Abstract
Biostimulants are an important alternative to improve and promote higher efficiency in cropping systems. Although the biostimulant industry has been developing for several years, there are still areas of opportunity for new sources of biostimulants as well as new ecofriendly extraction techniques that allow for a circular economy and the reuse of waste. Lignin is a heteropolymer that constitutes about 40% of the plant cell wall. A great source of lignin is agrowastes, giving it added value. Recently, its use has been tested in agronomy as a carrier of nutrients and pesticides. Walnuts are produced on a large scale in Northern Mexico, and the shell represents between 15 and 40% of its total weight. However, to obtain this biopolymer, to date, non-environmentally friendly techniques have been used; for this reason, it is necessary to find extraction alternatives to make this proposal sustainable. In this work, the obtaining and characterization of lignin through mild extraction conditions from nutshells and its evaluation as a biostimulant on the growth of tomato seedlings are reported. Lignin was extracted by hydrolysis with a mixture of acetic acid and distilled water (65:35 v/v). The results showed that it was possible to obtain 15% (w/w) lignin using mild solvents, evidenced by thermogravimetric analysis (TGA), proton magnetic nuclear resonance (H-RMN), and infrared (IR). Subsequently, lignin solutions were prepared at different concentrations, 0, 10, 50, and 100 ppm, and applied via foliar weekly to tomato seedlings. A greater fresh weight of the stem was found with 10 and 50 ppm, and the height and the fresh biomass increased with the three concentrations (10, 50, and 100 ppm), concluding that lignin extracted from nutshells using mild conditions can act as a plant biostimulant.
1. Introduction
Currently, there is a need to find efficient and non-polluting alternatives that allow for us to increase crop production in quantity and quality with less input. One of these alternatives has been the use of biostimulants of plant metabolism [].
These consist in the complex mixture of biological, organic, or inorganic substances that, when applied at low concentrations to plants, promote growth and production, improving the uptake of essential nutrients, increasing the concentration of antioxidants, and enhancing stress defense.
Due to the above, the development and application of biostimulants has been an increasingly demanded practice in modern agriculture, where the elucidation of the physiological and biochemical mechanisms of bioactive molecules has become necessary. Therefore, the development of new generation biostimulants will consist in synergistic molecules with specific action []. All this is also driven by the additional fact that it is an attractive trade, with profits that have exceeded USD 4 billion in 2024 and are estimated to have an annual growth of 11.5% [].
Lignin is an aromatic heteropolymer randomly formed by phenylpropane units that has high reactivity [], is very abundant in nature since it constitutes 40% of the cell wall of plants and even more of woody materials, and which can undergo various processes to generate high-value products, for instance, as additives, coating agents, absorbents, packaging materials and, recently in agronomy [], as carriers of macro- and micro-essential elements in plants, which are essential for the optimal development of crops. Lignin also presents beneficial effects such as the increase in stress tolerance and increase in crops due to still unknown mechanisms []. A great advantage of the use of this biopolymer as a biostimulant is that an important source is agriculture residues [].
In Northern Mexico, an economically significant crop is the walnut. The states with the highest production are Chihuahua, Coahuila, and Sonora. In the last 15 years, the sown area has increased by 80%, currently reaching 146,000 hectares planted []. This crop generates waste in 50% of its total production [,], and it has been reported that the lignin content in the nutshell ranges between 15 and 35% []; therefore, obtaining and purifying it represent a novel way to reduce the volume of residues and thus revalorize it through its application in plant production.
To achieve the extraction and separation of lignin from the raw material, there are a wide variety of physical, chemical, thermal, and biological methods []. However, to date, the most common techniques, such as pulp kraft and acid hydrolysis, use strong acids or bases at high temperatures, e.g., sulfuric or hydrochloric acids at temperatures between 180 and 250 °C, for long periods of time [], where the effect of sustainability and reuse of materials is diminished [].
However, recently, and in accordance with the need to apply green technologies with environmental responsibility, organic acids such as acetic, lactic, and oxalic acids have been used, obtaining yields of between 15 and 30% of lignin [].
As mentioned above, the objective of this research project was to promote the use of walnut residues as a raw material for obtaining and characterizing lignin through green technologies and their application as a biostimulant for the growth of tomato seedlings.
2. Materials and Methods
2.1. Nutshell and Reagents
The nutshells were collected from local producers in the city of San Buenaventura, Coahuila, Mexico. The walnut was of the Wichita variety.
The acetic acid (Sigma-Aldrich, ReagentPlus ≥ 99%, Toluca, Mexico) used in the lignin extraction was purchased from the supplier Sigma-Aldrich and used as received. The ethanol used in the lignin extraction technique was purchased from the supplier Supelco (Toluca, Mexico).
