Functional and Proteomic Analyses of a Putative Carbamoyl Phosphate Synthase Large Subunit in Relation to Virulence, Arginine and Pyrimidine Biosynthesis, and Siderophore Production in Erwinia amylovora
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Selection of carBEa:Tn5 and Generation of the Complemented Strain
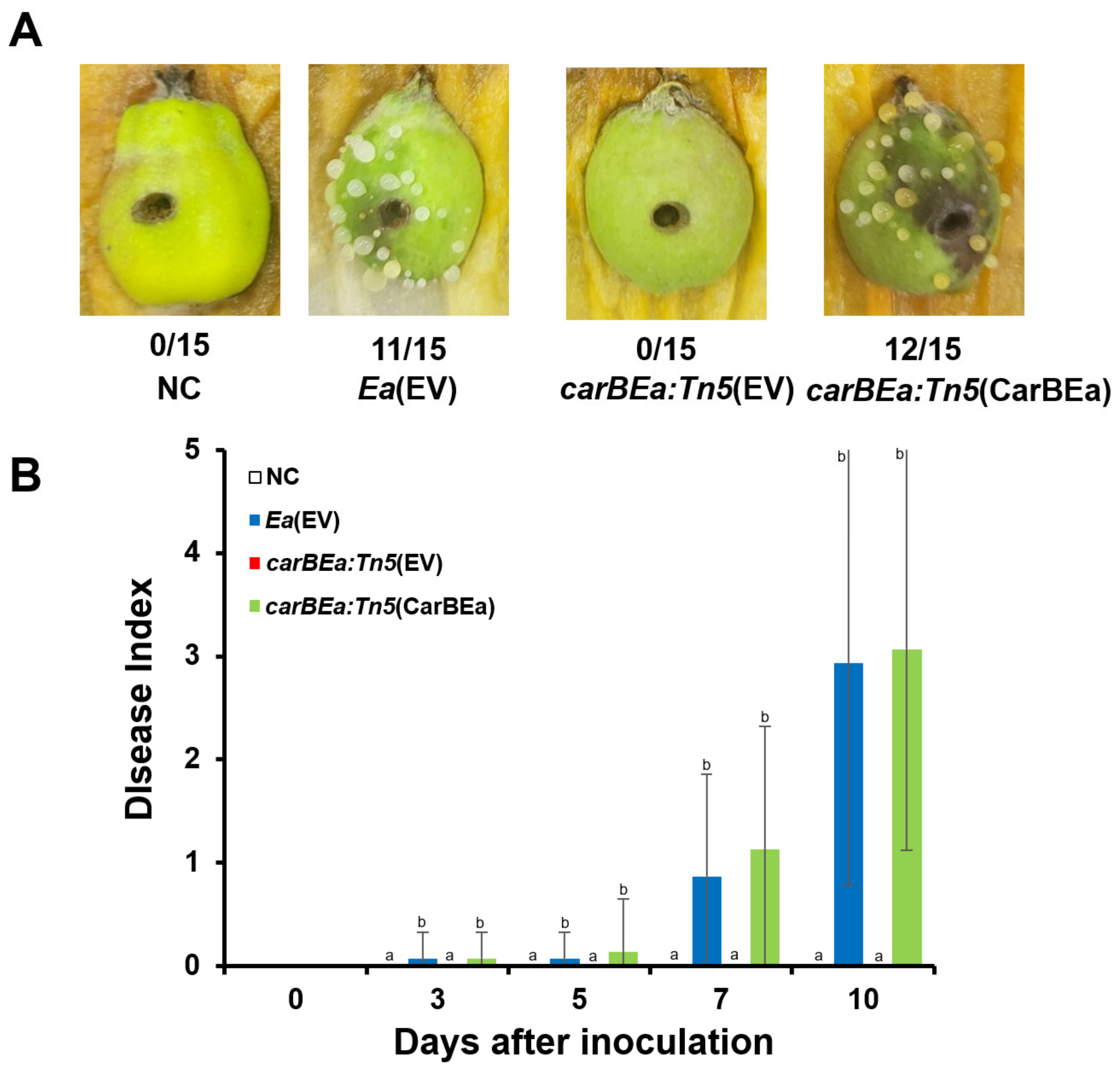
2.3. Pathogenicity Test
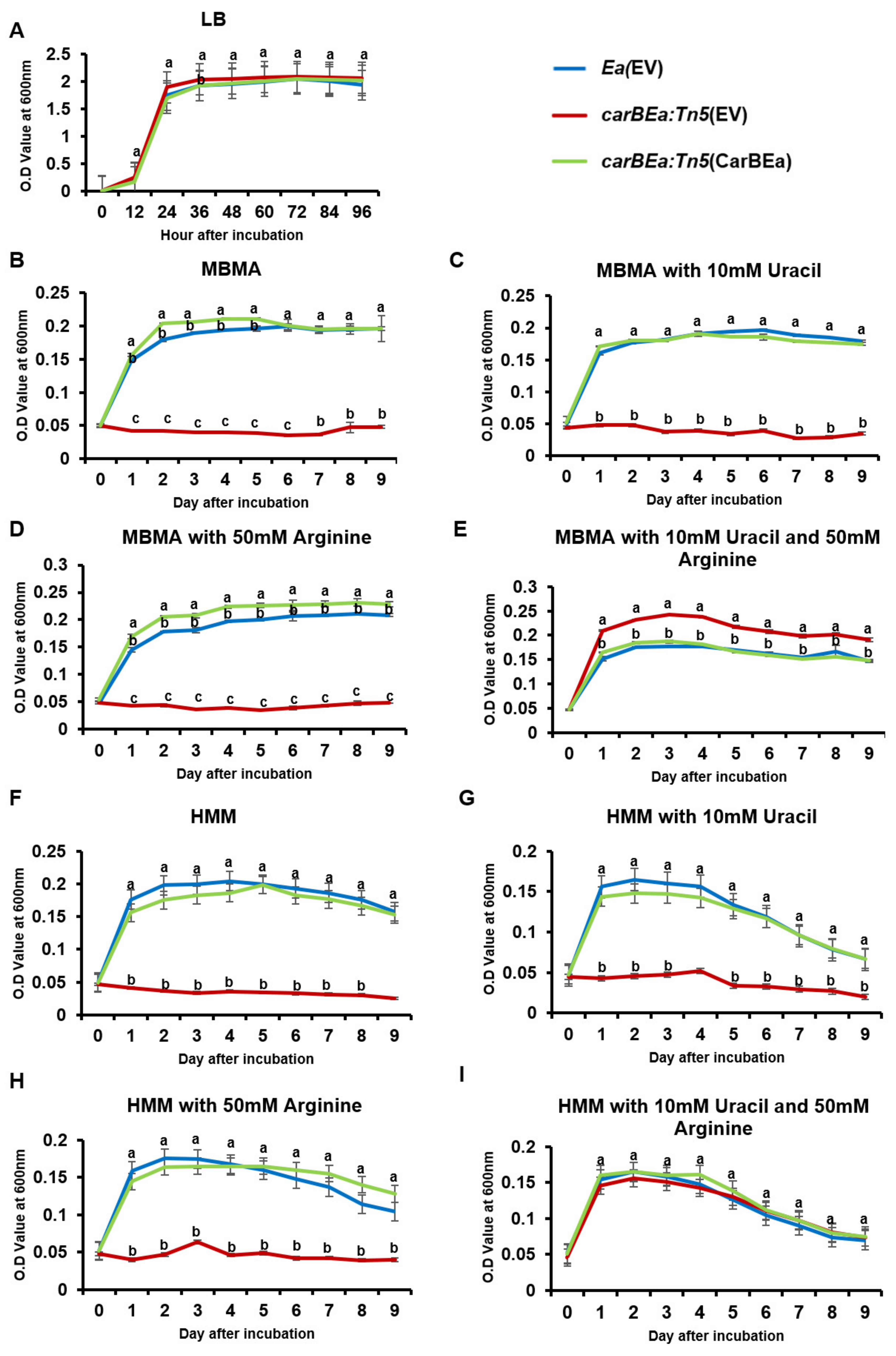
2.4. Growth and Auxotroph Assay
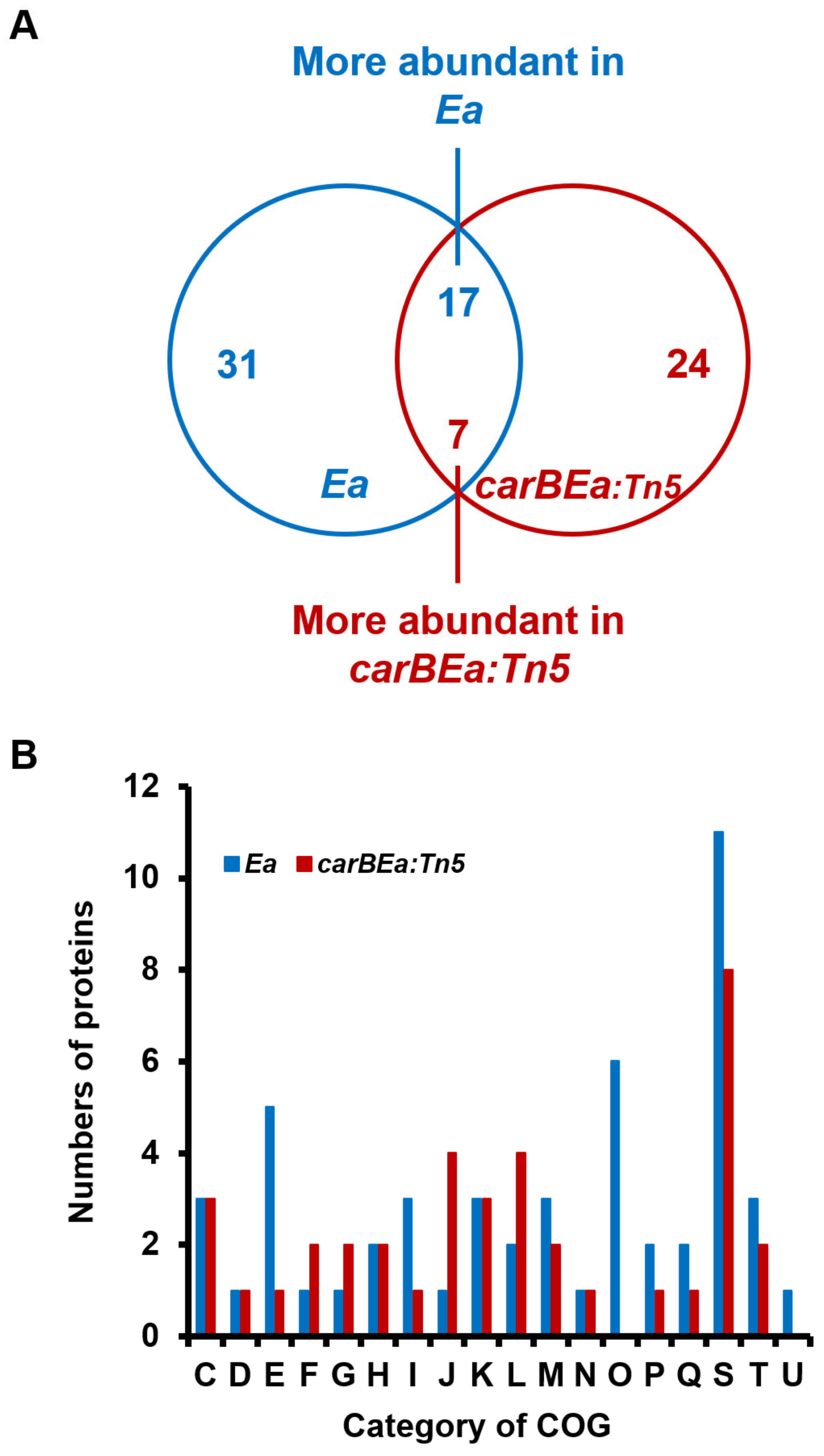
