Shading Impairs Mycorrhizal Benefits on Plant Growth, Leaf Gas Exchange, and Active Ingredients in Polygonum cuspidatum
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Plant Culture
2.3. Determination of Plant Growth, Root Morphology, and Leaf Gas Exchange
2.4. Determination of Root Mycorrhizal Colonization and Soil Mycelium Length
2.5. Determination of Active Ingredient Levels in Leaves and Roots
2.6. Statistical Analysis
3. Results
3.1. Changes in Root Mycorrhizal Colonization and Soil Mycelium Length
3.2. Changes in Biomass Production
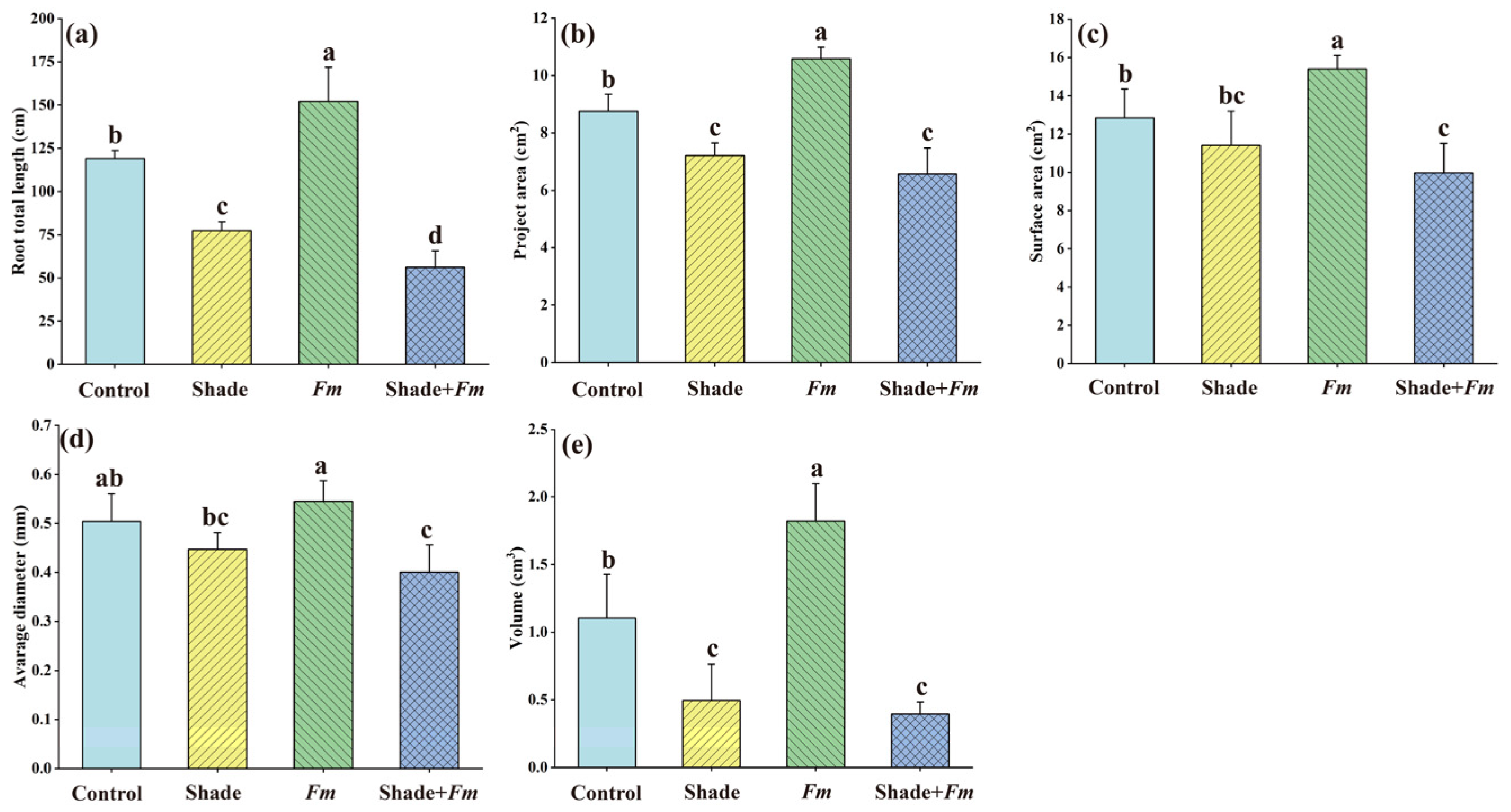
3.3. Changes in Root Morphological Variables
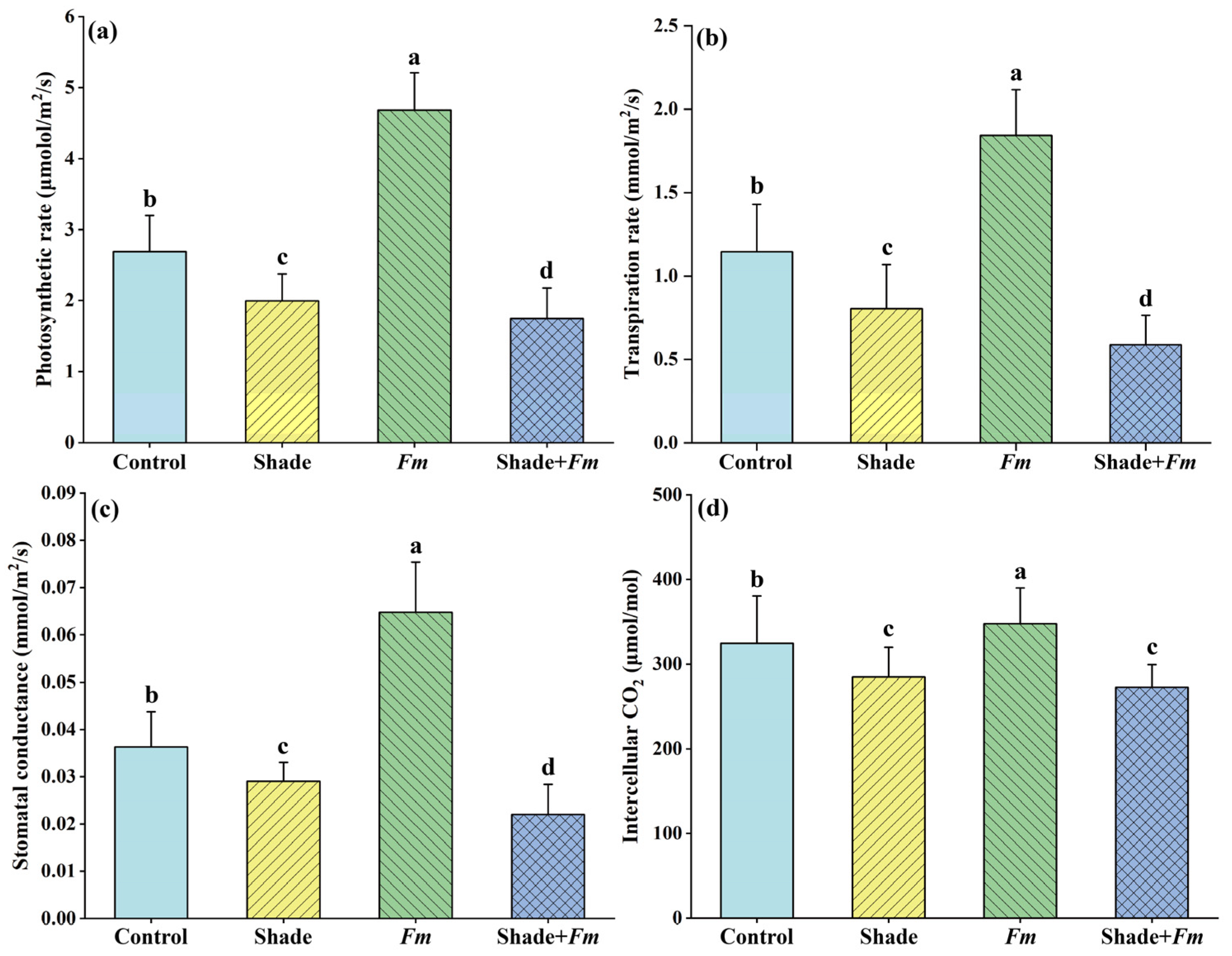
3.4. Changes in Leaf Gas Exchange
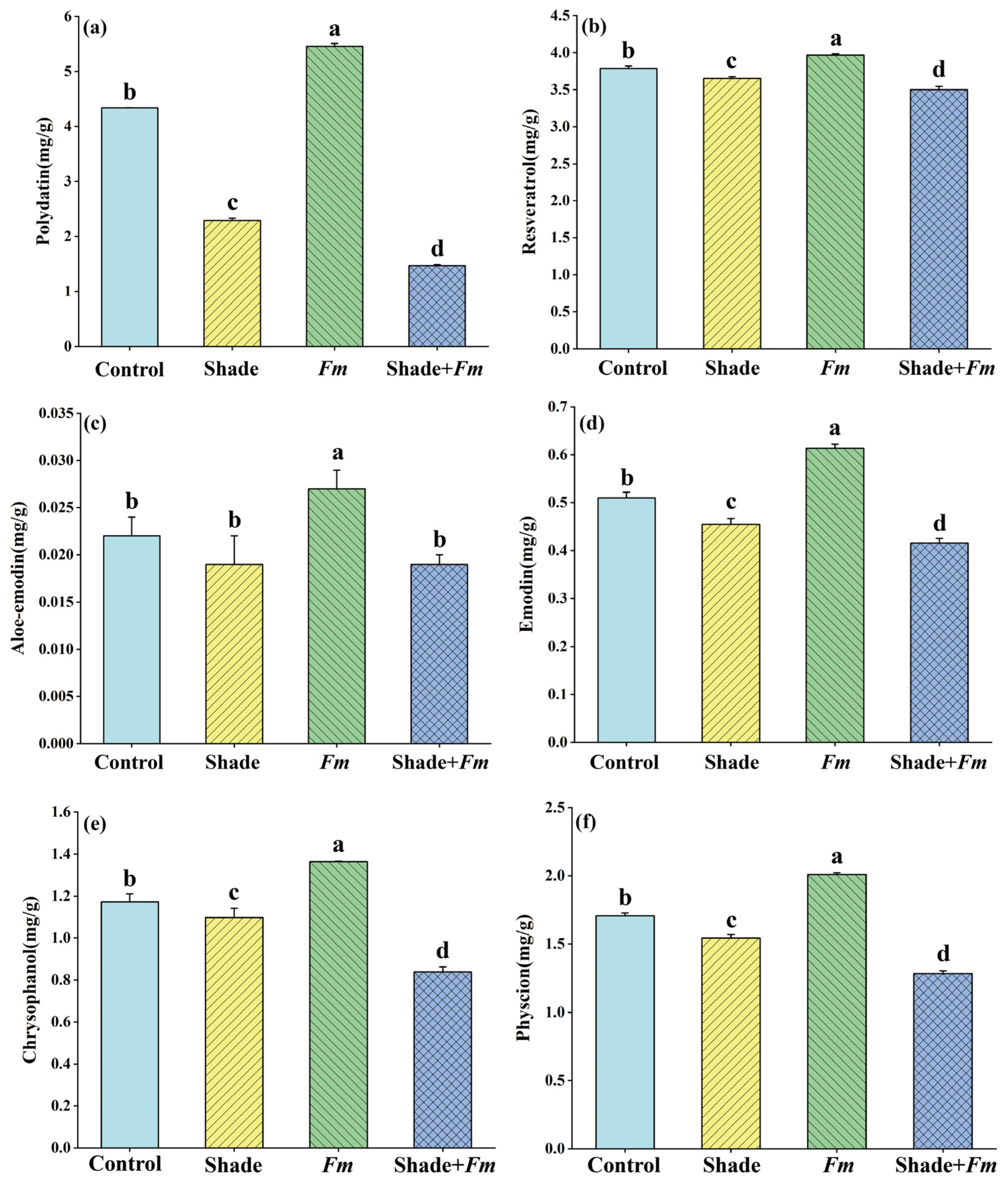
3.5. Changes in Active Ingredient Levels in Roots
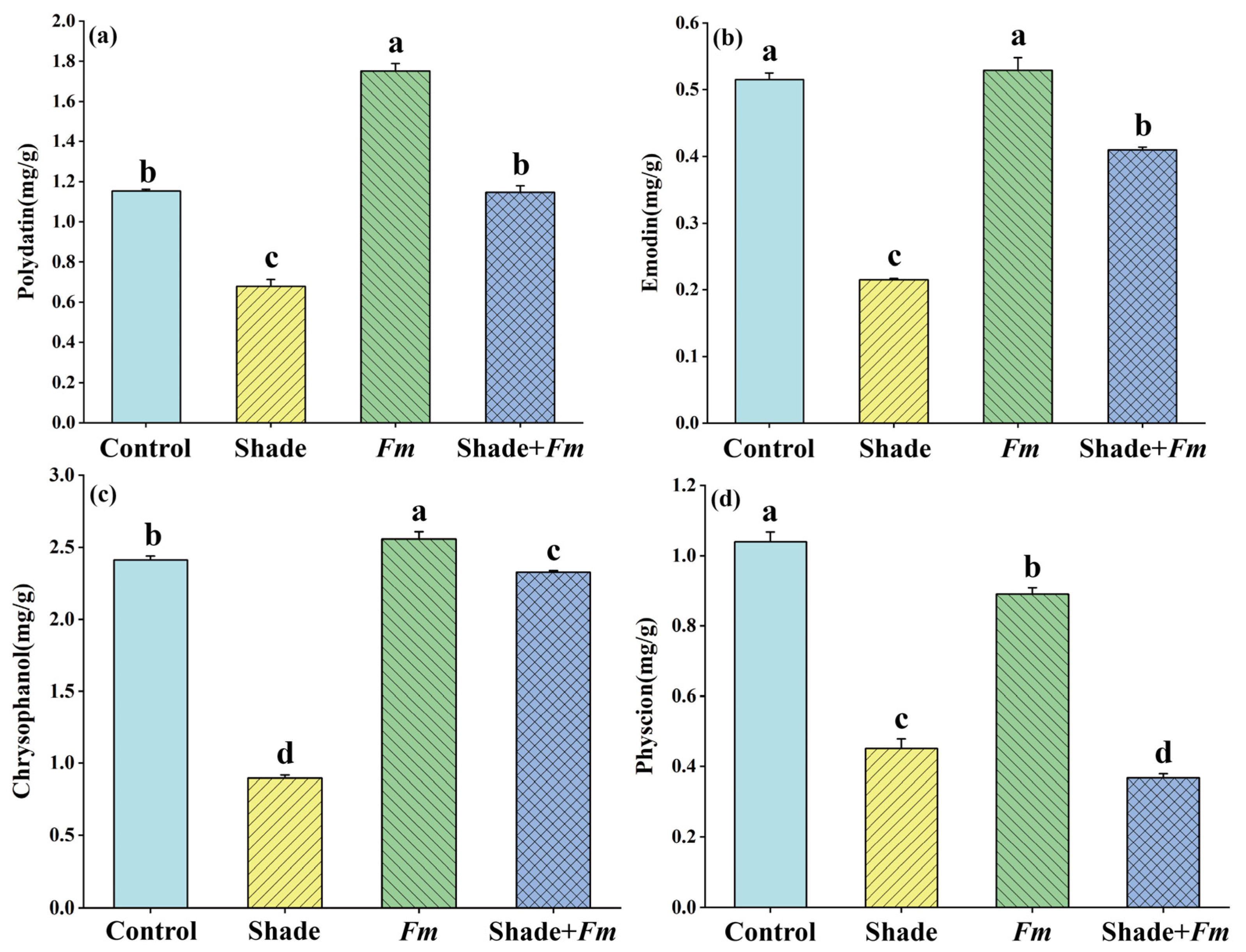
3.6. Changes in Active Ingredient Levels in Leaves
3.7. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurita, S.; Kashiwagi, T.; Ebisu, T.; Shimamura, T.; Ukeda, H. Content of resveratrol and glycoside and its contribution to the antioxidative capacity of Polygonum cuspidatum (Itadori) harvested in Kochi. Biosci. Biotech. Bioch. 2014, 78, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Leu, Y.L.; Hwang, T.L.; Hu, J.W.; Fang, J.Y. Anthraquinones from Polygonum cuspidatum as tyrosinase inhibitors for dermal use. Phytother. Res. 2008, 22, 552–556. [Google Scholar] [CrossRef]
- Alpert, P.; Simms, E.L. The relative advantages of plasticity and fixity in different environments: When is it good for a plant to adjust? Evol. Ecol. 2002, 16, 285–297. [Google Scholar] [CrossRef]
- Sultan, S.E. Phenotypic plasticity in plants: A case study in ecological development. Evol. Dev. 2003, 5, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L. Growth responses of 15 rain-forest tree species to a light gradient: The relative importance of morphological and physiological traits. Funct. Ecol. 1999, 13, 396–410. [Google Scholar] [CrossRef]