2.2. Quantification of Lignocellulosic Material from Nutshells
The lignocellulosic composition of the nutshell was determined using thermogravimetric analysis (TGA), weighing 12.2 mg of the sample, using the method described by Díez []. TGA Q500 equipment from TA Instruments was used (Waters, New Castle, DE, USA). The temperature range for the analysis was 20–700 °C, using a heating rate of 10 °C/min and a nitrogen flow of 50 mL/min.
2.3. Extraction of Lignin with Organic Solvents
The nutshell was crushed in a blade mill with a mesh of 1 mm in diameter and brought to dryness at 70 °C for 48 h. For the extraction of lignin, a hydrolysis treatment was carried out with a mixture of acetic acid (AC) and distilled water (AD), 65/35 (v/v). A total of 50 g of ground dried nutshell was added to a 4 L glass reactor, and the AC/AD mixture was added at a 1:10 (w/v) ratio and then heated to 80 °C, with constant stirring for 4 h. The lignocellulosic extract obtained from the hydrolysis was filtered by gravity in a porcelain Büchner funnel with a nylon mesh filter. The solid part was dried at 70 °C for 48 h. The filtered extract was precipitated 3 times in 95% ethanol, where hemicellulose was obtained. The soluble lignin was rotary evaporated to remove the ethanol and thus purify the lignin [], as in Figure 1.
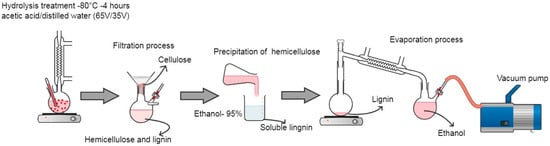
Figure 1.
Process of extraction and purification of lignin from nutshells.
2.4. Characterization of Lignin
2.4.1. Proton Nuclear Magnetic Resonance (1H-NMR)
The chemical structure of lignin was studied by 1H-NMR. The analysis was carried out on a 400 MHz Bruker nuclear magnetic resonance spectrometer (Billerica, MA, USA). Data acquisition and management were performed using TopSpin (Version 3.6.2) and MestReC software (6.0.2), respectively. The sample was dissolved in deuterated dimethylsulfoxide (DMSO-d Sigma-Aldrich, Toluca, Mexico) and placed in 5 mm diameter quartz tubes to be subsequently analyzed at room temperature.
2.4.2. Fourier Transform Infrared Spectroscopy (FTIR)
For the analysis by infrared spectroscopy (FTIR), the Nicole Is10 model (Thermo Fisher Scientific, Bohemia, NY, USA) was used. The data were collected with an absorbance scale from 4000 to 600 c−1 of the electromagnetic spectrum. A DTGS KBr detector was used. To process the spectra, the OMNIC program was used. The analyses were carried out in duplicate.
2.4.3. Scanning Electron Microscopy (SEM)
Using a JEOL model JCM6000 scanning electron microscope (JEOL, Tokyo, Japan) the chemical composition of the lignin was determined by X-ray energy dispersion spectrometry (EDS) coupled to SEM. Previously, the lignin was covered by a layer of gold.
2.4.4. Dynamic Light Scattering (DLS)
The particle diameter (Dp) of the lignin extracted from the walnut shell was obtained using the DLS technique (Malvern Zetasizer (nano zs), London, UK) particle analyzer at 25 °C. For this analysis, 2 solutions of 500 and 1000 ppm in methanol were used.
2.5. Seedling Growth
2.5.1. Location of the Experiment
The experiment was carried out in a medium-tech chapel greenhouse measuring 14 m long × 7 m wide in the Horticulture Department of Antonio Narro Autonomous University, Saltillo, Coahuila, Mexico, located at 25°21′12.8″ north latitude and 101°01′1.9″ west longitude.
2.5.2. Plant Material
To obtain the tomato seedlings, seeds of the Floradade variety were sown in a 128-cavity polystyrene germination tray with a 1:1 substrate of perlite and peat moss, placing 2 seeds per cavity. These seeds were placed in a bioclimatic chamber at a temperature of 28 °C in the dark until germination. Four weeks after sowing, they were transplanted into 500 mL polystyrene pots with peat moss and perlite in a 1:1 ratio; the photoperiod was 13 h light/11 h dark. The nutritive solution at 25% of the Steiner solution was manually applied daily, with a pH of 6.3 and an electrical conductivity of 2 dS/m, with the following composition: Ca (NO3)2·4H2O (9 mEq), KH2PO4 (1 mEq), MgSO4·7H2O (4 mEq), KNO3 (3 mEq), K2SO4 (3 mEq), Fe chelated (3 mg L−1), H3BO3 (0.5 mg L1), MnSO4 (0.7 mg L−1), ZnSO4 (0.09 mg L−1), and CuSO4 (0.02 mg L−1).
