Prospective Life Cycle Assessment of Microbial Sophorolipid Fermentation
Abstract
:1. Introduction
2. Materials and Methods
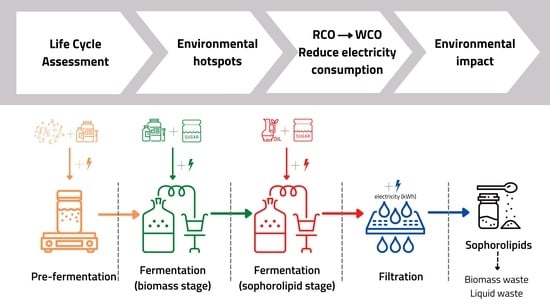
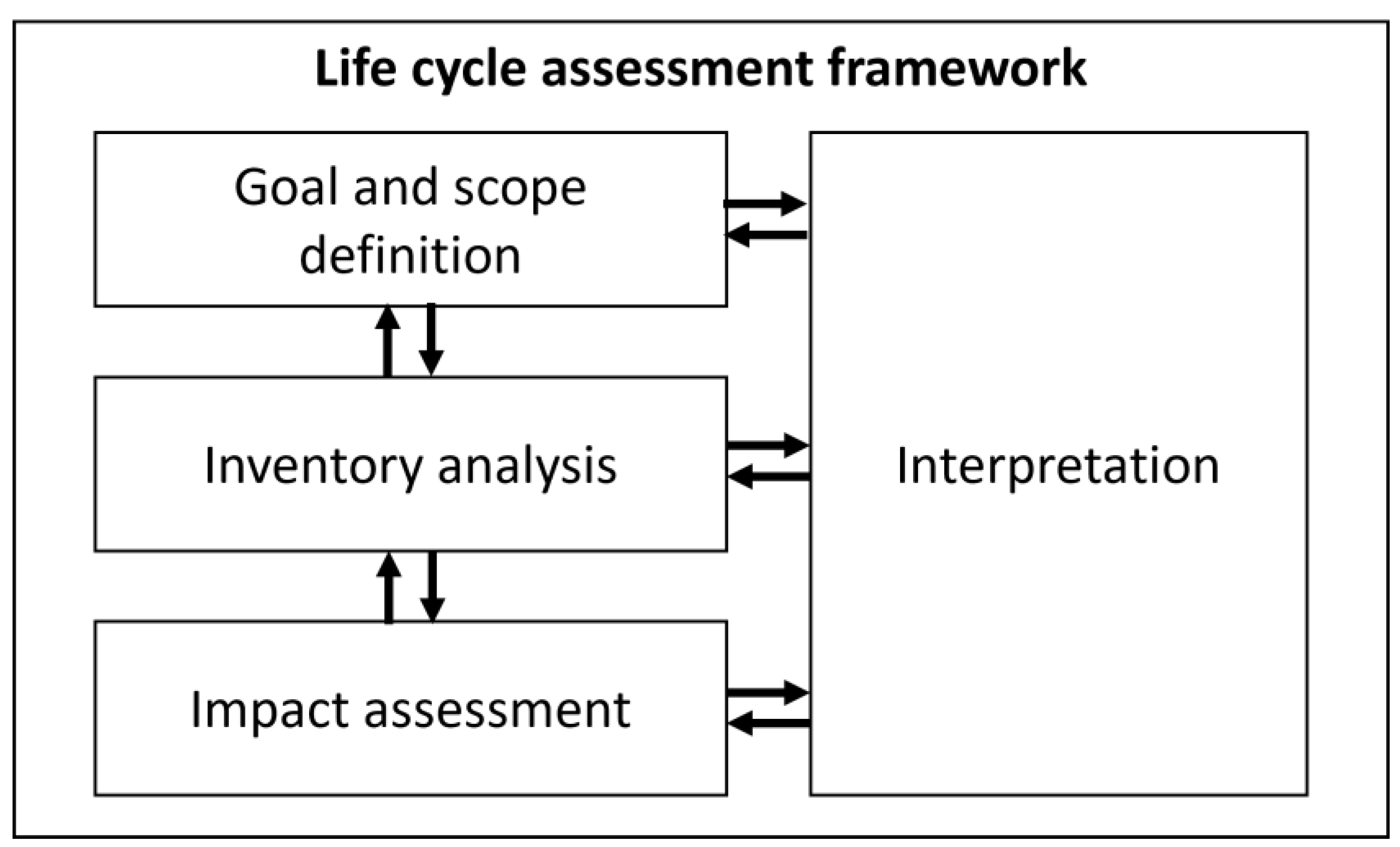
2.1. Life Cycle Assessment
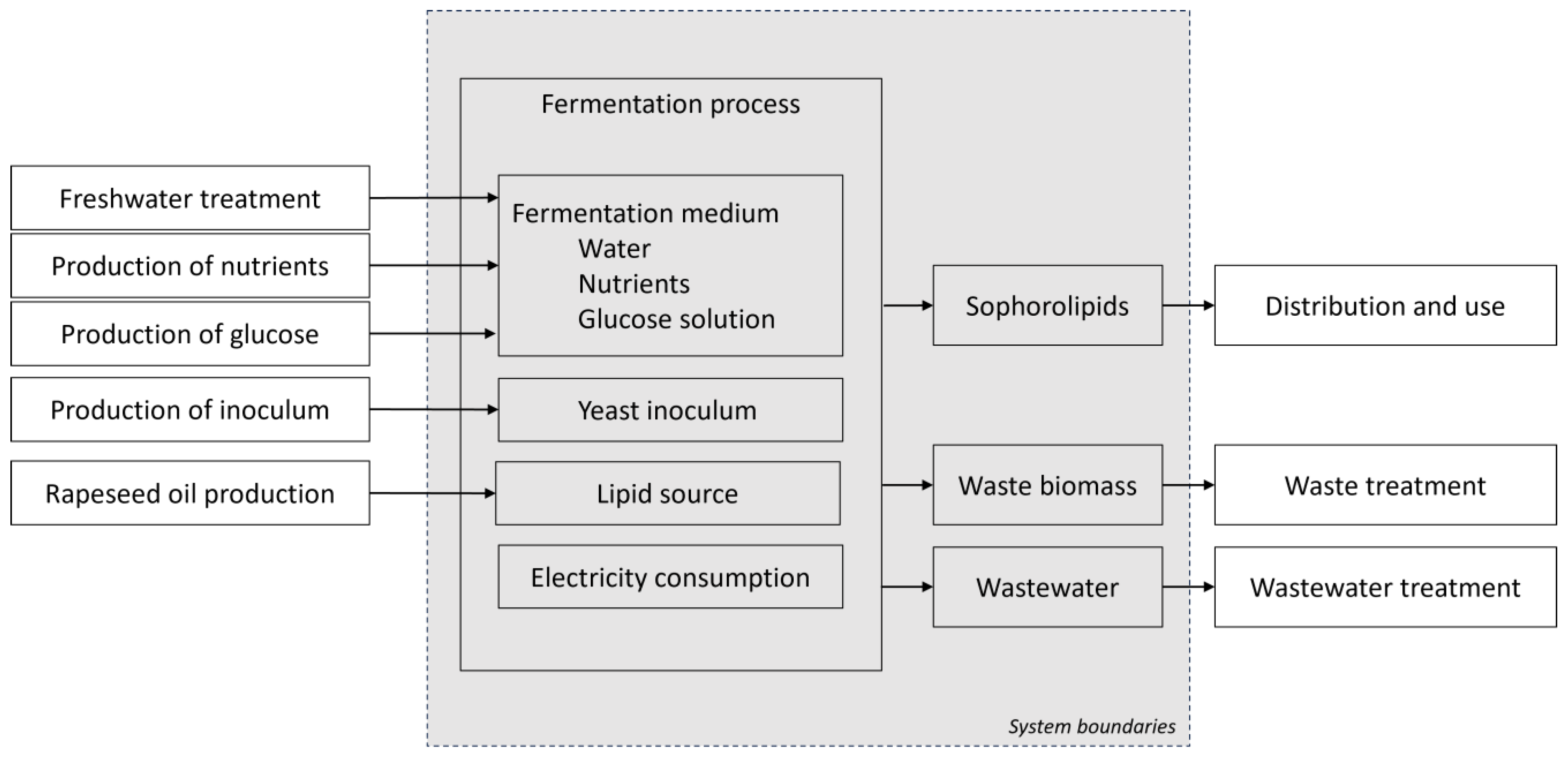
2.2. Goal and Scope Definition
2.3. Life Cycle Inventory Analysis
2.3.1. Yeast Strain and Medium
2.3.2. Lab-Scale Fed-Batch Fermentation
2.3.3. Quantitative Measurements of Biomass, Sophorolipids, and Residual Oil
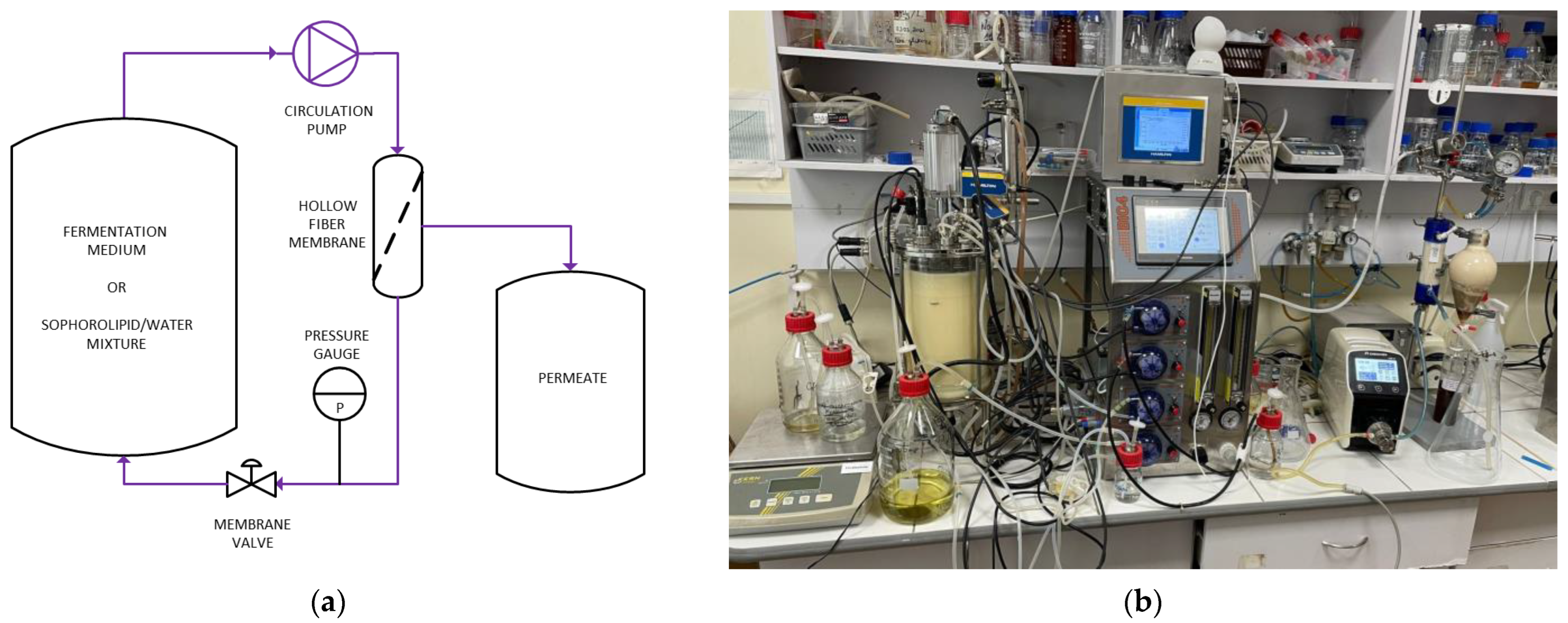
2.3.4. Separation of Biomass and Sophorolipids
2.4. Scenario Development
3. Life Cycle Impact Assessment and Interpretation
3.1. Environmental Hotspot Analysis
3.2. Solutions to Reduce Environmental Impact
4. Limitations and Future Research Prospects
5. Research Highlights and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waites, M.J.; Morgan, N.L.; Rockey, J.S.; Higton, G. Industrial Microbiology: An Introduction; Wiley-Blackwell: Hoboken, NJ, USA, 2013; ISBN 978-1-118-68739-0. [Google Scholar]
- Kosaric, N.; Sukan, F.V. (Eds.) Biosurfactants Production and Utilization—Processes, Technologies, and Economics; Taylor & Francis Group: Abingdon, UK, 2001; ISBN 978-0-632-05307-0. [Google Scholar]