2.5. Proteomic Analysis
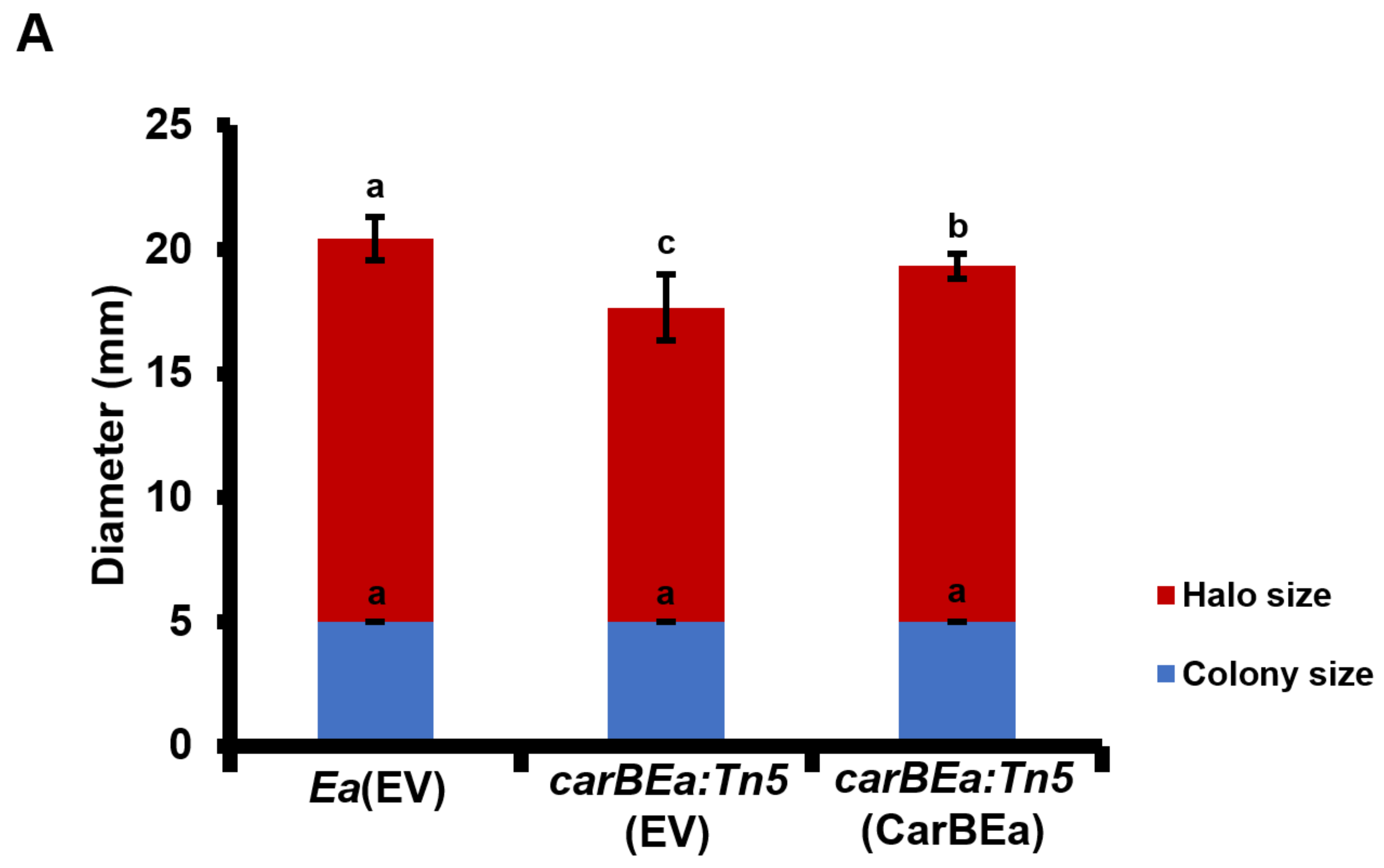
2.6. Siderophore Production Assay
2.7. Comparison of Amino Acid Sequences and In Silico 3D Modeling
2.8. Statistical Analysis
3. Results and Discussion
