Winemaking: “With One Stone, Two Birds”? A Holistic Review of the Bio-Functional Compounds, Applications and Health Benefits of Wine and Wineries’ By-Products
Abstract
1. Introduction
2. Bio-Functional Compounds and Health Benefits of the Fermented Alcoholic Beverage, Wine, and of Its By-Products
2.1. Composition, Nutritional Value, Bio-Functional Components and Functional Properties
2.1.1. Composition and Nutritional Value of Wine
2.1.2. Wineries’ By-Products—Composition and Nutritional Value
| Nutritional Components 1 | Red Wine | White Wine |
| Glycerol | 1–1.2 | 1–1.2 |
| Acids | 0.5–0.7 | 0.6–0.8 |
| Amino acids | 0.2–0.3 | 0.2–0.3 |
| Phenols | 0.1–0.3 | 0–0.02 |
| Sugars | 0.01–0.1 | 0.01–0.1 |
| Lipids | 0.01–0.03 | 0–0.02 |
| Vitamins | 0–0.02 | 0–0.02 |
| Phenolic Compounds 2 | Red Wine | White Wine |
| Gallic acid | 0–126 | 0–7 |
| Protocatechuic acid | 0–10 | 0–13 |
| Syringic acid | 0–23 | 0–2 |
| Caffeic acid | 0–77 | 0–7 |
| Coumaric acid | 0–40 | 0–6 |
| Hydroxybenzaldehydes | 0–46 | 2–6 |
| Resveratrol | 0–62 | 0–3 |
| Resveratrol 3-O-glucoside | 0–88 | 0–13 |
| Catechin | 14–390 | 0–46 |
| Epigallocatechin | 0–165 | 0–60 |
| Quercetin | 12–110 | 1–21 |
| Cyanidin | 0–12 | - |
| Malvidin | 12–541 | 0–4 |
| Proanthocyanidins | 100–560 | 0–2 |
| Tyrosols | 6–54 | 3–6 |
| Total phenolic content | 983–3624 | 89–434 |
| Antioxidant Capacity 3 | Red Wine | White Wine 2 |
| TAAc | 7.5–16.6 | |
| FRAP | 6.9–15.2 | |
| DPPH | 0.2–1.6 | 0.6–5.8 |
2.2. Bio-Functional Components and Associated Health Benefits
2.2.1. Wine and Winery By-Products’ Phenolic Bioactives with Antioxidant, Anti-Inflammatory and Antithrombotic Health-Promoting Effects
Phenolic Bioactives and Antioxidant Health-Promoting Bioactivities
Pleiotropic Health-Promoting Effects of Phenolic Bioactives
Limitations and Future Perspectives of Phenolic Compounds as Bioactive Ingredients with Health-Promoting Properties
2.2.2. Bioactive Lipid Compounds of Wine and Wineries’ By-Products
2.2.3. Bio-Functional Dietary Fibres from Wineries’ By-Products
3. Health Benefits of Moderate Wine Consumption and Detrimental Effects of Alcohol Abuse: A Coin with Two Sides
3.1. Health-Promoting Effects of Incorporating Moderate Wine Consumption in Diet
3.2. Alcohol-Containing Wine, Quantity Consumed and Detrimental/Beneficial Effects on Health: Is It Really a Debate or Is It a Matter of Re-Definitions and Re-Education?
3.3. Concluding Remarks on the Health-Promoting Effects of Wine Consumption in Moderation: From Ancient Times (Religion, Philosophy and Scientific Approaches) to Recent Scientific Evaluation
4. Recovery and Valorisation of Bioactive Compounds from Wineries’ By-Products as Ingredients for Developing Health-Promoting Functional Foods, Supplements and Nutraceuticals
4.1. The Importance of Wineries’ By-Products and Their Bioactives in the Functional Foods Sector
4.2. Characteristic Extraction Methods for the Recovery of Bioactive Compounds of Wineries’ By-Products
4.3. Applications of Wineries’ By-Products and Their Bioactives in the Food Industry as Ingredient(s) for the Fortification/Production of Existing/Novel Functional Food Products
4.3.1. Applications of Wineries’ By-Products and Their Bioactive Ingredients for the Fortification/Production of Functional Flour/Cereal-Based Foods
4.3.2. Applications of Wineries’ By-Products and Their Bioactive Ingredients for the Fortification/Production of Functional Dairy-Based Foods
4.3.3. Applications of Wineries’ By-Products and Their Bioactive Ingredients for the Fortification/Production of Functional Meat/Fish-Based Foods
4.3.4. Applications of Wineries’ By-Products and Their Bioactive Ingredients for the Fortification/Production of Other Plant-Based Functional Foods and Beverages
4.4. Health Benefits and Applications of Wineries’ By-Products and Their Bioactives as Ingredients of Bio-Functional Food Products, Supplements and Nutraceuticals
4.4.1. Antioxidant, Anti-Inflammatory and Antithrombotic Health-Promoting Effects of Grape Pomace and of Its Bioactives, Extracts and Relevant Bio-Functional Products
4.4.2. Antioxidant, Anti-Inflammatory and Antithrombotic Health-Promoting Effects of Grape Seeds and of Their Bioactives, Extracts and Relevant Bio-Functional Products
4.4.3. Anticancer Protective Effects of Wineries’ By-Products and of Their Bioactives, Extracts and Relevant Bio-Functional Products
4.4.4. Antimicrobial Protective Effects of Wineries’ By-Products and of Their Bioactives, Extracts and Relevant Bio-Functional Products
4.4.5. Biodelivery Systems to Improve the Bioavailability and Bio-Functionality of Wineries’ By-Products and of Their Bioactives, Extracts and Relevant Bio-Functional Products
4.5. Limitations in the Applications of Wineries’ By-Products and Their Bioactive Ingredients
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basalekou, M.; Kallithraka, S.; Kyraleou, M. Wine bioactive compounds. In Functional Foods and Their Implications for Health Promotion, 1st ed.; Zabetakis, I., Tsoupras, A., Lordan, R., Ramji, D., Eds.; Academic Press: Cambridge, MA, USA, 2023; Volume 13, pp. 341–363. [Google Scholar]
- Calabriso, N.; Scoditti, E.; Massaro, M.; Pellegrino, M.; Storelli, C.; Ingrosso, I.; Giovinazzo, G.; Carluccio, M.A. Multiple anti-inflammatory and anti-atherosclerotic properties of red wine polyphenolic extracts: Differential role of hydroxycinnamic acids, flavonols and stilbenes on endothelial inflammatory gene expression. Eur. J. Nutr. 2016, 55, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fidalgo, S.; Cárdeno, A.; Villegas, I.; Talero, E.; de la Lastra, C.A. Dietary supplementation of resveratrol attenuates chronic colonic inflammation in mice. Eur. J. Pharmac. 2010, 633, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; Pérez-Gregorio, R.; Soares, S.; Mateus, N.; De Freitas, V. Wine Flavonoids in Health and Disease Prevention. Molecules 2017, 22, 292. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.B.; Fragopoulou, E.; Nomikos, T.; Iatrou, C.; Antonopoulou, S.; Demopoulos, C.A. Characterization of the de novo biosynthetic enzyme of platelet activating factor, DDT-insensitive cholinephosphotransferase, of human mesangial cells. Mediators Inflamm. 2007, 2007, 27683. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.B.; Iatrou, C.; Frangia, C.; Demopoulos, C.A. The implication of platelet activating factor in cancer growth and metastasis: Potent beneficial role of PAF-inhibitors and antioxidants. Infect. Disord. Drug Targets 2009, 9, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Nomikos, T.; Tsantila, N.; Mitropoulou, A.; Zabetakis, I.; Demopoulos, C.A. Biological Activity of Total Lipids from Red and White Wine/Must. J. Agr. Food Chem. 2001, 49, 5186–5193. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Antonopoulou, S.; Demopoulos, C.A. Biologically Active Lipids with Antiatherogenic Properties from White Wine and Must. J. Agr. Food Chem. 2002, 50, 2684–2694. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Antonopoulou, S.; Tsoupras, A.; Tsantila, N.; Grypioti, A.; Gribilas, G.; Gritzapi, H.; Konsta, E.; Skandalou, E.; Papadopoulou, A. Antiatherogenic properties of red/white wine, musts, grape-skins, and yeast. In Proceedings of the 45th International Conference on the Bioscience of Lipids, University of Ioannina, Ioannina, Greece, 25–29 May 2004; p. 66. [Google Scholar]
- Fragopoulou, E.; Demopoulos, C.A.; Antonopoulou, S. Lipid Minor Constituents in Wines. A Biochemical Approach in the French Paradox. Int. J. Wine Res. 2009, 1, 131–143. [Google Scholar]
- Xanthopoulou, M.N.; Kalathara, K.; Melachroinou, S.; Arampatzi-Menenakou, K.; Antonopoulou, S.; Yannakoulia, M.; Fragopoulou, E. Wine Consumption Reduced Postprandial Platelet Sensitivity against Platelet Activating Factor in Healthy Men. Eur. J. Nutr. 2017, 56, 1485–1492. [Google Scholar] [CrossRef]
- Argyrou, C.; Vlachogianni, I.; Stamatakis, G.; Demopoulos, C.A.; Antonopoulou, S.; Fragopoulou, E. Postprandial Effects of Wine Consumption on Platelet Activating Factor Metabolic Enzymes. Prostaglandins Other Lipid Mediat. 2017, 130, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Fragopoulou, E.; Choleva, M.; Antonopoulou, S.; Demopoulos, C.A. Wine and its metabolic effects. A comprehensive review of clinical trials. Metabolism 2018, 83, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Gavriil, L.; Detopoulou, M.; Petsini, F.; Antonopoulou, S.; Fragopoulou, E. Consumption of plant extract supplement reduces platelet activating factor-induced platelet aggregation and increases platelet activating factor catabolism: A randomised, double-blind and placebo-controlled trial. Br. J. Nutr. 2019, 121, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Choleva, M.; Boulougouri, V.; Panara, A.; Panagopoulou, E.; Chiou, A.; Thomaidis, N.S.; Antonopoulou, S.; Fragopoulou, E. Evaluation of Anti-Platelet Activity of Grape Pomace Extracts. Food Funct. 2019, 10, 8069–8080. [Google Scholar] [CrossRef] [PubMed]
- Choleva, M.; Tsota, M.; Boulougouri, V.; Panara, A.; Thomaidis, N.; Antonopoulou, S.; Fragopoulou, E. Anti-platelet and anti-inflammatory properties of an ethanol-water red grape pomace extract. Proc. Nutr. Soc. 2020, 79, E370. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Antonopoulou, S. The French paradox three decades later: Role of inflammation and thrombosis. Clin. Chim. Acta 2020, 510, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Fragopoulou, E.; Argyrou, C.; Detopoulou, M.; Tsitsou, S.; Seremeti, S.; Yannakoulia, M.; Antonopoulou, S.; Kolovou, G.; Kalogeropoulos, P. The Effect of Moderate Wine Consumption on Cytokine Secretion by Peripheral Blood Mononuclear Cells: A Randomized Clinical Study in Coronary Heart Disease Patients. Cytokine 2021, 146, 155629. [Google Scholar] [CrossRef]
- Choleva, M.; Argyrou, C.; Detopoulou, M.; Donta, M.-E.; Gerogianni, A.; Moustou, E.; Papaemmanouil, A.; Skitsa, C.; Kolovou, G.; Kalogeropoulos, P.; et al. Effect of Moderate Wine Consumption on Oxidative Stress Markers in Coronary Heart Disease Patients. Nutrients 2022, 14, 1377. [Google Scholar] [CrossRef]
- Choleva, M.; Matalliotaki, E.; Antoniou, S.; Asimomyti, E.; Drouka, A.; Stefani, M.; Yannakoulia, M.; Fragopoulou, E. Postprandial Metabolic and Oxidative Stress Responses to Grape Pomace Extract in Healthy Normal and Overweight/Obese Women: A Randomized, Double-Blind, Placebo-Controlled Crossover Study. Nutrients 2023, 15, 156. [Google Scholar] [CrossRef]
- Karantonis, H.C.; Tsoupras, A.; Moran, D.; Zabetakis, I.; Nasopoulou, C. Olive, apple, and grape pomaces with antioxidant and anti-inflammatory bioactivities for functional foods. In Functional Foods and Their Implications for Health Promotion, 1st ed.; Zabetakis, I., Tsoupras, A., Lordan, R., Ramji, D., Eds.; Academic Press: Cambridge, MA, USA, 2023; Volume 5, pp. 131–159. [Google Scholar]
- Sabra, A.; Netticadan, T.; Wijekoon, C. Grape bioactive molecules, and the potential health benefits in reducing the risk of heart diseases. Food Chem. X 2021, 12, 100149. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation and Cardiovascular Diseases. In The Impact of Nutrition and Statins on Cardiovascular Diseases, 1st ed.; Zabetakis, I., Lordan, R., Tsoupras, A., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 3, pp. 53–117. [Google Scholar]
