Microbial Biostimulants and Seaweed Extract Synergistically Influence Seedling Growth and Morphology of Three Onion Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Cultivation
2.2. Greenhouse Temperature and Light Conditions
2.3. Biostimulants Application Treatments
2.4. Data Collection
2.5. Experiment Design and Statistical Analysis
3. Results and Discussion
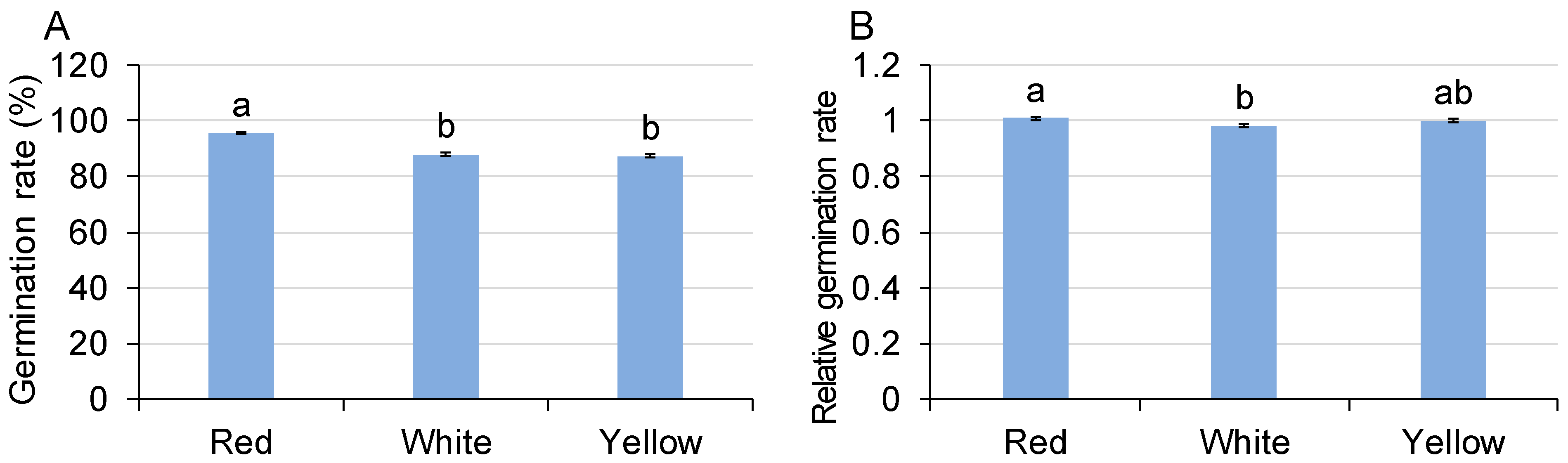
3.1. Germination
3.2. Shoot Morphology
3.3. Biomass
3.4. Root Morphology
3.5. Synergistic Effects of Microbial Biostimulants and Kelpak Seaweed Extract
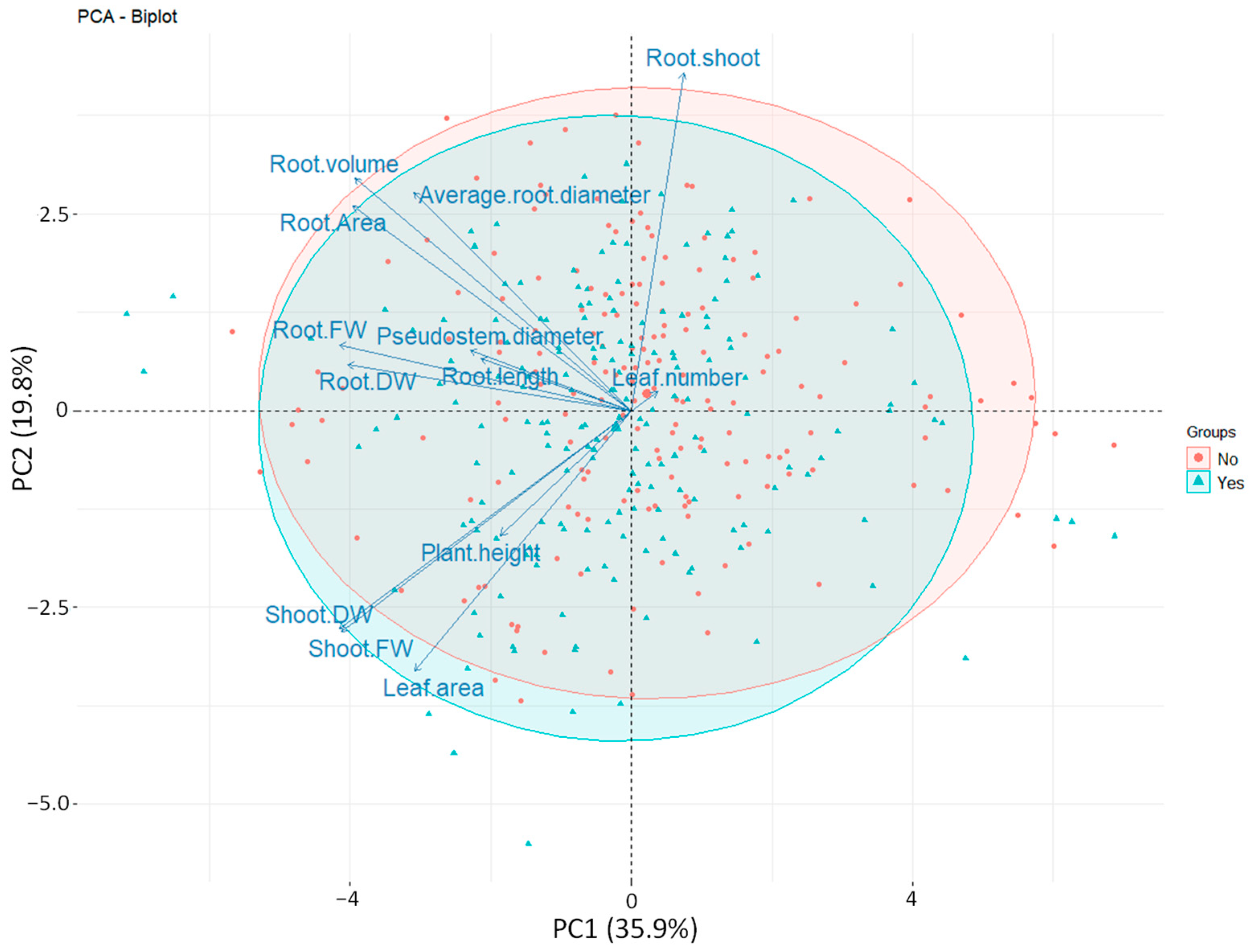
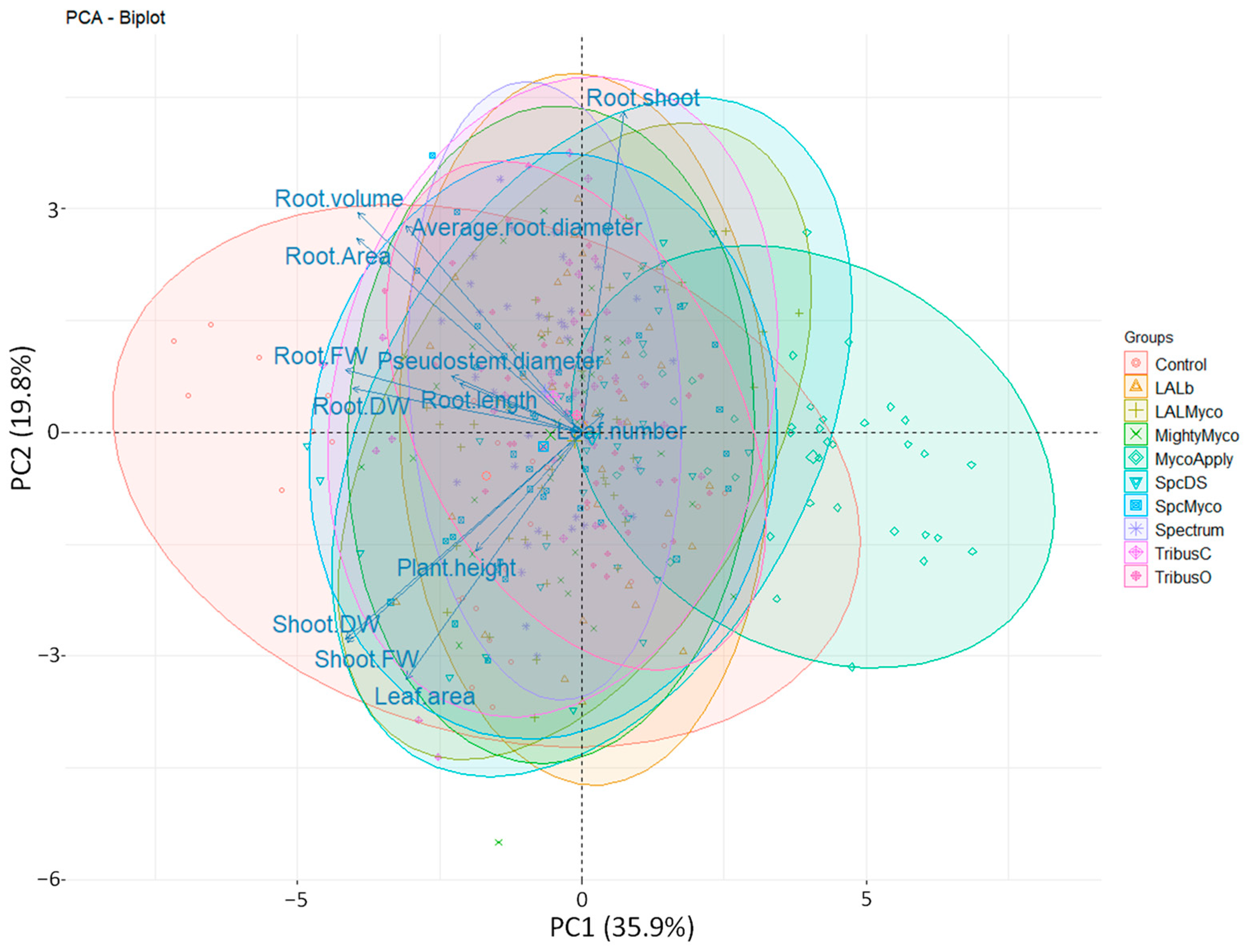
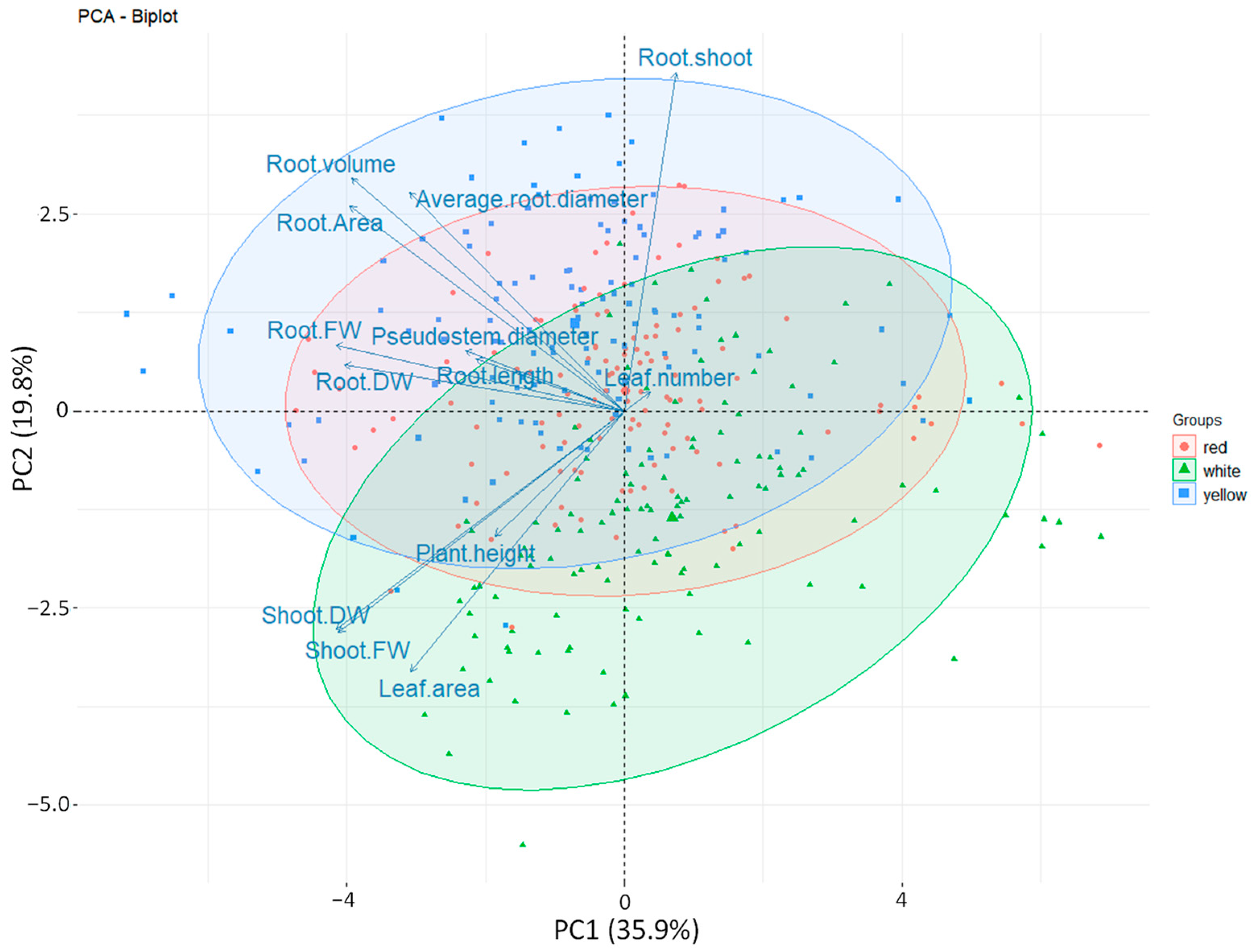
3.6. Principal Component Analysis (PCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kantor, L.; Blazejczyk, A. Food Availability (Per capita) Data System. Available online: https://www.ers.usda.gov/data-products/food-availability-per-capita-data-system/ (accessed on 7 May 2023).
- Sekara, A.; Pokluda, R.; Del Vacchio, L.; Somma, S.; Caruso, G. Interactions among Genotype, Environment and Agronomic Practices on Production and Quality of Storage Onion (Allium cepa L.)—A Review. Hortic. Sci. 2017, 44, 21–42. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration-Guidelines for Computing Crop Water Requirements-FAO Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998; Volume 300, p. D05109. [Google Scholar]
- Gravel, V.; Dorais, M.; Ménard, C. Organic Fertilization and Its Effect on Development of Sweet Pepper Transplants. HortScience 2012, 47, 198–204. [Google Scholar] [CrossRef]
- Qin, K.; Leskovar, D.I. Humic Substances Improve Vegetable Seedling Quality and Post-transplant Yield Performance under Stress Conditions. Agriculture 2020, 10, 254. [Google Scholar] [CrossRef]
- Leskovar, D.I.; Cantliffe, D.J. Comparison of Plant Establishment Method, Transplant, or Direct Seeding on Growth and Yield of Bell Pepper. J. Am. Soc. Hortic. Sci. 1993, 118, 17–22. [Google Scholar] [CrossRef]
- Ronga, D.; Vitti, A.; Zaccardelli, M.; Pane, C.; Caradonia, F.; Cardarelli, M.; Colla, G.; Rouphael, Y. Root Zone Management for Improving Seedling Quality of Organically Produced Horticultural Crops. Agronomy 2021, 11, 630. [Google Scholar] [CrossRef]
- Leszczynski, R.; Braccini, A.D.L.; Albrecht, L.P.; Scapim, C.A.; Piccinin, G.G.; Dan, L.G.D.M. Influence of Bio-Regulators on the Seed Germination and Seedling Growth of Onion Cultivars. Acta Sci. Agron. 2012, 34, 187–192. [Google Scholar] [CrossRef]
- Kȩpczyńska, E.; Piȩkna-Grochala, J.; Kȩpczynski, J. Effects of Matriconditioning on Onion Seed Germination, Seedling Emergence and Associated Physical and Metabolic Events. Plant Growth Regul. 2003, 41, 269–278. [Google Scholar] [CrossRef]
- Bolsunovsky, A.Y.; Dementyev, D.V.; Trofimova, E.A.; Iniatkina, E.M.; Kladko, Y.V.; Petrichenkov, M.V. Cytogenetic Effects of γ-Radiation in Onion (Allium cepa L.) Seedlings. Dokl. Biochem. Biophys. 2018, 481, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Brar, N.S.; Kaushik, P.; Dudi, B.S. Effect of Seed Priming Treatment on the Physiological Quality of Naturally Aged Onion (Allium cepa L.) Seeds. Appl. Ecol. Environ. Res. 2020, 18, 849–862. [Google Scholar] [CrossRef]
- Parajuli, R.; Thoma, G.; Matlock, M.D. Environmental Sustainability of Fruit and Vegetable Production Supply Chains in the Face of Climate Change: A Review. Sci. Total Environ. 2019, 650, 2863–2879. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Mor, V.S.; Tokas, J.; Punia, H.; Malik, S.; Malik, K.; Sangwan, S.; Tomar, S.; Singh, P.; Singh, N.; et al. Biostimulant-Treated Seedlings under Sustainable Agriculture: A Global Perspective Facing Climate Change. Agronomy 2021, 11, 14. [Google Scholar] [CrossRef]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Petropoulos, S.A. Biostimulants Application: A Low Input Cropping Management Tool for Sustainable Farming of Vegetables. Biomolecules 2021, 11, 698. [Google Scholar] [CrossRef] [PubMed]
- Wehner, M. Connecting Extreme Weather Events to Climate Change. Phys. Today 2023, 76, 40–46. [Google Scholar] [CrossRef]