3.1. Identification of the carBEa:Tn5 Strain and Amino Acid Sequence Analysis of CarBEa
3.2. CarBEa Is Required for Virulence in Ea
3.3. CarBEa Is Crucial for the Biosynthesis of Arginine and Uracil
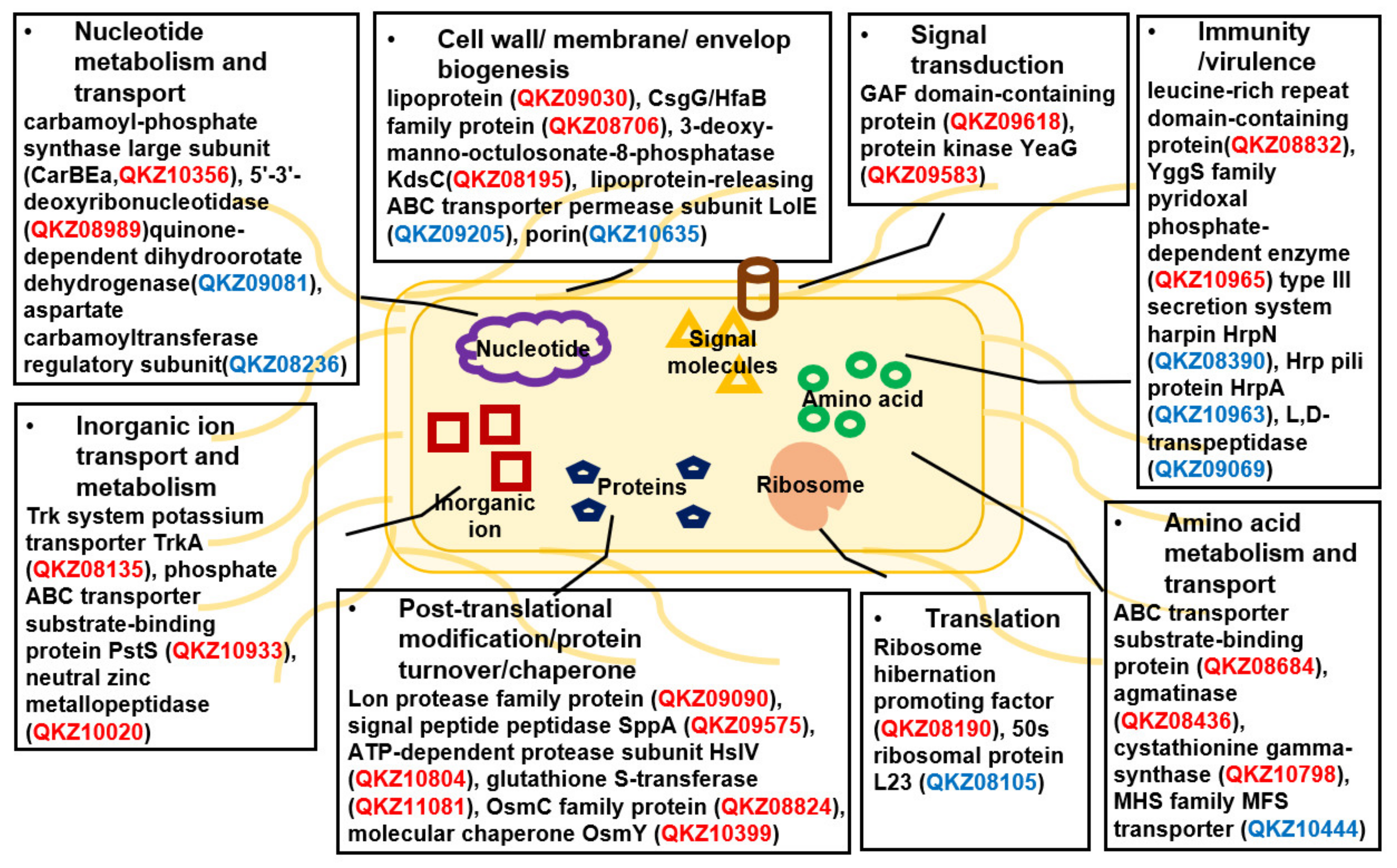