- Van Bussel, B.C.T.; Henry, R.M.A.; Schalkwijk, C.G.; Dekker, J.M.; Nijpels, G.; Feskens, E.J.M.; Stehouwer, C.D.A. Alcohol and red wine consumption, but not fruit, vegetables, fish or dairy products, are associated with less endothelial dysfunction and less low-grade inflammation: The Hoorn Study. Eur. J. Nutr. 2018, 57, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Queipo-Ortuño, M.I.; Boto-Ordóñez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andrés-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Pitsavos, C.; Arvaniti, F.; Stefanadis, C. Adherence to the Mediterranean Food Pattern Predicts the Prevalence of Hypertension, Hypercholesterolemia, Diabetes and Obesity, among Healthy Adults; the Accuracy of the MedDietScore. Prev. Med. 2007, 44, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Maiorino, M.I.; Bellastella, G.; Panagiotakos, D.B.; Giugliano, D. Mediterranean diet for type 2 diabetes: Cardiometabolic benefits. Endocrine 2017, 56, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Piano, M.R. Alcohol’s Effects on the Cardiovascular System. Alcohol Res. 2017, 38, 219–241. [Google Scholar] [PubMed]
- Markoski, M.M.; Garavaglia, J.; Oliveira, A.; Olivaes, J.; Marcadenti, A. Molecular Properties of Red Wine Compounds and Cardiometabolic Benefits. Nutr. Metabol. Insights 2016, 9, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Poklar Ulrih, N.; Opara, R.; Skrt, M.; Košmerl, T.; Wondra, M.; Abram, V.; Part, I. Polyphenols composition and antioxidant potential during ’Blaufränkisch’ grape maceration and red wine maturation, and the effects of trans-resveratrol addition. Food Chem. Toxicol. 2020, 137, 111122. [Google Scholar] [CrossRef]
- Xiang, L.; Xiao, L.; Wang, Y.; Li, H.; Huang, Z.; He, X. Health benefits of wine: Don’t expect resveratrol too much. Food Chem. 2014, 156, 258–263. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Rodrigues, E.; Gonzaga, L.V.; Caliari, V.; Genovese, M.I.; de Souza Schmidt Gonçalves, A.E.; Fett, R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem. 2011, 127, 174–179. [Google Scholar] [CrossRef]
- Onache, P.A.; Geana, E.-I.; Ciucure, C.T.; Florea, A.; Sumedrea, D.I.; Ionete, R.E.; Tița, O. Bioactive Phytochemical Composition of Grape Pomace Resulted from Different White and Red Grape Cultivars. Separations 2022, 9, 395. [Google Scholar] [CrossRef]
- Lingua, M.S.; Fabani, M.P.; Wunderlin, D.A.; Baroni, M.V. From grape to wine: Changes in phenolic composition and its influence on antioxidant activity. Food Chem. 2016, 208, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Göksel, Z.; Erdoğan, S.S.; Öztürk, A.; Atak, A.; Özer, C. Antioxidant Activity and Phenolic Content of Seed, Skin and Pulp Parts of 22 Grape (Vitis vinifera L.) Cultivars (4 Common and 18 Registered or Candidate for Registration). J. Food Proc. Preserv. 2015, 39, 1682–1691. [Google Scholar] [CrossRef]
- Ferri, M.; Bin, S.; Vallini, V.; Fava, F.; Michelini, E.; Roda, A.; Minnucci, G.; Bucchi, G.; Tassoni, A. Recovery of polyphenols from red grape pomace and assessment of their antioxidant and anti-cholesterol activities. New Biotechnol. 2016, 33, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Iora, S.R.F.; Maciel, G.M.; Zielinski, A.A.F.; da Silva, M.V.; Pontes, P.V.d.A.; Haminiuk, C.W.I.; Granato, D. Evaluation of the bioactive compounds and the antioxidant capacity of grape pomace. Int. J. Food Sci. Technol. 2015, 50, 62–69. [Google Scholar] [CrossRef]
- Yammine, S.; Delsart, C.; Vitrac, X.; Mietton Peuchot, M.; Ghidossi, R. Characterisation of polyphenols and antioxidant potential of red and white pomace by-product extracts using subcritical water extraction. OENO One 2020, 54, 263–278. [Google Scholar]
- Szabó, É.; Marosvölgyi, T.; Szilágyi, G.; Kőrösi, L.; Schmidt, J.; Csepregi, K.; Márk, L.; Bóna, Á. Correlations between Total Antioxidant Capacity, Polyphenol and Fatty Acid Content of Native Grape Seed and Pomace of Four Different Grape Varieties in Hungary. Antioxidants 2021, 10, 1101. [Google Scholar] [CrossRef] [PubMed]
- Chedea, V.S.; Macovei, Ș.O.; Bocșan, I.C.; Măgureanu, D.C.; Levai, A.M.; Buzoianu, A.D.; Pop, R.M. Grape Pomace Polyphenols as a Source of Compounds for Management of Oxidative Stress and Inflammation—A Possible Alternative for Non-Steroidal Anti-Inflammatory Drugs? Molecules 2022, 27, 6826. [Google Scholar] [CrossRef]
- Visioli, F.; Panaite, S.A.; Tomé-Carneiro, J. Wine’s Phenolic Compounds and Health: A Pythagorean View. Molecules 2020, 25, 4105. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Saucier, C.; Glories, Y. Grape and Wine Phenolics: History and Perspective. Am. J. Enol. Vitic. 2006, 57, 239–248. [Google Scholar] [CrossRef]
- Lago-Vanzela, E.; Alves Baffi, M.; Castilhos, M.; Ribeiro-Pinto, M.; Del Bianchi, V.; Ramos, A.; Stringheta, P.; Hermosín-Gutiérrez, I.; Da Silva, R. Phenolic compounds in grapes and wines: Chemical and biochemical characteristics and technological quality. In Grapes: Production, Phenolic Composition and Potential Biomedical Effects, 1st ed.; Câmara, J.S., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2014; Volume 3, pp. 1–18. [Google Scholar]
- Zhou, D.-D.; Li, J.; Xiong, R.-G.; Saimaiti, A.; Huang, S.-Y.; Wu, S.-X.; Yang, Z.-J.; Shang, A.; Zhao, C.-N.; Gan, R.-Y.; et al. Bioactive Compounds, Health Benefits and Food Applications of Grape. Foods 2022, 11, 2755. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Napolitano, A. Natural Phenol Polymers: Recent Advances in Food and Health Applications. Antioxidants 2017, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Cordova, A.C.; Sumpio, B.E. Polyphenols are medicine: Is it time to prescribe red wine for our patients? Int. J. Angiol. 2009, 18, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Snopek, L.; Mlcek, J.; Sochorova, L.; Baron, M.; Hlavacova, I.; Jurikova, T.; Kizek, R.; Sedlackova, E.; Sochor, J. Contribution of Red Wine Consumption to Human Health Protection. Molecules 2018, 23, 1684. [Google Scholar] [CrossRef] [PubMed]
- Hrelia, S.; Di Renzo, L.; Bavaresco, L.; Bernardi, E.; Malaguti, M.; Giacosa, A. Moderate Wine Consumption and Health: A Narrative Review. Nutrients 2023, 15, 175. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Fuhrman, B. Wine Flavonoids Protect against LDL Oxidation and Atherosclerosis. Ann. N. Y. Acad. Sci. 2002, 957, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Nigdikar, S.V.; Williams, N.R.; Griffin, B.A.; Howard, A.N. Consumption of red wine polyphenols reduces the susceptibility of low-density lipoproteins to oxidation in vivo. Am. J. Clin. Nutr. 1998, 68, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Apostolidou, C.; Adamopoulos, K.; Lymperaki, E.; Iliadis, S.; Papapreponis, P.; Kourtidou-Papadeli, C. Cardiovascular risk and benefits from antioxidant dietary intervention with red wine in asymptomatic hypercholesterolemics. Clin. Nutr. ESPEN 2015, 10, e224–e233. [Google Scholar] [CrossRef]
- Stranieri, C.; Guzzo, F.; Gambini, S.; Cominacini, L.; Fratta Pasini, A.M. Intracellular Polyphenol Wine Metabolites Oppose Oxidative Stress and Upregulate Nrf2/ARE Pathway. Antioxidants 2022, 11, 2055. [Google Scholar] [CrossRef]
- Schrieks, I.C.; van den Berg, R.; Sierksma, A.; Beulens, J.W.; Vaes, W.H.; Hendriks, H.F. Effect of red wine consumption on biomarkers of oxidative stress. Alcohol Alcohol. 2013, 48, 153–159. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Arranz, S.; Lamuela-Raventos, R.M.; Estruch, R. Effects of wine, alcohol and polyphenols on cardiovascular disease risk factors: Evidences from human studies. Alcohol Alcohol. 2013, 48, 270–277. [Google Scholar] [CrossRef]
- Mangge, H.; Becker, K.; Fuchs, D.; Gostner, J.M. Antioxidants, inflammation and cardiovascular disease. World J. Cardiol. 2014, 6, 462–477. [Google Scholar] [CrossRef] [PubMed]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [PubMed]
- Habauzit, V.; Morand, C. Evidence for a protective effect of polyphenols-containing foods on cardiovascular health: An update for clinicians. Ther. Adv. Chronic. Dis. 2012, 3, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Marsella, L.T.; Carraro, A.; Valente, R.; Gualtieri, P.; Gratteri, S.; Tomasi, D.; Gaiotti, F.; De Lorenzo, A. Changes in LDL Oxidative Status and Oxidative and Inflammatory Gene Expression after Red Wine Intake in Healthy People: A Randomized Trial. Mediat. Inflamm. 2015, 2015, 317348. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Cachofeiro, V.; Millán, J.; Lahera, V.; Nieto, M.L.; Martín, R.; Bello, E.; Alvarez-Sala, L.A. Red wine intake but not other alcoholic beverages increases total antioxidant capacity and improves pro-inflammatory profile after an oral fat diet in healthy volunteers. Rev. Clin. Esp. 2015, 215, 486–494. [Google Scholar] [CrossRef]
- Weseler, A.R.; Ruijters, E.J.B.; Drittij-Reijnders, M.-J.; Reesink, K.D.; Haenen, G.R.M.M.; Bast, A. Pleiotropic Benefit of Monomeric and Oligomeric Flavanols on Vascular Health—A Randomized Controlled Clinical Pilot Study. PLoS ONE 2011, 6, e28460. [Google Scholar] [CrossRef]
- Yang, H.; Xiao, L.; Yuan, Y.; Luo, X.; Jiang, M.; Ni, J.; Wang, N. Procyanidin B2 inhibits NLRP3 inflammasome activation in human vascular endothelial cells. Biochem. Pharmacol. 2014, 92, 599–606. [Google Scholar] [CrossRef]
- De Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the Lyon Diet Heart Study. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, J.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health benefits and molecular mechanisms of resveratrol: A narrative review. Foods 2020, 9, 340. [Google Scholar] [CrossRef]
- Khattar, S.; Khan, S.A.; Zaidi, S.A.A.; Darvishikolour, M.; Farooq, U.; Naseef, P.P.; Kurunian, M.S.; Khan, M.Z.; Shamim, A.; Khan, M.M.U.; et al. Resveratrol from Dietary Supplement to a Drug Candidate: An Assessment of Potential. Pharmaceuticals 2022, 15, 957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Li, C.X.; Kakar, M.U.; Khan, M.S.; Wu, P.F.; Amir, R.M.; Dai, D.F.; Naveed, M.; Li, Q.Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Zam, W.; Kumar, M.; Cardoso, S.M.; Pereira, O.R.; Ademiluyi, A.O.; Adeleke, O.; Moreira, A.C.; Živković, J. Phenolic bioactives as antiplatelet aggregation factors: The pivotal ingredients in maintaining cardiovascular health. Oxid. Med. Cell. Longev. 2021, 2021, 2195902. [Google Scholar] [CrossRef] [PubMed]
- Parsamanesh, N.; Asghari, A.; Sardari, S.; Tasbandi, A.; Jamialahmadi, T.; Xu, S.; Sahebkar, A. Resveratrol and endothelial function: A literature review. Pharmacol. Res. 2021, 170, 105725. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bernal, Ó.A.; Coria-Oliveros, A.J.; de la Rosa, L.A.; Rodrigo-García, J.; Del Rocío Martínez-Ruiz, N.; Sayago-Ayerdi, S.G.; Alvarez-Parrilla, E. Cardioprotective effect of red wine and grape pomace. Food Res. Int. 2021, 140, 110069. [Google Scholar] [CrossRef] [PubMed]
- Wiciński, M.; Socha, M.; Walczak, M.; Wódkiewicz, E.; Malinowski, B.; Rewerski, S.; Górski, K.; Pawlak-Osińska, K. Beneficial Effects of Resveratrol Administration-Focus on Potential Biochemical Mechanisms in Cardiovascular Conditions. Nutrients 2018, 10, 1813. [Google Scholar] [CrossRef] [PubMed]
- Kuršvietienė, L.; Stanevičienė, I.; Mongirdienė, A.; Bernatonienė, J. Multiplicity of effects and health benefits of resveratrol. Medicina 2016, 52, 148–155. [Google Scholar] [CrossRef]
- Riccioni, G.; Gammone, M.A.; Tettamanti, G.; Bergante, S.; Pluchinotta, F.R.; D’Orazio, N. Resveratrol and anti-atherogenic effects. Int. J. Food Sci. Nutr. 2015, 66, 603–610. [Google Scholar] [CrossRef]
- Tamer, F.; Tullemans, B.M.E.; Kuijpers, M.J.E.; Claushuis, T.A.M.; Heemskerk, J.W.M. Nutrition Phytochemicals Affecting Platelet Signaling and Responsiveness: Implications for Thrombosis and Hemostasis. Thromb. Haemost. 2022, 122, 879–894. [Google Scholar] [CrossRef]
- Shahcheraghi, S.H.; Salemi, F.; Small, S.; Syed, S.; Salari, F.; Alam, W.; Cheang, W.S.; Saso, L.; Khan, H. Resveratrol regulates inflammation and improves oxidative stress via Nrf2 signaling pathway: Therapeutic and biotechnological prospects. Phytother. Res. 2023, 37, 1590–1605. [Google Scholar] [CrossRef]