- Younes, N.A.; Anik, T.R.; Rahman, M.M.; Wardany, A.A.; Dawood, M.F.A.; Tran, L.S.P.; Abdel Latef, A.A.H.; Mostofa, M.G. Effects of Microbial Biostimulants (Trichoderma Album and Bacillus Megaterium) on Growth, Quality Attributes, and Yield of Onion under Field Conditions. Heliyon 2023, 9, e14203. [Google Scholar] [CrossRef] [PubMed]
- Vojnović, Đ.; Maksimović, I.; Tepić Horecki, A.; Žunić, D.; Adamović, B.; Milić, A.; Šumić, Z.; Sabadoš, V.; Ilin, Ž. Biostimulants Affect Differently Biomass and Antioxidant Status of Onion (Allium cepa) Depending on Production Method. Horticulturae 2023, 9, 1345. [Google Scholar] [CrossRef]
- Jiménez-Arias, D.; Morales-Sierra, S.; Borges, A.A.; Herrera, A.J.; Luis, J.C. New Biostimulants Screening Method for Crop Seedlings under Water Deficit Stress. Agronomy 2022, 12, 728. [Google Scholar] [CrossRef]
- Gemin, L.G.; Mógor, Á.F.; De Oliveira Amatussi, J.; Mógor, G. Microalgae Associated to Humic Acid as a Novel Biostimulant Improving Onion Growth and Yield. Sci. Hortic. 2019, 256, 108560. [Google Scholar] [CrossRef]
- Gupta, S.; Doležal, K.; Kulkarni, M.G.; Balázs, E.; Van Staden, J. Role of Non-Microbial Biostimulants in Regulation of Seed Germination and Seedling Establishment. Plant Growth Regul. 2022, 97, 271–313. [Google Scholar] [CrossRef]
- Quintero-Calderón, E.H.; Sánchez-Reinoso, A.D.; CháVez-Arias, C.C.; Garces-Varon, G.; Restrepo-Díaz, H. Rice Seedlings Showed a Higher Heat Tolerance through the Foliar Application of Biostimulants. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12120. [Google Scholar] [CrossRef]
- Schlemper, T.R.; Stürmer, S.L. On Farm Production of Arbuscular Mycorrhizal Fungi Inoculum Using Lignocellulosic Agrowastes. Mycorrhiza 2014, 24, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.H.; Eissenstat, D.M.; Drouillard, D.L. On the Relationship between a Plant’s Mycorrhizal Dependency and Rate of Vesicular-Arbuscular Mycorrhizal Colonization. Funct. Ecol. 1991, 5, 773–779. [Google Scholar] [CrossRef]
- Hung, S.H.W.; Huang, T.C.; Lai, Y.C.; Wu, I.C.; Liu, C.H.; Huarng, Y.F.; Hwang, H.H.; Chiang, E.P.I.; Kuo, C.H.; Huang, C.C. Endophytic Biostimulants for Smart Agriculture: Burkholderia Seminalis 869T2 Benefits Heading Leafy Vegetables In-Field Management in Taiwan. Agronomy 2023, 13, 967. [Google Scholar] [CrossRef]
- Abdelkader, M.; Voronina, L.; Puchkov, M.; Shcherbakova, N.; Pakina, E.; Zargar, M.; Lyashko, M. Seed Priming with Exogenous Amino Acids Improves Germination Rates and Enhances Photosynthetic Pigments of Onion Seedlings (Allium cepa L.). Horticulturae 2023, 9, 80. [Google Scholar] [CrossRef]
- Sobarzo-Bernal, O.; Gómez-Merino, F.C.; Alcántar-González, G.; Saucedo-Veloz, C.; Trejo-Téllez, L.I. Biostimulant Effects of Cerium on Seed Germination and Initial Growth of Tomato Seedlings. Agronomy 2021, 11, 1525. [Google Scholar] [CrossRef]
- Sultana, S.; Karim, R. Effects of Seedling Age and Potassium Fertilizer on Growth and Yield of Summer Onion. Asian J. Crop. Soil Sci. Plant Nutr. 2020, 4, 134–140. [Google Scholar] [CrossRef]
- Ghaafar Abdel, C.; Sameer Asaad, S.; Salh Mohammad, D. Minimum, Optimum, and Maximum Temperatures Required for Germination of Onion, Radish, Tomato, and Pepper. Int. J. Farming Allied Sci. 2016, 1, 25–45. [Google Scholar]
- Joshi, N.; Sawant, P. Response of Onion (Allium cepa L.) Seed Germination and Early Seedling Development to Salt Level. Int. J. Veg. Sci. 2012, 18, 3–19. [Google Scholar] [CrossRef]
- Muhie, S.; Memiş, N.; Özdamar, C.; Gökdaş, Z.; Demir, İ. Biostimulant Priming for Germination and Seedling Quality of Carrot Seeds under Drought, Salt and High Temperature Stress Conditions. Int. J. Agric. Environ. Food Sci. 2021, 5, 352–359. [Google Scholar] [CrossRef]
- Di Filippo-Herrera, D.A.; Muñoz-Ochoa, M.; Hernández-Herrera, R.M.; Hernández-Carmona, G. Biostimulant Activity of Individual and Blended Seaweed Extracts on the Germination and Growth of the Mung Bean. J. Appl. Phycol. 2019, 31, 2025–2037. [Google Scholar] [CrossRef]
