangsamer, Akira Thongtip, Supattana Janta, Praderm Wanichananan, Ornprapa Thepsilvisut, Hiroshi Ehara, and Preuk Chutimanukul. 2024. "Effects of Light Spectra on Growth, Physiological Responses, and Antioxidant Capacity in Five Radish Varieties in an Indoor Vertical Farming System" Horticulturae 10, no. 10: 1059. https://doi.org/10.3390/horticulturae10101059
APA StyleChutimanukul, P., Piew-ondee, P., Dangsamer, T., Thongtip, A., Janta, S., Wanichananan, P., Thepsilvisut, O., Ehara, H., & Chutimanukul, P. (2024). Effects of Light Spectra on Growth, Physiological Responses, and Antioxidant Capacity in Five Radish Varieties in an Indoor Vertical Farming System. Horticulturae, 10(10), 1059. https://doi.org/10.3390/horticulturae10101059