- Liepins, J.; Balina, K.; Soloha, R.; Berzina, I.; Lukasa, L.K.; Dace, E. Glycolipid Biosurfactant Production from Waste Cooking Oils by Yeast: Review of Substrates, Producers and Products. Fermentation 2021, 7, 136. [Google Scholar] [CrossRef]
- Sarubbo, L.A.; Maria da Gloria, C.S.; Durval, I.J.B.; Bezerra, K.G.O.; Ribeiro, B.G.; Silva, I.A.; Twigg, M.S.; Banat, I.M. Biosurfactants: Production, Properties, Applications, Trends, and General Perspectives. Biochem. Eng. J. 2022, 181, 108377. [Google Scholar] [CrossRef]
- Markande, A.R.; Patel, D.; Varjani, S. A Review on Biosurfactants: Properties, Applications and Current Developments. Bioresour. Technol. 2021, 330, 124963. [Google Scholar] [CrossRef] [PubMed]
- Kopsahelis, A.; Kourmentza, C.; Zafiri, C.; Kornaros, M. Gate-to-Gate Life Cycle Assessment of Biosurfactants and Bioplasticizers Production via Biotechnological Exploitation of Fats and Waste Oils. J. Chem. Technol. Biotechnol. 2018, 93, 2833–2841. [Google Scholar] [CrossRef]
- Baccile, N.; Babonneau, F.; Banat, I.M.; Ciesielska, K.; Cuvier, A.S.; Devreese, B.; Everaert, B.; Lydon, H.; Marchant, R.; Mitchell, C.A.; et al. Development of a Cradle-to-Grave Approach for Acetylated Acidic Sophorolipid Biosurfactants. ACS Sustain. Chem. Eng. 2017, 5, 1186–1198. [Google Scholar] [CrossRef]