- Lastochkina, O.; Garshina, D.; Ivanov, S.; Yuldashev, R.; Khafizova, R.; Allagulova, C.; Fedorova, K.; Avalbaev, A.; Maslennikova, D.; Bosacchi, M. Seed Priming with Endophytic Bacillus subtilis Modulates Physiological Responses of Two Different Triticum aestivum L. Cultivars under Drought Stress. Plants 2020, 9, 1810. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; Vetrano, F. Use of Microbial Biostimulants to Increase the Salinity Tolerance of Vegetable Transplants. Agronomy 2021, 11, 1143. [Google Scholar] [CrossRef]
- Zhang, Q.; Kong, Y.; Masabni, J.; Niu, G. Onion Peel Waste Has the Potential to Be Converted into a Useful Agricultural Product to Improve Vegetable Crop Growth. HortScience 2024, 59, 578–586. [Google Scholar] [CrossRef]
- Tarbell, T.J.; Koske, R.E. Evaluation of Commercial Arbuscular Mycorrhizal Inocula in a Sand/Peat Medium. Mycorrhiza 2007, 18, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y.; Xu, X. Competition between Roots and Microorganisms for Nitrogen: Mechanisms and Ecological Relevance. New Phytol. 2013, 198, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Pylak, M.; Oszust, K.; Frąc, M. Review Report on the Role of Bioproducts, Biopreparations, Biostimulants and Microbial Inoculants in Organic Production of Fruit. Rev. Environ. Sci. Biotechnol. 2019, 18, 597–616. [Google Scholar] [CrossRef]
- Szczepanek, M.; Wszelaczyńska, E.; Pobereżny, J.; Ochmian, I. Response of Onion (Allium cepa L.) to the Method of Seaweed Biostimulant Application. Acta Sci. Pol. Hortorum Cultus 2017, 16, 113–122. [Google Scholar]
- Godlewska, K.; Michalak, I.; Tuhy, Ł.; Chojnacka, K. Plant Growth Biostimulants Based on Different Methods of Seaweed Extraction with Water. Biomed Res. Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Mukherjee, A.; Patel, J.S. Seaweed Extract: Biostimulator of Plant Defense and Plant Productivity. Int. J. Environ. Sci. Technol. 2020, 17, 553–558. [Google Scholar] [CrossRef]
- Ngoroyemoto, N.; Kulkarni, M.G.; Stirk, W.A.; Gupta, S.; Finnie, J.F.; van Staden, J. Interactions Between Microorganisms and a Seaweed-Derived Biostimulant on the Growth and Biochemical Composition of Amaranthus hybridus L. Nat. Prod. Commun. 2020, 15, 1934578X20934228. [Google Scholar] [CrossRef]
- Mendez, A.; Martinez, S.; Leal, A.; Hernandez, A.; Garcia, J.; Sanchez, M. Synergism of Microorganisms and Seaweed Extract on Vegetative Growth, Yield and Quality of Cucumber Fruit. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 12888. [Google Scholar] [CrossRef]
- Sani, M.N.H.; Islam, M.N.; Uddain, J.; Chowdhury, M.S.N.; Subramaniam, S. Synergistic Effect of Microbial and Nonmicrobial Biostimulants on Growth, Yield, and Nutritional Quality of Organic Tomato. Crop Sci. 2020, 60, 2102–2114. [Google Scholar] [CrossRef]
- EL Boukhari, M.E.M.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in Seaweed Extract Based Biostimulants: Manufacturing Process and Beneficial Effect on Soil-Plant Systems. Plants 2020, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Wang, G.; Sui, J.; Liu, G.; Ma, F.; Bao, Z. Biostimulants Alleviate Temperature Stress in Tomato Seedlings. Sci. Hortic. 2022, 293, 110712. [Google Scholar] [CrossRef]
| Biostimulant Product | Abbreviation of Product | Major Ingredients | Manufacturer | 1st Application | 2nd Application |
|---|---|---|---|---|---|
| LALRISE Mycorrhizae | LALMyco | One species of mycorrhizal fungi | Lallemand, Montreal, Quebec, Canada | 4 g/L | 8 g/L |
| LALRISE Bacillus velezensis | LALb | One species of bacteria | Lallemand, Montreal, Quebec, Canada | 0.25 g/L | 0.5 g/L |
| Mighty Mycorrhizae | MightyMyco | Sixteen species of mycorrhizal fungi | Wildroot Organic, Marble Falls, TX, USA | 0.5 g/L | 1 g/L |
| MycoApply | MycoApply | Four species of mycorrhizal fungi | Mycorrhizal Applications, Grants Pass, OR, USA | 1.67 mL/L | 3.34 g/L |
| Spectrum DS | SpcDS | Twenty species of bacteria, for drought-stressed soils | Tainio Biology, Spokane, WA, USA | 0.5 g/L | 1 g/L |
| Spectrum Myco | SpcMyco | Nineteen species of bacteria, four species of mycorrhizal fungi | Tainio Biology, Spokane, WA, USA | 1.55 g/L | 3.1 g/L |
| Spectrum | Spctrum | Twenty species of bacteria | Tainio Biology, Spokane, WA, USA | 0.5 g/L | 1 g/L |
| Tribus Original | TribusO | Three species of bacteria | Impello Bioscience, Fort Collin, CO, USA | 0.26 mL/L | 0.52 mL/L |
