Impacts of Fermentation on the Phenolic Composition, Antioxidant Potential, and Volatile Compounds Profile of Commercially Roasted Coffee Beans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
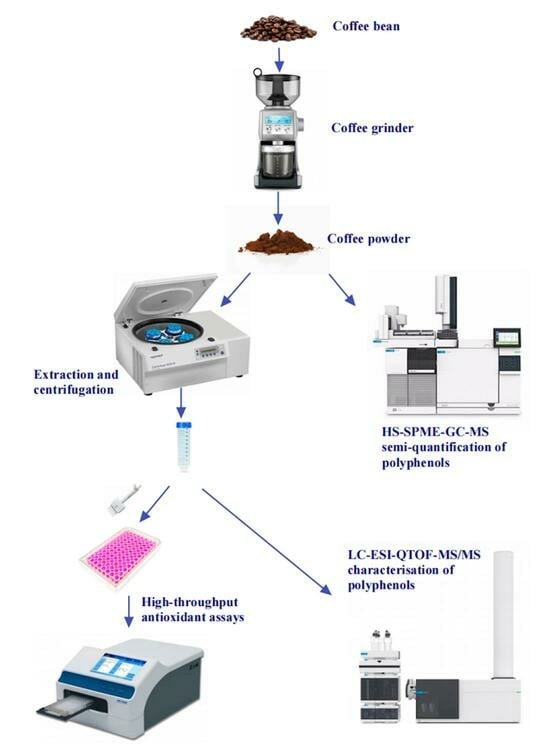
2.2. Sample Preparation
2.3. Extraction of Free and Bound Phenolic Compounds
2.4. Estimation of Phenolic Content and Antioxidant Potentials
2.4.1. Total Phenolic Content (TPC)
2.4.2. Total Flavonoid Content (TFC)
2.4.3. Total Condensed Tannins (TCT)
2.4.4. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
2.4.5. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS•+) Radical Cation-Based Assay
2.4.6. Ferric Reducing Antioxidant Power (FRAP) Assay
2.4.7. Hydroxyl Radical (•OH) Scavenging Activity Assay
2.4.8. Ferrous Ion Chelating Activity Assay
2.4.9. Reducing Power Assay (RPA)
2.5. Liquid Chromatography–Electrospray Ionization Quadrupole Time-of-Flight Mass Spectrometry (LC-ESI-QTOF-MS/MS)
2.6. Headspace Solid-Phase Microextraction Coupled to Gas Chromatography–Mass Spectrometry (HS-SPME-GC-MS)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Content Estimation Assays (TPC, TFC, TCT)
3.2. Antioxidant Activities of Coffee Bean Estimation
3.3. Correlation between Phenolic Compounds and Antioxidant Potential
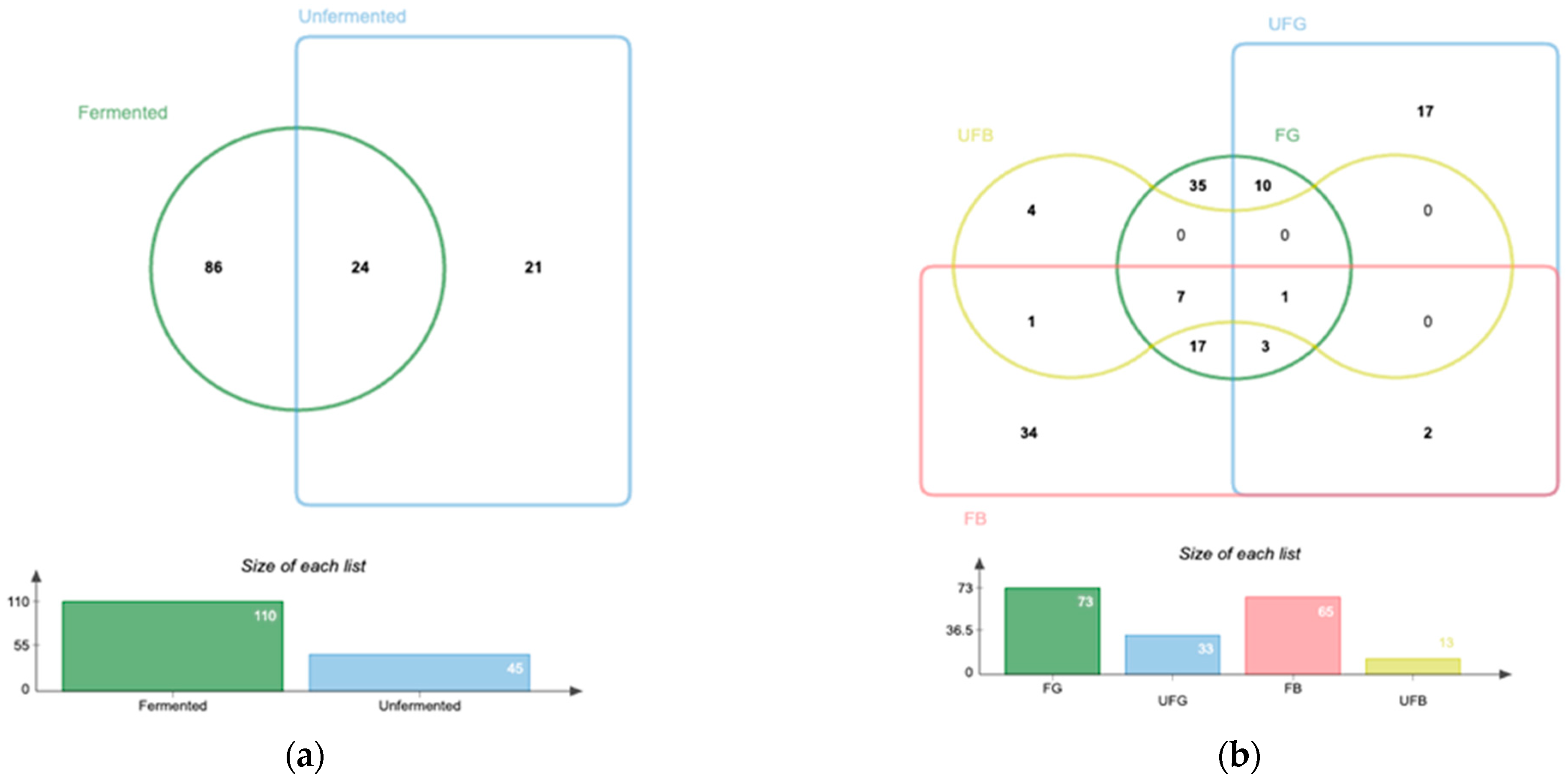
3.4. LS-ESI-QTOF-MS/MS Characterization of Phenolic Compounds in Roasted Fermented and Unfermented Coffee Beans
3.4.1. Phenolic Acids
Hydroxycinnamic Acids
3.4.2. Lignans and Stilbenes
3.4.3. Flavonoids and Isoflavonoids
3.4.4. Other Polyphenols
3.5. Volatile Compounds in Fermented and Unfermented Coffee Beans
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Febrianto, N.A.; Zhu, F. Coffee bean processing: Emerging methods and their effects on chemical, biological and sensory properties. Food Chem. 2023, 412, 135489. [Google Scholar] [CrossRef] [PubMed]
- de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Júnior, A.I.M.; Vásquez, Z.S.; Medeiros, A.B.; Vandenberghe, L.P.; Soccol, C.R. Exploring the impacts of postharvest processing on the aroma formation of coffee beans–A review. Food Chem. 2019, 272, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Sanz, C.; Maeztu, L.; Zapelena, M.J.; Bello, J.; Cid, C. Profiles of volatile compounds and sensory analysis of three blends of coffee: Influence of different proportions of Arabica and Robusta and influence of roasting coffee with sugar. J. Sci. Food Agric. 2002, 82, 840–847. [Google Scholar] [CrossRef]
- Clark, I.; Landolt, H.P. Coffee, caffeine, and sleep: A systematic review of epidemiological studies and randomized controlled trials. Sleep Med. Rev. 2017, 31, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Açıkalın, B.; Sanlier, N. Coffee and its effects on the immune system. Trends Food Sci. Technol. 2021, 114, 625–632. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. Braz. J. Plant Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Yeager, S.E.; Batali, M.E.; Guinard, J.-X.; Ristenpart, W.D. Acids in coffee: A review of sensory measurements and meta-analysis of chemical composition. Crit. Rev. Food Sci. Nutr. 2023, 63, 1010–1036. [Google Scholar] [CrossRef]