- Martinez-Garcia, J.F.; Rodriguez-Concepcion, M. Molecular mechanisms of shade tolerance in plants. New Phytol. 2023, 239, 1190–1202. [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. J. Sci. Food. Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef]
- Lv, J.; Wang, X.; Feng, Y.; Li, Y.; Zhao, H.; Wang, Y. Effects of shading on the photosynthetic characteristics and anatomical structure of Trollius chinensis Bunge. Acta. Ecol. Sin. 2012, 32, 6033–6043. [Google Scholar] [CrossRef]
- Goicoechea, N.; Baslam, M.; Erice, G.; Irigoyen, J.J. Increased photosynthetic acclimation in alfalfa associated with arbuscular mycorrhizal fungi (AMF) and cultivated in greenhouse under elevated CO2. J. Plant Physiol. 2014, 171, 1774–1781. [Google Scholar] [CrossRef]
- Konvalinková, T.; Püschel, D.; Řezáčová, V.; Gryndlerová, H.; Jansa, J. Carbon flow from plant to arbuscular mycorrhizal fungi is reduced under phosphorus fertilization. Plant Soil 2017, 419, 319–333. [Google Scholar] [CrossRef]
- Ding, Y.E.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Mycorrhizal fungi regulate daily rhythm of circadian clock in trifoliate orange under drought stress. Tree Physiol. 2021, 42, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Leifheit, E.F.; Veresoglou, S.D.; Lehmann, A.; Morris, E.K.; Rillig, M.C. Multiple factors influence the role of arbuscular mycorrhizal fungi in soil aggregation—A meta-analysis. Plant Soil 2014, 374, 523–537. [Google Scholar] [CrossRef]
- Rillig, M.C.; Mardatin, N.F.; Leifheit, E.F.; Antunes, P.M. Mycelium of arbuscular mycorrhizal fungi increases soil water repellency and is sufficient to maintain water-stable soil aggregates. Soil Biol. Biochem. 2010, 42, 1189–1191. [Google Scholar] [CrossRef]