2.6. Treatments of Lignin
The experiment consisted of applying three different concentrations of lignin, 10, 50, and 100 ppm, plus the control, by foliar spraying to 10 seedlings for each treatment. It began on the day of the transplant and was repeated once a week for four weeks. These seedlings had a completely randomized distribution.
2.7. Sampling
A completely random sampling was carried out five weeks after the transplant, one day after the application of the last treatment. Five complete seedlings were taken from each treatment to measure the following growth variables in a nondestructive way: number of leaves, number of compound leaves, leaf area (cm2), stem diameter (cm), seedling height (cm), and root length (cm). Destructively, the following were measured: fresh weight of leaves (g), root, stem and total fresh weight (g), dry weight of leaves, stem, and root, and total dry weight (g).
2.8. Experimental Design and Statistical Analyses
The experimental design was completely randomized, with four treatments and 10 repetitions per treatment, resulting in a total of 40 plants for the experiment; each plant was considered as an experimental unit. To verify the homogeneity of variance, the Levene test was carried out, along with the Shapiro-Wilk normality test.
The data were analyzed in univariate form, using a one-way analysis of variance (ANOVA) with five repetitions per treatment, followed by a least significant difference post hoc test (LSD, p ≤ 0.05) using the Infostat software package (2020 version).
3. Results
Thermogravimetric analysis (TGA) provided specific information on the loss of mass of the nutshell as a function of temperature when it was heated in a controlled atmosphere. The content of the lignocellulosic material is shown in Table 1, which was obtained using the DTG curves, which in turn are obtained from the first derivative of the TGA curve, following the methodology reported by [].

Table 1.
Quantification of lignocellulosic material in the nutshell.
Figure 2 shows the 1H-NMR spectrum of the lignin extracted from the nutshell, as well as the simplified chemical structure of lignin for the identification of the most important signals. Additionally, a comparison of the spectrum of the nut shell extraction product of this work and a spectrum of lignin reported in the literature [] is presented, and the great similarity of signals that exists between both spectra confirms the successful obtaining of lignin from the extraction method under the mild conditions reported in this work.
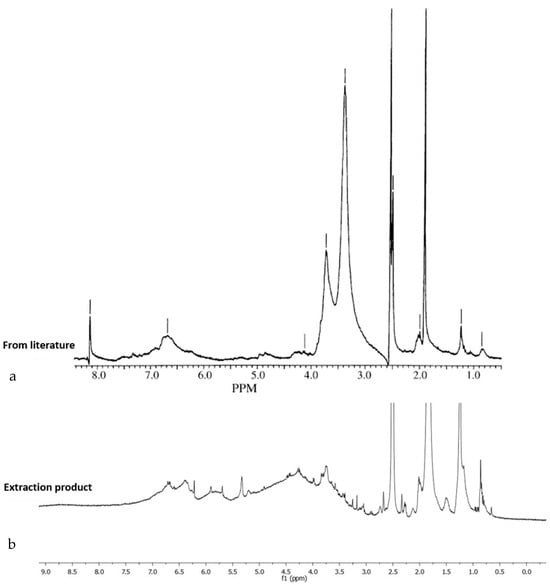
Figure 2.
1H-NMR spectrum of the lignin from literature (a) Xu et al. [] and (b) 1H-NMR spectrum of the lignin sample extracted from nutshells.
Being a natural polymer, the structure of lignin is not well defined, and this was evidenced by the appearance of various signals, of which the most important were identified; in the region of 6.2 to 7.5 ppm, the signals corresponded to the aromatic protons of lignin, specifically those attributed to the structures known as “syringyl propane” and “guaiacyl propane”. On the other hand, the signal covers approximately 3.5 to 4.0 ppm. This is attributed to the protons of the methoxy groups (CH3-O) that are distributed in different positions throughout the general structure of lignin. There are some aliphatic hydrocarbon groups in the complex structure of lignin, mainly in the side chains of lignin, and these are observable in the region of 0.8 to 2.0 ppm of the spectrum. On the other hand, the lignin sample analyzed by FTIR showed the presence of the characteristic functional groups of this polymer, such as the phenolic hydroxyl and aliphatic structures, in the broad band of 3500 to 3350 cm−1. The bands centered at approximately 2.927 to 2.848 cm−1 arise predominantly from the stretching of the carbon-hydrogen bonds in the methoxy groups (CH3-O), methylene groups (CH2), and methine groups (CH) of the side chains. The bands observed in the range of 1700 cm−1 indicate the presence of unconjugated carbonyl groups. Specifically, the bands at 1716 and 1711 cm−1 are attributed to the esterification of the phenol and alcohol groups of the propane chain (Cα and Cγ) that have been previously reported for extraction processes using acetic and formic acid. The vibration of the aromatic skeleton of lignin was recorded in the band range of 1559–1607 cm−1. The signal at 1240 cm−1 is due to the stretching of the guaiacyl units (G), where deformations of these units can also be seen at 1020 cm−1. The signals in the range of 820 cm−1 represent the vibrations of the CH plane of the guaiacyl units. Finally, asymmetric flexions of the HCCH groups are observed at approximately 730 cm−1. Figure 3 shows the FTIR spectrum of the lignin sample extracted from the walnut shell, and all the signals described above can be observed. The results obtained from this analysis are in accordance with previously published research [,].