3.4. Comparative Proteomic Analysis
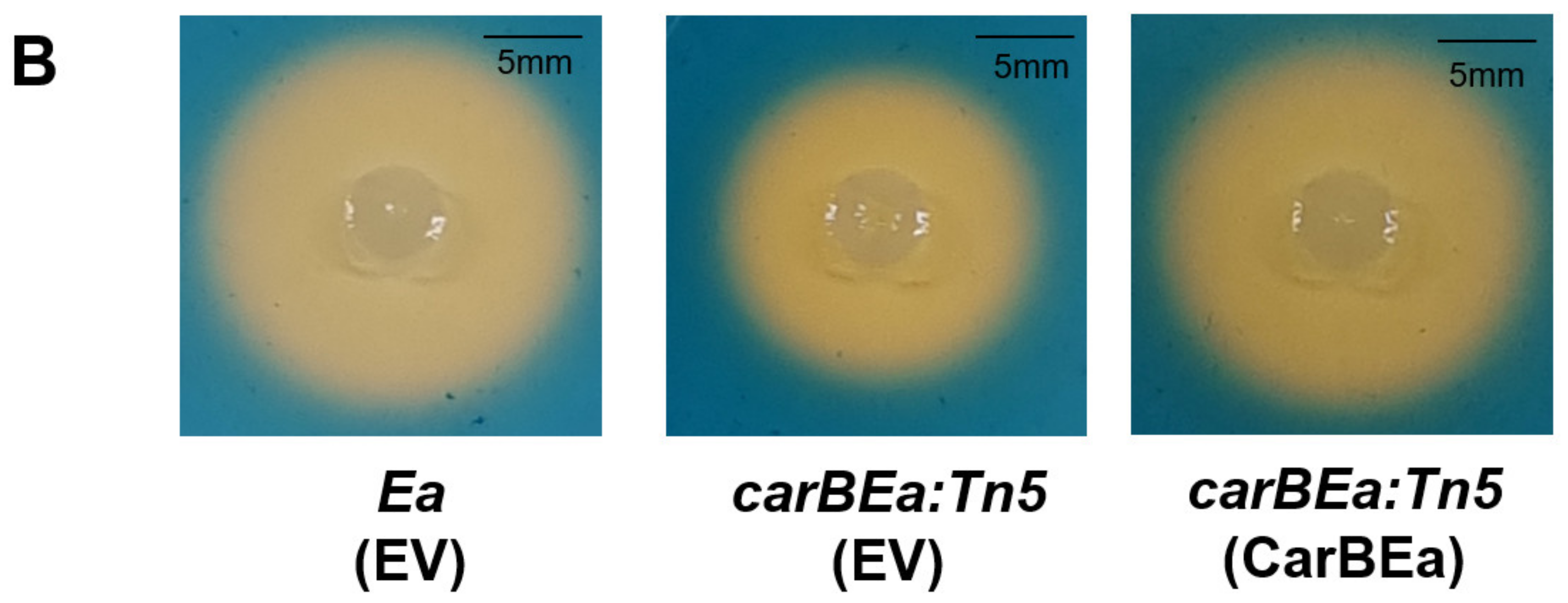
3.5. CarBEa Is Involved in Siderophore Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Data. 2022. Available online: https://www.fao.org/faostat (accessed on 1 June 2024).