- Elhalis, H.; Cox, J.; Frank, D.; Zhao, J. The role of wet fermentation in enhancing coffee flavor, aroma and sensory quality. Eur. Food Res. Technol. 2021, 247, 485–498. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Machado, E.M.; Martins, S.; Teixeira, J.A. Production, composition, and application of coffee and its industrial residues. Food Bioprocess Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef]
- Wu, H.; Lu, P.; Liu, Z.; Sharifi-Rad, J.; Suleria, H.A.R. Impact of roasting on the phenolic and volatile compounds in coffee beans. Food Sci. Nutr. 2022, 10, 2408–2425. [Google Scholar] [CrossRef]
- Jiang, Y.; Subbiah, V.; Wu, H.; Bk, A.; Sharifi-Rad, J.; Suleria, H.A.R. Phenolic Profiling of Berries Waste and Determination of Their Antioxidant Potential. J. Food Qual. 2022, 2022, 5605739. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Barrow, C.J.; Dunshea, F.R. Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Qi, Y.; Shi, L.; Giovani, M.; Zaki, N.A.A.; Guo, S.; Suleria, H.A.R. Screening of Phenolic Compounds in Rejected Avocado and Determination of Their Antioxidant Potential. Processes 2022, 10, 1747. [Google Scholar] [CrossRef]
- Hu, T.; Subbiah, V.; Wu, H.; Bk, A.; Rauf, A.; Alhumaydhi, F.A.; Suleria, H.A.R. Determination and Characterization of Phenolic Compounds from Australia-Grown Sweet Cherries (Prunus avium L.) and Their Potential Antioxidant Properties. ACS Omega 2021, 6, 34687–34699. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.Y.; Vo, G.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Mango rejects and mango waste: Characterization and quantification of phenolic compounds and their antioxidant potential. J. Food Process. Preserv. 2021, 45, e15618. [Google Scholar] [CrossRef]
- Zhu, C.; Chou, O.; Lee, F.Y.; Wang, Z.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Characterization of Phenolics in Rejected Kiwifruit and Their Antioxidant Potential. Processes 2021, 9, 781. [Google Scholar] [CrossRef]
- Wang, Z.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. A Comparative Investigation on Phenolic Composition, Characterization and Antioxidant Potentials of Five Different Australian Grown Pear Varieties. Antioxidants 2021, 10, 151. [Google Scholar] [CrossRef]
- Peng, D.; Zahid, H.F.; Ajlouni, S.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Profiling of Australian Mango Peel By-Product Polyphenols and Their Potential Antioxidant Activities. Processes 2019, 7, 764. [Google Scholar] [CrossRef]
- Rocchetti, G.; Braceschi, G.P.; Odello, L.; Bertuzzi, T.; Trevisan, M.; Lucini, L. Identification of markers of sensory quality in ground coffee: An untargeted metabolomics approach. Metabolomics 2020, 16, 127. [Google Scholar] [CrossRef]
- Kwak, H.S.; Jeong, Y.; Kim, M. Effect of Yeast Fermentation of Green Coffee Beans on Antioxidant Activity and Consumer Acceptability. J. Food Qual. 2018, 2018, 5967130. [Google Scholar] [CrossRef]
- Fritsch, C.; Heinrich, V.; Vogel, R.F.; Toelstede, S. Phenolic acid degradation potential and growth behavior of lactic acid bacteria in sunflower substrates. Food Microbiol. 2016, 57, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Alnsour, L.; Issa, R.; Awwad, S.; Albals, D.; Al-Momani, I. Quantification of Total Phenols and Antioxidants in Coffee Samples of Different Origins and Evaluation of the Effect of Degree of Roasting on Their Levels. Molecules 2022, 27, 1591. [Google Scholar] [CrossRef]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Miyagusuku-Cruzado, G.; García-Cano, I.; Rocha-Mendoza, D.; Jiménez-Flores, R.; Giusti, M.M. Monitoring hydroxycinnamic acid decarboxylation by lactic acid bacteria using high-throughput UV-Vis spectroscopy. Molecules 2020, 25, 3142. [Google Scholar] [CrossRef]