- Almeida, D.; Da Silva, R.S.; Brasileiro, P.; Luna, J.; Silva, M.D.G.; Rufino, R.; Costa, A.; Sarubbo, L. Application of a Biosurfactant from Candida Tropicalis Ucp 0996 Produced in Low-Cost Substrates for Hydrophobic Contaminants Removal. Chem. Eng. Trans. 2018, 64, 541–546. [Google Scholar] [CrossRef]
- Briem, A.K.; Bippus, L.; Oraby, A.; Noll, P.; Zibek, S.; Albrecht, S. Environmental Impacts of Biosurfactants from a Life Cycle Perspective: A Systematic Literature Review. In Advances in Biochemical Engineering/Biotechnology; Springer Science and Business Media Deutschland GmbH: Berlin, Germany, 2022; Volume 181, pp. 235–269. [Google Scholar]
- Jala, R.C.R.; Vudhgiri, S.; Kumar, C.G. A Comprehensive Review on Natural Occurrence, Synthesis and Biological Activities of Glycolipids. Carbohydr. Res. 2022, 516, 108556. [Google Scholar] [CrossRef]
- Schonhoff, A.; Ihling, N.; Schreiber, A.; Zapp, P. Environmental Impacts of Biosurfactant Production Based on Substrates from the Sugar Industry. ACS Sustain. Chem. Eng. 2022, 10, 9345–9358. [Google Scholar] [CrossRef]
- Standard ISO 14040; Environmental Management—Life Cycle Assessment—Principles and Framework, 2nd ed. International Standard Organization: Geneva, Switzerland, 2006.