| Tribus Continuum | TribusC | Four species of bacteria | Impello Bioscience, Fort Collin, CO, USA | 0.26 mL/L | 0.52 mL/L |
| Kelpak | Kelp | Extracted from kelp Ecklonia maxima | Kelp Products International, Cape Town, South Africa | 1% (v/v) | 1% (v/v) |
| Parameters | Micro | Kelp | CV | Micro × Kelp | Micro × CV | Kelp × CV | Micro × Kelp × CV | |
|---|---|---|---|---|---|---|---|---|
| Germination | Germination rate | NS | NS | *** | NS | NS | NS | NS |
| Relative germination rate | NS | NS | * | NS | NS | * | NS | |
| Shoot morphology | Leaf number | *** | NS | *** | ** | NS | NS | NS |
| Plant height | *** | *** | *** | *** | *** | *** | *** | |
| Pseudostem diameter | *** | NS | *** | *** | * | NS | * | |
| Leaf area | *** | *** | *** | *** | *** | *** | *** | |
| Biomass | Shoot fresh weight | *** | *** | NS | *** | ** | ** | *** |
| Shoot dry weight | *** | ** | ** | *** | ** | * | *** | |
| Root fresh weight | *** | NS | *** | * | NS | NS | ** | |
| Root dry weight | *** | NS | *** | ** | * | NS | *** | |
| Root/shoot ratio | *** | ** | *** | *** | NS | NS | NS | |
| Root morphology | Total length | *** | * | * | *** | NS | NS | *** |
| Projected area | *** | NS | *** | *** | NS | NS | *** | |
| Root diameter | *** | * | *** | ** | * | NS | ** | |
| Root volume | *** | NS | *** | *** | ** | NS | *** |
| Cultivar | Biostimulant | Leaf Number | Plant Height (cm) | Pseudostem Diameter (cm) | Leaf Area (cm2) |
|---|---|---|---|---|---|
| Red | Control | 3.05 ± 0.07 a | 25.56 ± 0.42 a | 0.49 ± 0.02 abc | 12.90 ± 0.33 a |
| LALMyco | 2.60 ± 0.11 b | 24.12 ± 0.62 ab | 0.53 ± 0.03 a | 11.34 ± 0.47 b | |
| LALb | 3.00 ± 0.00 a | 23.78 ± 0.31 bc | 0.43 ± 0.01 bcd | 10.65 ± 0.20 bc | |
| MightyMyco | 2.90 ± 0.07 a | 22.33 ± 0.35 cd | 0.43 ± 0.01 cd | 10.97 ± 0.32 bc | |
| MycoApply | 3.00 ± 0.00 a | 22.70 ± 0.31 bcd | 0.37 ± 0.01 d | 8.62 ± 0.21 d | |
| SpcDS | 3.00 ± 0.00 a | 23.11 ± 0.29 bc | 0.51 ± 0.02 ab | 10.66 ± 0.26 bc | |
| SpcMyco | 3.00 ± 0.00 a | 23.53 ± 0.37 bc | 0.50 ± 0.02 abc | 10.64 ± 0.39 bc | |
| Spctrum | 3.00 ± 0.00 a | 23.07 ± 0.19 bc | 0.53 ± 0.03 a | 11.37 ± 0.29 b | |
| TribusO | 2.90 ± 0.07 a | 21.24 ± 0.28 d | 0.50 ± 0.02 abc | 9.74 ± 0.26 cd | |
| TribusC | 2.90 ± 0.07 a | 23.48 ± 0.32 bc | 0.49 ± 0.02 abc | 10.73 ± 0.37 bc | |
| Mean | 2.94 ± 0.02 | 23.29 ± 0.14 | 0.48 ± 0.01 | 10.76 ± 0.12 | |
| White | Control | 2.85 ± 0.11 a | 29.50 ± 0.32 a | 0.56 ± 0.03 a | 14.48 ± 0.30 a |
| LALMyco | 2.20 ± 0.09 b | 23.30 ± 0.49 c | 0.53 ± 0.02 a | 11.18 ± 0.52 c | |
| LALb | 2.75 ± 0.12 a | 27.21 ± 0.52 b | 0.51 ± 0.01 ab | 12.96 ± 0.37 ab | |
| MightyMyco | 2.60 ± 0.11 ab | 23.71 ± 0.35 c | 0.50 ± 0.01 ab | 10.58 ± 0.25 cd | |
| MycoApply | 2.65 ± 0.11 ab | 23.16 ± 0.44 c | 0.42 ± 0.02 b | 9.28 ± 0.34 d | |
| SpcDS | 2.75 ± 0.10 a | 24.33 ± 0.39 c | 0.58 ± 0.02 a | 11.62 ± 0.35 bc | |
| SpcMyco | 2.75 ± 0.12 a | 23.12 ± 0.38 c | 0.57 ± 0.02 a | 10.76 ± 0.32 cd | |
| Spctrum | 2.65 ± 0.11 b | 24.80 ± 0.54 c | 0.58 ± 0.03 a | 11.72 ± 0.38 bc | |
| TribusO | 2.45 ± 0.15 ab | 24.74 ± 0.74 c | 0.53 ± 0.01 a | 10.51 ± 0.52 cd | |
| TribusC | 2.45 ± 0.11 ab | 24.01 ± 0.51 c | 0.53 ± 0.02 a | 11.25 ± 0.27 c | |
| Mean | 2.61 ± 0.04 | 24.79 ± 0.20 | 0.53 ± 0.01 | 11.43 ± 0.15 | |
| Yellow | Control | 2.80 ± 0.09 ab | 25.96 ± 0.42 b | 0.56 ± 0.02 a | 12.26 ± 0.53 ab |
| LALMyco | 2.40 ± 0.11 b | 25.00 ± 0.37 bc | 0.53 ± 0.01 ab | 10.22 ± 0.43 cd | |
| LALb | 2.65 ± 0.11 ab | 25.32 ± 0.43 bc | 0.57 ± 0.01 a | 13.36 ± 0.85 a | |
| MightyMyco | 2.55 ± 0.11 ab | 24.29 ± 0.32 bc | 0.54 ± 0.02 ab | 10.69 ± 0.27 bcd | |
| MycoApply | 2.90 ± 0.07 a | 25.08 ± 0.31 bc | 0.47 ± 0.02 b | 8.92 ± 0.25 d | |
| SpcDS | 2.80 ± 0.09 ab | 28.11 ± 0.36 a | 0.61 ± 0.02 a | 13.03 ± 0.32 a | |
| SpcMyco | 2.75 ± 0.10 ab | 25.42 ± 0.49 bc | 0.56 ± 0.03 ab | 10.89 ± 0.34 bc | |
| Spctrum | 2.65 ± 0.11 ab | 24.63 ± 0.46 bc | 0.53 ± 0.01 ab | 10.36 ± 0.22 cd | |
| TribusO | 2.55 ± 0.11 ab | 25.24 ± 0.33 bc | 0.55 ± 0.02 ab | 10.47 ± 0.24 bcd | |
| TribusC | 2.70 ± 0.11 ab | 23.74 ± 0.31 c | 0.58 ± 0.02 a | 9.54 ± 0.25 cd | |
| Mean | 2.68 ± 0.03 | 25.28 ± 0.14 | 0.55 ± 0.01 | 10.97 ± 0.16 | |