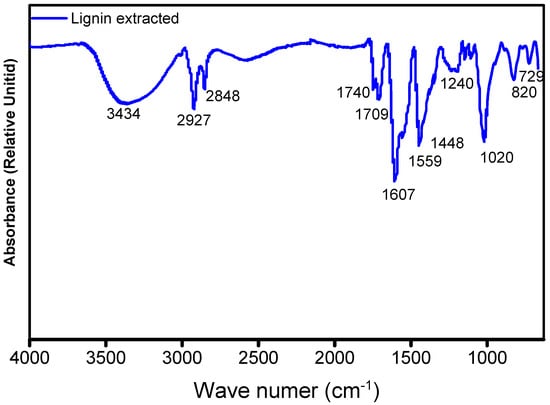
Figure 3.
FTIR spectrum of lignin extracted from the nutshells.
Figure 4 shows the SEM micrographs of the lignin extracted from the nutshells at two different magnifications, obtained with secondary electrons. In the micrographs, an irregular morphology in shape and size can be observed. This type of morphology is very similar to that which is obtained in soda lignin [], or the kraft lignin from the supplier Sigma Aldrich, that presents defined morphologies of rounded or hemispherical shape, according to what was reported by Kohnke []. According to the previous studies, spherical morphologies have greater thermodynamic stability than the other forms of particles []. The morphology evidenced under these treatment conditions can be explained by the milling process. The lignocellulosic liquor obtained after treatment is subjected to evaporation where a thin film is formed, which then undergoes a grinding process that could cause this type of irregular morphology in shape and size.
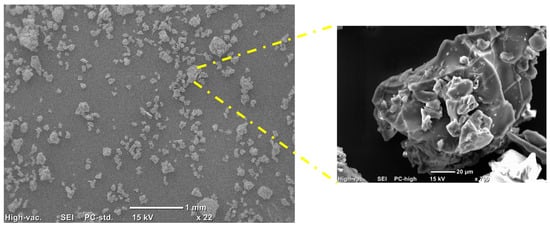
Figure 4.
SEM micrographs of lignin obtained from nutshells.
On the other hand, the micrograph at higher magnifications shows a rough surface without the presence of microdefects such as cracks or porosity. In addition, it is possible to observe some agglomerations of particles that are promoted by the grinding process.
The elemental analysis obtained by EDS is presented in Figure 5. This analysis showed that lignin is mainly composed of carbon and oxygen, with small traces of chlorine, alumina, magnesium, sodium, potassium, and calcium. The presence of these elements is mainly due to traces of ash that remain in the lignin from the extraction biomass. The predominant presence of carbon and oxygen is in accordance with the structure of lignin since it presents functional groups based on carbon and carbon-carbon, carbon-oxygen, and carbon-hydrogen bonds. Lignin generally has a high carbon content that can vary between 50 and 75% by weight [], which is consistent with that which has been reported in this work, showing a carbon content of 76.5%.
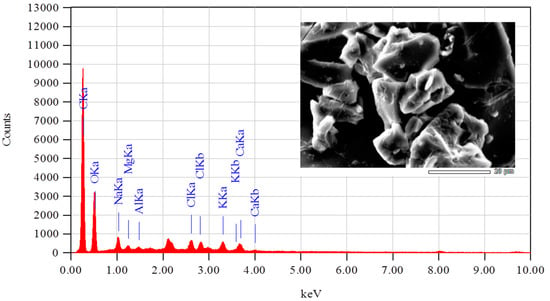
Figure 5.
EDS spectrum of lignin obtained from nutshells.
Finally, regarding the characterization of the extracted lignin, Figure 6 shows the particle diameter distribution of lignin obtained by DLS, which measures fluctuations in scattering intensity as a function of time. A distribution ranging from 10 to 70 nm in particle diameter can be observed for the lignin concentrations analyzed (500 and 1000 ppm); however, both concentrations exhibit particle diameters of the same order of magnitude, with average diameters of 47 and 33 nm.