- Habte, M.; Osorio, N.W. Arbuscular Mycorrhizas: Producing and Applying Arbuscular Mycorrhizal Inoculum; University of Hawaii: Honolulu, HI, USA, 2001. [Google Scholar]
- Tuomi, J.; Kytöviita, M.M.; Härdling, R. Cost efficiency of nutrient acquisition and the advantage of mycorrhizal symbiosis for the host plant. Oikos 2001, 92, 62–70. [Google Scholar] [CrossRef]
- Zheng, C.; Ji, B.; Zhang, J.; Zhang, F.; Bever, J.D. Shading decreases plant carbon preferential allocation towards the most beneficial mycorrhizal mutualist. New Phytol. 2015, 205, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Menezes, K.M.S.; Silva, D.K.A.; Queiroz, M.A.A.; Félix, W.P.; Yano-Melo, A.M. Arbuscular mycorrhizal fungal communities in buffelgrass pasture under intercropping and shading systems in Brazilian semiarid conditions. Agric. Ecosyst. Environ. 2016, 230, 55–67. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, X.; Li, S. Effects of arbuscular mycorrhizal fungi on rice growth under different flooding and shading regimes. Front. Microbiol. 2021, 12, 756752. [Google Scholar] [CrossRef]
- Shukla, A.; Kumar, A.; Jha, A.; Chaturvedi, O.P.; Prasad, R.; Gupta, A. Effects of shade on arbuscular mycorrhizal colonization and growth of crops and tree seedlings in Central India. Agrofor. Syst. 2009, 76, 95–109. [Google Scholar] [CrossRef]
- Thokchom, S.D.; Gupta, S.; Kapoor, R. An appraisal of arbuscular mycorrhiza-mediated augmentation in production of secondary metabolites in medicinal plants. J. Appl. Res. Med. Arom. 2023, 37, 100515. [Google Scholar] [CrossRef]