| Overall Mean | 2.74 ± 0.02 | 24.45 ± 0.10 | 0.52 ± 0.00 | 11.06 ± 0.08 | |
| Cultivar | Biostimulant | Leaf Number | Plant Height (cm) | Pseudostem Diameter (cm) | Leaf Area (cm2) |
|---|---|---|---|---|---|
| Red | Control | 3.00 ± 0.00 | 23.89 ± 0.41 ab | 0.48 ± 0.02 abc | 11.31 ± 0.31 bc |
| LALMyco | 3.00 ± 0.00 | 25.26 ± 0.47 a | 0.54 ± 0.02 ab | 11.92 ± 0.37 b | |
| LALb | 2.80 ± 0.09 | 23.07 ± 0.41 bc | 0.47 ± 0.02 bc | 10.30 ± 0.22 cd | |
| MightyMyco | 2.70 ± 0.11 | 23.98 ± 0.43 ab | 0.49 ± 0.01 abc | 11.65 ± 0.28 b | |
| MycoApply | 2.90 ± 0.07 | 24.56 ± 0.29 ab | 0.43 ± 0.01 c | 11.16 ± 0.32 bc | |
| SpcDS | 2.90 ± 0.07 | 22.13 ± 0.22 c | 0.48 ± 0.02 abc | 9.05 ± 0.19 d | |
| SpcMyco | 2.95 ± 0.09 | 25.32 ± 0.28 a | 0.57 ± 0.03 a | 13.34 ± 0.36 a | |
| Spctrum | 3.00 ± 0.00 | 23.16 ± 0.20 bc | 0.51 ± 0.02 abc | 11.51 ± 0.28 bc | |
| TribusO | 2.85 ± 0.08 | 25.20 ± 0.30 a | 0.53 ± 0.03 ab | 11.95 ± 0.25 b | |
| TribusC | 2.90 ± 0.07 | 23.80 ± 0.35 ab | 0.48 ± 0.02 abc | 11.74 ± 0.35 b | |
| Mean | 2.90 ± 0.02 | 24.04 ± 0.13 | 0.50 ± 0.01 | 11.39 ± 0.12 | |
| White | Control | 2.65 ± 0.11 | 25.55 ± 0.48 bc | 0.51 ± 0.02 abc | 11.14 ± 0.39 efg |
| LALMyco | 2.50 ± 0.11 | 27.14 ± 0.55 abc | 0.58 ± 0.02 a | 14.27 ± 0.44 ab | |
| LALb | 2.55 ± 0.11 | 24.61 ± 0.69 c | 0.49 ± 0.01 bc | 10.65 ± 0.28 fg | |
| MightyMyco | 2.60 ± 0.11 | 29.10 ± 0.56 a | 0.52 ± 0.01 abc | 14.94 ± 0.50 a | |
| MycoApply | 2.60 ± 0.13 | 24.84 ± 0.51 c | 0.46 ± 0.01 c | 10.23 ± 0.37 g | |
| SpcDS | 2.70 ± 0.11 | 25.78 ± 0.43 bc | 0.53 ± 0.02 abc | 12.26 ± 0.31 cdef | |
| SpcMyco | 2.45 ± 0.11 | 26.88 ± 0.47 abc | 0.59 ± 0.02 a | 13.06 ± 0.28 bcd | |
| Spctrum | 2.85 ± 0.08 | 27.18 ± 0.47 abc | 0.56 ± 0.02 ab | 12.68 ± 0.38 bcde | |
| TribusO | 2.40 ± 0.11 | 26.73 ± 0.51 abc | 0.53 ± 0.02 abc | 11.93 ± 0.23 defg | |
| TribusC | 2.85 ± 0.15 | 28.08 ± 0.99 ab | 0.56 ± 0.02 ab | 13.95 ± 0.74 abc | |
| Mean | 2.62 ± 0.04 | 26.59 ± 0.20 | 0.53 ± 0.01 | 12.51 ± 0.17 | |
| Yellow | Control | 2.65 ± 0.11 ab | 27.91 ± 0.38 a | 0.58 ± 0.02 ab | 12.71 ± 0.45 a |
| LALMyco | 2.50 ± 0.11 ab | 26.30 ± 0.35 b | 0.60 ± 0.02 a | 11.54 ± 0.47 abc | |
| LALb | 2.65 ± 0.11 ab | 23.80 ± 0.33 de | 0.58 ± 0.02 ab | 10.36 ± 0.33 bcde | |
| MightyMyco | 2.75 ± 0.10 ab | 24.97 ± 0.35 bcd | 0.48 ± 0.01 c | 10.23 ± 0.30 bcde | |
| MycoApply | 2.80 ± 0.09 ab | 25.48 ± 0.36 bc | 0.48 ± 0.02 c | 10.16 ± 0.47 cde | |
| SpcDS | 2.95 ± 0.05 a | 23.27 ± 0.28 e | 0.50 ± 0.01 bc | 9.10 ± 0.21 e | |
| SpcMyco | 2.80 ± 0.09 ab | 26.08 ± 0.37 b | 0.57 ± 0.02 ab | 11.78 ± 0.33 ab | |
| Spctrum | 2.60 ± 0.11 ab | 24.32 ± 0.30 cde | 0.58 ± 0.02 ab | 9.78 ± 0.26 de | |
| TribusO | 2.45 ± 0.11 b | 26.19 ± 0.32 b | 0.60 ± 0.02 a | 11.01 ± 0.29 bcd | |
| TribusC | 2.70 ± 0.11 ab | 24.94 ± 0.40 bcd | 0.54 ± 0.02 abc | 11.17 ± 0.36 abcd | |
| Mean | 2.69 ± 0.03 | 25.32 ± 0.14 | 0.55 ± 0.01 | 10.78 ± 0.13 | |
| Overall mean | 2.73 ± 0.02 | 25.32 ± 0.10 | 0.53 ± 0.00 | 11.56 ± 0.09 | |
| Cultivar | Biostimulant | Shoot FW (g) | Shoot DW (mg) | Root FW (g) | Root DW (mg) | Root/Shoot |
|---|---|---|---|---|---|---|
| Red | Control | 1.25 ± 0.04 a | 79.06 ± 3.02 a | 0.40 ± 0.02 a | 20.14 ± 0.69 ab | 25.56 ± 0.82 |
| LALMyco | 1.01 ± 0.07 b | 73.98 ± 6.52 a | 0.39 ± 0.02 ab | 21.06 ± 0.94 a | 29.10 ± 1.55 | |
| LALb | 0.97 ± 0.02 b | 70.02 ± 1.66 a | 0.35 ± 0.00 ab | 18.68 ± 0.63 ab | 26.66 ± 0.54 | |
| MightyMyco | 0.99 ± 0.02 b | 69.83 ± 2.45 a | 0.35 ± 0.01 ab | 18.18 ± 0.49 ab | 26.12 ± 0.72 | |
| MycoApply | 0.75 ± 0.02 c | 49.11 ± 1.67 b | 0.27 ± 0.01 c | 14.22 ± 0.44 c | 29.00 ± 0.54 | |
| SpcDS | 1.03 ± 0.02 b | 66.50 ± 1.68 a | 0.35 ± 0.01 ab | 18.04 ± 0.50 b | 27.14 ± 0.53 | |
| SpcMyco | 0.99 ± 0.04 b | 68.63 ± 3.85 a | 0.34 ± 0.02 b | 19.07 ± 0.46 ab | 28.11 ± 1.25 | |
| Spctrum | 1.08 ± 0.01 b | 71.27 ± 1.31 a | 0.37 ± 0.01 ab | 20.35 ± 0.68 ab | 28.54 ± 0.70 | |
| TribusO | 0.96 ± 0.05 b | 65.80 ± 2.07 a | 0.39 ± 0.01 ab | 19.50 ± 0.26 ab | 29.76 ± 0.92 | |
| TribusC | 0.98 ± 0.03 b | 71.55 ± 1.77 a | 0.37 ± 0.01 ab | 19.91 ± 0.87 ab | 27.86 ± 1.17 | |
| Mean | 1.00 ± 0.02 | 68.57 ± 1.30 | 0.36 ± 0.01 | 18.92 ± 0.30 | 27.79 ± 0.32 | |