- Rius, C.; Abu-Taha, M.; Hermenegildo, C.; Piqueras, L.; Cerda-Nicolas, J.M.; Issekutz, A.C.; Estañ, L.; Cortijo, J.; Morcillo, E.J.; Orallo, F.; et al. Trans- but Not Cis-Resveratrol impairs angiotensin-II-mediated vascular inflammation through inhibition of NF-κB activation and peroxisome proliferator-activated Receptor-γ upregulation. J. Immunol. 2010, 185, 3718–3727. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Smith, K.; Labinskyy, N.; Orosz, Z.; Rivera, A.; Ungvari, Z. Resveratrol attenuates TNF-alpha-induced activation of coronary arterial endothelial cells: Role of NF-kappaB inhibition. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1694–H1699. [Google Scholar] [CrossRef] [PubMed]
- Toaldo, I.M.; Van Camp, J.; Gonzales, G.B.; Kamiloglu, S.; Bordignon-Luiz, M.T.; Smagghe, G.; Raes, K.; Capanoglu, E.; Grootaert, C. Resveratrol improves TNF-α-induced endothelial dysfunction in a coculture model of a Caco-2 with an endothelial cell line. J. Nutr. Biochem. 2016, 36, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Chalons, P.; Amor, S.; Courtaut, F.; Cantos-Villar, E.; Richard, T.; Auger, C.; Chabert, P.; Schni-Kerth, V.; Aires, V.; Delmas, D. Study of potential anti-inflammatory effects of red wine extract and resveratrol through a modulation of interleukin-1-beta in macrophages. Nutrients 2018, 10, 1856. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Ogasawara, Y.; Hayashi, H.; Inoue, K.; Sakashita, H. Resveratrol Inhibits Proliferation and Induces Autophagy by Blocking SREBP1 Expression in Oral Cancer Cells. Molecules 2022, 27, 8250. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, A.; Claps, F.; Pastore, A.L.; Perotti, A.; Biagini, A.; Sallicandro, L.; Gentile, R.; Caglioti, C.; Palazzetti, F.; Fioretti, B. Focus on the Use of Resveratrol in Bladder Cancer. Int. J. Mol. Sci. 2023, 24, 4562. [Google Scholar] [CrossRef] [PubMed]
- Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Beneficial Effects of Red Wine Polyphenols on Human Health: Comprehensive Review. Curr. Issues Mol. Biol. 2023, 45, 782–798. [Google Scholar] [CrossRef]
- Chimento, A.; D’Amico, M.; De Luca, A.; Conforti, F.L.; Pezzi, V.; De Amicis, F. Resveratrol, Epigallocatechin Gallate and Curcumin for Cancer Therapy: Challenges from Their Pro-Apoptotic Properties. Life 2023, 13, 261. [Google Scholar] [CrossRef]
- Lalani, A.R.; Fakhari, F.; Radgoudarzi, S.; Rastegar-Pouyani, N.; Moloudi, K.; Khodamoradi, E.; Taeb, S.; Najafi, M. Immunoregulation by resveratrol; implications for normal tissue protection and tumour suppression. Clin. Exp. Pharmacol. Physiol. 2023, 50, 353–368. [Google Scholar] [CrossRef]
- Angellotti, G.; Di Prima, G.; Belfiore, E.; Campisi, G.; De Caro, V. Chemopreventive and Anticancer Role of Resveratrol against Oral Squamous Cell Carcinoma. Pharmaceutics 2023, 15, 275. [Google Scholar] [CrossRef]
- Gupta, D.S.; Gadi, V.; Kaur, G.; Chintamaneni, M.; Tuli, H.; Ramniwas, S.; Sethi, G. Resveratrol and Its Role in the Management of B-Cell Malignancies-A Recent Update. Biomedicines 2023, 11, 221. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Fan, F.; Hou, X.; Gao, C.; Meng, L.; Meng, S.; Huang, S.; Wu, H. Resveratrol suppresses pulmonary tumor metastasis by inhibiting platelet-mediated angiogenic responses. J. Surg. Res. 2017, 217, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Bae, J.U.; Kim, I.S.; Chang, C.L.; Oh, S.O.; Kim, C.D. SIRT1 prevents pulmonary thrombus formation induced by arachidonic acid via downregulation of PAF receptor expression in platelets. Platelets 2016, 27, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Michno, A.; Grużewska, K.; Ronowska, A.; Gul-Hinc, S.; Zyśk, M.; Jankowska-Kulawy, A. Resveratrol Inhibits Metabolism and Affects Blood Platelet Function in Type 2 Diabetes. Nutrients 2022, 14, 1633. [Google Scholar] [CrossRef] [PubMed]
- Crescente, M.; Jessen, G.; Momi, S.; Höltje, H.D.; Gresele, P.; Cerletti, C.; de Gaetano, G. Interactions of gallic acid, resveratrol, quercetin and aspirin at the platelet cyclooxygenase-1 level. Functional and modelling studies. Thromb. Haem. 2009, 102, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Marumo, M.; Ekawa, K.; Wakabayashi, I. Resveratrol inhibits Ca2+ signals and aggregation of platelets. Environ. Health Prev. Med. 2020, 25, 70. [Google Scholar] [CrossRef] [PubMed]
- Vlachogianni, I.C.; Fragopoulou, E.; Stamatakis, G.M.; Kostakis, I.K.; Antonopoulou, S. Platelet Activating Factor (PAF) biosynthesis is inhibited by phenolic compounds in U-937 cells under inflammatory conditions. Prostaglandins Other Lipid Mediat. 2015, 121, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Eräsalo, H.; Hämäläinen, M.; Leppänen, T.; Mäki-Opas, I.; Laavola, M.; Haavikko, R.; Yli-Kauhaluoma, J.; Moilanen, E. Natural Stilbenoids Have Anti-Inflammatory Properties in Vivo and Down-Regulate the Production of Inflammatory Mediators NO, IL6, and MCP1 Possibly in a PI3K/Akt-Dependent Manner. J. Nat. Prod. 2018, 81, 1131–1142. [Google Scholar] [CrossRef]
- Dutra, L.A.; Guanaes, J.F.O.; Johmann, N.; Lopes Pires, M.E.; Chin, C.M.; Marcondes, S.; Dos Santos, J.L. Synthesis, antiplatelet and antithrombotic activities of resveratrol derivatives with NO-donor properties. Bioorg. Med. Chem. Lett. 2017, 27, 2450–2453. [Google Scholar] [CrossRef]
- Deng, Y.H.; Alex, D.; Huang, H.Q.; Wang, N.; Yu, N.; Wang, Y.T.; Leung, G.P.; Lee, S.M. Inhibition of TNF-α-mediated endothelial cell-monocyte cell adhesion and adhesion molecules expression by the resveratrol derivative, trans-3,5,4’-trimethoxystilbene. Phytother. Res. 2011, 25, 451–457. [Google Scholar] [CrossRef]
- Nash, V.; Ranadheera, C.S.; Georgousopoulou, E.N.; Mellor, D.D.; Panagiotakos, D.B.; McKune, A.J.; Kellett, J.; Naumovski, N. The effects of grape and red wine polyphenols on gut microbiota—A systematic review. Food Res. Int. 2018, 113, 277–287. [Google Scholar] [CrossRef]
- Dueñas, M.; Cueva, C.; Muñoz-González, I.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolomé, B. Studies on Modulation of Gut Microbiota by Wine Polyphenols: From Isolated Cultures to Omic Approaches. Antioxidants 2015, 4, 1–21. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef]
- Haas, E.A.; Saad, M.J.A.; Santos, A.; Vitulo, N.; Lemos, W.J.F.; Martins, A.M.A.; Picossi, C.R.C.; Favarato, D.; Gaspar, R.S.; Magro, D.O.; et al. WineFlora Study. A red wine intervention does not modify plasma trimethylamine N-oxide but is associated with broad shifts in the plasma metabolome and gut microbiota composition. Am. J. Clin. Nutr. 2022, 116, 1515–1529. [Google Scholar] [CrossRef] [PubMed]
- Suo, H.; Shishir, M.R.I.; Xiao, J.; Wang, M.; Chen, F.; Cheng, K.-W. Red Wine High-Molecular-Weight Polyphenolic Complex: An Emerging Modulator of Human Metabolic Disease Risk and Gut Microbiota. J. Agr. Food Chem. 2021, 69, 10907–10919. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Yin, N.; Wele, P.; Li, F.; Dave, S.; Lin, J.; Xiao, H.; Wu, X. Resveratrol in disease prevention and health promotion: A role of the gut microbiome. Crit. Rev. Food Sci. Nutr. 2023, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Brummell, C.; Kealy, C.; Vitkaitis, K.; Redfern, S.; Zabetakis, I. Cardio-Protective Properties and Health Benefits of Fish Lipid Bioactives; The Effects of Thermal Processing. Mar. Drugs 2022, 20, 187. [Google Scholar] [CrossRef]
- Nunez, D.; Randon, J.; Gandhi, C.; Siafaka-Kapadai, A.; Olson, M.S.; Hanahan, D.J. The inibition of platelet-activating factor-induced platelet activation by oleic acid is associated with a decrease in polyphosphoinositide metabolism. J. Biol. Chem. 1990, 265, 18330–18338. [Google Scholar] [CrossRef]
- Perdomo, L.; Beneit, N.; Otero, Y.F.; Escribano, O.; Diaz-Castroverde, S.; Gómez-Hernández, A.; Benito, M. Protective role of oleic acid against cardiovascular insulin resistance and in the early and late cellular atherosclerotic process. Cardiovasc. Diabetol. 2015, 14, 75–87. [Google Scholar] [CrossRef]
- Delgado, G.E.; Krämer, B.K.; Lorkowski, S.; März, W.; Von Schacky, C.; Kleber, M.E. Individual omega-9 monounsaturated fatty acids and mortality—The Ludwigshafen Risk and Cardiovascular Health Study. J. Clin. Lipidol. 2017, 11, 126–135. [Google Scholar] [CrossRef]
- Holy, E.W.; Forestier, M.; Richter, E.K.; Akhmedov, A.; Leiber, F.; Camici, G.C.; Mocharla, P.; Lüscher, T.F.; Beer, J.H.; Tanner, F.C. Dietary α-linolenic acid inhibits arterial thrombus formation, tissue factor expression, and platelet activation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Bazán-Salinas, I.L.; Matías-Pérez, D.; Pérez-Campos, E.; Pérez-Campos Mayoral, L.; García-Montalvo, I.A. Reduction of platelet aggregation from ingestion of oleic and linoleic acids found in Vitis vinifera and Arachis hypogaea Oils. Am. J. Ther. 2016, 23, e1315–e1319. [Google Scholar] [CrossRef] [PubMed]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Rombaut, N.; Savoire, R.; Thomasset, B.; Castello, J.; Van Hecke, E.; Lanoisellé, J.-L. Optimization of oil yield and oil total phenolic content during grape seed cold screw pressing. Ind. Crops Prod. 2015, 63, 26–33. [Google Scholar] [CrossRef]
- Garavaglia, J.; Markoski, M.M.; Oliveira, A.; Marcadenti, A. Grape Seed Oil Compounds: Biological and Chemical Actions for Health. Nutr. Metab. Insights 2016, 9, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Matthäus, B. Virgin grape seed oil: Is it really a nutritional highlight? Eur. J. Lipid. Sci. Technol. 2008, 110, 645–650. [Google Scholar] [CrossRef]
- Moran, D.; Fleming, M.; Daly, E.; Gaughan, N.; Zabetakis, I.; Traas, C.; Tsoupras, A. Anti-Platelet Properties of Apple Must/Skin Yeasts and of Their Fermented Apple Cider Products. Beverages 2021, 7, 54. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Harrington, J.; Pienaar, R.; Devaney, K.; Heaney, S.; Koidis, A.; Zabetakis, I. The Effects of Oxidation on the Antithrombotic Properties of Tea Lipids against PAF, Thrombin, Collagen, and ADP. Foods 2020, 9, 385. [Google Scholar] [CrossRef]
- Janssen, I.; Landay, A.L.; Ruppert, K.; Powell, L.H. Moderate wine consumption is associated with lower hemostatic and inflammatory risk factors over 8 years: The study of women’s health across the nation (SWAN). Nutr. Aging 2014, 2, 91–99. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Kouli, G.-M.; Magripis, E.; Kyrou, I.; Georgousopoulou, E.N.; Chrysohoou, C.; Tsigos, C.; Tousoulis, D.; Pitsavos, C. Beer, wine consumption, and 10-year CVD incidence: The ATTICA study. Eur. J. Clin. Nutr. 2019, 73, 1015–1023. [Google Scholar] [CrossRef]
- Taborsky, M.; Ostadal, P.; Adam, T.; Moravec, O.; Gloger, V.; Schee, A.; Skala, T. Red or white wine consumption effect on atherosclerosis in healthy individuals (In Vino Veritas study). Bratisl. Lekárske Listy 2017, 118, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Salazar, H.M.; de Deus Mendonça, R.; Laclaustra, M.; Moreno-Franco, B.; Åkesson, A.; Guallar-Castillón, P.; Donat-Vargas, C. The intake of flavonoids, stilbenes, and tyrosols, mainly consumed through red wine and virgin olive oil, is associated with lower carotid and femoral subclinical atherosclerosis and coronary calcium. Eur. J. Nutr. 2022, 61, 2697–2709. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Llorach, R.; Rotches-Ribalta, M.; Guillén, M.; Casas, R.; Arranz, S.; Valderas-Martinez, P.; Portoles, O.; Corella, D. Differential effects of polyphenols and alcohol of red wine on the expression of adhesion molecules and inflammatory cytokines related to atherosclerosis: A randomized clinical trial. Am. J. Clin. Nutr. 2011, 95, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Canali, R.; Comitato, R.; Ambra, R.; Virgili, F. Red wine metabolites modulate NF-κB, activator protein-1 and cAMP response element-binding proteins in human endothelial cells. Br. J. Nutr. 2010, 103, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Nallasamy, P.; Kang, Z.Y.; Sun, X.; Anandh Babu, P.V.; Liu, D.; Jia, Z. Natural compound resveratrol attenuates TNF-alpha-induced vascular dysfunction in mice and human endothelial cells: The involvement of the NF-κB signaling pathway. Int. J. Mol. Sci. 2021, 22, 12486. [Google Scholar] [CrossRef] [PubMed]