- Lee, L.W.; Tay, G.Y.; Cheong, M.W.; Curran, P.; Yu, B.; Liu, S.Q. Modulation of the volatile and non-volatile profiles of coffee fermented with Yarrowia lipolytica: I. Green coffee. LWT 2017, 77, 225–232. [Google Scholar] [CrossRef]
- La Mantia, A.; Ianni, F.; Schoubben, A.; Cespi, M.; Lisjak, K.; Guarnaccia, D.; Sardella, R.; Blasi, P. Effect of Cocoa Roasting on Chocolate Polyphenols Evolution. Antioxidants 2023, 12, 469. [Google Scholar] [CrossRef]
- Adetuyi, F.; Ibrahim, T. Effect of fermentation time on the phenolic, flavonoid and vitamin C contents and antioxidant activities of okra (Abelmoschus esculentus) seeds. Niger. Food J. 2014, 32, 128–137. [Google Scholar] [CrossRef]
- Shang, Y.F.; Cao, H.; Ma, Y.L.; Zhang, C.; Ma, F.; Wang, C.X.; Ni, X.L.; Lee, W.J.; Wei, Z.J. Effect of lactic acid bacteria fermentation on tannins removal in Xuan Mugua fruits. Food Chem. 2019, 274, 118–122. [Google Scholar] [CrossRef]
- Chávez-González, M.L.; Guyot, S.; Rodríguez-Herrera, R.; Prado-Barragán, A.; Aguilar, C.N. Production profiles of phenolics from fungal tannic acid biodegradation in submerged and solid-state fermentation. Process Biochem. 2014, 49, 541–546. [Google Scholar] [CrossRef]
- Rodríguez, H.; de las Rivas, B.; Gómez-Cordovés, C.; Muñoz, R. Degradation of tannic acid by cell-free extracts of Lactobacillus plantarum. Food Chem. 2008, 107, 664–670. [Google Scholar] [CrossRef]
- Król, K.; Gantner, M.; Tatarak, A.; Hallmann, E. The content of polyphenols in coffee beans as roasting, origin and storage effect. Eur. Food Res. Technol. 2020, 246, 33–39. [Google Scholar] [CrossRef]
- Mehari, B.; Chandravanshi, B.S.; Redi-Abshiro, M.; Combrinck, S.; McCrindle, R.; Atlabachew, M. Polyphenol contents of green coffee beans from different regions of Ethiopia. Int. J. Food Prop. 2021, 24, 17–27. [Google Scholar] [CrossRef]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of methods to determine antioxidant capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant property of coffee components: Assessment of methods that define mechanisms of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef]
- Du, J.; Zhong, B.; Subbiah, V.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS Profiling and Antioxidant Activity of Phenolics from Custard Apple Fruit and By-Products. Separations 2021, 8, 62. [Google Scholar] [CrossRef]
- Abu, F.; Mat Taib, C.N.; Mohd Moklas, M.A.; Mohd Akhir, S. Antioxidant properties of crude extract, partition extract, and fermented medium of Dendrobium sabin flower. Evid.-Based Complement. Altern. Med. 2017, 2017, 2907219. [Google Scholar] [CrossRef]
- Subbiah, V.; Zhong, B.; Nawaz, M.A.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Screening of Phenolic Compounds in Australian Grown Berries by LC-ESI-QTOF-MS/MS and Determination of Their Antioxidant Potential. Antioxidants 2021, 10, 26. [Google Scholar] [CrossRef]
- Chou, O.; Ali, A.; Subbiah, V.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS Characterisation of Phenolics in Herbal Tea Infusion and Their Antioxidant Potential. Fermentation 2021, 7, 73. [Google Scholar] [CrossRef]