| White | Control | 1.25 ± 0.05 a | 82.52 ± 1.37 a | 0.36 ± 0.01 a | 17.30 ± 0.51 a | 20.95 ± 0.35 b |
| LALMyco | 0.89 ± 0.08 bc | 62.30 ± 7.20 bc | 0.32 ± 0.03 a | 16.22 ± 0.96 a | 26.91 ± 1.68 a | |
| LALb | 1.10 ± 0.05 ab | 77.22 ± 5.61 ab | 0.32 ± 0.02 a | 17.40 ± 1.10 a | 22.71 ± 0.94 ab | |
| MightyMyco | 0.93 ± 0.02 bc | 64.97 ± 1.82 abc | 0.29 ± 0.01 ab | 15.49 ± 0.20 a | 23.90 ± 0.48 ab | |
| MycoApply | 0.76 ± 0.02 c | 46.99 ± 2.56 c | 0.22 ± 0.01 b | 11.39 ± 0.68 b | 24.32 ± 0.95 ab | |
| SpcDS | 1.05 ± 0.05 ab | 68.24 ± 2.75 ab | 0.30 ± 0.02 ab | 15.95 ± 0.52 a | 23.47 ± 0.78 ab | |
| SpcMyco | 0.99 ± 0.04 bc | 68.01 ± 4.12 ab | 0.31 ± 0.01 ab | 17.24 ± 0.68 a | 25.57 ± 0.88 ab | |
| Spctrum | 1.04 ± 0.09 ab | 69.20 ± 5.18 ab | 0.34 ± 0.03 a | 18.34 ± 1.36 a | 26.66 ± 1.22 a | |
| TribusO | 0.94 ± 0.05 bc | 64.98 ± 1.82 abc | 0.33 ± 0.01 a | 16.67 ± 0.73 a | 25.72 ± 1.26 ab | |
| TribusC | 0.96 ± 0.02 bc | 66.52 ± 1.92 ab | 0.34 ± 0.01 a | 17.86 ± 0.58 a | 26.99 ± 1.31 a | |
| Mean | 0.99 ± 0.02 | 67.09 ± 1.62 | 0.31 ± 0.01 | 16.38 ± 0.34 | 24.72 ± 0.39 | |
| Yellow | Control | 1.23 ± 0.09 ab | 71.97 ± 5.00 ab | 0.42 ± 0.03 a | 19.44 ± 0.74 a | 27.40 ± 1.28 |
| LALMyco | 0.90 ± 0.06 c | 62.13 ± 5.31 abc | 0.38 ± 0.03 ab | 18.95 ± 1.17 a | 30.89 ± 1.26 | |
| LALb | 1.08 ± 0.09 abc | 72.83 ± 4.75 ab | 0.40 ± 0.02 a | 20.02 ± 0.77 a | 27.80 ± 1.11 | |
| MightyMyco | 1.03 ± 0.06 abc | 71.28 ± 6.33 ab | 0.39 ± 0.02 a | 20.29 ± 1.26 a | 28.85 ± 0.97 | |
| MycoApply | 0.82 ± 0.03 c | 49.48 ± 3.53 c | 0.30 ± 0.02 b | 14.94 ± 0.67 b | 30.64 ± 1.64 | |
| SpcDS | 1.26 ± 0.10 a | 78.31 ± 5.22 a | 0.41 ± 0.01 a | 21.03 ± 0.54 a | 27.43 ± 1.91 | |
| SpcMyco | 1.05 ± 0.04 abc | 69.65 ± 1.98 ab | 0.42 ± 0.01 a | 20.79 ± 0.58 a | 29.89 ± 0.64 | |
| Spctrum | 0.95 ± 0.02 bc | 59.71 ± 1.43 abc | 0.38 ± 0.01 ab | 19.30 ± 0.69 a | 32.38 ± 1.14 | |
| TribusO | 1.00 ± 0.04 abc | 66.62 ± 3.02 abc | 0.41 ± 0.01 a | 19.65 ± 0.63 a | 29.72 ± 1.35 | |
| TribusC | 0.82 ± 0.02 c | 57.33 ± 1.50 bc | 0.37 ± 0.01 ab | 18.09 ± 0.25 ab | 31.68 ± 1.07 | |
| Mean | 1.01 ± 0.03 | 65.93 ± 1.62 | 0.39 ± 0.01 | 19.25 ± 0.31 | 29.67 ± 0.43 | |
| Overall mean | 1.00 ± 0.01 | 67.20 ± 0.88 | 0.35 ± 0.00 | 18.18 ± 0.21 | 27.39 ± 0.27 | |
| Cultivar | Biostimulant | Shoot FW (g) | Shoot DW (mg) | Root FW (g) | Root DW (mg) | Root/Shoot |
|---|---|---|---|---|---|---|
| Red | Control | 1.03 ± 0.02 abcd | 69.12 ± 2.36 bcd | 0.35 ± 0.01 abc | 18.07 ± 0.63 bc | 26.23 ± 0.90 ab |
| LALMyco | 1.11 ± 0.06 abc | 75.20 ± 2.97 abc | 0.35 ± 0.01 abc | 18.09 ± 0.39 bc | 24.17 ± 0.64 b | |
| LALb | 0.95 ± 0.02 bcd | 67.67 ± 2.04 bcd | 0.34 ± 0.01 bc | 17.74 ± 0.59 bc | 26.29 ± 0.95 ab | |
| MightyMyco | 1.16 ± 0.05 ab | 86.33 ± 4.82 a | 0.41 ± 0.03 a | 23.21 ± 1.94 a | 27.05 ± 2.14 ab | |
| MycoApply | 0.84 ± 0.04 d | 54.86 ± 3.07 d | 0.28 ± 0.01 c | 15.16 ± 0.81 c | 27.76 ± 1.17 ab | |
| SpcDS | 0.91 ± 0.04 cd | 62.16 ± 2.73 cd | 0.33 ± 0.01 abc | 18.18 ± 0.98 bc | 29.28 ± 1.23 a | |
| SpcMyco | 1.24 ± 0.07 a | 79.50 ± 4.43 ab | 0.39 ± 0.02 ab | 20.67 ± 1.19 ab | 26.04 ± 0.78 ab | |
| Spctrum | 1.08 ± 0.04 abc | 72.48 ± 3.91 abc | 0.39 ± 0.02 ab | 19.97 ± 0.86 ab | 27.68 ± 0.81 ab | |
| TribusO | 1.12 ± 0.04 abc | 75.53 ± 2.35 abc | 0.39 ± 0.01 ab | 20.11 ± 0.71 ab | 26.67 ± 0.84 ab | |
| TribusC | 1.15 ± 0.07 ab | 78.18 ± 3.35 ab | 0.38 ± 0.01 ab | 20.62 ± 0.67 ab | 26.45 ± 0.48 ab | |
| Mean | 1.06 ± 0.02 | 72.10 ± 1.48 | 0.36 ± 0.01 | 19.18 ± 0.39 | 26.76 ± 0.36 | |
| White | Control | 1.11 ± 0.07 ab | 71.98 ± 5.66 ab | 0.33 ± 0.02 abc | 17.05 ± 0.89 bc | 23.99 ± 0.98 |
| LALMyco | 1.13 ± 0.04 ab | 77.56 ± 3.17 ab | 0.32 ± 0.02 abc | 17.33 ± 0.44 bc | 22.44 ± 0.53 | |
| LALb | 0.91 ± 0.03 bc | 64.48 ± 2.81 bc | 0.30 ± 0.01 c | 15.75 ± 0.30 c | 24.70 ± 1.33 | |
| MightyMyco | 1.17 ± 0.07 ab | 78.29 ± 3.91 ab | 0.34 ± 0.01 abc | 18.16 ± 0.25 abc | 23.42 ± 0.97 | |
| MycoApply | 0.81 ± 0.03 c | 49.13 ± 2.50 c | 0.23 ± 0.01 d | 11.63 ± 0.34 d | 23.82 ± 0.59 | |
| SpcDS | 1.30 ± 0.06 a | 85.08 ± 3.48 a | 0.39 ± 0.01 a | 20.51 ± 0.59 a | 24.21 ± 0.74 | |
| SpcMyco | 1.24 ± 0.03 a | 83.01 ± 0.79 a | 0.37 ± 0.02 ab | 19.30 ± 0.43 ab | 23.27 ± 0.60 | |