- Kechagias, S.; Zanjani, S.; Gjellan, S.; Leinhard, O.D.; Kihlberg, J.; Smedby, O.; Johansson, L.; Kullberg, J.; Ahlström, H.; Lindström, T.; et al. Effects of moderate red wine consumption on liver fat and blood lipids: A prospective randomized study. Ann. Med. 2011, 43, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Roth, I.; Casas, R.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Consumption of aged white wine modulates cardiovascular risk factors via circulating endothelial progenitor cells and inflammatory biomarkers. Clin. Nutr. 2019, 38, 1036–1044. [Google Scholar] [CrossRef]
- Huang, P.H.; Chen, Y.H.; Tsai, H.Y.; Chen, J.S.; Wu, T.C.; Lin, F.Y.; Sata, M.; Chen, J.W.; Lin, S.J. Intake of Red Wine Increases the Number and Functional Capacity of Circulating Endothelial Progenitor Cells by Enhancing Nitric Oxide Bioavailability. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 869–877. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; Yáñez-Gascón, M.J.; García-Almagro, F.J.; Ruiz-Ros, J.A.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: A triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovasc. Drugs Ther. 2013, 27, 37–48. [Google Scholar] [CrossRef]
- Cosmi, F.; Di Giulio, P.; Masson, S.; Finzi, A.; Marfisi, R.M.; Cosmi, D.; Scarano, M.; Tognoni, G.; Maggioni, A.P.; Porcu, M.; et al. Regular Wine Consumption in Chronic Heart Failure: Impact on Outcomes, Quality of Life, and Circulating Biomarkers. Circ. Heart Fail. 2015, 8, 428–437. [Google Scholar] [CrossRef]
- Downer, M.K.; Kenfield, S.A.; Stampfer, M.J.; Wilson, K.M.; Dickerman, B.A.; Giovannucci, E.L.; Rimm, E.B.; Wang, M.; Mucci, L.A.; Willett, W.C.; et al. Alcohol Intake and Risk of Lethal Prostate Cancer in the Health Professionals Follow-Up Study. J. Clin. Oncol. 2019, 37, 1499–1511. [Google Scholar] [CrossRef] [PubMed]
- Crockett, S.D.; Long, M.D.; Dellon, E.S.; Martin, C.F.; Galanko, J.A.; Sandler, R.S. Inverse relationship between moderate alcohol intake and rectal cancer: Analysis of the North Carolina Colon Cancer Study. Dis. Colon. Rectum. 2011, 54, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Song, C.; Suo, C.; Fan, H.; Zhang, T.; Jin, L.; Chen, X. Alcohol consumption and hepatocellular carcinoma: Novel insights from a prospective cohort study and nonlinear Mendelian randomization analysis. BMC Med. 2022, 20, 413. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, M.; Mao, Y.; Jia, Z.; Liu, H.; Yang, G.; Wang, S.; Sun, B.; Zhang, H. Very-light alcohol consumption suppresses breast tumor progression in a mouse model. Food Funct. 2022, 13, 3391–3404. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, S.M.; Kaiser, A.; Behrendt, I.; Eichner, G.; Fasshauer, M. Association of alcohol types, coffee and tea intake with mortality: Prospective cohort study of UK Biobank participants. Br. J. Nutr. 2022, 129, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Turati, F.; Carioli, G.; Bravi, F.; Ferraroni, M.; Serraino, D.; Montella, M.; Giacosa, A.; Toffolutti, F.; Negri, E.; Levi, F.; et al. Mediterranean Diet and Breast Cancer Risk. Nutrients 2018, 10, 326. [Google Scholar] [CrossRef] [PubMed]
- Shufelt, C.; Merz, C.N.; Yang, Y.; Kirschner, J.; Polk, D.; Stanczyk, F.; Paul-Labrador, M.; Braunstein, D. Red versus white wine as a nutritional aromatase inhibitor in premenopausal women: A pilot study. J. Womens Health 2012, 21, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Mellinger, J.L.; Trivedi, P.J. Alcohol Consumption in Patients with Non-alcoholic Fatty Liver Disease: Convenient vs. Inconvenient Truths. Am. J. Gastroenterol. 2018, 113, 1437–1439. [Google Scholar] [CrossRef]
- Zhu, W.; Qin, W.; Zhang, K.; Rottinghaus, G.E.; Chen, Y.C.; Kliethermes, B.; Sauter, E.R. Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr. Cancer. 2012, 64, 393–400. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Bulló, M.; Salas-Salvadó, J.; Corella, D.; Fitó, M.; Gea, A.; Gómez-Gracia, E.; Lapetra, J.; et al. Moderate red wine consumption is associated with a lower prevalence of the metabolic syndrome in the PREDIMED population. Br. J. Nutr. 2015, 113, S121–S130. [Google Scholar] [CrossRef]
- Larsen, B.A.; Klinedinst, B.S.; Le, S.T.; Pappas, C.; Wolf, T.; Meier, N.F.; Lim, Y.L.; Willette, A.A. Beer, wine, and spirits differentially influence body composition in older white adults-a United Kingdom Biobank study. Obes. Sci. Pract. 2022, 8, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Gepner, Y.; Golan, R.; Harman-Boehm, I.; Henkin, Y.; Schwarzfuchs, D.; Shelef, I.; Durst, R.; Kovsan, J.; Bolotin, A.; Leitersdorf, E.; et al. Effects of Initiating Moderate Alcohol Intake on Cardiometabolic Risk in Adults With Type 2 Diabetes: A 2-Year Randomized, Controlled Trial. Ann. Intern. Med. 2015, 163, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Golan, R.; Shai, I.; Gepner, Y.; Harman-Boehm, I.; Schwarzfuchs, D.; Spence, J.D.; Parraga, G.; Buchanan, D.; Witkow, S.; Friger, M.; et al. Effect of wine on carotid atherosclerosis in type 2 diabetes: A 2-year randomized controlled trial. Eur. J. Clin. Nutr. 2018, 72, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, X.; Li, X.; Heianza, Y.; Qi, L. Moderate alcohol drinking with meals is related to lower incidence of type 2 diabetes. Am. J. Clin. Nutr. 2022, 116, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Valderas-Martinez, P.; Casas, R.; Arranz, S.; Guillén, M.; Lamuela-Raventós, R.M.; Llorach, R.; Andres-Lacueva, C.; et al. Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: A randomized clinical trial. Clin. Nutr. 2013, 32, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Stagling, S.; Lundberg, A.; Nystrom, F.H. A cross-over study of postprandial effects from moist snuff and red wine on metabolic rate, appetite-related hormones and glucose. Drug Alcohol Depend. 2022, 236, 109479. [Google Scholar] [CrossRef]
- Sattarinezhad, A.; Roozbeh, J.; Shirazi Yeganeh, B.; Omrani, G.R.; Shams, M. Resveratrol reduces albuminuria in diabetic nephropathy: A randomized double-blind placebo-controlled clinical trial. Diabetes Metab. 2019, 45, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Liu, Y.; Ren, G.; Yin, L.; Liang, X.; Geng, T.; Dang, H.; An, R. Resveratrol limits diabetes-associated cognitive decline in rats by preventing oxidative stress and inflammation and modulating hippocampal structural synaptic plasticity. Brain Res. 2016, 1650, 1–9. [Google Scholar] [CrossRef]
- Fischer, K.; Melo van Lent, D.; Wolfsgruber, S.; Weinhold, L.; Kleineidam, L.; Bickel, H.; Scherer, M.; Eisele, M.; van den Bussche, H.; Wiese, B.; et al. Prospective Associations between Single Foods, Alzheimer’s Dementia and Memory Decline in the Elderly. Nutrients 2018, 10, 852. [Google Scholar] [CrossRef]
- Mendes, D.; Oliveira, M.M.; Moreira, P.I.; Coutinho, J.; Nunes, F.M.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Videira, R.A. Beneficial effects of white wine polyphenols-enriched diet on Alzheimer’s disease-like pathology. J. Nutr. Biochem. 2018, 55, 165–177. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Wan, Y.; Tan, C.; Li, J.; Tan, L.; Yu, J.T. Alcohol consumption and dementia risk: A dose-response meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Ferruzzi, M.G.; Janle, E.M.; Wang, J.; Gong, B.; Chen, T.Y.; Lobo, J.; Cooper, B.; Wu, Q.L.; Talcott, S.T.; et al. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. FASEB J. 2013, 27, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Valls-Pedret, C.; Lamuela-Raventós, R.M.; Medina-Remón, A.; Quintana, M.; Corella, D.; Pintó, X.; Martínez-González, M.Á.; Estruch, R.; Ros, E. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J. Alzheimers Dis. 2012, 29, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Smyth, A.; O’Donnell, M.; Rangarajan, S.; Hankey, G.J.; Oveisgharan, S.; Canavan, M.; McDermott, C.; Xavier, D.; Zhang, H.; Damasceno, A.; et al. INTERSTROKE Investigators. Alcohol Intake as a Risk Factor for Acute Stroke: The INTERSTROKE Study. Neurology 2023, 100, e142–e153. [Google Scholar] [CrossRef] [PubMed]
- Kaluza, J.; Harris, H.R.; Linden, A.; Wolk, A. Alcohol Consumption and Risk of Chronic Obstructive Pulmonary Disease: A Prospective Cohort Study of Men. Am. J. Epidemiol. 2019, 188, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Castellano, S.; Ray, S.; Grosso, G.; Galvano, F. Dietary Polyphenol Intake and Depression: Results from the Mediterranean Healthy Eating, Lifestyle and Aging (MEAL) Study. Molecules 2018, 23, 999. [Google Scholar] [CrossRef]
- Barbería-Latasa, M.; Bes-Rastrollo, M.; Pérez-Araluce, R.; Martínez-González, M.Á.; Gea, A. Mediterranean Alcohol-Drinking Patterns and All-Cause Mortality in Women More Than 55 Years Old and Men More Than 50 Years Old in the "Seguimiento Universidad de Navarra" (SUN) Cohort. Nutrients 2022, 14, 5310. [Google Scholar] [CrossRef] [PubMed]
- Carballo-Casla, A.; Ortolá, R.; García-Esquinas, E.; Oliveira, A.; Sotos-Prieto, M.; Lopes, C.; Lopez-Garcia, E.; Rodríguez-Artalejo, F. The Southern European Atlantic Diet and all-cause mortality in older adults. BMC Med. 2021, 19, 36. [Google Scholar] [CrossRef]
- Grønbaek, M.; Becker, U.; Johansen, D.; Gottschau, A.; Schnohr, P.; Hein, H.O.; Jensen, G.; Sørensen, T.I. Type of alcohol consumed and mortality from all causes, coronary heart disease, and cancer. Ann. Intern. Med. 2000, 133, 411–419. [Google Scholar] [CrossRef]
- Noguer, M.A.; Cerezo, A.B.; Donoso Navarro, E.; Garcia-Parrilla, M.C. Intake of alcohol-free red wine modulates antioxidant enzyme activities in a human intervention study. Pharmacol. Res. 2012, 65, 609–614. [Google Scholar] [CrossRef]
- Giovannucci, E.; Stampfer, M.J.; Colditz, G.A.; Manson, J.E.; Rosner, B.A.; Longnecker, M.P.; Speizer, F.E.; Willett, W.C. Recall and selection bias in reporting past alcohol consumption among breast cancer cases. Cancer Causes Control 1993, 4, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, W.Q.; Qureshi, A.A.; Cho, E. Alcohol consumption and risk of cutaneous basal cell carcinoma in women and men: 3 prospective cohort studies. Am. J. Clin. Nutr. 2015, 102, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, N.; Bouras, E.; van den Brandt, P.A.; Muller, D.C.; Papadopoulou, A.; Heath, A.K.; Critselis, E.; Gunter, M.J.; Vineis, P.; Ferrari, P.; et al. A Prospective Diet-Wide Association Study for Risk of Colorectal Cancer in EPIC. Clin. Gastroenterol. Hepatol. 2022, 20, 864–873.e13. [Google Scholar] [CrossRef] [PubMed]
- Mahamat-Saleh, Y.; Al-Rahmoun, M.; Severi, G.; Ghiasvand, R.; Veierod, M.B.; Caini, S.; Palli, D.; Botteri, E.; Sacerdote, C.; Ricceri, F.; et al. Baseline and lifetime alcohol consumption and risk of skin cancer in the European Prospective Investigation into Cancer and Nutrition cohort (EPIC). Int. J. Cancer 2023, 152, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Heath, A.K.; Muller, D.C.; Brandt, P.A.v.D.; Papadimitriou, N.; Critselis, E.; Gunter, M.; Vineis, P.; Weiderpass, E.; Fagherazzi, G.; Boeing, H.; et al. Nutrient-wide association study of 92 foods and nutrients and breast cancer risk. Breast Cancer Res. 2020, 22, 5. [Google Scholar] [CrossRef] [PubMed]
- Naudin, S.; Li, K.; Jaouen, T.; Assi, N.; Kyrø, C.; Tjønneland, A.; Overvad, K.; Boutron-Ruault, M.-C.; Rebours, V.; Védié, A.-L.; et al. Lifetime and baseline alcohol intakes and risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition study. Int. J. Cancer 2018, 143, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Seidenberg, A.B.; Wiseman, K.P.; Klein, W.M.P. Do Beliefs about Alcohol and Cancer Risk Vary by Alcoholic Beverage Type and Heart Disease Risk Beliefs? Cancer Epidemiol. Biomark. Prev. 2023, 32, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Hay, J.L.; Kiviniemi, M.T.; Orom, H.; Waters, E.A. Moving beyond the "Health Halo" of Alcohol: What Will it Take to Achieve Population Awareness of the Cancer Risks of Alcohol? Cancer Epidemiol. Biomark. Prev. 2023, 32, 9–11. [Google Scholar] [CrossRef]