- Delpierre, M.; Quist, J.; Mertens, J.; Prieur-Vernat, A.; Cucurachi, S. Assessing the Environmental Impacts of Wind-Based Hydrogen Production in the Netherlands Using Ex-Ante LCA and Scenarios Analysis. J. Clean. Prod. 2021, 299, 126866. [Google Scholar] [CrossRef]
- Gálvez-Martos, J.L.; Greses, S.; Magdalena, J.A.; Iribarren, D.; Tomás-Pejó, E.; González-Fernández, C. Life Cycle Assessment of Volatile Fatty Acids Production from Protein- and Carbohydrate-Rich Organic Wastes. Bioresour. Technol. 2021, 321, 124528. [Google Scholar] [CrossRef]
- Saavedra del Oso, M.; Mauricio-Iglesias, M.; Hospido, A.; Steubing, B. Prospective LCA to Provide Environmental Guidance for Developing Waste-to-PHA Biorefineries. J. Clean. Prod. 2023, 383, 135331. [Google Scholar] [CrossRef]
- Cucurachi, S.; Steubing, B.; Siebler, F.; Navarre, N.; Caldeira, C.; Sala, S. Prospective LCA Methodology for Novel and Emerging Technologies for BIO-Based Products; Publications Office of the European Union: Luxembourg, 2022. [Google Scholar]
- Thonemann, N.; Schulte, A.; Maga, D. How to Conduct Prospective Life Cycle Assessment for Emerging Technologies? A Systematic Review and Methodological Guidance. Sustainability 2020, 12, 1192. [Google Scholar] [CrossRef]
- Hu, X.; Subramanian, K.; Wang, H.; Roelants, S.L.K.W.; To, M.H.; Soetaert, W.; Kaur, G.; Lin, C.S.K.; Chopra, S.S. Guiding Environmental Sustainability of Emerging Bioconversion Technology for Waste-Derived Sophorolipid Production by Adopting a Dynamic Life Cycle Assessment (DLCA) Approach. Environ. Pollut. 2021, 269, 116101. [Google Scholar] [CrossRef]
- Ögmundarson, Ó.; Herrgård, M.J.; Forster, J.; Hauschild, M.Z.; Fantke, P. Addressing Environmental Sustainability of Biochemicals. Nat. Sustain. 2020, 3, 167–174. [Google Scholar] [CrossRef]
- Yeo, J.; Chopra, S.S.; Zhang, L.; An, A.K. Life Cycle Assessment (LCA) of Food Waste Treatment in Hong Kong: On-Site Fermentation Methodology. J. Environ. Manag. 2019, 240, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Gaur, V.K.; Sharma, P.; Sirohi, R.; Varjani, S.; Taherzadeh, M.J.; Chang, J.S.; Yong Ng, H.; Wong, J.W.C.; Kim, S.H. Production of Biosurfactants from Agro-Industrial Waste and Waste Cooking Oil in a Circular Bioeconomy: An Overview. Bioresour. Technol. 2022, 343, 126059. [Google Scholar] [CrossRef] [PubMed]
- Stalidzans, E.; Dace, E. Sustainable Metabolic Engineering for Sustainability Optimisation of Industrial Biotechnology. Comput. Struct. Biotechnol. J. 2021, 19, 4770–4776. [Google Scholar] [CrossRef] [PubMed]
- Standard ISO 14044; Environmental Management—Life Cycle Assessment—Requirements and Guidelines, 1st ed. International Standard Organization: Geneva, Switzerland, 2006.