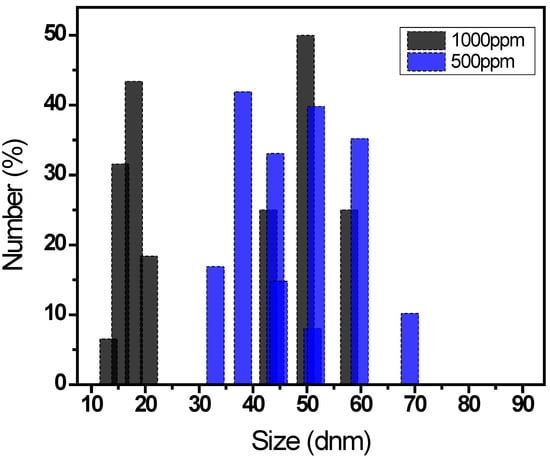
Figure 6.
Particle size distribution of lignin obtained from nutshells.
Once the presence of lignin extracted from the walnut shell was demonstrated by a set of characterization techniques, it was evaluated as a possible biostimulant in tomato seedlings. For this, the application of the three concentrations of lignin evaluated (10, 50 and 100 ppm) showed an increase in the height of the seedlings compared to the control seedlings, and the following trend was observed: the higher the concentration of lignin was, the lower the increase, as the treatment of 10 ppm of lignin showed an increase of 34.98%, while that of 50 ppm showed a 31% increase, and that of 100 ppm only showed an increase of 17.62%. This trend was contrary to what happened with respect to the number of composite leaves, where the higher the concentration of lignin, the greater the number of leaves. In this sense, the tests carried out with 50 and 100 ppm of biostimulant showed statistically significant increases of 26 and 38%, respectively. The foliar area and the diameter of the stem did not change after the application of the treatments. All the comparative results are reported in Table 2.

Table 2.
Results obtained on nondestructive variables of seedling growth.
Table 3 shows the results obtained from the fresh and dry biomass variables of each plant organ, as well as the total biomass. The application of the three concentrations of lignin (10, 50, and 100 ppm) promoted an increase in the total dry weight and the dry weight of the radicle. Additionally, the application of lignin at a concentration of 50 ppm showed an increase in the fresh and dry weights of the stem.

Table 3.
Results obtained on the destructive variables of seedling growth.
4. Discussion
Lignocellulosic material is the largest source of carbon on Earth, translating into enormous potential for renewable energy with limitless applications. This material can be derived from agricultural residues, adding value and reducing the production of pollutants []. However, this material is made up of the following three polymers: cellulose (40–50% w/w), hemicellulose (25–35% w/w), and lignin (15–30% w/w), tightly bonded through strong interactions []. Several physical, chemical, thermal, and biological methods are employed to extract and separate these components []. To date, the most widely used techniques involve strong acids or bases at high temperatures for extended periods, such as kraft pulping and inorganic acid hydrolysis, leading to diminished sustainability and material reuse [,,]. As extensively documented, lignin is an aromatic biopolymer, mainly composed of three monolignols, sinapyl alcohol, coniferyl alcohol, and p-coumaryl alcohol, rich in highly reactive functional groups like phenolic, hydroxyl, carboxylic, benzoic, etc. This reactivity makes lignin susceptible to modifications and substitutions [], resulting in exceptional physicochemical and mechanical properties, including antioxidant, antimicrobial, and thermal stabilizer []. Consequently, exploring lignin applications in high-demand crop production has recently gained scientific interest []. Lignin has been tested as a carrier of fertilizers and pesticides, forming bonds with ions like Zn2+, Ca2+, Mg2+, and Cu2+, through active groups like hydroxyl, carbonyl, and methoxy, enabling controlled release and efficient uptake and therefore enhancing its agronomical application [].
In this research, lignin was successfully extracted, purified, and characterized by TGA, H-RMN, FTIR, SEM, and DLS from nutshells using a mixture of organic acid (acetic acid) and water. Cellulose and hemicellulose were hydrolyzed and removed, recovering 15% (w/w) of pure lignin [].
Subsequently, the purified lignin from nutshells was applied via foliar at three concentrations (10, 50, and 100 ppm) on tomato seedlings. The results demonstrated increased height, fresh root weight, and total dry weight with all three concentrations. Furthermore, the 50 ppm concentration showed higher fresh and dry stem weights, along with an increase in the number of compound leaves. One of the most noticeable indicators of plant metabolic stimulation was an improvement in growth, with biomass as the most prominent factor []. It has been established that the application of lignin or the modified forms of it depends on the tissue, plant species, molecular form, and the concentration. However, the concentration has been tested in a wide range, for example, in soil, it was applied between 5 and 20 ppm, resulting in an improvement in the biomass content of ryegrass []; on the other hand, lignosulfonates from 10 ppm [] to 1000 ppm [] have been applied directly to plants, complexing the ions and resulting in improvements in the growth observed in both cases.