| Spctrum | 1.17 ± 0.03 ab | 75.65 ± 3.46 ab | 0.32 ± 0.01 bc | 17.73 ± 0.62 bc | 23.57 ± 0.86 | |
| TribusO | 1.06 ± 0.03 abc | 71.10 ± 0.95 ab | 0.33 ± 0.02 abc | 16.63 ± 0.59 c | 23.40 ± 0.86 | |
| TribusC | 1.10 ± 0.12 ab | 72.03 ± 6.83 ab | 0.32 ± 0.01 abc | 16.89 ± 0.54 bc | 24.32 ± 1.95 | |
| Mean | 1.10 ± 0.02 | 72.83 ± 1.68 | 0.32 ± 0.01 | 17.10 ± 0.33 | 23.71 ± 0.31 | |
| Yellow | Control | 1.27 ± 0.05 a | 82.15 ± 4.60 a | 0.43 ± 0.02 a | 21.12 ± 0.72 a | 25.87 ± 0.63 c |
| LALMyco | 1.03 ± 0.04 bcd | 69.23 ± 2.77 ab | 0.39 ± 0.01 a | 19.22 ± 0.54 ab | 27.91 ± 1.01 bc | |
| LALb | 0.91 ± 0.04 cd | 61.70 ± 3.37 bcd | 0.37 ± 0.01 ab | 18.65 ± 0.45 ab | 30.50 ± 1.03 ab | |
| MightyMyco | 1.01 ± 0.06 bcd | 69.13 ± 4.12 ab | 0.39 ± 0.02 a | 20.05 ± 1.11 ab | 29.07 ± 0.59 abc | |
| MycoApply | 0.85 ± 0.03 d | 50.73 ± 2.79 d | 0.30 ± 0.01 b | 14.06 ± 0.50 c | 27.89 ± 0.82 bc | |
| SpcDS | 0.85 ± 0.02 d | 52.99 ± 1.36 cd | 0.36 ± 0.01 ab | 17.39 ± 0.28 b | 32.92 ± 0.95 a | |
| SpcMyco | 1.13 ± 0.03 ab | 71.69 ± 1.85 ab | 0.40 ± 0.02 a | 20.92 ± 1.10 a | 29.23 ± 1.51 abc | |
| Spctrum | 0.99 ± 0.03 bcd | 64.92 ± 1.23 bc | 0.39 ± 0.02 a | 20.06 ± 0.50 ab | 30.91 ± 0.70 ab | |
| TribusO | 1.06 ± 0.02 bc | 70.55 ± 2.45 ab | 0.43 ± 0.01 a | 20.59 ± 0.74 a | 29.23 ± 0.80 abc | |
| TribusC | 1.04 ± 0.05 bc | 68.53 ± 2.02 b | 0.39 ± 0.01 a | 19.93 ± 0.30 ab | 29.20 ± 0.92 abc | |
| Mean | 1.02 ± 0.02 | 66.16 ± 1.41 | 0.39 ± 0.01 | 19.20 ± 0.33 | 29.27 ± 0.36 | |
| Overall mean | 1.06 ± 0.01 | 70.36 ± 0.91 | 0.36 ± 0.00 | 18.49 ± 0.21 | 26.58 ± 0.26 | |
| Cultivar | Biostimulant | Length (cm) | Area (cm2) | AvgDiam (mm) | RootVolume (cm3) |
|---|---|---|---|---|---|
| Red | Control | 64.14 ± 1.82 a | 5.07 ± 0.19 a | 0.79 ± 0.02 abc | 0.32 ± 0.02 a |
| LALMyco | 55.83 ± 2.35 abc | 4.74 ± 0.16 ab | 0.86 ± 0.02 a | 0.32 ± 0.01 a | |
| LALb | 56.67 ± 1.40 abc | 4.36 ± 0.09 b | 0.77 ± 0.01 bc | 0.26 ± 0.01 b | |
| MightyMyco | 54.82 ± 2.08 bc | 4.37 ± 0.14 b | 0.80 ± 0.02 abc | 0.28 ± 0.01 ab | |
| MycoApply | 51.62 ± 1.54 c | 3.35 ± 0.12 c | 0.65 ± 0.02 d | 0.17 ± 0.01 c | |
| SpcDS | 56.53 ± 2.12 abc | 4.27 ± 0.15 b | 0.76 ± 0.02 bc | 0.26 ± 0.01 b | |
| SpcMyco | 52.98 ± 1.81 bc | 4.18 ± 0.09 b | 0.80 ± 0.02 abc | 0.26 ± 0.01 b | |
| Spctrum | 57.90 ± 1.94 abc | 4.59 ± 0.12 ab | 0.80 ± 0.02 abc | 0.29 ± 0.01 ab | |
| TribusO | 53.60 ± 2.03 bc | 4.38 ± 0.11 b | 0.83 ± 0.02 ab | 0.28 ± 0.01 ab | |
| TribusC | 61.52 ± 2.38 ab | 4.41 ± 0.11 b | 0.73 ± 0.03 cd | 0.25 ± 0.01 b | |
| Mean | 56.56 ± 0.66 | 4.37 ± 0.05 | 0.78 ± 0.01 | 0.27 ± 0.00 | |
| White | Control | 66.07 ± 1.97 a | 4.52 ± 0.13 a | 0.69 ± 0.02 ab | 0.25 ± 0.01 a |
| LALMyco | 56.15 ± 1.78 b | 4.21 ± 0.11 abc | 0.76 ± 0.02 a | 0.25 ± 0.01 a | |
| LALb | 55.41 ± 1.67 b | 4.04 ± 0.11 abc | 0.73 ± 0.02 ab | 0.23 ± 0.01 a | |
| MightyMyco | 54.61 ± 1.51 b | 3.93 ± 0.10 bc | 0.72 ± 0.02 ab | 0.22 ± 0.01 a | |
| MycoApply | 51.92 ± 1.79 b | 3.38 ± 0.12 d | 0.66 ± 0.02 b | 0.18 ± 0.01 b | |
| SpcDS | 54.04 ± 2.18 b | 3.84 ± 0.10 cd | 0.72 ± 0.02 ab | 0.22 ± 0.01 ab | |
| SpcMyco | 54.00 ± 1.37 b | 4.01 ± 0.11 abc | 0.74 ± 0.02 a | 0.24 ± 0.01 a | |
| Spctrum | 59.84 ± 2.38 ab | 4.42 ± 0.17 ab | 0.74 ± 0.02 a | 0.26 ± 0.01 a | |
| TribusO | 54.56 ± 1.66 b | 3.87 ± 0.12 cd | 0.71 ± 0.02 ab | 0.22 ± 0.01 ab | |
| TribusC | 56.60 ± 1.67 b | 4.10 ± 0.10 abc | 0.73 ± 0.01 ab | 0.23 ± 0.01 a | |
| Mean | 56.32 ± 0.62 | 4.03 ± 0.04 | 0.72 ± 0.01 | 0.23 ± 0.00 | |
| Yellow | Control | 62.22 ± 1.99 a | 4.98 ± 0.20 ab | 0.80 ± 0.01 abcd | 0.31 ± 0.02 ab |
| LALMyco | 57.06 ± 2.49 ab | 4.89 ± 0.14 abc | 0.87 ± 0.02 a | 0.33 ± 0.01 ab | |
| LALb | 57.05 ± 2.32 ab | 4.71 ± 0.18 abc | 0.83 ± 0.01 ab | 0.31 ± 0.01 abc | |
| MightyMyco | 60.58 ± 1.49 a | 5.16 ± 0.13 a | 0.85 ± 0.02 ab | 0.35 ± 0.01 a | |
| MycoApply | 51.74 ± 1.16 b | 3.74 ± 0.08 d | 0.73 ± 0.02 d | 0.21 ± 0.01 d | |
| SpcDS | 61.61 ± 2.01 a | 4.91 ± 0.15 abc | 0.80 ± 0.02 abcd | 0.31 ± 0.01 ab | |
| SpcMyco | 61.22 ± 1.46 a | 5.04 ± 0.14 ab | 0.83 ± 0.02 abc | 0.33 ± 0.02 ab | |
| Spctrum | 61.68 ± 1.55 a | 4.77 ± 0.11 abc | 0.78 ± 0.02 bcd | 0.29 ± 0.01 abc | |