- Zhao, Y.; Cartabia, A.; Lalaymia, I.; Declerck, S. Arbuscular mycorrhizal fungi and production of secondary metabolites in medicinal plants. Mycorrhiza 2022, 32, 221–256. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, D.; Zhang, Y.; Naz, M.; Dai, Z.; Qi, S.; Du, D. Impacts of arbuscular mycorrhizal fungi on metabolites of an invasive weed Wedelia trilobata. Microorganisms 2024, 12, 701. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Ames, R.N.; Bethlenfalvay, G.J. Localized increase in nodule activity but no competitive interaction of cowpea rhizobia due to pre-establishment of vesicular-arbuscular mycorrhiza. New Phytol. 1987, 106, 207–215. [Google Scholar] [CrossRef]
- Sun, R.T.; Zhang, Z.Z.; Liu, M.Y.; Feng, X.C.; Zhou, N.; Feng, H.D.; Hashem, A.; Abd_Allah, E.F.; Harsonowati, W.; Wu, Q.S. Arbuscular mycorrhizal fungi and phosphorus supply accelerate main medicinal component production of Polygonum cuspidatum. Front. Microbiol. 2022, 13, 1006140. [Google Scholar] [CrossRef] [PubMed]
- Olsson, P.A.; Rahm, J.; Aliasgharzad, N. Carbon dynamics in mycorrhizal symbioses is linked to carbon costs and phosphorus benefits. FEMS Microbiol. Ecol. 2010, 72, 125–131. [Google Scholar] [CrossRef]
- Heinemeyer, A.; Ridgway, K.P.; Edwards, E.J.; Benham, D.G.; Young, J.P.W.; Fitter, A.H. Impact of soil warming and shading on colonization and community structure of arbuscular mycorrhizal fungi in roots of a native grassland community. Glob. Chang. Bio. 2004, 10, 52–64. [Google Scholar] [CrossRef]
- Shukla, A.; Kumar, A.; Chaturvedi, O.P.; Nagori, T.; Kumar, N.; Gupta, A. Efficacy of rhizobial and phosphate-solubilizing bacteria and arbuscular mycorrhizal fungi to ameliorate shade response on six pulse crops. Agrofor. Syst. 2018, 92, 499–509. [Google Scholar] [CrossRef]
- Shi, G.; Liu, Y.; Johnson, N.C.; Olsson, P.A.; Mao, L.; Cheng, G.; Jiang, S.; An, L.; Du, G.; Feng, H. Interactive influence of light intensity and soil fertility on root-associated arbuscular mycorrhizal fungi. Plant Soil 2014, 378, 173–188. [Google Scholar] [CrossRef]