The lignin-like biostimulant effect could be related to the reactivity of functional groups like hydroxyls and aliphatic aromatics, which were evidenced by 1H-NMR analysis [].
Similar results were reported by [], where lignin application at 10 and 50 ppm promoted increased root growth in maize seedlings, as well as stem and leaf growth, correlating this effect with the phenol content in lignin and arguing that these groups might impact plant redox metabolism, enhancing photosynthetic rate and consequently biomass production [].
Other authors have indicated that the possible mechanism of action of lignin on plant metabolism is a mimic process of signaling like regulators such as indoleacetic acid []; similarly, Ertani et al. [] observed that the application of modified lignin on maize seedlings led to an increased leaf weight and root size, enhanced enzymatic activity of RUBISCO, glutamate, and glutamine synthetase, along with higher total protein content, chlorophyll, and monosaccharides. This biostimulant effect might be due to elicited hormone-like gibberellin properties.
Another possibility is that the monolignols from lignin can be used in the synthesis of molecules related to plant defense, photosynthesis, and cell wall structure. However, elucidating the specific way through which lignin acts as a plant metabolic bio-stimulant requires in-depth studies in metabolomics, genomics, ionomics, and different plant species.
5. Conclusions
It was possible to obtain and characterize lignin from the nutshells by hydrolysis under mild conditions, and the biostimulant effect of this was evidenced through applying it via foliar weekly at low concentrations. An increase in the growth of the tomato seedlings was observed, along with a greater number of compound leaves, and the fresh weight of the stem, the height, and the fresh biomass were also increased.
These results show that the lignin derived from nutshells has significant potential for use as a biostimulant; however, it is necessary to carry out further studies in a boarder spectrum of plant species and conditions.
Author Contributions
Conceptualization, J.M.-M. and A.B.-M.; methodology, J.A.D.-E. and F.J.E.-M.; software, F.J.E.-M. and J.A.D.-E.; validation, A.A.-V., J.M.-M. and J.A.D.-E.; formal analysis, J.M.-M. and A.A.-V.; investigation, J.M.-M. and A.A.-V.; resources, A.B.-M.; data curation, J.M.-M.; writing—original draft preparation, J.M.-M.; writing—review and editing, J.M.-M., A.B.-M. and F.J.E.-M.; visualization, J.A.D.-E.; supervision, A.B.-M.; project administration, J.M.-M.; funding acquisition, A.B.-M. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by infrastructure of Antonio Narro Autonomous Agrarian University and Center for Research in Applied Chemistry.
Data Availability Statement
The original contributions presented in this study are included in the article. For further inquiries, please contact the corresponding authors.
Acknowledgments
To the technical staff of CIQA, Jesús Cepeda for their support in the characterization by SEM, Guadalupe Méndez and Myrna Salinas for the thermal characterization, Guadalupe Tellez for the characterization by FTIR, Maricela García for the characterization by NMR and Ricardo Mendoza for support in the sample preparation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 871, 426696. [Google Scholar] [CrossRef]
- Bajpai, S.; Shukla, P.S.; Prithiviraj, B.; Critchley, A.T.; Nivetha, N. Editorial: Development of next generation bio stimulants for sustainable agriculture. Front. Plant Sci. 2024, 15, 8–10. [Google Scholar] [CrossRef]
- Research, P. Biostimulants Market Size, Share, and Trends 2024 to 2034. Available online: https://www.precedenceresearch.com/biostimulants-market (accessed on 10 September 2024).