| TribusO | 56.34 ± 1.62 ab | 4.44 ± 0.12 bc | 0.79 ± 0.02 abcd | 0.28 ± 0.01 bc | |
| TribusC | 58.06 ± 1.60 ab | 4.28 ± 0.10 cd | 0.74 ± 0.02 cd | 0.25 ± 0.01 cd | |
| Mean | 58.76 ± 0.60 | 4.69 ± 0.05 | 0.80 ± 0.01 | 0.30 ± 0.00 | |
| Total Mean | 57.21 ± 0.37 | 4.36 ± 0.03 | 0.77 ± 0.00 | 0.27 ± 0.00 | |
| Cultivar | Biostimulant | Length (cm) | Area (cm2) | AvgDiam (mm) | RootVolume (cm3) |
|---|---|---|---|---|---|
| Red | Control | 55.09 ± 1.47 ab | 4.48 ± 0.14 abc | 0.81 ± 0.02 ab | 0.29 ± 0.01 ab |
| LALMyco | 56.79 ± 1.50 ab | 4.46 ± 0.11 abc | 0.79 ± 0.02 ab | 0.28 ± 0.01 abc | |
| LALb | 52.93 ± 1.96 b | 4.12 ± 0.08 bc | 0.79 ± 0.02 ab | 0.26 ± 0.01 abc | |
| MightyMyco | 56.30 ± 1.79 ab | 4.42 ± 0.11 abc | 0.79 ± 0.02 ab | 0.27 ± 0.01 abc | |
| MycoApply | 55.07 ± 1.57 ab | 3.97 ± 0.11 c | 0.73 ± 0.02 b | 0.23 ± 0.01 c | |
| SpcDS | 52.89 ± 2.25 b | 4.08 ± 0.13 bc | 0.78 ± 0.02 ab | 0.25 ± 0.01 bc | |
| SpcMyco | 62.00 ± 2.00 a | 4.90 ± 0.14 a | 0.80 ± 0.02 ab | 0.31 ± 0.01 a | |
| Spctrum | 51.66 ± 1.73 b | 4.28 ± 0.12 bc | 0.83 ± 0.02 a | 0.28 ± 0.01 ab | |
| TribusO | 55.62 ± 1.93 ab | 4.54 ± 0.11 ab | 0.83 ± 0.03 a | 0.30 ± 0.01 ab | |
| TribusC | 57.96 ± 1.51 ab | 4.60 ± 0.14 ab | 0.80 ± 0.03 ab | 0.29 ± 0.02 ab | |
| Mean | 55.63 ± 0.59 | 4.39 ± 0.04 | 0.80 ± 0.01 | 0.28 ± 0.00 | |
| White | Control | 52.83 ± 1.22 bc | 3.96 ± 0.09 ab | 0.75 ± 0.02 ab | 0.24 ± 0.01 abc |
| LALMyco | 52.13 ± 1.66 c | 4.03 ± 0.11 ab | 0.78 ± 0.02 a | 0.25 ± 0.01 abc | |
| LALb | 54.30 ± 1.56 bc | 3.87 ± 0.08 b | 0.72 ± 0.01 ab | 0.22 ± 0.01 c | |
| MightyMyco | 58.87 ± 1.62 abc | 4.41 ± 0.09 a | 0.75 ± 0.01 ab | 0.26 ± 0.01 ab | |
| MycoApply | 51.69 ± 1.92 c | 3.18 ± 0.14 c | 0.62 ± 0.02 c | 0.16 ± 0.01 d | |
| SpcDS | 59.51 ± 1.39 ab | 4.38 ± 0.09 a | 0.74 ± 0.02 ab | 0.26 ± 0.01 abc | |
| SpcMyco | 56.94 ± 1.85 abc | 4.44 ± 0.14 a | 0.78 ± 0.02 a | 0.27 ± 0.01 a | |
| Spctrum | 58.25 ± 1.66 abc | 4.10 ± 0.14 ab | 0.70 ± 0.02 b | 0.23 ± 0.01 bc | |
| TribusO | 56.57 ± 1.42 abc | 4.06 ± 0.11 ab | 0.72 ± 0.02 ab | 0.23 ± 0.01 bc | |
| TribusC | 61.78 ± 1.53 a | 4.39 ± 0.11 a | 0.71 ± 0.01 ab | 0.25 ± 0.01 abc | |
| Mean | 56.29 ± 0.54 | 4.08 ± 0.04 | 0.73 ± 0.01 | 0.24 ± 0.00 | |
| Yellow | Control | 58.01 ± 1.86 | 5.26 ± 0.24 a | 0.91 ± 0.03 a | 0.38 ± 0.03 a |
| LALMyco | 59.45 ± 1.30 | 4.75 ± 0.10 ab | 0.80 ± 0.01 bc | 0.30 ± 0.01 b | |
| LALb | 52.88 ± 1.76 | 4.24 ± 0.11 bc | 0.81 ± 0.02 b | 0.27 ± 0.01 bc | |
| MightyMyco | 54.66 ± 1.82 | 4.26 ± 0.09 bc | 0.79 ± 0.02 bc | 0.26 ± 0.01 bc | |
| MycoApply | 55.56 ± 1.53 | 3.97 ± 0.10 c | 0.72 ± 0.02 c | 0.23 ± 0.01 c | |
| SpcDS | 52.85 ± 1.41 | 4.45 ± 0.12 bc | 0.85 ± 0.02 ab | 0.30 ± 0.01 b | |
| SpcMyco | 57.66 ± 1.59 | 4.59 ± 0.15 b | 0.80 ± 0.02 bc | 0.29 ± 0.01 b | |
| Spctrum | 54.56 ± 1.71 | 4.56 ± 0.14 bc | 0.84 ± 0.02 ab | 0.30 ± 0.01 b | |
| TribusO | 59.64 ± 2.05 | 4.85 ± 0.11 ab | 0.82 ± 0.02 ab | 0.31 ± 0.01 b | |
| TribusC | 59.98 ± 2.04 | 4.74 ± 0.14 ab | 0.80 ± 0.02 bc | 0.30 ± 0.01 b | |
| Mean | 56.53 ± 0.56 | 4.57 ± 0.05 | 0.81 ± 0.01 | 0.29 ± 0.00 | |
| Total mean | 56.15 ± 0.33 | 4.35 ± 0.03 | 0.78 ± 0.00 | 0.27 ± 0.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Masabni, J.; Niu, G. Microbial Biostimulants and Seaweed Extract Synergistically Influence Seedling Growth and Morphology of Three Onion Cultivars. Horticulturae 2024, 10, 800. https://doi.org/10.3390/horticulturae10080800
Zhang Q, Masabni J, Niu G. Microbial Biostimulants and Seaweed Extract Synergistically Influence Seedling Growth and Morphology of Three Onion Cultivars. Horticulturae. 2024; 10(8):800. https://doi.org/10.3390/horticulturae10080800
Chicago/Turabian StyleZhang, Qianwen, Joseph Masabni, and Genhua Niu. 2024. "Microbial Biostimulants and Seaweed Extract Synergistically Influence Seedling Growth and Morphology of Three Onion Cultivars" Horticulturae 10, no. 8: 800. https://doi.org/10.3390/horticulturae10080800
APA StyleZhang, Q., Masabni, J., & Niu, G. (2024). Microbial Biostimulants and Seaweed Extract Synergistically Influence Seedling Growth and Morphology of Three Onion Cultivars. Horticulturae, 10(8), 800. https://doi.org/10.3390/horticulturae10080800