- Pré Sustainability. SimaPro Database Manual: Methods Library; Pré Sustainability: Amersfoort, The Netherlands, 2020; p. 98. [Google Scholar]
- Ekvall, T.; Assefa, G.; Björklund, A.; Eriksson, O.; Finnveden, G. What Life-Cycle Assessment Does and Does Not Do in Assessments of Waste Management. Waste Manag. 2007, 27, 989–996. [Google Scholar] [CrossRef]
- Schrijvers, D.; Loubet, P.; Sonnemann, G. Consistent Allocation Using Archetypes of LCA Goal and Scope Definitions. Sustainability 2020, 12, 5587. [Google Scholar] [CrossRef]
- Fridrihsone, A. Life Cycle Assessment of Polyol Monomers for Polyurethane; RTU Press: Riga, Latvia, 2020. [Google Scholar]
- Kim, Y.B.; Yun, H.S.; Kim, E.K. Enhanced Sophorolipid Production by Feeding-Rate-Controlled Fed-Batch Culture. Bioresour. Technol. 2009, 100, 6028–6032. [Google Scholar] [CrossRef]
- Aru, O.O.; Ikechukwu, O. Biodegradation Life Cycle Assessment of the Environmental Impact of Biosurfactant Production from Oil Waste by a Diculture of Azotobacter Vinelandii And. J. Bioremedation Biodegrad. 2018, 9, 435. [Google Scholar] [CrossRef]
- Gronlund, C.J.; Humbert, S.; Shaked, S.; O’Neill, M.S.; Jolliet, O. Characterizing the Burden of Disease of Particulate Matter for Life Cycle Impact Assessment. Air Qual. Atmos. Health 2015, 8, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Meesters, K.P.H.; Corré, W.J.; Conijn, J.G.; Patel, M.K.; Bos, H.L. Sustainability Aspects of Biobased Products: Comparison of Different Crops and Products from the Vegetable Oil Platform; Food & Biobased Research: Wageningen, The Netherlands, 2012; ISBN 9789461739827. [Google Scholar]
- Liepins, J.; Lukasa, L.K.; Berzina, I. Local Waste Oil Valorisation for Sophorolipid Synthesis Using Yeast Starmerella Bombicola. In Proceedings of the Book of Abstracts International Conference Biosurfactants 2022, Virtual, 21–22 October 2022; p. 99. [Google Scholar]