- Duell, E.J.; Travier, N.; Lujan-Barroso, L.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C.; Morois, S.; Palli, D.; Krogh, V.; Panico, S.; Tumino, R.; et al. Alcohol consumption and gastric cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Am. J. Clin. Nutr. 2011, 94, 1266–1275. [Google Scholar] [CrossRef]
- Cote, D.J.; Smith, T.R.; Kaiser, U.B.; Laws, E.R., Jr.; Stampfer, M.J. Alcohol intake and risk of pituitary adenoma. Cancer Causes Control 2022, 33, 353–361. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Liu, Y.L.; Zheng, Y.; Ivey, K.; Willett, W.C.; Stampfer, M.J.; Rimm, E.B.; Cassidy, A. Change in habitual intakes of flavonoid-rich foods and mortality in US males and females. BMC Med. 2023, 21, 181. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Guo, H.; Fang, Y.; Zhou, G. The mechanisms of wine phenolic compounds for preclinical anticancer therapeutics. Food Nutr. Res. 2021, 65. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.H.; Bybee, K.A.; Lavie, C.J. Alcohol and cardiovascular health: The razor-sharp double-edged sword. J. Am. Coll. Cardiol. 2007, 50, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, E.L.; DiNicolantonio, J.J.; O’Keefe, J.H.; Lavie, C.J. Alcohol and CV Health: Jekyll and Hyde J-Curves. Prog. Cardiov. Dis. 2018, 61, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Poli, A. Is drinking wine in moderation good for health or not? Eur. Heart J. Suppl. 2022, 24, I119–I122. [Google Scholar] [CrossRef] [PubMed]
- Rifler, J.P. Is a Meal without Wine Good for Health? Diseases 2018, 6, 105. [Google Scholar] [CrossRef] [PubMed]
- Gerardi, G.; Cavia-Saiz, M.; Muñiz, P. From winery by-product to healthy product: Bioavailability, redox signaling and oxidative stress modulation by wine pomace product. Crit. Rev. Food Sci. Nutr. 2022, 62, 7427–7448. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, J.Y.; Deng, Z.X.; Pan, X.Q.; Xie, X.F.; Peng, C. Effective utilization of food wastes: Bioactivity of grape seed extraction and its application in food industry. J. Funct. Foods 2020, 73, 104113. [Google Scholar] [CrossRef]
- Yang, C.; Han, Y.; Tian, X.; Sajid, M.; Mehmood, S.; Wang, H.; Li, H. Phenolic composition of grape pomace and its metabolism. Crit. Rev. Food Sci. Nutr. 2022, 17, 1–17. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. The Potential of Grape Pomace Varieties as a Dietary Source of Pectic Substances. Foods 2021, 10, 867. [Google Scholar] [CrossRef]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive Phenolic Compounds From Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, T.; Chowdhary, P.; Chaurasia, D.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Sustainable green processing of grape pomace for the production of value-added products: An overview. Environ. Technol. Innov. 2021, 23, 101592. [Google Scholar] [CrossRef]
- Chowdhary, P.; Gupta, A.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Current trends and possibilities for exploitation of Grape pomace as a potential source for value addition. Environ. Pollut. 2021, 278, 116796. [Google Scholar] [CrossRef] [PubMed]
- Drevelegka, I.; Goula, A.M. Recovery of grape pomace phenolic compounds through optimized extraction and adsorption processes. Chem. Eng. Process.-Process Intensif. 2020, 149, 107845. [Google Scholar] [CrossRef]
- Barba, F.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Jokić, S.; Velić, D.; Bilić, M.; Bucić-Kojić, A.; Planinić, M.; Tomas, S. Modelling of the Process of Solid-Liquid Extraction of Total Polyphenols from Soybeans. Czech J. Food Sci. 2010, 28, 7. [Google Scholar] [CrossRef]
- Yang, C.L.; Shang, K.; Lin, C.C.; Wang, C.; Shi, X.Q.; Wang, H.; Li, H. Processing technologies, phytochemical constituents, and biological activities of grape seed oil (gso): A review. Trends Food Sci. Technol. 2021, 116, 1074–1083. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A Extr. Technol. 2010, 1217, 2495–2511. [Google Scholar] [CrossRef]
- Pinelo, M.; Ruiz-Rodríguez, A.; Sineiro, J.; Señoráns, F.J.; Reglero, G.; Núñez, M.J. Supercritical fluid and solid–liquid extraction of phenolic antioxidants from grape pomace: A comparative study. Eur. Food Res. Technol. 2007, 226, 199–205. [Google Scholar] [CrossRef]
- Jeong, J.; Jung, H.; Lee, S.; Lee, H.; Hwang, K.; Kim, T. Anti-oxidant, anti-proliferative and anti-inflammatory activities of the extracts from black raspberry fruits and wine. Food Chem. 2010, 123, 338–344. [Google Scholar] [CrossRef]
- Casas, L.; Mantell, C.; Rodríguez, M.; de la Ossa, E.J.M.; Roldán, A.; Ory, I.D.; Caro, I.; Blandino, A. Extraction of resveratrol from the pomace of Palomino fino grapes by supercritical carbon dioxide. J. Food Eng. 2010, 96, 304–308. [Google Scholar] [CrossRef]
- Liazid, A.; Guerrero, R.F.; Cantos, E.; Palma, M.; Barroso, C.G. Microwave assisted extraction of anthocyanins from grape skins. Food Chem. 2011, 124, 1238–1243. [Google Scholar] [CrossRef]
- Yu, H.B.; Ding, L.F.; Wang, Z.; Shi, L.X. Study on Extraction of Polyphenol from Grape Peel Microwave-Assisted Activity. Adv. Mater. Res. 2014, 864–867, 520–525. [Google Scholar] [CrossRef]
- Al Bittar, S.; Périno-Issartier, S.; Dangles, O.; Chemat, F. An innovative grape juice enriched in polyphenols by microwave-assisted extraction. Food Chem. 2013, 141, 3268–3272. [Google Scholar] [CrossRef] [PubMed]
- Da Porto, C.; Porretto, E.; Decorti, D. Comparison of ultrasound-assisted extraction with conventional extraction methods of oil and polyphenols from grape (Vitis vinifera L.) seeds. Ultrason. Sonochemistry 2013, 20, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Ind. Crops Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Wang, J.; Wang, K.; Wang, Y.; Lin, S.; Zhao, P.; Jones, G. A novel application of pulsed electric field (PEF) processing for improving glutathione (GSH) antioxidant activity. Food Chem. 2014, 161, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Jeyamkondan, S.; Jayas, D.S.; Holley, R.A. Pulsed electric field processing of foods: A review. J. Food Prot. 1999, 62, 1088–1096. [Google Scholar] [CrossRef]
- Brianceau, S.; Turk, M.; Vitrac, X.; Vorobiev, E. Combined densification and pulsed electric field treatment for selective polyphenols recovery from fermented grape pomace. Innov. Food Sci. Emerg. Technol. App. Food Process 2015, 29, 2–8. [Google Scholar] [CrossRef]
- Wan, J.; Coventry, J.; Swiergon, P.; Sanguansri, P.; Versteeg, C. Advances in innovative processing technologies for microbial inactivation and enhancement of food safety-pulsed electric field and low-temperature plasma. Trends Food Sci. Technol. Nat. Safe Foods 2009, 20, 414–424. [Google Scholar] [CrossRef]
- Barba, F.J.; Brianceau, S.; Turk, M.; Boussetta, N.; Vorobiev, E. Effect of Alternative Physical Treatments (Ultrasounds, Pulsed Electric Fields, and High-Voltage Electrical Discharges) on Selective Recovery of Bio-compounds from Fermented Grape Pomace. Food Bioprocess Technol. 2015, 8, 1139–1148. [Google Scholar] [CrossRef]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Dordoni, R.; Duserm Garrido, G.; Marinoni, L.; Torri, L.; Piochi, M.; Spigno, G. Enrichment of Whole Wheat Cocoa Biscuits with Encapsulated Grape Skin Extract. Int. J. Food Sci. 2019, 2019, 9161840. [Google Scholar] [CrossRef] [PubMed]
- Pasqualone, A.; Bianco, A.M.; Paradiso, V.M. Production trials to improve the nutritional quality of biscuits and to enrich them with natural anthocyanins. CyTA –J. Food 2013, 11, 301–308. [Google Scholar] [CrossRef][Green Version]
- Mildner-Szkudlarz, S.; Bajerska, J.; Zawirska-Wojtasiak, R.; Górecka, D. White grape pomace as a source of dietary fibre and polyphenols and its effect on physical and nutraceutical characteristics of wheat biscuits. J. Sci. Food Agric. 2013, 93, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Acun, S.; Gül, H. Effects of grape pomace and grape seed flours on cookie quality. Qual. Assur. Saf. Crops Foods 2014, 6, 81–88. [Google Scholar] [CrossRef]
- Bender, A.B.B.; Speroni, C.S.; Salvador, P.R.; Loureiro, B.B.; Lovatto, N.M.; Goulart, F.R.; Lovatto, M.T.; Miranda, M.Z.; Silva, L.P.; Penna, N.G. Grape Pomace Skins and the Effects of Its Inclusion in the Technological Properties of Muffins. J. Culin. Sci. Technol. 2017, 15, 143–157. [Google Scholar] [CrossRef]
- Šporin, M.; Avbelj, M.; Kovač, B.; Možina, S.S. Quality characteristics of wheat flour dough and bread containing grape pomace flour. Food Sci. Technol. Int. 2018, 24, 251–263. [Google Scholar] [CrossRef]
- Tolve, R.; Simonato, B.; Rainero, G.; Bianchi, F.; Rizzi, C.; Cervini, M.; Giuberti, G. Wheat Bread Fortification by Grape Pomace Powder: Nutritional, Technological, Antioxidant, and Sensory Properties. Foods 2021, 10, 75. [Google Scholar] [CrossRef]
- Bianchi, F.; Lomuscio, E.; Rizzi, C.; Simonato, B. Predicted Shelf-Life, Thermodynamic Study and Antioxidant Capacity of Breadsticks Fortified with Grape Pomace Powders. Foods 2021, 10, 2815. [Google Scholar] [CrossRef]
- Nowak, J.Z. Oxidative stress, polyunsaturated fatty acids-derived oxidation products and bisretinoids as potential inducers of CNS diseases: Focus on age-related macular degeneration. Pharmacol. Rep. 2013, 65, 288–304. [Google Scholar] [CrossRef] [PubMed]
- Hoye, C.; Ross, C. Total Phenolic Content, Consumer Acceptance, and Instrumental Analysis of Bread Made with Grape Seed Flour. J. Food Sci. 2011, 76, S428–S436. [Google Scholar] [CrossRef] [PubMed]
- Lavelli, V.; Kerr, W.L.; García-Lomillo, J.; González-SanJosé, M.L. Applications of Recovered Bioactive Compounds in Food Products. In Handbook of Grape Processing By-Products; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 10, pp. 233–266. [Google Scholar]
- Tolve, R.; Pasini, G.; Vignale, F.; Favati, F.; Simonato, B. Effect of Grape Pomace Addition on the Technological, Sensory, and Nutritional Properties of Durum Wheat Pasta. Foods 2020, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, V.; Christiano, F.D.P.; Marczak, L.D.F.; Tessaro, I.C.; Thys, R.C.S. The effect of the incorporation of grape marc powder in fettuccini pasta properties. LWT-Food Sci. Technol. 2014, 58, 497–501. [Google Scholar] [CrossRef]
- Lavelli, V.; Torri, L.; Zeppa, G.; Fiori, L.; Spigno, G. Recovery Of Winemaking By-Products For Innovative Food Applications. Ital. J. Food Sci. 2016, 28, 542–564. [Google Scholar]
- Balli, D.; Cecchi, L.; Innocenti, M.; Bellumori, M.; Mulinacci, N. Food by-products valorisation: Grape pomace and olive pomace (pâté) as sources of phenolic compounds and fiber for enrichment of tagliatelle pasta. Food Chem. 2021, 355, 129642. [Google Scholar] [CrossRef] [PubMed]
- Codină, G.G.; Zaharia, D.; Stroe, S.-G.; Ropciuc, S. Influence of calcium ions addition from gluconate and lactate salts on refined wheat flour dough rheological properties. CyTA-J. Food 2018, 16, 884–891. [Google Scholar] [CrossRef]
- Mironeasa, S.; Codina, G.; Mironeasa, C. The effects of wheat flour substitution with grape seed flour on the rheological parameters of the dough assessed by Mixolab. J. Texture Stud. 2012, 43, 40–48. [Google Scholar] [CrossRef]
- Rashwan, A.K.; Osman, A.I.; Chen, W. Natural nutraceuticals for enhancing yogurt properties: A review. Environ. Chem. Lett. 2023, 21, 1907–1931. [Google Scholar] [CrossRef]
- Barakat, H.; Hassan, M.F.Y. Chemical, Nutritional, Rheological, and Organoleptical Characterizations of Stirred Pumpkin-Yoghurt. Food Nutr. Sci. 2017, 8, 746–759. [Google Scholar] [CrossRef]
- O’Connell, J.E.; Fox, P.F. Significance and applications of phenolic compounds in the production and quality of milk and dairy products: A review. Int. Dairy J. 2001, 11, 103–120. [Google Scholar] [CrossRef]