- Rich, M.K.; Nouri, E.; Courty, P.E.; Reinhardt, D. Diet of arbuscular mycorrhizal fungi: Bread and butter? Trends. Plant Sci. 2017, 22, 652–660. [Google Scholar] [CrossRef]
- Johnson, N.C. Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol. 2010, 185, 631–647. [Google Scholar] [CrossRef]
- Konvalinková, T.; Jansa, J. Lights off for arbuscular mycorrhiza: On its symbiotic functioning under light deprivation. Front. Plant Sci. 2016, 7, 782. [Google Scholar] [CrossRef]
- Schubert, A.; Wyss, P.; Wiemken, A. Occurrence of trehalose in vesicular-arbuscular mycorrhizal fungi and in mycorrhizal roots. J. Plant Physiol. 1992, 140, 41–45. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Islam, M.; Hossain, M.; Hossain, T.; Miah, M. Effects of shade and nitrogen levels on quality Bangladhonia production. Bangl. J. Agric. Res. 1970, 34, 205–213. [Google Scholar] [CrossRef]
- Corré, W.J. Growth and morphogenesis of sun and shade plants ii. the influence of light quality. Acta Bot. Neerl. 1983, 32, 185–202. [Google Scholar] [CrossRef]
- Deng, C.; Sun, R.T.; Ma, Q.; Yang, Q.H.; Zhou, N.; Hashem, A.; Al-Arjani, A.B.F.; Abd_Allah, E.F.; Wu, Q.S. Mycorrhizal effects on active components and associated gene expressions in leaves of Polygonum cuspidatum under P stress. Agronomy 2022, 12, 2970. [Google Scholar] [CrossRef]
- Kiers, E.T.; Duhamel, M.; Beesetty, Y.; Mensah, J.A.; Franken, O.; Verbruggen, E.; Fellbaum, C.R.; Kowalchuk, G.A.; Hart, M.M.; Bago, A.; et al. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 2011, 333, 880–882. [Google Scholar] [CrossRef]
- Corrêa, A.; Cruz, C.; Pérez-Tienda, J.; Ferrol, N. Shedding light onto nutrient responses of arbuscular mycorrhizal plants: Nutrient interactions may lead to unpredicted outcomes of the symbiosis. Plant Sci. 2014, 221–222, 29–41. [Google Scholar] [CrossRef]