- Kaur, H.; Wang, L.; Stawniak, N.; Sloan, R.; van Erp, H.; Eastmond, P.; Bancroft, I. The Impact of Reducing Fatty Acid Desaturation on the Composition and Thermal Stability of Rapeseed Oil. Plant Biotechnol. J. 2020, 18, 983–991. [Google Scholar] [CrossRef]
- Ashby, R.D.; Solaiman, D.K.Y.; Foglia, T.A. Property Control of Sophorolipids: Influence of Fatty Acid Substrate and Blending. Biotechnol. Lett. 2008, 30, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Jurjevic, M.; Badia, D. Utilization of Restaurant Waste Oil as a Precursor for Sophorolipid Production. Biotechnol. Prog. 2007, 23, 512–515. [Google Scholar] [CrossRef]



| Key Parameter | Unit | Value | Data Source | |
|---|---|---|---|---|
| Pre-fermentation | INPUT | |||
| Yeast inoculum | g | 100 | New process—Yeast inoculum | |
| Fermentation medium | g | 1900 | New process—Fermentation medium | |
| Length of process | h | 24 | ||
| Power usage | kW | 0.16 | ecoinvent v3.8, Medium voltage, LV electricity mix | |
| OUTPUT | ||||
| Pre-fermented substrate | kg | 2 | ||
| Biomass growth stage (A) | INPUT | |||
| Pre-fermented substrate | kg | 2 | ||
| Fermentation medium | kg | 1 | New process—Fermentation medium | |
| Length of process | h | 48 | ||
| Power usage | kW | 0.3 | ecoinvent v3.8, Medium voltage, LV electricity mix | |
| OUTPUT | ||||
| Fermented biomass A | kg | 2.1 | ||
| Sophorolipid production stage (B) | INPUT | |||
| Fermented biomass A | kg | 2.1 | ||
| Rapeseed oil (RCO) | kg | 0.4 | New process—Rapeseed oil [23] | |
| Glucose solution | kg | 0.24 | New process—Glucose solution | |
| Length of process | h | 161 | ||
| Power usage | kW | 0.42 | ecoinvent v3.8, Medium voltage, LV electricity mix | |
| OUTPUT | ||||
| Fermented substrate B | kg | 2.74 | ||
| Downstream processing | INPUT | |||
| Fermented substrate B | kg | 2.74 | ||