- Cho, A.R.; Park, K.W.; Kim, K.M.; Kim, S.Y.; Han, J. Influence of Roasting Conditions on the Antioxidant Characteristics of Colombian Coffee (Coffea arabica L.) Beans. J. Food Biochem. 2014, 38, 271–280. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Chan, M.Z.A.; Lau, H.; Lim, S.Y.; Li, S.F.Y.; Liu, S.-Q. Untargeted LC-QTOF-MS/MS based metabolomics approach for revealing bioactive components in probiotic fermented coffee brews. Food Res. Int. 2021, 149, 110656. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, C.; Jänsch, A.; Ehrmann, M.A.; Toelstede, S.; Vogel, R.F. Characterization of cinnamoyl esterases from different Lactobacilli and Bifidobacteria. Curr. Microbiol. 2017, 74, 247–256. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Ho, K.V.; Schreiber, K.L.; Park, J.; Vo, P.H.; Lei, Z.; Sumner, L.W.; Brown, C.R.; Lin, C.H. Identification and Quantification of Bioactive Molecules Inhibiting Pro-inflammatory Cytokine Production in Spent Coffee Grounds Using Metabolomics Analyses. Front. Pharmacol. 2020, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-K.; Yoo, H.S.; Shibamoto, T. Role of Roasting Conditions in the Level of Chlorogenic Acid Content in Coffee Beans: Correlation with Coffee Acidity. J. Agric. Food Chem. 2009, 57, 5365–5369. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, S.; Navarini, L.; Khamitova, G.; Sagratini, G.; Vittori, S.; Caprioli, G. Quantification of lignans in 30 ground coffee samples and evaluation of theirs extraction yield in espresso coffee by HPLC-MS/MS triple quadrupole. Int. J. Food Sci. Nutr. 2020, 71, 193–200. [Google Scholar] [CrossRef]
- Heinonen, S.; Nurmi, T.; Liukkonen, K.; Poutanen, K.; Wähälä, K.; Deyama, T.; Nishibe, S.; Adlercreutz, H. In vitro metabolism of plant lignans: New precursors of mammalian lignans enterolactone and enterodiol. J. Agric. Food Chem. 2001, 49, 3178–3186. [Google Scholar] [CrossRef]
- Landete, J. Plant and mammalian lignans: A review of source, intake, metabolism, intestinal bacteria and health. Food Res. Int. 2012, 46, 410–424. [Google Scholar] [CrossRef]
- Zou, X.; Bk, A.; Rauf, A.; Saeed, M.; Al-Awthan, Y.S.; Al-Duais, M.A.; Bahattab, O.; Hamayoon Khan, M.; Suleria, H.A.R. Screening of Polyphenols in Tobacco (Nicotiana tabacum) and Determination of Their Antioxidant Activity in Different Tobacco Varieties. ACS Omega 2021, 6, 25361–25371. [Google Scholar] [CrossRef]
- Yang, S.; Shan, L.; Luo, H.; Sheng, X.; Du, J.; Li, Y. Rapid classification and identification of chemical components of schisandra chinensis by uplc-q-tof/ms combined with data post-processing. Molecules 2017, 22, 1778. [Google Scholar] [CrossRef] [PubMed]
- Ci, X.; Ren, R.; Xu, K.; Li, H.; Yu, Q.; Song, Y.; Wang, D.; Li, R.; Deng, X. Schisantherin A Exhibits Anti-inflammatory Properties by Down-Regulating NF-κB and MAPK Signaling Pathways in Lipopolysaccharide-Treated RAW 264.7 Cells. Inflammation 2010, 33, 126–136. [Google Scholar] [CrossRef]
- Androutsopoulos, V.P.; Ruparelia, K.C.; Papakyriakou, A.; Filippakis, H.; Tsatsakis, A.M.; Spandidos, D.A. Anticancer effects of the metabolic products of the resveratrol analogue, DMU-212: Structural requirements for potency. Eur. J. Med. Chem. 2011, 46, 2586–2595. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, Z.; Yao, S.; Li, S.; Yang, W.; Jiang, B.; Liu, X.; Wu, W.; Qv, H.; Guo, D.-A. Neutral Loss Ion Mapping Experiment Combined with Precursor Mass List and Dynamic Exclusion for Screening Unstable Malonyl Glucoside Conjugates. J. Am. Soc. Mass Spectrom. 2016, 27, 99–107. [Google Scholar] [CrossRef]