- Gavito, M.E.; Jakobsen, I.; Mikkelsen, T.N.; Mora, F. Direct Evidence for modulation of photosynthesis by an arbuscular mycorrhiza-induced carbon sink strength. New Phytol. 2019, 223, 896–907. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, W.; Xie, Q.; Liu, N.A.; Liu, L.; Wang, D.; Zhang, X.; Yang, C.; Chen, X.Y.; Tang, D.Z.; et al. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 2017, 356, 1172–1175. [Google Scholar] [CrossRef]
- Keymer, A.; Pimprikar, P.; Wewer, V.; Huber, C.; Brands, M.; Bucerius, S.L.; Delaux, P.M.; Klingl, V.; von Röpenack-Lahaye, E.; Wang, T.L.; et al. Lipid transfer from plants to arbuscular mycorrhiza fungi. elife 2017, 6, e29107. [Google Scholar] [CrossRef]
- Salmeron-Santiago, I.A.; Martínez-Trujillo, M.; Valdez-Alarcón, J.J.; Pedraza-Santos, M.E.; Santoyo, G.; Pozo, M.J.; Chávez-Bárcenas, A.T. An updated review on the modulation of carbon partitioning and allocation in arbuscular mycorrhizal plants. Microorganisms 2022, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Konvalinková, T.; Püschel, D.; Janoušková, M.; Gryndler, M.; Jansa, J. Duration and intensity of shade differentially affects mycorrhizal growth- and phosphorus uptake responses of Medicago truncatula. Front. Plant Sci. 2015, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Chen, Y.T.; Chiu, C.C.; Liao, W.T.; Liu, Y.C.; David Wang, H.M. Polygonum cuspidatum extracts as bioactive antioxidaion, anti-tyrosinase, immune stimulation and anticancer agents. J. Biosci. Bioeng. 2015, 119, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Wang, H.; Liu, M.; Li, B.; Chen, X.; Ma, Y.T.; Yan, Z.Y. Effects of native arbuscular mycorrhizae isolated on root biomass and secondary metabolites of Salvia miltiorrhiza bge. Front. Plant Sci. 2021, 12, 617892. [Google Scholar] [CrossRef]