| Electricity consumption | kWh | 0.3 | ecoinvent v3.8, Medium voltage, LV electricity mix | |
| OUTPUT | ||||
| Sophorolipids | g | 537.9 | ||
| Water + salt residuals | kg | 2.17 | ecoinvent v3.8, Wastewater from ADof whey {GLO} | |
| Biomass | g | 32.3 |
| Data | Unit | Value | Data Source |
|---|---|---|---|
| Input | |||
| Yeast (inoculum) | g | 10 | Not included in study |
| Glucose | g | 30 | ecoinvent v3.8, Glucose {GLO} |
| Yeast extract | g | 5 | Not included in study |
| KH2PO4 | g | 1 | ecoinvent v3.8, Assumed as Chemical, inorganic {GLO} |
| MgSO4 × 7H2O | g | 0.5 | ecoinvent v3.8, Assumed as Magnesium sulfate {GLO} |
| CaCl2 × 2H2O | g | 0.1 | ecoinvent v3.8, Assumed as Calcium chloride {GLO} |
| NaCl | g | 0.1 | ecoinvent v3.8, Sodium chloride, powder {GLO} |
| Meat peptone | g | 0.7 | Not included in study |
| Distilled H2O | g | 952.6 | New process—Distilled water |
| Electricity | kWh | 28.8 | ecoinvent v3.8, Medium voltage, LV electricity mix |
| Output | |||
| Inoculum | g | 1000 | Assumed that 1 L = 1000 g |
| Data | Unit | Value | Data Source |
|---|---|---|---|
| Input | |||
| Glucose | g | 400 | ecoinvent v3.8, Glucose {GLO}| |
| Distilled water | g | 560 | New process—Distilled water |
| Output | |||
| Glucose solution | g | 1000 | Assumed that 1 L = 1000 g |
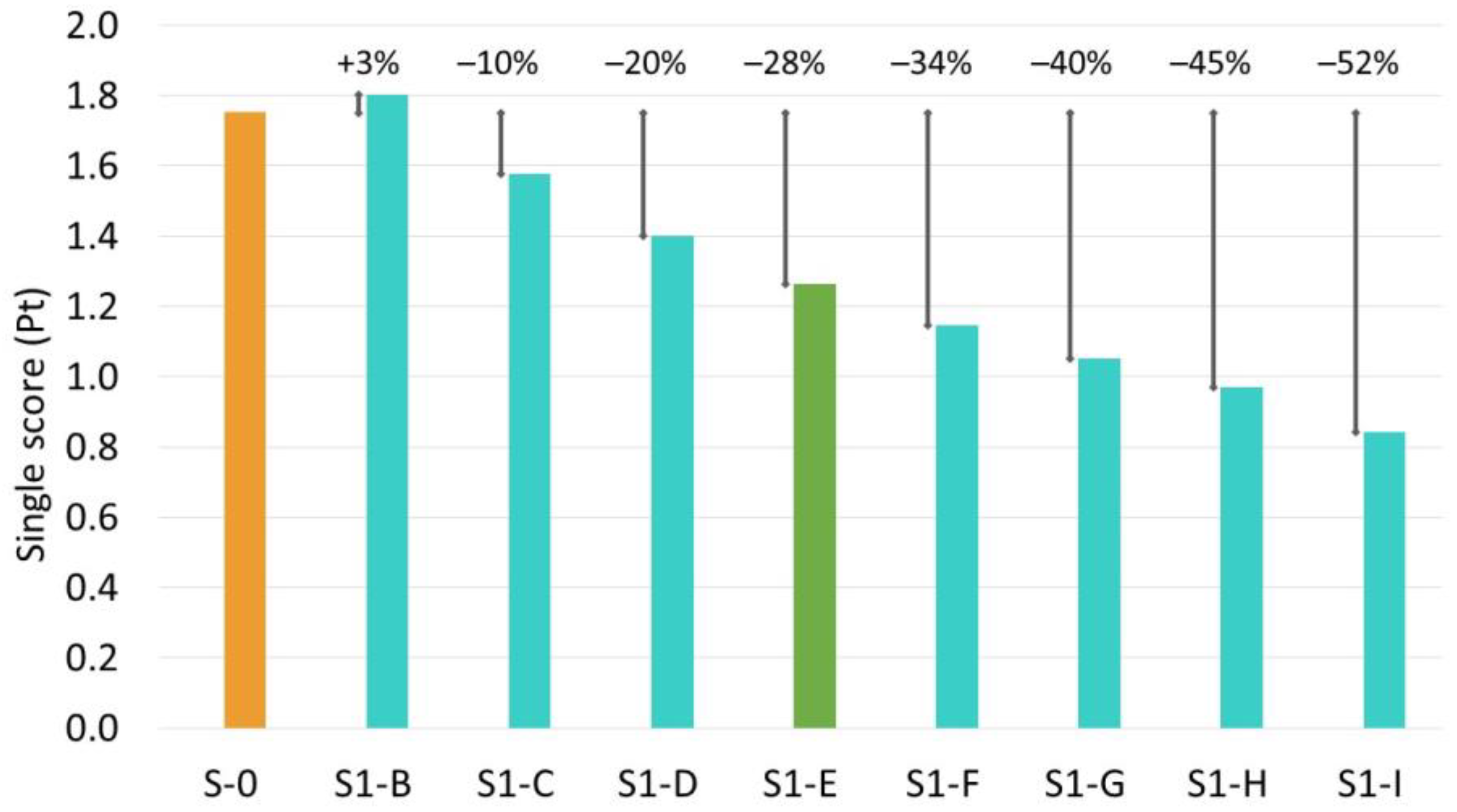
| Scenario | S-0 | S1-B | S1-C | S1-D | S1-E | S1-F | S1-G | S1-H | S1-I |
|---|---|---|---|---|---|---|---|---|---|
| Type of substrate | RCO | WCO | WCO | WCO | WCO | WCO | WCO | WCO | WCO |
| Sophorolipid titre compared to the titre in base scenario | Base scenario | −30% | −20% | −10% | 0% | 10% | 20% | 30% | 50% |
| SL titre (g L−1) | 196.3 | 137.4 | 157.1 | 176.7 | 196.3 | 215.9 | 235.6 | 255.2 | 294.5 |
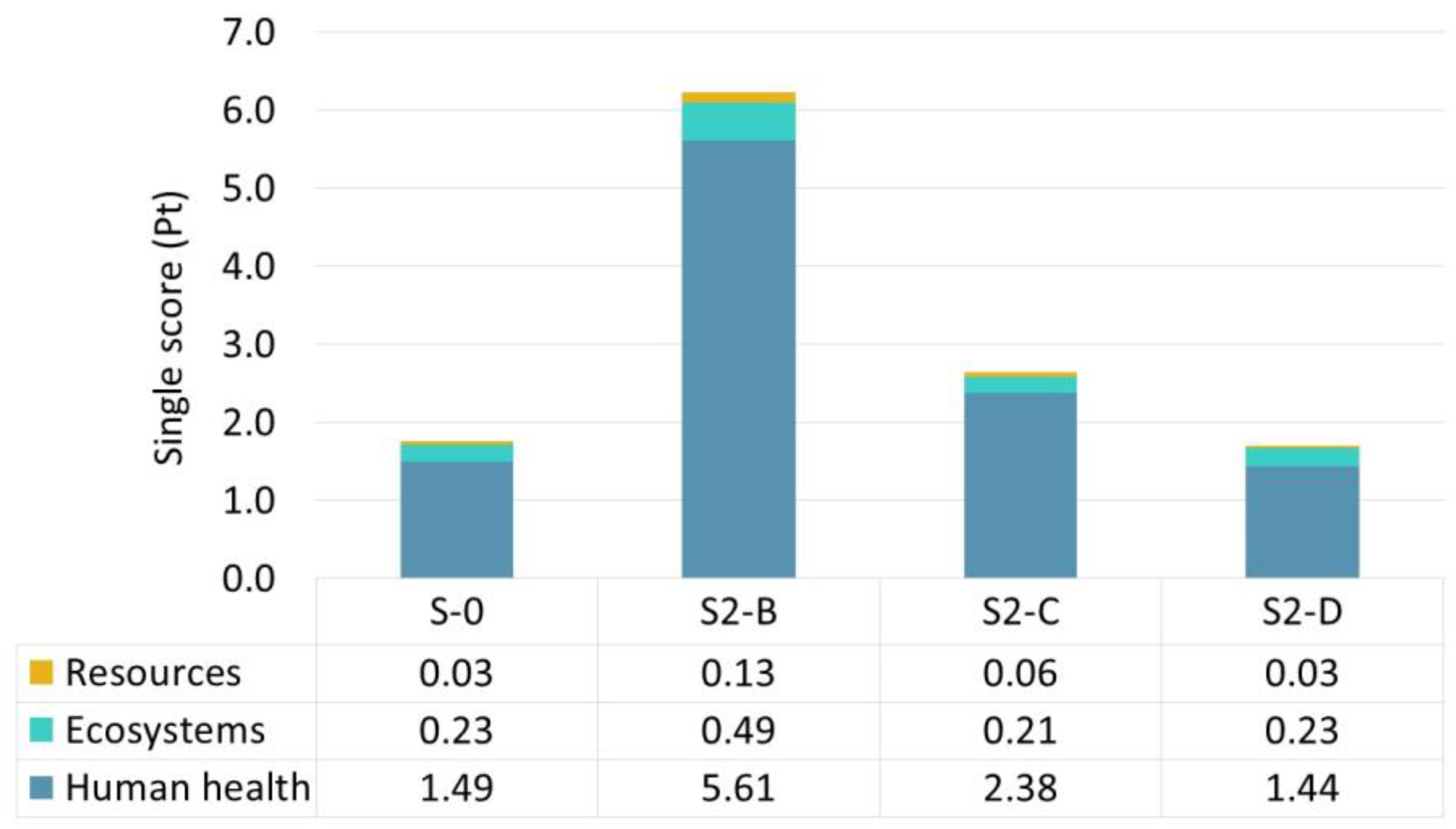
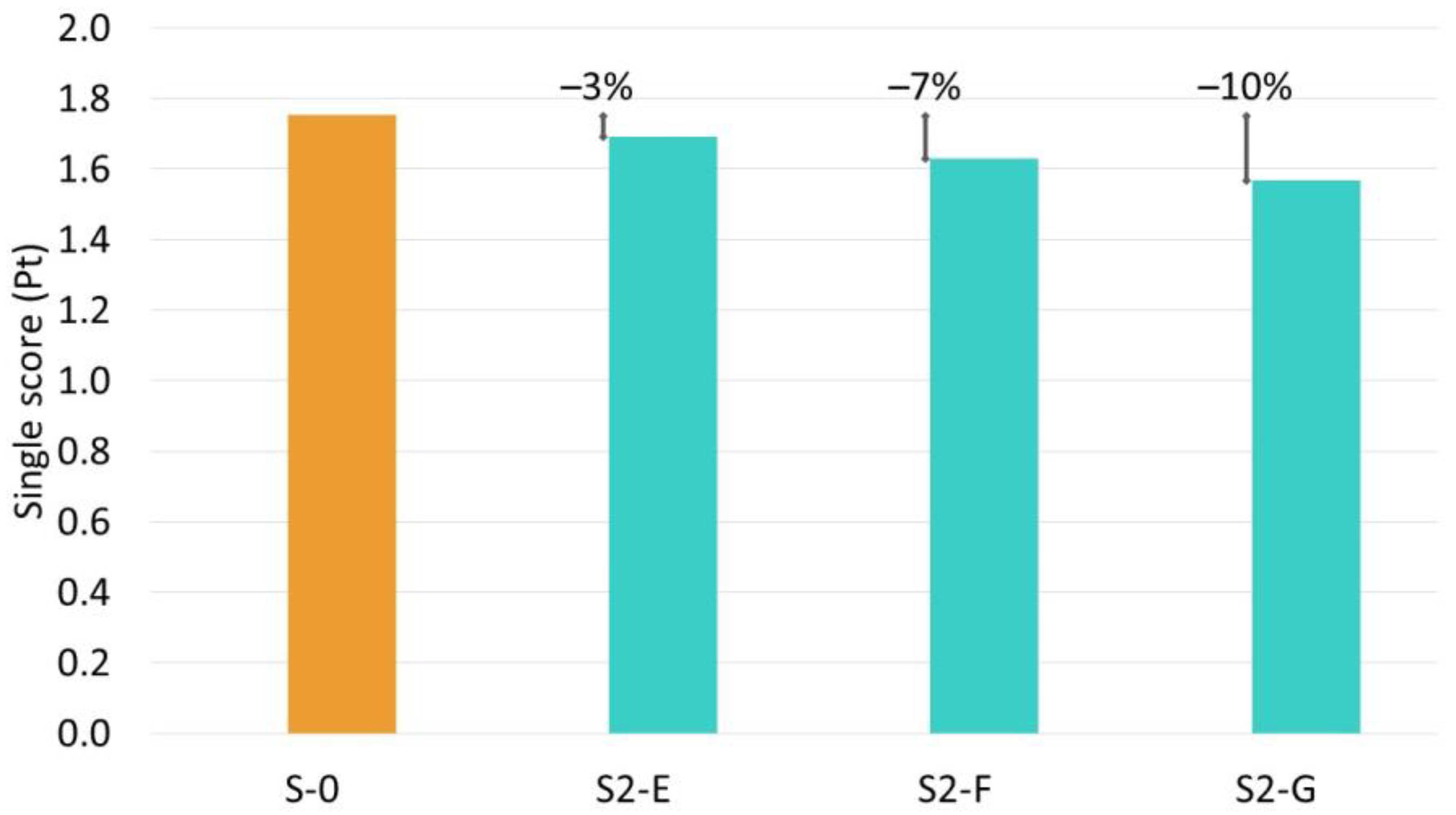
| Scenario | S-0 | S2-B | S2-C | S2-D | S2-E | S2-F | S2-G |