- Bennett, R.N.; Mellon, F.A.; Foidl, N.; Pratt, J.H.; Dupont, M.S.; Perkins, L.; Kroon, P.A. Profiling Glucosinolates and Phenolics in Vegetative and Reproductive Tissues of the Multi-Purpose Trees Moringa oleifera L. (Horseradish Tree) and Moringa stenopetala L. J. Agric. Food Chem. 2003, 51, 3546–3553. [Google Scholar] [CrossRef]
- Carvalho, D.O.; Curto, A.F.; Guido, L.F. Determination of phenolic content in different barley varieties and corresponding malts by liquid chromatography-diode array detection-electrospray ionization tandem mass spectrometry. Antioxidants 2015, 4, 563–576. [Google Scholar] [CrossRef]
- Plumb, G.W.; de Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C.; Williamson, G. Antioxidant properties of gallocatechin and prodelphinidins from pomegranate peel. Redox Rep. 2002, 7, 41–46. [Google Scholar] [CrossRef]
- Tang, J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Characterization of Phenolic Compounds from Medicinal Plants (Hops and Juniper Berries) and Their Antioxidant Activity. Foods 2019, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Langa, S.; Peirotén, Á.; Curiel, J.A.; de la Bastida, A.R.; Landete, J.M. Isoflavone Metabolism by Lactic Acid Bacteria and Its Application in the Development of Fermented Soy Food with Beneficial Effects on Human Health. Foods 2023, 12, 1293. [Google Scholar] [CrossRef] [PubMed]
- Avallone, S.; Brillouet, J.M.; Guyot, B.; Olguin, E.; Guiraud, J.P. Involvement of pectolytic micro-organisms in coffee fermentation. Int. J. Food Sci. Technol. 2002, 37, 191–198. [Google Scholar] [CrossRef]
- Figueroa Campos, G.A.; Sagu, S.T.; Saravia Celis, P.; Rawel, H.M. Comparison of Batch and Continuous Wet-Processing of Coffee: Changes in the Main Compounds in Beans, By-Products and Wastewater. Foods 2020, 9, 1135. [Google Scholar] [CrossRef] [PubMed]
- Louzada Pereira, L.; Guarçoni, R.; Moreira, T.; Brioschi, D., Jr.; Marcate, J.; Sousa, L.; Moreli, A.; Grancieri Debona, D.; Caten, C. Sensory Profile of Fermented Arabica Coffee in the Perception of American Cupping Tasters. Agric. Sci. 2019, 10, 321–329. [Google Scholar] [CrossRef]
- Martinez, S.J.; Bressani, A.P.P.; Dias, D.R.; Simão, J.B.P.; Schwan, R.F. Effect of Bacterial and Yeast Starters on the Formation of Volatile and Organic Acid Compounds in Coffee Beans and Selection of Flavors Markers Precursors During Wet Fermentation. Front. Microbiol. 2019, 10, 1287. [Google Scholar] [CrossRef]
- Akiyama, M.; Murakami, K.; Ikeda, M.; Iwatsuki, K.; Wada, A.; Tokuno, K.; Onishi, M.; Iwabuchi, H. Analysis of the headspace volatiles of freshly brewed Arabica coffee using solid-phase microextraction. J. Food Sci. 2007, 72, C388–C396. [Google Scholar] [CrossRef]
- Wang, F.; Shen, H.; Liu, T.; Yang, X.; Yang, Y.; Guo, Y. Formation of Pyrazines in Maillard Model Systems: Effects of Structures of Lysine-Containing Dipeptides/Tripeptides. Foods 2021, 10, 273. [Google Scholar] [CrossRef] [PubMed]
- Pavesi Arisseto, A.; Vicente, E.; Soares Ueno, M.; Verdiani Tfouni, S.A.; De Figueiredo Toledo, M.C. Furan Levels in Coffee as Influenced by Species, Roast Degree, and Brewing Procedures. J. Agric. Food Chem. 2011, 59, 3118–3124. [Google Scholar] [CrossRef] [PubMed]