- Lei, A.Q.; Zhou, J.H.; Rong, Z.Y.; Alqahtani, M.D.; Gao, X.B.; Wu, Q.S. Mycorrhiza-triggered changes in leaf food quality and secondary metabolite profile in tea at low temperatures. Rhizosphere 2024, 29, 100840. [Google Scholar] [CrossRef]
- Liu, X.Q.; Cheng, S.; Aroca, R.; Zou, Y.N.; Wu, Q.S. Arbuscular mycorrhizal fungi induce flavonoid synthesis for mitigating oxidative damage of trifoliate orange under water stress. Environ. Exp. Bot. 2022, 204, 105089. [Google Scholar] [CrossRef]
- Amani Machiani, M.; Javanmard, A.; Morshedloo, M.R.; Aghaee, A.; Maggi, F. Funneliformis mosseae Inoculation under water deficit stress improves the yield and phytochemical characteristics of thyme in intercropping with soybean. Sci. Rep. 2021, 11, 15279. [Google Scholar] [CrossRef]
- Amanifar, S.; Toghranegar, Z. The The efficiency of arbuscular mycorrhiza for improving tolerance of Valeriana officinali L. and enhancing valerenic acid accumulation under salinity stress. Ind. Crop. Prod. 2020, 147, 112234. [Google Scholar] [CrossRef]
- Lang, M.; Li, X.; Zheng, C.; Li, H.; Zhang, J. Shading mediates the response of mycorrhizal maize (Zea mays L.) seedlings under varying levels of phosphorus. Appl. Soil Ecol. 2021, 166, 104060. [Google Scholar] [CrossRef]
- Stonor, R.N.; Smith, S.E.; Manjarrez, M.; Facelli, E.; Smith, F.A. Mycorrhizal responses in wheat: Shading decreases growth but does not lower the contribution of the fungal phosphate uptake pathway. Mycorrhiza 2014, 24, 465–472. [Google Scholar] [CrossRef]


| Variables | AMF | Shading | Interaction | Variables | AMF | Shading | Interaction |
|---|---|---|---|---|---|---|---|
| Height | NS | ** | ** | Stomatal conductance | ** | ** | ** |
| Stem diameter | NS | * | NS | Intercellular CO2 level | * | ** | NS |
| Leaf number | NS | ** | NS | Root polydain | ** | ** | ** |
| Leaf biomass | ** | ** | NS | Root resveratrol | NS | ** | ** |