- Mahmud, M.; Shamim Hasan, M.; Riajul Islam Sardar, M.; Adnan Shafin, A.; Sohanur Rahman, M.; Mosaddek Hossen, M.; Md Hasan, C.S.; Islam Sardar, M.R.; Adnan Shafin, A.; Rahman, M.S.; et al. Brief Review on Applications of Lignin. J. Chem. Rev 2023, 5, 56–82. [Google Scholar]
- Abejón, R.; Pérez-Acebo, H.; Clavijo, L. Alternatives for chemical and biochemical lignin valorization: Hot topics from a bibliometric analysis of the research published during the 2000–2016 period. Processes 2018, 6, 98. [Google Scholar] [CrossRef]
- Ahmad, U.M.; Ji, N.; Li, H.; Wu, Q.; Song, C.; Liu, Q.; Ma, D.; Lu, X. Can lignin be transformed into agrochemicals? Recent advances in the agricultural applications of lignin. Ind. Crop. Prod. 2021, 170, 113646. [Google Scholar] [CrossRef]
- Lim, S.F.; Matu, S.U. Utilization of agro-wastes to produce biofertilizer. Int. J. Energy Environ. Eng. 2015, 6, 31–35. [Google Scholar] [CrossRef]
- Orona Castillo, I.; Sangerman-Jarquín, D.M.; Cervantes Vázquez, M.G.; Espinoza Arellano, J.d.J.; Núñez Moreno, J.H. producción y comercialización de nuez pecanera en México. Rev. Mex. Cienc. Agrícolas 2019, 10, 1797–1808. [Google Scholar] [CrossRef]
- Suarez-Jacobo, A.; Obregon, E.; Urzua, E.; García-Fajardo, J.A. Retos y Oportunidades para el Aprovechamiento de la Nuez Pecanera en México; Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco (CIATEJ): Guadalajara, México, 2016. [Google Scholar]
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef]
- de Prá Andrade, M.; Piazza, D.; Poletto, M. Pecan nutshell: Morphological, chemical and thermal characterization. J. Mater. Res. Technol. 2021, 13, 2229–2238. [Google Scholar] [CrossRef]
- Liao, J.J.; Latif, N.H.A.; Trache, D.; Brosse, N.; Hussin, M.H. Current advancement on the isolation, characterization and application of lignin. Int. J. Biol. Macromol. 2020, 162, 985–1024. [Google Scholar] [CrossRef]
- Chio, C.; Sain, M.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Sturgeon, M.R.; Kim, S.; Lawrence, K.; Paton, R.S.; Chmely, S.C.; Nimlos, M.; Foust, T.D.; Beckham, G.T. A Mechanistic investigation of acid-catalyzed cleavage of aryl-ether linkages: Implications for lignin depolymerization in acidic environments. ACS Sustain. Chem. Eng. 2014, 2, 472–485. [Google Scholar] [CrossRef]
- Hong, S.; Shen, X.J.; Pang, B.; Xue, Z.; Cao, X.F.; Wen, J.L.; Sun, Z.H.; Lam, S.S.; Yuan, T.Q.; Sun, R.C. In-depth interpretation of the structural changes of lignin and formation of diketones during acidic deep eutectic solvent pretreatment. Green Chem. 2020, 22, 1851–1858. [Google Scholar] [CrossRef]
- Díez, D.; Urueña, A.; Piñero, R.; Barrio, A.; Tamminen, T. Determination of hemicellulose, cellulose, and Lignin Content in Different Types of Biomasses by Thermogravimetric Analysis and Pseudocomponent Kinetic Model. Processes 2020, 8, 1048. [Google Scholar] [CrossRef]
- Xu, F.; Sun, J.X.; Sun, R.; Fowler, P.; Baird, M.S. Comparative study of organosolv lignins from wheat straw. Ind. Crop. Prod. 2006, 23, 180–193. [Google Scholar] [CrossRef]
- Florian, T.D.M.; Villani, N.; Aguedo, M.; Jacquet, N.; Thomas, H.G.; Gerin, P.; Magali, D.; Richel, A. Chemical composition analysis and structural features of banana rachis lignin extracted by two organosolv methods. Ind. Crop. Prod. 2019, 132, 269–274. [Google Scholar] [CrossRef]
- Del Buono, D.; Luzi, F.; Puglia, D. Lignin nanoparticles: A promising tool to improve maize physiological, biochemical, and chemical traits. Nanomaterials 2021, 11, 846. [Google Scholar] [CrossRef]
- Pe, J.A.; Mun, J.S.; Mun, S.P. Thermal Characterization of Kraft Lignin Prepared from Mixed Hardwoods. BioResources 2023, 18, 926–936. [Google Scholar] [CrossRef]
- Köhnke, J.; Gierlinger, N.; Mateu, B.P.; Unterweger, C.; Solt, P.; Mahler, A.K.; Schwaiger, E.; Liebner, F.; Gindl-Altmutter, W. Comparison of four technical lignins as a resource for electrically conductive carbon particles. BioResources 2019, 14, 1091–1109. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Abdel-Fatah, S.M. Development and functionalization of magnetic nanoparticles as powerful and green catalysts for organic synthesis. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 55–67. [Google Scholar] [CrossRef]