- Khan, A.; Svara, A.; Wang, N. Comparing apples and oranges: Advances in disease resistance breeding of woody perennial fruit crops. Annu. Rev. Phytopathol. 2024, 62, 263–287. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef]
- Roberts, R.G.; Hale, C.N.; van der Zwet, T.; Miller, C.E.; Redlin, S.C. The potential for spread of Erwinia amylovora and fire blight via commercial apple fruit; a critical review and risk assessment. Crop Prot. 1998, 17, 19–28. [Google Scholar] [CrossRef]
- Eastgate, J.A. Erwinia amylovora: The molecular basis of fireblight disease. Mol. Plant Pathol. 2000, 1, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.J.; Luz, J.P.; Santos, C.; Tavares, F. CRISPR genotyping as complementary tool for epidemiological surveillance of outbreaks. PLoS ONE 2021, 16, e0250280. [Google Scholar] [CrossRef]
- Ismailova, E.; Shemshura, O.; Sadanov, A.; Baimakhanova, G.; Turlybayeva, Z.; Kuldybayev, N.; Yelubayeva, A.; Kopzhassarov, B.; Issina, Z.; Temreshev, I.; et al. Monitoring studies of the occurrence of fire blight pathogen in Kazakhstan and identification of antagonistic microorganisms suppressing its development. Braz. J. Biol. 2024, 84, e285493. [Google Scholar] [CrossRef]
- Lee, H.J.; Seon, S.W.; Suh, S.-J.; Hyun, I.-H. Recent spread and potential pathways for fire blight in South Korea. EPPO Bull. 2022, 52, 135–140. [Google Scholar] [CrossRef]
- Pedroncelli, A.; Puopolo, G. This tree is on fire: A review on the ecology of the causal agent of fire blight disease. J. Plant Pathol. 2023, 106, 823–837. [Google Scholar] [CrossRef]
- Park, D.H.; Yu, J.G.; Oh, E.J.; Han, K.S.; Yea, M.C.; Lee, S.J.; Myung, I.S.; Shim, H.S.; Oh, C.S. First report of fire blight disease on Asian pear caused by in Korea. Plant Dis. 2016, 100, 1946. [Google Scholar] [CrossRef]
- Ahn, M.I.; Yang, H.J.; Yun, S.C. Development of K-Maryblyt for fire blight control in apple and pear trees in Korea. Plant Pathol. J. 2024, 40, 290–298. [Google Scholar] [CrossRef]
- Song, S.; Kim, B.; Kim, K.P.; Roh, E. Novel detection protocol for Erwinia amylovora in orchard soil after removal of infected trees. Plant Pathol. J. 2024, 40, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, S.Y.; Park, J.; Song, S.; Kim, K.P.; Roh, E. Bacteriophage cocktail comprising Fifi044 and Fifi318 for biocontrol of Erwinia amylovora. Plant Pathol. J. 2024, 40, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Nyunoya, H.; Lusty, C.J. The carB gene of Escherichia coli: A duplicated gene coding for the large subunit of carbamoyl-phosphate synthetase. Proc. Natl. Acad. Sci. USA 1983, 80, 4629–4633. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.H.; Lu, C.D.; Walthall, D.A.; Brown, T.M.; Houghton, J.E.; Abdelal, A.T. Structure and regulation of the carAB operon in Pseudomonas aeruginosa and Pseudomonas stutzeri: No untranslated region exists. J. Bacteriol. 1994, 176, 2532–2542. [Google Scholar] [CrossRef][Green Version]
- Raushel, F.M.; Thoden, J.B.; Holden, H.M. The amidotransferase family of enzymes: Molecular machines for the production and delivery of ammonia. Biochemistry 1999, 38, 7891–7899. [Google Scholar] [CrossRef]
- Holden, H.M.; Thoden, J.B.; Raushel, F.M. Carbamoyl phosphate synthetase: An amazing biochemical odyssey from substrate to product. Cell. Mol. Life Sci. 1999, 56, 507–522. [Google Scholar] [CrossRef]
- Arioli, S.; Monnet, C.; Guglielmetti, S.; Mora, D. Carbamoylphosphate synthetase activity is essential for the optimal growth of Streptococcus thermophilus in milk. J. Appl. Microbiol. 2009, 107, 348–354. [Google Scholar] [CrossRef]
- Fox, B.A.; Bzik, D.J. Pyrimidine biosynthesis is required for virulence of Toxoplasma gondii. Nature 2002, 415, 926–929. [Google Scholar] [CrossRef]
- Guo, J.; Song, X.; Zou, L.F.; Zou, H.S.; Chen, G.Y. The small and large subunits of carbamoyl-phosphate synthase exhibit diverse contributions to pathogenicity in Xanthomonas citri subsp. citri. J. Integr. Agric. 2015, 14, 1338–1347. [Google Scholar] [CrossRef][Green Version]