- Baggenstoss, J.; Poisson, L.; Kaegi, R.; Perren, R.; Escher, F. Coffee roasting and aroma formation: Application of different time−temperature conditions. J. Agric. Food Chem. 2008, 56, 5836–5846. [Google Scholar] [CrossRef]
- Elhalis, H.; Cox, J.; Zhao, J. Ecological diversity, evolution and metabolism of microbial communities in the wet fermentation of Australian coffee beans. Int. J. Food Microbiol. 2020, 321, 108544. [Google Scholar] [CrossRef]
- Kim, J.-S.; Park, S.-E.; Kim, E.-J.; Seo, S.-H.; Son, H.-S. Investigation of metabolite differences in green coffee beans fermented with various microbes. LWT 2022, 172, 114202. [Google Scholar] [CrossRef]
- Krogerus, K.; Gibson, B.R. Influence of valine and other amino acids on total diacetyl and 2,3-pentanedione levels during fermentation of brewer’s wort. Appl. Microbiol. Biotechnol. 2013, 97, 6919–6930. [Google Scholar] [CrossRef]
| Fermented Coffee Bean | Unfermented Coffee Bean | |||
|---|---|---|---|---|
| Geisha | Bourbon | Geisha | Bourbon | |
| Free Phenolic | ||||
| TPC (mg GAE/g) | 33.52 ± 2.31 a | 29.95 ± 1.75 b | 29.19 ± 0.74 abc | 23.82 ± 0.81 c |
| TFC (mg QE/g) | 0.42 ± 0.07 a | 0.35 ± 0.06 ab | 0.35 ± 0.08 ab | 0.25 ± 0.08 b |
| TCT (mg CE/g) | 3.49 ± 0.38 a | 3.18 ± 0.23 a | 4.91 ± 0.74 b | 4.45 ± 0.81 b |
| DPPH (mg TE/g) | 116.79 ± 0.55 a | 91.34 ± 0.23 b | 32.81 ± 2.97 c | 32.68 ± 3.20 c |
| FRAP (mg TE/g) | 110.06 ± 0.55 a | 86.83 ± 1.97 b | 62.39 ± 4.95 c | 30.99 ± 0.16 d |
| ABTS (mg TE/g) | 285.45 ± 1.77 a | 266.61 ± 3.84 a | 144.41 ± 1.94 b | 256.27 ± 1.06 ab |
| OH-RSA (mg TE/g) | 255.08 ± 3.91 a | 279.66 ± 1.18 a | 230.51 ± 3.35 ab | 196.61 ± 4.82 b |
| FICA (mg EE/g) | 0.79 ± 0.13 a | 0.77 ± 0.42 a | 0.68 ± 0.10 ab | 0.63 ± 0.29 b |
| RPA (mg TE/g) | 45.37 ± 0.61 ab | 52.95 ± 3.90 a | 29.56 ± 1.47 c | 34.16 ± 0.15 b |
| TAC (GAE mg/g) | 6.48 ± 0.28 a | 5.83 ± 0.66 b | 5.89 ± 0.27 ab | 5.20 ± 0.36 b |
| Bound Phenolic | ||||
| TPC (mg GAE/g) | 7.59 ± 0.12 a | 6.97 ± 0.08 a | 3.14 ± 0.12 b | 2.56 ± 0.15 b |
| TFC (mg QE/g) | 0.07 ± 0.01 a | 0.04 ± 0.16 a | - | - |
| TCT (mg CE/g) | - | - | - | - |
| DPPH (mg TE/g) | 13.30 ± 2.30 a | 9.79 ± 0.70 ab | 0.60 ± 0.86 b | 0.53 ± 0.15 b |
| FRAP (mg TE/g) | 12.89 ± 1.23 ab | 20.00 ± 0.78 a | 5.56 ± 0.87 b | 8.08 ± 0.12 b |
| ABTS (mg TE/g) | 75.63 ± 1.03 a | 73.62 ± 0.38 a | 41.81 ± 1.13 b | 8.89 ± 3.11 c |
| OH-RSA (mg TE/g) | 53.25 ± 1.53 a | 49.15 ± 1.38 ab | 48.31 ± 1.38 ab | 28.25 ± 1.44 b |
| FICA (mg EE/g) | 0.79 ± 0.16 ab | 0.97 ± 0.15 a | 0.78 ± 0.06 ab | 0.72 ± 0.20 b |
| RPA (mg TE/g) | 8.01 ± 0.83 a | 9.31 ± 0.05 a | 3.59 ± 0.06 b | 2.63 ± 0.54 b |
| TAC (GAE mg/g) | 0.99 ± 0.13 a | 0.94 ± 0.02 a | 0.75 ± 0.08 b | 0.81 ± 0.18 ab |
| TPC | TFC | TCT | DPPH | FRAP | ABTS·+ | ·OH | FICA | RPA | TAC | |
| TPC | 1 | |||||||||
| TFC | 0.997 * | 1 | ||||||||
| TCT | 0.918 * | 0.900 * | 1 | |||||||
| DPPH | 0.859 * | 0.873 * | 0.605 | 1 | ||||||
| FRAP | 0.918 * | 0.935 * | 0.702 | 0.966 * | 1 | |||||
| ABTS·+ | 0.917 * | 0.900 * | 0.808 | 0.876 * | 0.837 * | 1 | ||||
| ·OH | 0.986 * | 0.979 * | 0.904 * | 0.856 * | 0.905 * | 0.919 * | 1 | |||
| FICA | −0.357 | −0.348 | −0.601 | −0.061 | −0.092 | −0.290 | −0.382 | 1 | ||
| RPA | 0.953 * | 0.942 * | 0.820 | 0.908 * | 0.909 * | 0.960 * | 0.975 * | −0.279 | 1 | |