|---|---|---|---|---|---|---|---|
| Biomass growth stage | |||||||
| Glucose solution (kg) | 0 | 1.14↑ | 1.14↑ | N/A | 0 | 0 | 0 |
| Length of the process (h) | 48 | 138↑ | 138↑ | N/A | 48 | 48 | 48 |
| Power usage (kW) | 0.3 | 0.3 | 0.3 | N/A | 0.3 | 0.3 | 0.3 |
| Sophorolipid production stage | |||||||
| Glucose solution (kg) | 0.24 | 0.36↑ | 0.36↑ | N/A | 0.24 | 0.24 | 0.24 |
| Oil (kg) | 0.4 | 0.36↓ | 0.36↓ | N/A | 0.4 | 0.4 | 0.4 |
| Length of the process (h) | 161 | 130↓ | 130↓ | N/A | 161 | 161 | 161 |
| Power usage (kW) | 0.42 | 0.42 | 0.42 | N/A | 0.42 | 0.42 | 0.42 |
| Electricity consumption compared to base scenario (%) | 0% | N/A | N/A | +10%↑ | −5%↓ | −10%↓ | −15%↓ |
| Total volume of the fermentation substrate (L) | 2.74 | N/A | N/A | 3.99↑ | N/A | N/A | N/A |
| SL titre (g L−1) | 196.3 | 83.2↓ | 196.1 | 196.1 | 196.1 | 196.1 | 196.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balina, K.; Soloha, R.; Suleiko, A.; Dubencovs, K.; Liepins, J.; Dace, E. Prospective Life Cycle Assessment of Microbial Sophorolipid Fermentation. Fermentation 2023, 9, 839. https://doi.org/10.3390/fermentation9090839
Balina K, Soloha R, Suleiko A, Dubencovs K, Liepins J, Dace E. Prospective Life Cycle Assessment of Microbial Sophorolipid Fermentation. Fermentation. 2023; 9(9):839. https://doi.org/10.3390/fermentation9090839
Chicago/Turabian StyleBalina, Karina, Raimonda Soloha, Arturs Suleiko, Konstantins Dubencovs, Janis Liepins, and Elina Dace. 2023. "Prospective Life Cycle Assessment of Microbial Sophorolipid Fermentation" Fermentation 9, no. 9: 839. https://doi.org/10.3390/fermentation9090839
APA StyleBalina, K., Soloha, R., Suleiko, A., Dubencovs, K., Liepins, J., & Dace, E. (2023). Prospective Life Cycle Assessment of Microbial Sophorolipid Fermentation. Fermentation, 9(9), 839. https://doi.org/10.3390/fermentation9090839