- Zhou, N.; Thilakarathna, W.P.D.W.; He, Q.S. A Review: Depolymerization of Lignin to Generate High-Value Bio-Products: Opportunities, Challenges, and Prospects. Front. Energy Res. 2022, 9, 758744. [Google Scholar] [CrossRef]
- Hilbig, J.; Alves, V.R.; Müller, C.M.O.; Micke, G.A.; Vitali, L.; Pedrosa, R.C.; Block, J.M. Ultrasonic-assisted extraction combined with sample preparation and analysis using LC-ESI-MS/MS allowed the identification of 24 new phenolic compounds in pecan nut shell [Carya illinoinensis (Wangenh) C. Koch] extracts. Food Res. Int. 2018, 106, 549–557. [Google Scholar] [CrossRef]
- Jardim, J.M.; Hart, P.W.; Lucia, L.; Jameel, H. Insights into the potential of hardwood kraft lignin to be a green platform material for emergence of the biorefinery. Polymers 2020, 12, 1795. [Google Scholar] [CrossRef]
- Boarino, A.; Klok, H.A. Opportunities and Challenges for Lignin Valorization in Food Packaging, Antimicrobial, and Agricultural Applications. Biomacromolecules 2022, 24, 1065–1077. [Google Scholar] [CrossRef]
- Ruwoldt, J. A Critical Review of the Physicochemical Properties of Lignosulfonates: Chemical Structure and Behavior in Aqueous Solution, at Surfaces and Interfaces. Surfaces 2020, 3, 622–648. [Google Scholar] [CrossRef]
- Vasile, C.; Baican, M. Lignins as Promising Renewable Biopolymers and Bioactive Compounds for High-Performance Materials. Polymers 2023, 15, 3177. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mosier, N.; Ladisch, M. Valorization of Lignin from Aqueous-Based Lignocellulosic Biorefineries. In Trends in Biotechnology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–15. [Google Scholar] [CrossRef]
- Eraghi Kazzaz, A.; Hosseinpour Feizi, Z.; Fatehi, P. Grafting strategies for hydroxy groups of lignin for producing materials. Green Chem. 2019, 21, 5714–5752. [Google Scholar] [CrossRef]
- Da Cunha, K.P.V.; Do Nascimento, C.W.A. Silicon effects on metal tolerance and structural changes in Maize (Zea mays L.) grown on a cadmium and zinc enriched soil. Water. Air. Soil Pollut. 2009, 197, 323–330. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Gebremikael, M.; Vandendaele, R.; Alarcon, M.; Torregrosa, R.; De Neve, S. The effect of lignin application on plant growth and soil biological quality. EGU Gen. Assem. 2020, 2020, EGU2020-19535. [Google Scholar]
- Kevers, C.; Soteras, G.; Baccou, J.C.; Gaspar, T. Lignosulfonates: Novel promoting additives for plant tissue cultures. Vitr. Cell. Dev. Biol. Plant 1999, 35, 413–416. [Google Scholar] [CrossRef]
- Docquier, S.; Kevers, C.; Lambé, P.; Gaspar, T.; Dommes, J. Beneficial use of lignosulfonates in in vitro plant cultures: Stimulation of growth, of multiplication and of rooting. Plant Cell Tissue Organ Cult. 2007, 90, 285–291. [Google Scholar] [CrossRef]
- Tarrés, Q.; Aguado, R.; Domínguez-Robles, J.; Larrañeta, E.; Delgado-Aguilar, M. Valorization of Kraft Lignin from Black Liquor in the Production of Composite Materials with Poly(caprolactone) and Natural Stone Groundwood Fibers. Polymers 2022, 14, 5178. [Google Scholar] [CrossRef]
- Savy, D.; Cozzolino, V.; Nebbioso, A.; Drosos, M.; Nuzzo, A.; Mazzei, P.; Piccolo, A. Humic-like bioactivity on emergence and early growth of maize (Zea mays L.) of water-soluble lignins isolated from biomass for energy. Plant Soil 2016, 402, 221–233. [Google Scholar] [CrossRef]
- Kok, A.D.X.; Wan Abdullah, W.M.A.N.; Tang, C.N.; Low, L.Y.; Yuswan, M.H.; Ong-Abdullah, J.; Tan, N.P.; Lai, K.S. Sodium lignosulfonate improves shoot growth of Oryza sativa via enhancement of photosynthetic activity and reduced accumulation of reactive oxygen species. Sci. Rep. 2021, 11, 13226. [Google Scholar] [CrossRef]
- Savy, D.; Cozzolino, V. Novel fertilising products from lignin and its derivatives to enhance plant development and increase the sustainability of crop production. J. Clean. Prod. 2022, 366, 132832. [Google Scholar] [CrossRef]
- Ertani, A.; Francioso, O.; Nardi, S. Mini review: Fruit residues as plant biostimulants for bio-based product recovery. AIMS Agric. Food 2017, 2, 251–257. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).