| TAC | 0.992 * | 0.985 * | 0.949 * | 0.824 | 0.884 * | 0.910 * | 0.985 * | −0.451 | 0.943 * | 1 |
| No. | Proposed Compounds | Molecular Formula | RT (min) | Ionization (ESI+/ESI−) | Molecular Weight | Theoretical (m/z) | Observed (m/z) | Error (ppm) | MS2 Product Ions | Coffee Samples |
|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic acid | ||||||||||
| Hydroxycinnamic acids | ||||||||||
| 1 | p-Coumaroyl tartaric acid | C13H12O8 | 9.185 | [M−H]- | 296.0541 | 295.0468 | 295.0470 | 0.7 | 115 | FG |
| 2 | 3-Caffeoylquinic acid | C16H18O9 | 25.788 | ** [M−H]- | 354.0942 | 353.0869 | 353.0871 | 0.6 | 253, 190, 144 | * FB, UFG |
| 3 | 5-5′-Dehydrodiferulic acid | C20H18O8 | 27.889 | [M+H]+ | 386.0999 | 387.1072 | 387.1073 | 0.3 | 369 | * FB, FG |
| Lignans | ||||||||||
| 4 | Enterolactone | C18H18O4 | 16.535 | [M+H]+ | 298.1192 | 299.1265 | 299.1263 | −0.7 | 281, 187, 165 | FG |
| 5 | 7-Oxomatairesinol | C20H20O7 | 45.824 | [M+H]+ | 372.122 | 373.1293 | 373.1297 | 1.1 | 358, 343, 328, 325 | FB |
| 6 | Schisandrol B | C23H28O7 | 89.329 | [M+H]+ | 416.1825 | 417.1898 | 417.1892 | −1.4 | 224, 193, 165 | FB |
| 7 | Schisantherin A | C30H32O9 | 86.857 | [M+H]+ | 536.2054 | 537.2127 | 537.2130 | 0.6 | 519, 415, 385, 371 | * FG, FB |
| Stilbenes | ||||||||||
| 8 | 4-Hydroxy-3,5,4′-trimethoxystilbene | C17H18O4 | 39.862 | [M+H]+ | 286.1191 | 287.1264 | 287.1264 | 0.0 | 271, 241, 225 | * FB, FG |
| Flavonoids | ||||||||||
| Flavonols | ||||||||||
| 9 | Quercetin 3-O-(6″-malonyl-glucoside) | C24H22O15 | 24.64 | [M+H]+ | 550.0956 | 551.1029 | 551.1028 | −0.2 | 303 | * FB, FG |
| 10 | Prodelphinidin dimer B3 | C30H26O14 | 34.76 | [M+H]+ | 610.1332 | 611.1405 | 611.1394 | −1.8 | 469, 311, 291 | FB |
| 11 | Quercetin 3-O-xylosyl-rutinoside | C32H38O20 | 40.43 | [M+H]+ | 742.1962 | 743.2035 | 743.2009 | −3.5 | 479, 317 | FG |
| Flavones | ||||||||||
| 12 | Gardenin B | C19H18O7 | 88.235 | [M+H]+ | 358.1066 | 359.1139 | 359.1148 | 2.5 | 344, 329, 311 | FB |
| Anthocyanins | ||||||||||
| 13 | Pelargonidin 3-O-rutinoside | C27H31O14 | 21.545 | [M+H]+ | 579.1701 | 580.1774 | 580.1775 | 0.2 | 271, 433 | * FB, FG |
| Isoflavonoids | ||||||||||
| 14 | 6″-O-Malonylgenistin | C24H22O13 | 4.081 | [M+H]+ | 518.107 | 519.1143 | 519.1118 | −4.8 | 271 | UFG |
| 15 | 6″-O-Acetylglycitin | C24H24O11 | 10.044 | [M+H]+ | 488.1327 | 489.14 | 489.1397 | −0.6 | 285, 270 | UFG |
| Other polyphenols | ||||||||||
| Hydroxycoumarins | ||||||||||
| 16 | Esculin | C15H16O9 | 26.85 | [M+H]+ | 340.0794 | 341.0867 | 341.0855 | −3.5 | 179, 151 | FG |
| 17 | Coumarin | C9H6O2 | 24.106 | [M+H]+ | 146.0369 | 147.0442 | 147.0442 | 0.0 | 103, 91 | * UFG, FG |
| Furanocoumarins | ||||||||||
| 18 | Isopimpinellin | C13H10O5 | 80.679 | [M+H]+ | 246.0539 | 247.0612 | 247.0611 | −0.4 | 232, 217, 205, 203 | * FG, FB |
| No. | Compound Name | Molecular Formula | RT (mins) | Aroma | Content (mg/g) | |||
|---|---|---|---|---|---|---|---|---|
| Fermented | Unfermented | |||||||
| Geisha | Bourbon | Geisha | Bourbon | |||||
| Phenols | ||||||||
| 1 | Phenol | C6H6O | 31.02 | Phenolic/Rubbery | 0.07 ± 0.01 | 0.10 ± 0.03 | 0.05 ± 0.03 | 0.09 ± 0.01 |
| 2 | 1,6-Octadien-3-ol, 3,7-dimethyl- (linalool) | C10H18O2 | 20.97 | Citrus/Floral/Woody/Green | 0.09 ± 0.03 | 0.05 ± 0.03 | 0.07 ± 0.03 | 0.03 ± 0.01 |
| 3 | 2,3-Butanediol | C4H10O2 | 21.43 | Fruity/Creamy/Buttery | 0.41 ± 0.09 | 0.23 ± 0.12 | 0.20 ± 0.02 | 0.12 ± 0.19 |