| Stem biomass | ** | ** | NS | Root aloe-emodin | * | ** | * |
| Root biomass | ** | ** | ** | Root emodin | ** | ** | ** |
| Root total length | ** | ** | NS | Root chrysophanol | NS | ** | ** |
| Project area | ** | ** | NS | Root physcion | NS | ** | ** |
| Surface area | * | ** | * | Leaf polydain | ** | ** | ** |
| Average diameter | ** | NS | NS | Leaf emodin | ** | ** | ** |
| Volume | * | ** | ** | Leaf chrysophanol | ** | ** | ** |
| Photosynthetic rate | ** | ** | ** | Leaf physcion | ** | ** | * |
| Transpiration rate | ** | ** | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, C.; Zhang, Z.-Z.; da Silva, F.S.B.; Hashem, A.; Abd_Allah, E.F.; Zou, Y.-N.; Wu, Q.-S. Shading Impairs Mycorrhizal Benefits on Plant Growth, Leaf Gas Exchange, and Active Ingredients in Polygonum cuspidatum. Horticulturae 2024, 10, 1078. https://doi.org/10.3390/horticulturae10101078
Deng C, Zhang Z-Z, da Silva FSB, Hashem A, Abd_Allah EF, Zou Y-N, Wu Q-S. Shading Impairs Mycorrhizal Benefits on Plant Growth, Leaf Gas Exchange, and Active Ingredients in Polygonum cuspidatum. Horticulturae. 2024; 10(10):1078. https://doi.org/10.3390/horticulturae10101078
Chicago/Turabian StyleDeng, Ci, Ze-Zhi Zhang, Fábio Sérgio Barbosa da Silva, Abeer Hashem, Elsayed Fathi Abd_Allah, Ying-Ning Zou, and Qiang-Sheng Wu. 2024. "Shading Impairs Mycorrhizal Benefits on Plant Growth, Leaf Gas Exchange, and Active Ingredients in Polygonum cuspidatum" Horticulturae 10, no. 10: 1078. https://doi.org/10.3390/horticulturae10101078
APA StyleDeng, C., Zhang, Z.-Z., da Silva, F. S. B., Hashem, A., Abd_Allah, E. F., Zou, Y.-N., & Wu, Q.-S. (2024). Shading Impairs Mycorrhizal Benefits on Plant Growth, Leaf Gas Exchange, and Active Ingredients in Polygonum cuspidatum. Horticulturae, 10(10), 1078. https://doi.org/10.3390/horticulturae10101078