- Demirkol, M.; Tarakci, Z. Effect of grape (Vitis labrusca L.) pomace dried by different methods on physicochemical, microbiological and bioactive properties of yoghurt. LWT Food Sci. Technol. 2018, 97, 770–777. [Google Scholar] [CrossRef]
- Tseng, A.; Zhao, Y. Wine grape pomace as antioxidant dietary fibre for enhancing nutritional value and improving storability of yogurt and salad dressing. Food Chem. 2013, 138, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.A.; do Egypto, R.D.; de Souza, E.L.; Voss, G.B.; Borges, G.D.; dos Santos Lima, M.; Pintado, M.M.; da Silva Vasconcelos, M.A. Incorporation of phenolic-rich ingredients from integral valorization of Isabel grape improves the nutritional, functional and sensory characteristics of probiotic goat milk yogurt. Food Chem. 2022, 369, 130957. [Google Scholar] [CrossRef] [PubMed]
- Marchiani, R.; Bertolino, M.; Ghirardello, D.; McSweeney, P.L.H.; Zeppa, G. Physicochemical and nutritional qualities of grape pomace powder-fortified semi-hard cheeses. J. Food Sci. Technol. 2016, 53, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Tami, S.H.; Aly, E.; Darwish, A.A.; Mohamed, E.S. Buffalo stirred yoghurt fortified with grape seed extract: New insights into its functional properties. Food Biosci. 2022, 47, 101752. [Google Scholar] [CrossRef]
- Gaglio, R.; Restivo, I.; Barbera, M.; Barbaccia, P.; Ponte, M.; Tesoriere, L.; Bonanno, A.; Attanzio, A.; Di Grigoli, A.; Francesca, N.; et al. Effect on the Antioxidant, Lipoperoxyl Radical Scavenger Capacity, Nutritional, Sensory and Microbiological Traits of an Ovine Stretched Cheese Produced with Grape Pomace Powder Addition. Antioxidants 2021, 10, 306. [Google Scholar] [CrossRef]
- Chouchouli, V.; Kalogeropoulos, N.; Konteles, S.J.; Karvela, E.; Makris, D.P.; Karathanos, V.T. Fortification of yoghurts with grape (Vitis vinifera) seed extracts. LWT-Food Sci. Technol. 2013, 53, 522–529. [Google Scholar] [CrossRef]
- Marchiani, R.; Bertolino, M.; Belviso, S.; Giordano, M.; Ghirardello, D.; Torri, L.; Piochi, M.; Zeppa, G. Yogurt Enrichment with Grape Pomace: Effect of Grape Cultivar on Physicochemical, Microbiological and Sensory Properties. J. Food Qual. 2016, 39, 77–89. [Google Scholar] [CrossRef]
- Karaaslan, M.; Ozden, M.; Vardin, H.; Turkoglu, H. Phenolic fortification of yogurt using grape and callus extracts. LWT-Food Sci. Technol. 2011, 44, 1065–1072. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Wang, Q.Y.; Wang, S.J. Effects of rosemary extract, grape seed extract and green tea polyphenol on the formation of n-Nitrosamines and quality of western-Style smoked sausage. J. Food Process Preserv. 2020, 44, e14459. [Google Scholar] [CrossRef]
- García-Lomillo, J.; González-SanJosé, M.L. Applications of Wine Pomace in the Food Industry: Approaches and Functions. Compreh. Rev. Food Sci. Food Saf. 2017, 16, 3–22. [Google Scholar] [CrossRef]
- García-Lomillo, J.; Gonzalez-SanJose, M.L.; Del Pino-García, R.; Ortega-Heras, M.; Muñiz-Rodríguez, P. Antioxidant effect of seasonings derived from wine pomace on lipid oxidation in refrigerated and frozen beef patties. LWT 2017, 77, 85–91. [Google Scholar] [CrossRef]
- Ryu, K.; Shim, K.; Shin, D. Effect of Grape Pomace Powder Addition on TBARS and Color of Cooked Pork Sausages during Storage. Korean J. Food Sci. Res. 2014, 34, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Riazi, F.; Zeynali, F.; Hoseini, E.; Behmadi, H. Effect of Dry Red Grape Pomace as a Nitrite Substitute on the Microbiological and Physicochemical Properties and Residual Nitrite of Dry-cured Sausage. Nutr. Food Sci. Res. 2016, 3, 37–44. [Google Scholar] [CrossRef]
- Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Intern. 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Bobko, M.; Haščí k, P.; Kročko, M.; Trembecká, L.; Mendelova, A.; Tkáčová, J.; Czako, P.; Tóth, T. Effect of grape seed extract on quality of raw-cooked meat products. Potr. Slovak J. Food Sci. 2017, 11, 517–521. [Google Scholar] [CrossRef][Green Version]
- Guerra-Rivas, C.; Vieira, C.; Rubio, B.; Martínez, B.; Gallardo, B.; Mantecón, A.R.; Lavín, P.; Manso, T. Effects of grape pomace in growing lamb diets compared with vitamin E and grape seed extract on meat shelf life. Meat Sci. 2016, 116, 221–229. [Google Scholar] [CrossRef]
- Selani, M.M.; Contreras-Castillo, C.J.; Shirahigue, L.D.; Gallo, C.R.; Plata-Oviedo, M.; Montes-Villanueva, N.D. Wine industry residues extracts as natural antioxidants in raw and cooked chicken meat during frozen storage. Meat Sci. 2011, 88, 397–403. [Google Scholar] [CrossRef]
- Shirahigue, L.D.; Plata-Oviedo, M.; De Alencar, S.M.; D’Arce, M.A.B.R.; De Souza Vieira, T.M.F.; Oldoni, T.L.C.; Contreras-Castillo, C.J. Wine industry residue as antioxidant in cooked chicken meat. Int. J. Food Sci. Technol. 2010, 45, 863–870. [Google Scholar] [CrossRef]
- Sáyago-Ayerdi, S.G.; Brenes, A.; Goñi, I. Effect of grape antioxidante dietary fibre on the lípido oxidation of raw and cooked Chicken hamburgers. LW-Food Sci. Technol. 2009, 42, 971–976. [Google Scholar] [CrossRef]
- Sánchez-Alonso, I.; Jiménez-Escrig, A.; Saura-Calixto, F.; Borderías, A.J. Effect of grape antioxidant dietary fibre on the prevention of lipid oxidation in miced fish: Evaluation by different methodologies. Food Chem. 2007, 101, 372–378. [Google Scholar] [CrossRef]
- Özvural, E.; Vural, H. Grape seed flour is a viable ingredient to improve the nutritional profile and reduce lipid oxidation of frankfurters. Meat Sci. 2011, 88, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Choi, J.; Han, D.; Kim, H.; Lee, M.; Kim, H.; Lee, J.; Chung, H.; Kim, C. Optimization of replacing pork back fat with grape seed oil and rice bran fibre for reduced-fat meat emulsion systems. Meat Sci. 2010, 84, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Özalp Özen, B.; Eren, M.; Pala, A.; Özmen, İ.; Soyer, A. Effect of plant extracts on lipid oxidation during frozen storage of minced fish muscle. Int. J. Food Sci. Technol. 2011, 46, 724–731. [Google Scholar] [CrossRef]
- Cilli, L.; Contini, L.; Sinnecker, P.; Lopes, P.; Andréo, M.; Neiva, C.; Nascimento, M.; Yoshida, C.; Venturini, A. Effects of grape pomace flour on quality parameters of salmon burger. J. Food Process Preserv. 2020, 44, e14329. [Google Scholar] [CrossRef]
- Pazos, M.; Gallardo, J.M.; Torres, J.L.; Medina, I. Activity of grape polyphenols as inhibitors of the oxidation of fish lipids and frozen fish muscle. Food Chem. 2005, 92, 547–557. [Google Scholar] [CrossRef]
- Sánchez-Alonso, I.; Jiménez-Escrig, A.; Saura-Calixto, F.; Borderías, A.J. Antioxidant protection of white grape pomace on restructured fish products during frozen storage. LWT-Food Sci. Technol. 2008, 41, 42–50. [Google Scholar] [CrossRef]
- Sánchez-Alonso, I.; Borderías, J.; Larsson, K.; Undeland, I. Inhibition of hemoglobin-mediated oxidation of regular and lipid-fortified washed cod mince by a white grape dietary fibre. J. Agric. Food Chem. 2007, 55, 5299–5305. [Google Scholar] [CrossRef]
- Urquiaga, I.; Troncoso, D.; Mackenna, M.J.; Urzúa, C.; Pérez, D.; Dicenta, S.; de la Cerda, P.M.; Amigo, L.; Carreño, J.C.; Echeverría, G.; et al. The Consumption of Beef Burgers Prepared with Wine Grape Pomace Flour Improves Fasting Glucose, Plasma Antioxidant Levels, and Oxidative Damage Markers in Humans: A Controlled Trial. Nutrients 2018, 10, 1388. [Google Scholar] [CrossRef]
- Lavelli, V.; Sri Harsha, P.S.C.; Torri, L.; Zeppa, G. Use of winemaking by-products as an ingredient for tomato puree: The effect of particle size on product quality. Food Chem. 2014, 152, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.A.T.; Bucknall, M.P.; Arcot, J. Interferences of anthocyanins with the uptake of lycopene in Caco-2 cells, and their interactive effects on anti-oxidation and anti-inflammation in vitro and ex vivo. Food Chem. 2019, 276, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Dordoni, R.; Cantaboni, S.; Spigno, G. Walnut paste: Oxidative stability and effect of grape skin extract addition. Heliyon 2019, 5, e02506. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Chemistry, Biochemistry, and Safety of Acrylamide. A Review. J. Agric. Food Chem. 2003, 51, 4504–4526. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yagiz, Y.; Marshall, S.; Li, Z.; Simonne, A.; Lu, J.; Marshall, M.R. Application of muscadine grape (Vitis rotundifolia Michx.) pomace extract to reduce carcinogenic acrylamide. Food Chem. 2015, 182, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Hu, X.; Tao, N.; Wang, M. Impact and inhibitory mechanism of phenolic compounds on the formation of toxic Maillard reaction products in food. Front. Agr. Sci. Eng. 2018, 5, 321. [Google Scholar] [CrossRef]
- Rózek, A.; Achaerandio, I.; Güell, C.; López, F.; Ferrando, M. Use of commercial grape phenolic extracts to supplement solid foodstuff. LWT Food Sci. Technol. 2010, 43, 623–631. [Google Scholar] [CrossRef]
- Guerrero, R.L.F.; Smith, P.; Bindon, K.A. Application of insoluble fibres in the fining of wine phenolics. J. Agric. Food Chem. 2013, 61, 4424–4432. [Google Scholar] [CrossRef]
- Shinagawa, F.B.; de Santana, F.C.; Torres, L.R.O.; Mancini, J. Grape seed oil: A potential functional food? Food Sci. Technol. 2015, 35, 399–406. [Google Scholar] [CrossRef]
- Auger, C.; Gérain, P.; Laurent-Bichon, F.; Portet, K.; Bornet, A.; Caporiccio, B.; Rouanet, J.M. Phenolics from commercialized grape extracts prevent early atherosclerotic lesions in hamsters by mechanisms other than antioxidant effect. J. Agric. Food Chem. 2004, 52, 5297–5302. [Google Scholar] [CrossRef]
- Tournour, H.H.; Segundo, M.A.; Magalhães, L.M.; Barreiros, L.; Queiroz, J.; Cunha, L.M. Valorization of grape pomace: Extraction of bioactive phenolics with antioxidant properties. Ind. Crops Prod. 2015, 74, 397–406. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, R.; Justo, M.L.; Claro, C.M.; Vila, E.; Parrado, J.; Herrera, M.D.; de Sotomayor, M.A. Endothelium-dependent vasodilator and antioxidant properties of a novel enzymatic extract of grape pomace from wine industrial waste. Food Chem. 2012, 135, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Scola, G.; Conte, D.; Spada, P.W.D.-S.; Dani, C.; Vanderlinde, R.; Funchal, C.; Salvador, M. Flavan-3-ol Compounds from Wine Wastes with in Vitro and in Vivo Antioxidant Activity. Nutrients 2012, 2, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Ed Nignpense, B.; Chinkwo, K.A.; Blanchard, C.L.; Santhakumar, A.B. Polyphenols: Modulators of Platelet Function and Platelet Microparticle Generation? Int. J. Mol. Sci. 2019, 21, 146. [Google Scholar] [CrossRef] [PubMed]
- De Lange, D.W.; van Golde, P.H.; Scholman, W.L.G.; Kraaijenhagen, R.J.; Akkerman, J.W.N.; Van De Wiel, A. Red wine and red wine polyphenolic compounds but not alcohol inhibit ADP-induced platelet aggregation. Eur. J. Intern. Med. 2003, 14, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Olas, B.; Wachowicz, B.; Stochmal, A.; Oleszek, W. The polyphenol-rich extract from grape seeds inhibits platelet signaling pathways triggered by both proteolytic and non-proteolytic agonists. Platelets 2012, 23, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Sut, A.; Kosiorek, A.; Saluk-Bijak, J.; Golanski, J. Dual anticoagulant/antiplatelet activity of polyphenolic grape seeds extract. Nutrients 2019, 11, 93. [Google Scholar] [CrossRef]
- Shanmuganayagam, D.; Beahm, M.R.; Osman, H.E.; Krueger, C.G.; Reed, J.D.; Folts, J.D. Grape seed and grape skin extracts elicit a greater antiplatelet effect when used in combination than when used individually in dogs and humans. J. Nutr. 2002, 132, 3592–3598. [Google Scholar] [CrossRef]
- Moschona, A.; Liakopoulou-Kyriakides, M. Encapsulation of biological active phenolic compounds extracted from wine wastes in alginate-chitosan microbeads. J. Microencapsul. 2018, 35, 229–240. [Google Scholar] [CrossRef]