| Pyrazines | ||||||||
| 4 | Pyrazine | C4H4N2 | 11.36 | Nutty/Roasted | 1.25 ± 0.17 | 1.32 ± 0.44 | 1.21 ± 0.06 | 1.11 ± 0.33 |
| 5 | Pyrazine, 2,5-dimethyl- | C6H8N2 | 14.76 | Nutty/Peanut/Musty/Earthy | 0.10 ± 0.27 | 0.93 ± 0.20 | 0.99 ± 0.36 | 0.64 ± 0.01 |
| 6 | Pyrazine, 2,3-dimethyl- | C6H8N2 | 15.44 | Butter/Coffee/Caramellic/Roasted | 0.33 ± 0.04 | 0.27 ± 0.03 | 0.25 ± 0.01 | 0.16 ± 0.06 |
| 7 | Pyrazine, 2-ethyl-5-methyl- | C7H10N2 | 16.75 | Coffee/Roasted/Nutty | 0.42 ± 0.09 | 0.41 ± 0.10 | 0.38 ± 0.15 | 0.28 ± 0.02 |
| 8 | Pyrazine, 2,6-diethyl- | C8H12ON2 | 17.93 | Nutty/Hazelnut | 0.06 ± 0.01 | 0.06 ± 0.02 | 0.04 ± 0.02 | 0.04 ± 0.01 |
| Acid and esters | ||||||||
| 9 | Acetic acid | C2H4O2 | 18.24 | Sour/Overripe fruit | 9.80 ± 2.61 | 8.31 ± 0.93 | 9.13 ± 0.55 | 7.73 ± 1.00 |
| 10 | Butanoic acid, 3-methyl- | C5H10O2 | 23.83 | Mentholic/Fruity | 1.18 ± 0.17 | 1.32 ± 0.44 | 1.21 ± 0.06 | 1.34 ± 0.33 |
| 11 | Propanoic acid | C3H6O2 | 20.61 | Acidic/Cheesy/Vinegar/Oniony | 0.26 ± 0.01 | 0.34 ± 0.03 | 0.35 ± 0.01 | - |
| 12 | 2-Butenoic acid, 3-methyl- | C5H8O2 | 26.67 | Green/Phenolic/Dairy | 0.28 ± 0.06 | 0.24 ± 0.06 | 0.28 ± 0.02 | 0.32 ± 0.11 |
| Furan and Furanic compounds | ||||||||
| 13 | Furfural | C5H4O2 | 18.56 | Bready/Woody/Baked | 4.57 ± 0.13 | 5.34 ± 0.67 | 4.11 ± 0.92 | 5.25 ± 1.35 |
| 14 | 2-Furanmethanol | C5H6O2 | 23.62 | Sweet/Caramellic/Bready/Coffee | 5.02 ± 0.37 | 4.97 ± 0.52 | 4.52 ± 0.40 | 4.86 ± 0.92 |
| 15 | 2-Furancarboxaldehyde, 5-methyl- | C6H6O2 | 21.37 | Caramellic/Spice/Maple/Bready | 2.70 ± 0.41 | 3.28 ± 0.56 | 2.25 ± 0.47 | 3.14 ± 0.93 |
| Ketones | ||||||||
| 16 | Butyrolactone | C4H6O2 | 22.65 | Creamy/Oily/Caramellic | 0.58 ± 0.13 | 0.55 ± 0.04 | 0.50 ± 0.01 | 0.44 ± 0.11 |
| 17 | 2-Cyclopenten-1-one, 3-ethyl-2-hydroxy- | C7H10O2 | 28.86 | Caramellic/Maple/Brown/Toasted | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.07 ± 0.01 |
| Other compounds | ||||||||
| 18 | Anethole | C10H12O | 27.41 | Licorice/Anise/Spice/Sweet | 0.95 ± 0.13 | 1.05 ± 0.16 | 0.80 ± 0.10 | 0.99 ± 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.; Wu, H.; Shi, L.; Barrow, C.; Dunshea, F.R.; Suleria, H.A.R. Impacts of Fermentation on the Phenolic Composition, Antioxidant Potential, and Volatile Compounds Profile of Commercially Roasted Coffee Beans. Fermentation 2023, 9, 918. https://doi.org/10.3390/fermentation9100918
Tan Y, Wu H, Shi L, Barrow C, Dunshea FR, Suleria HAR. Impacts of Fermentation on the Phenolic Composition, Antioxidant Potential, and Volatile Compounds Profile of Commercially Roasted Coffee Beans. Fermentation. 2023; 9(10):918. https://doi.org/10.3390/fermentation9100918
Chicago/Turabian StyleTan, Yuanyuan, Hanjing Wu, Linghong Shi, Colin Barrow, Frank R. Dunshea, and Hafiz A. R. Suleria. 2023. "Impacts of Fermentation on the Phenolic Composition, Antioxidant Potential, and Volatile Compounds Profile of Commercially Roasted Coffee Beans" Fermentation 9, no. 10: 918. https://doi.org/10.3390/fermentation9100918
APA StyleTan, Y., Wu, H., Shi, L., Barrow, C., Dunshea, F. R., & Suleria, H. A. R. (2023). Impacts of Fermentation on the Phenolic Composition, Antioxidant Potential, and Volatile Compounds Profile of Commercially Roasted Coffee Beans. Fermentation, 9(10), 918. https://doi.org/10.3390/fermentation9100918