- Zhuo, T.; Rou, W.; Song, X.; Guo, J.; Fan, X.; Kamau, G.G.; Zou, H. Molecular study on the carAB operon reveals that carB gene is required for swimming and biofilm formation in Xanthomonas citri subsp. citri. BMC Microbiol. 2015, 15, 225. [Google Scholar] [CrossRef]
- Klee, S.M.; Sinn, J.P.; Finley, M.; Allman, E.L.; Smith, P.B.; Aimufua, O.; Sitther, V.; Lehman, B.L.; Krawczyk, T.; Peter, K.A.; et al. Erwinia amylovora auxotrophic mutant exometabolomics and virulence on apples. Appl. Environ. Microbiol. 2019, 85, e00935-19. [Google Scholar] [CrossRef]
- Kang, I.J.; Park, D.H.; Lee, Y.K.; Han, S.W.; Kwak, Y.S.; Oh, C.S. Complete genome sequence of Erwinia amylovora strain TS3128, a Korean strain isolated in an Asian pear orchard in 2015. Microbiol. Resour. Announc. 2021, 10, e0069421. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, J.; Lee, J.; Cho, Y.; Kang, I.J.; Han, S.W. Comparing protein expression in Erwinia amylovora strain TS3128 cultured under three sets of environmental conditions. Plant Pathol. J. 2022, 38, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.S.; Yuan, L.A.; Guo, W.; Li, Y.R.; Che, Y.Z.; Zou, L.F.; Chen, G.Y. Construction of a Tn5-tagged mutant library of Xanthomonas oryzae pv. oryzicola as an invaluable resource for functional genomics. Curr. Microbiol. 2011, 62, 908–916. [Google Scholar] [CrossRef]
- Singer, T.; Burke, E. High-throughput TAIL-PCR as a tool to identify DNA flanking insertions. Methods Mol. Biol. 2003, 236, 241–272. [Google Scholar] [CrossRef]
- Heo, L.; Cho, Y.; Choi, J.; Lee, J.; Han, Y.; Han, S.W. Proteomic and phenotypic analyses of a putative YggS family pyridoxal phosphate-dependent enzyme in Acidovorax citrulli. Plant Pathol. J. 2023, 39, 235–244. [Google Scholar] [CrossRef]
- Heo, L.; Han, Y.; Cho, Y.; Choi, J.; Lee, J.; Han, S.W. A putative glucose 6-phosphate isomerase has pleiotropic functions on virulence and other mechanisms in Acidovorax citrulli. Front. Plant Sci. 2023, 14, 1275438. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; Garcia-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.; Cho, Y.; Choi, J.; Han, S.W. A putative 2,3-bisphosphoglycerate-dependent phosphoglycerate mutase is involved in the virulence, carbohydrate metabolism, biofilm formation, twitching halo, and osmotic tolerance in. Front. Plant Sci. 2022, 13, 1039420. [Google Scholar] [CrossRef]
- Elias, J.E.; Gygi, S.P. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Methods 2007, 4, 207–214. [Google Scholar] [CrossRef]
- Choi, H.; Fermin, D.; Nesvizhskii, A.I. Significance analysis of spectral count data in label-free shotgun proteomics. Mol. Cell. Proteom. 2008, 7, 2373–2385. [Google Scholar] [CrossRef]
- Tatusov, R.L.; Galperin, M.Y.; Natale, D.A.; Koonin, E.V. The COG database: A tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000, 28, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Do, E.; Kim, M.; Park, H.J.; Lee, J.; Han, S.W. A lysR-type transcriptional regulator LcrX is involved in virulence, biofilm formation, swimming motility, siderophore secretion, and growth in sugar sources in Xanthomonas axonopodis pv. glycines. Front. Plant Sci. 2020, 10, 1657. [Google Scholar] [CrossRef]
- Louden, B.C.; Haarmann, D.; Lynne, A.M. Use of blue agar CAS assay for siderophore detection. J. Microbiol. Biol. Educ. 2011, 12, 51–53. [Google Scholar] [CrossRef]
- Roy, A.; Kucukural, A.; Zhang, Y. I-TASSER: A unified platform for automated protein structure and function prediction. Nat. Protoc. 2010, 5, 725–738. [Google Scholar] [CrossRef]
- Kwaga, J.; Allan, B.J.; Van der Hurk, J.; Seida, H.; Potter, A.A. A carAB mutant of avian pathogenic Escherichia coli serogroup O2 is attenuated and effective as a live oral vaccine against colibacillosis in turkeys. Infect. Immun. 1994, 62, 3766–3772. [Google Scholar] [CrossRef]
- Mahan, M.J.; Slauch, J.M.; Mekalanos, J.J. Selection of bacterial virulence genes that are specifically induced in host tissues. Science 1993, 259, 686–688. [Google Scholar] [CrossRef]
- Shi, D.S.; Caldovic, L.; Tuchman, M. Sources and fates of carbamyl phosphate: A labile energy-rich molecule with multiple facets. Biology 2018, 7, 34. [Google Scholar] [CrossRef]