- Rivera, K.; Salas-Pérez, F.; Echeverría, G.; Urquiaga, I.; Dicenta, S.; Pérez, D.; de la Cerda, P.; González, L.; Andia, M.E.; Uribe, S.; et al. Red Wine Grape Pomace Attenuates Atherosclerosis and Myocardial Damage and Increases Survival in Association with Improved Plasma Antioxidant Activity in a Murine Model of Lethal Ischemic Heart Disease. Nutrients 2019, 11, 2135. [Google Scholar] [CrossRef]
- Hogan, S.; Zhang, L.; Li, J.; Sun, S.; Canning, C.; Zhou, K. Antioxidant rich grape pomace extract suppresses postprandial hyperglycemia in diabetic mice by specifically inhibiting alpha-glucosidase. Nutr. Metab. 2010, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Urquiaga, I.; D’Acuña, S.; Pérez, D.; Dicenta, S.; Echeverría, G.; Rigotti, A.; Leighton, F. Wine grape pomace flour improves blood pressure, fasting glucose and protein damage in humans: A randomized controlled trial. Biol. Res. 2015, 48, 49. [Google Scholar] [CrossRef] [PubMed]
- Draijer, R.; de Graaf, Y.; Slettenaar, M.; de Groot, E.; Wright, C. Consumption of a Polyphenol-Rich Grape-Wine Extract Lowers Ambulatory Blood Pressure in Mildly Hypertensive Subjects. Nutrients 2015, 7, 3138–3153. [Google Scholar] [CrossRef] [PubMed]
- Del Pino-García, R.; Gerardi, G.; Rivero-Pérez, M.D.; González-SanJosé, M.L.; García-Lomillo, J.; Muñiz, P. Wine pomace seasoning attenuates hyperglycaemia-induced endothelial dysfunction and oxidative damage in endothelial cells. J. Funct. Foods 2016, 22, 431–445. [Google Scholar] [CrossRef]
- Flammer, A.J.; Sudano, I.; Wolfrum, M.; Thomas, R.; Enseleit, F.; Périat, D.; Kaiser, P.; Hirt, A.; Hermann, M.; Serafini, M.; et al. Cardiovascular effects of flavanol-rich chocolate in patients with heart failure. Eur. Heart J. 2012, 33, 2172–2180. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mateos, R.; Goya, L.; Amigo-Benavent, M.; Sarriá, B.; Bravo, L. A phenolic extract from grape by-products and its main hydroxybenzoic acids protect Caco-2 cells against pro-oxidant induced toxicity. Food Chem. Toxicol. 2016, 88, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; García-Almagro, F.J.; Avilés-Plaza, F.; Parra, S.; Yáñez-Gascón, M.J.; Ruiz-Ros, J.A.; García-Conesa, M.T.; Tomás-Barberán, F.A.; et al. Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and ApoB in patients undergoing primary prevention of cardiovascular disease: A triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Mol. Nutr. Food Res. 2012, 56, 810–821. [Google Scholar] [CrossRef]
- De Oliveira, W.P.; Biasoto, A.C.T.; Marques, V.F.; dos Santos, I.M.; Magalhães, K.; Correa, L.C.; Shahidi, F. Phenolics from Winemaking By-Products Better Decrease VLDL-Cholesterol and Triacylglycerol Levels than Those of Red Wine in Wistar Rats. J. Food Sci. 2017, 82, 2432–2437. [Google Scholar] [CrossRef]
- Razavi, S.-M.; Gholamin, S.; Eskandari, A.; Mohsenian, N.; Ghorbanihaghjo, A.; Delazar, A.; Argani, H. Red grape seed extract improves lipid profiles and decreases oxidized low-density lipoprotein in patients with mild hyperlipidemia. J. Med. Food 2013, 16, 255–258. [Google Scholar] [CrossRef]
- Park, E.; Edirisinghe, I.; Choy, Y.Y.; Waterhouse, A.; Burton-Freeman, B. Effects of grape seed extract beverage on blood pressure and metabolic indices in individuals with pre-Hypertension: A randomised, double-Blinded, two-Arm, parallel, placebo-Controlled trial. Br. J. Nutr. 2016, 115, 226–238. [Google Scholar] [CrossRef]
- Pasini, F.; Chinnici, F.; Caboni, M.F.; Verardo, V. Recovery of oligomeric proanthocyanidins and other phenolic compounds with established bioactivity from grape seed by-Products. Molecules 2019, 24, 677. [Google Scholar] [CrossRef] [PubMed]
- Sapwarobol, S.; Adisakwattana, S.; Changpeng, S.; Ratanawachirin, W.; Tanruttanawong, K.; Boonyarit, W. Postprandial blood glucose response to grape seed extract in healthy participants: A pilot study. Pharmacogn. Mag. 2012, 8, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Montagut, G.; Onnockx, S.; Vaqué, M.; Bladé, C.; Blay, M.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, M.J.; Arola, L.; Pirson, I. Oligomers of grape-seed procyanidin extract activate the insulin receptor and key targets of the insulin signaling pathway differently from insulin. J. Nutr. Biochem. 2010, 21, 476–481. [Google Scholar] [CrossRef]
- Kaur, M.; Agarwal, C.; Agarwa, R. Anticancer and cancer chemopreventive potential of grape seed extract and other grape-based products. J. Nutr. 2009, 139, 1806S–1812S. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Condron, M.M.; Ho, L.; Wang, J.; Zhao, W.; Pasinetti, G.M.; Teplow, D.B. Effects of grape seed-derived polyphenols on amyloid beta-protein self-assembly and cytotoxicity. J. Biol. Chem. 2008, 283, 32176–32187. [Google Scholar] [CrossRef] [PubMed]
- Charradi, K.; Mahmoudi, M.; Bedhiafi, T.; Jebari, K.; El May, M.; Limam, F.; Aouani, E. Safety evaluation, anti-oxidative and anti-inflammatory effects of subchronically dietary supplemented high dosing grape seed powder (GSP) to healthy rat. Biomed. Pharmacother. 2018, 107, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Cádiz-Gurrea, M.D.L.L.; Borrás-Linares, I.; Lozano-Sánchez, J.; Joven, J.; Fernández-Arroyo, S.; Segura-Carretero, A. Cocoa and Grape Seed Byproducts as a Source of Antioxidant and Anti-Inflammatory Proanthocyanidins. Int. J. Mol. Sci. 2017, 18, 376. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharm. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Kruger, M.J.; Davies, N.; Myburgh, K.H.; Lecour, S. Proanthocyanidins, anthocyanins and cardiovascular diseases. Food Res. Int. 2014, 59, 41–52. [Google Scholar] [CrossRef]
- Iriondo-DeHond, M.; Blázquez-Duff, J.M.; Del Castillo, M.D.; Miguel, E. Nutritional Quality, Sensory Analysis and Shelf Life Stability of Yogurts Containing Inulin-Type Fructans and Winery Byproducts for Sustainable Health. Foods 2020, 9, 1199. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Huang, B.; Choi, S.-K.; Seo, J.-S. Anti-obesity effect of resveratrol-amplified grape skin extracts on 3T3-L1 adipocytes differentiation. Nutr. Res. Pract. 2012, 6, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.F.M.; Moawed, F.S.M.; Mohamed, M.A. Protective mechanism of grape seed oil on carbon tetrachloride-induced brain damage in γ-irradiated rats. J. Photoch. Photobio. 2015, 153, 317–323. [Google Scholar] [CrossRef]
- Ismail, A.F.M.; Salem, A.A.M.; Eassawy, M.M.T. Hepatoprotective effect of grape seed oil against carbon tetrachloride induced oxidative stress in liver of γ-irradiated rat. J. Photoch. Photobio. 2016, 160, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Asadi, F.; Shahriari, A.; Chahardah-Cheric, M. Effect of long-term optimal ingestion of canola oil, grape seed oil, corn oil and yogurt butter on serum, muscle and liver cholesterol status in rats. Food Chem. Toxicol. 2010, 48, 2454–2457. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.V.; Martinez, M.; Stamos, M.J.; Moyer, M.P.; Planutis, K.; Hope, C.; Holcombe, R.F. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Manag. Res. 2009, 1, 25–37. [Google Scholar]
- Elshaer, M.; Chen, Y.; Wang, X.J.; Tang, X. Resveratrol: An overview of its anti-cancer mechanisms. Life Sci. 2018, 207, 340–349. [Google Scholar] [CrossRef]
- Wang, L.-S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef]
- Lala, G.; Malik, M.; Zhao, C.; He, J.; Kwon, Y.; Giusti, M.M.; Magnuson, B.A. Anthocyanin-rich extracts inhibit multiple biomarkers of colon cancer in rats. Nutr. Cancer 2006, 54, 84–93. [Google Scholar] [CrossRef]
- Tian, Q.; Xu, Z.; Sun, X.; Deavila, J.; Du, M.; Zhu, M. Grape pomace inhibits colon carcinogenesis by suppressing cell proliferation and inducing epigenetic modifications. J. Nutr. Biochem. 2020, 84, 108443. [Google Scholar] [CrossRef]
- Mišković Špoljarić, K.; Šelo, G.; Pešut, E.; Martinović, J.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Antioxidant and antiproliferative potentials of phenolic-rich extracts from biotransformed grape pomace in colorectal Cancer. BMC Complement. Med. Ther. 2023, 23, 29. [Google Scholar] [CrossRef]
- Singletary, K.W.; Jung, K.J.; Giusti, M. Anthocyanin-rich grape extract blocks breast cell DNA damage. J. Med. Food. 2007, 10, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Song, S.; Wei, Z.; Huang, Y.; Zhang, Y.; Lu, J. The comparative study among different fractions of muscadine grape ’Noble’ pomace extracts regarding anti-oxidative activities, cell cycle arrest and apoptosis in breast cancer. Food Nutr. Res. 2017, 61, 1412795. [Google Scholar] [CrossRef]
- Silva, A.; Silva, V.; Igrejas, G.; Gaivao, I.; Aires, A.; Klibi, N.; Dapkevicius, M.; Valentao, P.; Falco, V.; Poeta, P. Valorization of Winemaking By-Products as a Novel Source of Antibacterial Properties: New Strategies to Fight Antibiotic Resistance. Molecules 2021, 26, 2331. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.-Z.; Brooks, J.D.; Corke, H. Potential application of spice and herb extracts as natural preservatives in cheese. J. Med. Food 2011, 14, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Mattio, L.M.; Dallavalle, S.; Musso, L.; Filardi, R.; Franzetti, L.; Pellegrino, L.; D’Incecco, P.; Mora, D.; Pinto, A.; Arioli, S. Antimicrobial activity of resveratrol-derived monomers and dimers against foodborne pathogens. Sci. Rep. 2019, 9, 19525. [Google Scholar] [CrossRef] [PubMed]
- De Vries, K.; Strydom, M.; Steenkamp, V. Bioavailability of resveratrol: Possibilities for enhancement. J. Herb. Med. 2018, 11, 71–77. [Google Scholar] [CrossRef]
- Tsali, A.; Goula, A.M. Valorization of grape pomace: Encapsulation and storage stability of its phenolic extract. Powder Technol. 2018, 340, 194–207. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; McClements, D.J.; Lorenzo, J.M. Encapsulation of Bioactive Phytochemicals in Plant-Based Matrices and Application as Additives in Meat and Meat Products. Molecules 2021, 26, 3984. [Google Scholar] [CrossRef]
- Glampedaki, P.; Dutschk, V. Stability studies of cosmetic emulsions prepared from natural products such as wine, grape seed oil and mastic resin. Colloid. Surf. A 2014, 460, 306–311. [Google Scholar] [CrossRef]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Battaglini, M.; Desii, A.; Lavarello, C.; Genchi, G.; Petretto, A.; Ciofani, G. Liposomes loaded with polyphenol-rich grape pomace extracts protect from neurodegeneration in a rotenone-based in vitro model of Parkinson’s disease. Biomater. Sci. 2021, 9, 8171–8188. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; McClements, D.J. Nutraceutical delivery systems: Resveratrol encapsulation in grape seed oil nanoemulsions formed by spontaneous emulsification. Food Chem. 2015, 167, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Hübner, A.A.; Sarruf, F.D.; Oliveira, C.A.; Neto, A.V.; Fischer, D.C.H.; Kato, E.T.M.; Lourenço, F.R.; Baby, A.R.; Bacchi, E.M. Safety and Photoprotective Efficacy of a Sunscreen System Based on Grape Pomace (Vitis vinifera L.) Phenolics from Winemaking. Pharmaceutics 2020, 12, 1148. [Google Scholar] [CrossRef]
- Jakobek, L.; Matić, P. Non-covalent dietary fiber—Polyphenol interactions and their influence on polyphenol bioaccessibility. Trends Food Sci. Technol. 2019, 83, 235–247. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Serrano, J.; Tabernero, M.; Arranz, S.; Díaz-Rubio, M.E.; García-Diz, L.; Goñi, I.; Saura-Calixto, F. Bioavailability of Phenolic Antioxidants Associated with Dietary Fiber: Plasma Antioxidant Capacity After Acute and Long-Term Intake in Humans. Plant Foods Hum. Nutr. 2009, 64, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.S.; Pinho, M.N.; Cabral, L. Solid-liquid extraction and concentration with processes of membrane technology of soluble fibers from wine grape pomace. Técnico Lisboa. 2013. Available online: https://api.semanticscholar.org/CorpusID:189801481 (accessed on 7 September 2023).