- Liu, X.Y.; Cai, Y.C.; Zhang, X.; Zhang, H.F.; Zheng, X.B.; Zhang, Z.G. Carbamoyl phosphate synthetase subunit MoCpa2 affects development and pathogenicity by modulating arginine biosynthesis in Magnaporthe oryzae. Front. Microbiol. 2016, 7, 2023. [Google Scholar] [CrossRef]
- Khanapur, M.; Alvala, M.; Prabhakar, M.; Kumar, K.S.; Edwin, R.K.; Saranya, P.S.V.K.S.; Patel, R.K.; Bulusu, G.; Misra, P.; Pal, M. Mycobacterium tuberculosis chorismate mutase: A potential target for TB. Bioorg. Med. Chem. 2017, 25, 1725–1736. [Google Scholar] [CrossRef]
- Brzovic, P.; Holbrook, E.L.; Greene, R.C.; Dunn, M.F. Reaction mechanism of Escherichia coli cystathionine gamma-synthase: Direct evidence for a pyridoxamine derivative of vinylglyoxylate as a key intermediate in pyridoxal phosphate dependent gamma-elimination and gamma-replacement reactions. Biochemistry 1990, 29, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Xia, L.; Chen, L.; Liao, Y.; Chen, B.; Liu, Y.; Gong, W.; Tian, Y.; Hu, B. yggS encoding pyridoxal 5′-phosphate binding protein is required for Acidovorax citrulli virulence. Front. Microbiol. 2021, 12, 783862. [Google Scholar] [CrossRef] [PubMed]
- Maturana, P.; Orellana, M.S.; Herrera, S.M.; Martinez, I.; Figueroa, M.; Martinez-Oyanedel, J.; Castro-Fernandez, V.; Uribe, E. Crystal structure of Escherichia coli agmatinase: Catalytic mechanism and residues relevant for substrate specificity. Int. J. Mol. Sci. 2021, 22, 4769. [Google Scholar] [CrossRef] [PubMed]
- Laube, G.; Bernstein, H.G. Agmatine: Multifunctional arginine metabolite and magic bullet in clinical neuroscience? Biochem. J. 2017, 474, 2619–2640. [Google Scholar] [CrossRef]
- Frawley, E.R.; Fang, F.C. The ins and outs of bacterial iron metabolism. Mol. Microbiol. 2014, 93, 609–616. [Google Scholar] [CrossRef]
- Chen, R.; Liu, N.; Ren, Y.; Cui, T. Transcriptomic and biochemical analysis of metabolic remodeling in Bacillus subtilis MSC4 under Benzo[a]pyrene stress. Chemosphere 2024, 353, 141637. [Google Scholar] [CrossRef]
- Dietl, A.M.; Binder, U.; Bauer, I.; Shadkchan, Y.; Osherov, N.; Haas, H. Arginine auxotrophy affects siderophore biosynthesis and attenuates virulence of Aspergillus fumigatus. Genes 2020, 11, 423. [Google Scholar] [CrossRef]
- Monti, S.M.; De Simone, G.; D’Ambrosio, K. L-histidinol dehydrogenase as a new target for old diseases. Curr. Top. Med. Chem. 2016, 16, 2369–2378. [Google Scholar] [CrossRef]
- Kovach, M.E.; Elzer, P.H.; Hill, D.S.; Robertson, G.T.; Farris, M.A.; Roop, R.M.; Peterson, K.M. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 1995, 166, 175–176. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Lee, S.Y.; Kim, D.; Lee, S.; Choi, J.; Cho, Y.; Lee, J.; Roh, E.; Han, S.-W. Functional and Proteomic Analyses of a Putative Carbamoyl Phosphate Synthase Large Subunit in Relation to Virulence, Arginine and Pyrimidine Biosynthesis, and Siderophore Production in Erwinia amylovora. Horticulturae 2024, 10, 1080. https://doi.org/10.3390/horticulturae10101080
Han Y, Lee SY, Kim D, Lee S, Choi J, Cho Y, Lee J, Roh E, Han S-W. Functional and Proteomic Analyses of a Putative Carbamoyl Phosphate Synthase Large Subunit in Relation to Virulence, Arginine and Pyrimidine Biosynthesis, and Siderophore Production in Erwinia amylovora. Horticulturae. 2024; 10(10):1080. https://doi.org/10.3390/horticulturae10101080
Chicago/Turabian StyleHan, Yoobin, Seung Yeup Lee, Dohyun Kim, Suhyun Lee, Junhyeok Choi, Yongmin Cho, Jeongwook Lee, Eunjung Roh, and Sang-Wook Han. 2024. "Functional and Proteomic Analyses of a Putative Carbamoyl Phosphate Synthase Large Subunit in Relation to Virulence, Arginine and Pyrimidine Biosynthesis, and Siderophore Production in Erwinia amylovora" Horticulturae 10, no. 10: 1080. https://doi.org/10.3390/horticulturae10101080
APA StyleHan, Y., Lee, S. Y., Kim, D., Lee, S., Choi, J., Cho, Y., Lee, J., Roh, E., & Han, S.-W. (2024). Functional and Proteomic Analyses of a Putative Carbamoyl Phosphate Synthase Large Subunit in Relation to Virulence, Arginine and Pyrimidine Biosynthesis, and Siderophore Production in Erwinia amylovora. Horticulturae, 10(10), 1080. https://doi.org/10.3390/horticulturae10101080