- Zhang, L.; Zhu, M.; Shi, T.; Guo, C.; Huang, Y.; Chen, Y.; Xie, M. Recovery of dietary fiber and polyphenol from grape juice pomace and evaluation of their functional properties and polyphenol compositions. Food Funct. 2017, 8, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Du, B.; Zheng, L.; Li, J. Advance on the bioactivity and potential applications of dietary fibre from grape pomace. Food Chem. 2015, 186, 207–212. [Google Scholar] [CrossRef]
- Valiente, C.; Arrigoni, E.; Esteban, R.; Amadò, R. Grape Pomace as a Potential Food Fiber. J. Food Sci. 1995, 60, 818–820. [Google Scholar] [CrossRef]
- Dias, J.F.; Simbras, B.D.; Beres, C.; dos Santos, K.O.; Cabral, L.M.C.; Miguel, M.A.L. Acid Lactic Bacteria as a Bio-Preservant for Grape Pomace Beverage. Front. Sust. Food Syst. 2018, 2, 58. [Google Scholar] [CrossRef]
- Ageyeva, N.; Tikhonova, A.; Burtsev, B.; Biryukova, S.; Globa, E. Grape pomace treatment methods and their effects on storage. Foods Raw. Mat. 2021, 9, 215–223. [Google Scholar] [CrossRef]
- Ayed, N.; Yu, H.-L.; Lacroix, M. Using gamma irradiation for the recovery of anthocyanins from grape pomace. Rad. Phys. Chem. 2000, 57, 277–279. [Google Scholar] [CrossRef]
- Tseng, A.; Zhao, Y. Effect of different drying methods and storage time on the retention of bioactive compounds and antibacterial activity of wine grape pomace (Pinot Noir and Merlot). J. Food Sci. 2012, 77, H192–H201. [Google Scholar] [CrossRef] [PubMed]
- Sokač, T.; Gunjević, V.; Pušek, A.; Tušek, A.J.; Dujmić, F.; Brnčić, M.; Ganić, K.K.; Jakovljević, T.; Uher, D.; Mitrić, G.; et al. Comparison of Drying Methods and Their Effect on the Stability of Graševina Grape Pomace Biologically Active Compounds. Foods 2022, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- MohdMaidin, N.; Oruna-Concha, M.J.; Jauregi, P. Surfactant TWEEN20 provides stabilisation effect on anthocyanins extracted from red grape pomace. Food Chem. 2019, 271, 224–231. [Google Scholar] [CrossRef]
- Torri, L.; Piochi, M.; Marchiani, R.; Zeppa, G.; Dinnella, C.; Monteleone, E. A sensory- and consumer-based approach to optimize cheese enrichment with grape skin powders. J. Dairy Sci. 2016, 99, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Brandão, E.; Guerreiro, C.; Soares, S.; Mateus, N.; de Freitas, V. Tannins in Food: Insights into the Molecular Perception of Astringency and Bitter Taste. Molecules 2020, 25, 2590. [Google Scholar] [CrossRef]
- McRae, J.M.; Kennedy, J.A. Wine and Grape Tannin Interactions with Salivary Proteins and Their Impact on Astringency: A Review of Current Research. Molecules 2011, 16, 2348–2364. [Google Scholar] [CrossRef]
- Canett-Romero, R.; Osuna, A.; Sánchez, M.; Castro, R.; León-Martínez, L.; León-Gálvez, R. Characterization of cookies made with deseeded grape pomace. Arch. Latinoam. Nutr. 2004, 54, 93–99. [Google Scholar]
- Caponio, G.R.; Noviello, M.; Calabrese, F.M.; Gambacorta, G.; Giannelli, G.; De Angelis, M. Effects of Grape Pomace Polyphenols and In Vitro Gastrointestinal Digestion on Antimicrobial Activity: Recovery of Bioactive Compounds. Antioxidants 2022, 11, 567. [Google Scholar] [CrossRef]
- Čuš, F.; Česnik, H.B.; Bolta, Š.V.; Gregorčič, A. Pesticide residues in grapes and during vinification process. Food Control 2010, 21, 1512–1518. [Google Scholar] [CrossRef]
- Hou, X.; Xu, Z.; Zhao, Y.; Liu, D. Rapid analysis and residue evaluation of six fungicides in grape wine-making and drying. J. Food Comp. Anal. 2020, 89, 103465. [Google Scholar] [CrossRef]
- Moncalvo, A.; Marinoni, L.; Dordoni, R.; Duserm Garrido, G.; Lavelli, V.; Spigno, G. Waste grape skins: Evaluation of safety aspects for the production of functional powders and extracts for the food sector. Food Addit. Contam. Part A 2016, 33, 1116–1126. [Google Scholar] [CrossRef]
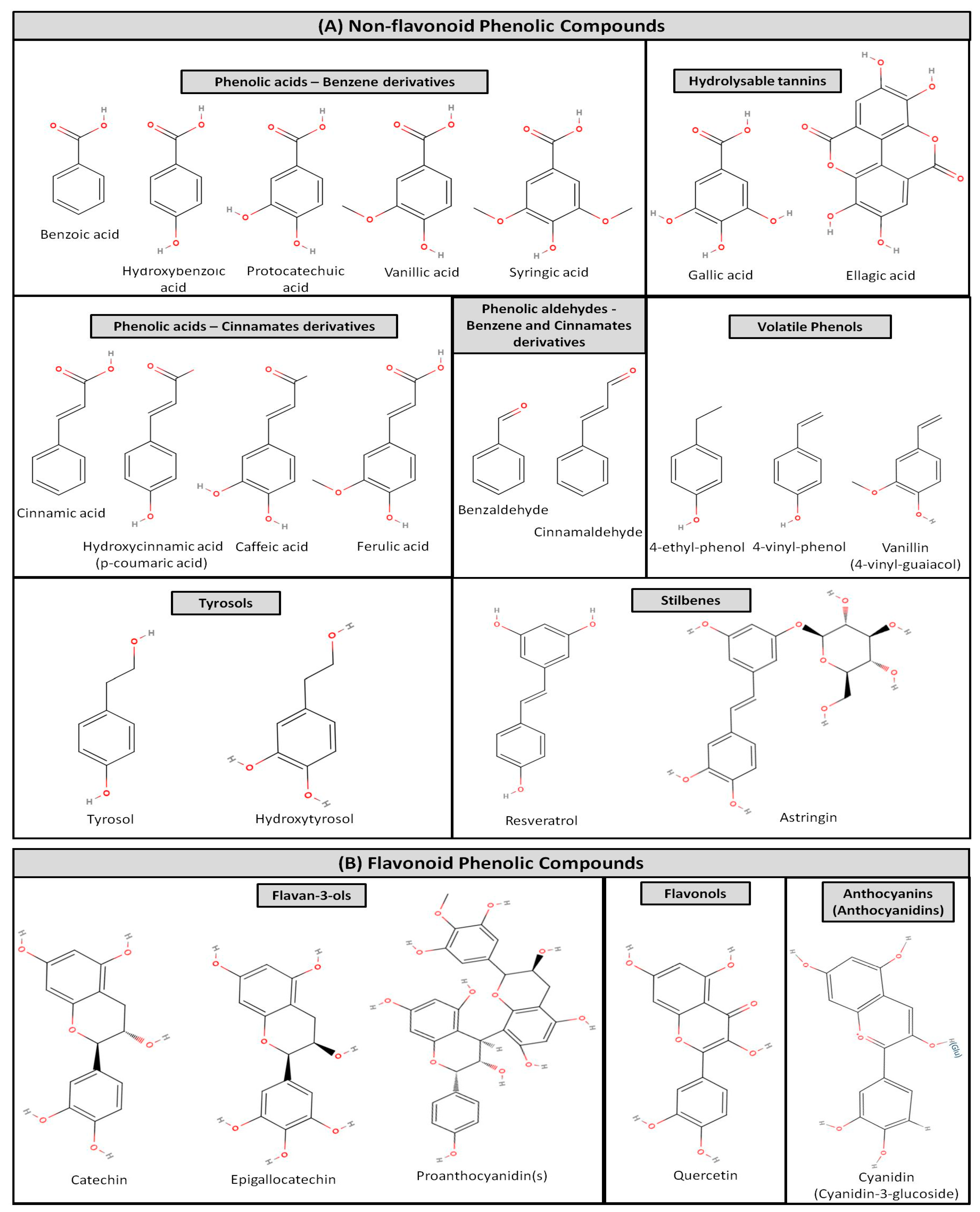



| Nutritional Compounds | Quantity (g/100 g DW) * | Phenolic Compounds | Quantity (mg/100 g DW) * |
|---|---|---|---|
| Ash | 1.7–9.1 | Flavonoids | 1121.3–1723.4 |
| Protein | 3.6–14.2 | Anthocyanins | 490.7–2983.5 |
| Lipids | 1.1–13.9 | Tannins | 350.0–625.2 |
| Total dietary fibre | 17.3–88.7 | Procyanidin | 1545.2–2582.8 |
| Insoluble fibre | 16.4–63.7 | Catechin | 78.8–195.0 |
| Carbohydrates | 12.2–40.5 | Epicatechin | 18.1–150.0 |
| Gallic acid | 4.6–18.7 | ||
| Quercetin | 157.7–1518.0 | ||
| Resveratrol | 0.3–383.1 | ||
| Total polyphenolic content | 0.28–16.2 * | - | 18.5–77.0 ** |
| Antioxidant activity | TAAc | 14.6–75.1 ** | |
| ABTS | 191.2–85.1 *** | ||
| DPPH | 185.6–510.1 *** | ||
| FRAP | 116.5–250.4 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsoupras, A.; Ni, V.L.J.; O’Mahony, É.; Karali, M. Winemaking: “With One Stone, Two Birds”? A Holistic Review of the Bio-Functional Compounds, Applications and Health Benefits of Wine and Wineries’ By-Products. Fermentation 2023, 9, 838. https://doi.org/10.3390/fermentation9090838
Tsoupras A, Ni VLJ, O’Mahony É, Karali M. Winemaking: “With One Stone, Two Birds”? A Holistic Review of the Bio-Functional Compounds, Applications and Health Benefits of Wine and Wineries’ By-Products. Fermentation. 2023; 9(9):838. https://doi.org/10.3390/fermentation9090838
Chicago/Turabian StyleTsoupras, Alexandros, Victoria Ling Jun Ni, Éimhín O’Mahony, and Maria Karali. 2023. "Winemaking: “With One Stone, Two Birds”? A Holistic Review of the Bio-Functional Compounds, Applications and Health Benefits of Wine and Wineries’ By-Products" Fermentation 9, no. 9: 838. https://doi.org/10.3390/fermentation9090838
APA StyleTsoupras, A., Ni, V. L. J., O’Mahony, É., & Karali, M. (2023). Winemaking: “With One Stone, Two Birds”? A Holistic Review of the Bio-Functional Compounds, Applications and Health Benefits of Wine and Wineries’ By-Products. Fermentation, 9(9), 838. https://doi.org/10.3390/fermentation9090838







